Abstract
A new species of caligid copepod, Caligus adanensis sp. nov., is described based on one male and three female specimens collected from the body surface of garfish, Belone belone (Linnaeus, 1760), caught in the Mediterranean Sea, off the south-eastern coast of Turkey. The new species belongs in the Caligus macarovi group of species as established by Boxshall and Gurney (1980) and recently updated in Boxshall (2018), as it shares the following set of characters: (i) leg 4 with 2-segmented exopod, carrying only three apical spines on distal segment; (ii) distal exopodal segment of leg 1 bearing three plumose setae posteriorly plus four distal elements, with spine 1 naked, spines 2 and 3 each with accessory process, spines 1 to 3 of similar length, and seta 4 about twice the length of the others; (iii) females with a 1-segmented abdomen but males with a 2-segmented abdomen; (iv) male maxilliped with a myxal process opposing the tip of the subchela; (v) maxilla with spinules on the posterodistal margin of the brachium; and (vi) unisensillate papillae associated with the post-antennal process. The new species differs from other members of the group by a combination of characteristics that include (1) a large, prominent cuticular swelling present near the basal segment of the female antenna; (2) antenna with posterior process on the proximal segment; (3) the sternal furca has long, slightly divergent tines that are slightly expanded and incurved at the tip; (4) the second endopodal segment of leg 3 carries a small subtriangular, papilliform process on its ventral surface; (5) the male maxilliped has a prominent bilobate myxal process bearing corrugations on the surface of the larger lobe and two unequal, spiniform projections on the subchela in addition to the proximal seta at the base of the claw; and (6) body proportions of the new species differs from its congeners.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The parasitic copepod family Caligidae Burmeister, 1835 (Copepoda: Siphonostomatoida) currently comprises a total of 505 valid species belonging to 30 valid genera (Table 1). All caligid species are referred to as “sea lice” and some are known to cause significant commercial losses in marine finfish aquaculture (Johnson et al. 2004). Among the 30 caligid genera, Caligus O. F. Müller, 1785 contains approximately 53% of the valid species in the family and it is the most speciose taxon of parasitic copepods on marine fishes. The genus currently comprises 268 valid species (Rodrigues et al. 2018).
Garfish, Belone belone (Linnaeus, 1760), is an economically important fish species in the Mediterranean due to its high market value (~ 10–12 €/kg). This epipelagic fish is widespread throughout the Mediterranean Sea, Black Sea, Baltic Sea, Sea of Azov, and northeast Atlantic waters. Early studies on the egg and larval development of B. belone revealed that the newly hatched larvae are excellent experimental organisms for rearing trials, as they accept a large variety of food, and exhibit rapid growth and high survival rates under laboratory conditions (Rosenthal and Folds 1973; Westernhagen 1974). In recent years, studies have been published on the determination of the reproductive cycle, diet composition, age, growth, and mortality of garfish (Sever et al. 2009; Zorica et al. 2011; Zorica and Čikeš Keč 2013; Çoker et al. 2013). Garfish can be considered as one of the most promising species for future marine aquaculture and the successful commercialization of garfish farming will require improved knowledge of diseases and the development of parasite and disease management strategies. In this study, a new species of Caligus is described based on specimens collected from the body surface of garfish caught in Iskenderun Bay, in the north-eastern Mediterranean Sea, off the Turkish coast.
Materials and methods
Five individuals of Belone belone (total body length 62–80 cm) caught using a rod and reel by one of us (Y. S.) on 15 November 2017 in north-eastern Mediterranean waters, off Yumurtalık, Iskenderun Bay, Turkey (Fig. 1), were examined for copepods. Water depth and coordinates of the sampling area were determined by the GPS Aquameter (Aquaread, UK), salinity was measured using a refractometer (Atago, Japan), and surface water temperature was measured using a digital thermometer (Akuamaks, Turkey). The copepods were removed during the macroscopic examination of the fish and were immediately preserved in 70% ethanol. Fixed specimens were cleared in lactic acid for 2 h prior to examination using a Nikon SMZ 800N dissecting microscope and an Olympus BX51 compound microscope. Subsequently, specimens were mounted as temporary preparations in a drop of lactic acid on a cavity slide for conducting measurements and drawings. Measurements were made using an ocular micrometer and drawings were made with the aid of a drawing tube. All measurements are in millimetres. The scientific and common names of fishes follow Froese and Pauly (2018) and the morphological terminology for the copepods follows Boxshall (1990) and Huys and Boxshall (1991). The holotype female (NHMUK 2018.187) and allotype male (NHMUK 2018.188) are stored in the collection of the Natural History Museum, London, and the remaining paratype females (n = 2) (CUMAP-COP/2018-3) are stored in the collection of Aquatic Parasitology Museum at the Faculty of Fisheries in Cukurova University, Adana, Turkey.
Results
Family: Caligidae Burmeister, 1835
Genus: Caligus Müller, 1785
Caligus adanensis sp. nov.
Type host: Belone belone (Linnaeus, 1760) (Belonidae).
Type locality: North-eastern Mediterranean waters, off Yumurtalık in İskenderun Bay, Turkey; (36° 44′ 31.05″ N; 35° 44′ 56.54″ E); depth range, 5–12 m; mean surface water temperature, 15.5°C; salinity, 35 ppt.
Site on host: Dorsolateral body surface, near the base of the dorsal fin.
Prevalence: 20% (1 fish infected out of a total of 5 examined).
Type material: Holotype female (NHMUK 2018.187) and allotype male (NHMUK 2018.188).
Etymology: The species name refers to the city of Adana, Turkey, close to where the material was collected.
Description (Figs. 2, 3, 4, 5, and 6)
Adult female
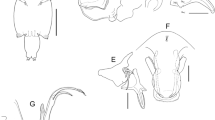
Body (Fig. 2a, b) comprising caligiform cephalothorax incorporating first to third pedigerous somites, free fourth pedigerous somite, genital complex, and 1-segmented abdomen bearing paired caudal rami. Total body length, 4.88 mm (4.79–4.97) (n = 3) including caudal rami. Dorsal cephalothoracic shield slightly longer than wide, 2.47–2.55 × 2.22–2.28 (2.51 × 2.25). Thoracic zone of shield wider than long, 1.00–1.60 × 1.70–2.3 (1.30 × 2.00), with posterior margin extending well beyond lateral zones. Fourth pedigerous somite wider than long, 0.16–0.23 × 0.37–0.43 (0.20 × 0.40), distinctly separated from the cephalothorax and genital complex; leg 4 located ventrally on fourth pedigerous somite (Fig. 2b). Genital complex subcircular, longer than wide, 2.09–2.19 × 1.92–1.95 (2.14 × 1.93), about 4.2 times longer than the abdomen, and with slightly lobate posterolateral corners. Free abdomen 1-segmented, slightly longer than wide, 0.49–0.55 × 0.44–0.52 (0.52 × 0.48), anterior part 1.36 times wider than posterior part. Combined length of genital complex and abdomen about 1.2 times longer than cephalothorax. Caudal ramus (Fig. 2c) subrectangular, 1.66 times longer than wide, 0.17–0.23 × 0.1–0.14 (0.20 × 0.12), armed with six plumose setae; outer dorsal seta smallest.
Antennule (Fig. 2d) 2-segmented; proximal segment with 25 plumose setae arrayed along anteroventral surface plus 2 naked dorsal setae; distal segment armed with 1 subterminal seta on posterior margin and 11 setae plus 2 aesthetascs around apex.
Antenna (Fig. 2e) uniramous, 3-segmented. With subrectangular cuticular process near base; proximal segment with posteriorly directed spinous process; middle segment subrectangular, bearing corrugated adhesion pad on dorsal surface; distal segment forming sharply recurved claw, with distal seta located at mid-length and large, proximal cuticular swelling carrying apical seta. Post-antennal process (Fig. 2f) weakly curved, ornamented with 2 unisensillate papillae on basal part, plus single unisensillate papilla on adjacent ventral cephalothoracic surface.
Mandible (Fig. 3a) stylet-like, with 12 inner teeth near apex.
Maxillule (Fig.3b) comprising anterior papilla bearing 3 unequal, naked setae and slender dentiform posterior process.
Sternal furca (Fig. 3c) with subrectangular box and long, slightly divergent tines with incurved tips.
Maxilla (Fig. 3d) 2-segmented, brachiform; proximal segment (lacertus) large, unarmed; slender distal segment (brachium) ornamented with minute spinules distally along posterior margin (Fig. 3e), and bearing small subterminal hyaline membrane on inner margin plus short canna and long calamus distally.
Maxilliped (Fig. 3f) subchelate; protopodal segment (corpus) slender, unarmed; distal subchela representing fused endopodal segments plus claw armed with small seta at base.
Spine (Roman numerals) and seta (Arabic numerals) formula of rami of legs 1–4 as follows:
Exopod | Endopod | |
|---|---|---|
L 1 | I-0; III,1,3 | Vestigial |
L 2 | I-1; I-1; II,I,5 | 0-1; 0-2; 6 |
L 3 | I-0; I-1; III,4 | 0-1; 6 |
L 4 | I-0; III | Absent |
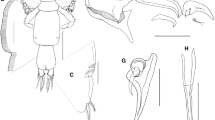
Leg 1 (Fig. 3g) biramous, with 2-segmented exopod and unsegmented, vestigial endopod. Sympod armed with lateral plumose seta and inner plumose seta, and ornamented with ventral patch of spinules. First exopodal segment robust, about 2.3 times longer than wide, ornamented with row of setules along posterior margin and armed with small spine at outer distal corner. Distal exopodal segment with 3 plumose setae posteriorly plus 4 terminal elements (Fig. 3h); outermost element (spine 1) with fine serrations along inner margin, and slightly more than half length of spine 2; middle 2 elements (spines 2 and 3) subequal, each bearing slender accessory process; innermost element (seta 4) about twice as long as spines; and finely serrated along inner margin. Endopod naked, vestigial.
Leg 2 (Fig. 4a) biramous, with distinct coxa and basis; coxa short, bearing long plumose inner seta and with sensillum on ventral surface; basis armed with short naked seta on outer distal corner plus extensive marginal membrane along posterior margin, sensillum near mid-length of posterior margin. Exopod (Fig. 4b) 3-segmented; first segment with inner plumose seta, outer spine reflexed obliquely over surface of second segment and row of setules on inner margin; second segment with inner plumose seta and outer distal spine extending obliquely across to posterior margin of third segment; spines on first and second exopodal segments each with hyaline membrane along outer margin. Third exopodal segment with 3 outer spines and five inner plumose setae; first spine simple and smallest, second spine with broad flanges bilaterally and third spine with marginal membrane laterally and pinnules medially and five plumose setae. Endopod (Fig. 4c) 3-segmented; first segment with inner plumose seta and tuft of fine pinnules distolaterally; second segment elongate, with 2 inner plumose setae, and rows of fine spinules along outer edge; third segment with 6 distal plumose setae.
Leg 3 (Fig. 4d) with intercoxal sclerite, coxa and basis fused into flattened apron-like sympod ornamented with extended strips of hyaline membrane along lateral and posterior margins. Inner coxal seta and outer basal seta both pinnate. Exopod 3-segmented, first segment with outer spine slightly shorter than second segment, extending parallel with longitudinal axis of ramus; second segment with outer spine, inner plumose seta and setules along outer margin; third segment with outer row of setules and 3 outer spines (first two spines equal in length, third spine slightly longer than other two spines) plus 4 short pinnate setae. Endopod (Figs. 4d and 5a) 2-segmented; first segment forming flap-like velum between rami, with row of fine setules along posterior margin and long, inner pinnate seta; compound distal segment with 6 pinnate setae and bearing row of long setules along outer margin and prominent subtriangular process on ventral surface.
Leg 4 (Fig. 5b) uniramous. Protopodal segment with outer seta derived from basis. Exopod 2-segmented; first segment armed with long slender, bilaterally flanged, outer distal spine; second segment with 3 apical spines increasing in length from outer to inner; each spine with pecten at base.
Leg 5 (Fig. 5c) located ventrally near posterolateral corners of genital complex and represented by 2 papillae; outer papilla bearing single plumose seta; inner (exopodal) papilla bearing 2 unequal plumose setae.
Adult male (Fig. 6a)
Total body length 3.78 mm (n = 1); cephalothoracic shield 1.2 times longer than wide (2.14 × 1.85) excluding hyaline membranes. Thoracic zone of shield just wider than long (1.11 × 1.25). Fourth pedigerous somite wider than long (0.33 × 0.50) and distinctly divided from genital complex. Genital complex about 1.26 times longer than wide (0.72 × 0.57) with slightly convex lateral margins. Abdomen about 63% of genital complex, comprising 2 somites; first somite 1.9 times wider than long (0.14 × 0.27), with convex lateral margins; anal somite as long as wide (0.31 × 0.31), about 2.2 times longer than first abdominal somite; combined length of genital complex and abdomen slightly more than half of cephalothorax (54%). Caudal rami 1.5 times longer than wide, armed with 6 plumose setae.
Antennule as in female.
Antenna (Fig. 6b) 3-segmented; proximal segment elongate, with corrugated adhesion pad on medio-ventral surface and prominent triangular projection on outer distal margin; middle segment largest with corrugated pads on medial and distal surfaces; distal segment (Fig. 6c) with 3 overlapping plates (smallest upper plate subtriangular, middle plate subcircular and widest, lower plate narrow, and longest) and 2 basal setae. Post-antennal process (Fig. 6d) more curved than that of female.
Maxillule (Fig. 6e) with small dentiform knob located medially on the posterior process.
Mandible and maxilla as in female.
Maxilliped (Fig. 6f) with massive corpus produced into conspicuous bilobate process distally on myxal area, opposing tip of claw; surface of larger lobe (Fig. 6g) covered with corrugations; smaller, more proximal lobe naked, overlapping larger lobe anteriorly. Subchela armed with small seta at base of claw plus 2 unequal cuticular processes (Fig. 6g); proximal process smallest; longer distal process located on opposite side of seta.
Sternal furca and legs 1–4 as in female.
Leg 5 (Fig. 6h) represented by 2 papillae located on posterolateral margin of genital complex; outer papilla with 1 plumose seta and inner papilla with 2 plumose setae.
Leg 6 (Fig. 6h) represented by single papilla on posteroventral side of genital complex, bearing 2 setae; outer seta with hyaline membrane bilaterally, inner seta pinnate.
Remarks
Since the establishment of Caligus by Müller (1785), the genus has grown to include 268 species. The large number of species in the genus makes accurate species identification of newly collected specimens a challenging endeavour. Navigating through the multitude of species of Caligus is facilitated by grouping species that share similar sets of taxonomic characters. This method was first applied by the establishment of the Caligus macarovi and C. productus groups by Boxshall and Gurney (1980). Boxshall and El-Rashidy (2009) expanded on the diagnosis of the productus group. In his recent study, Boxshall (2018) updated the list of members of these two groups and added three additional species groups, namely the C. bonito, C. confusus, and C. diaphanus groups.
The new species described herein has a 3-segmented leg 4 with the first and second exopodal segments bearing 1 and 3 distal spines, respectively. There are 65 valid species within the genus Caligus that share this leg 4 configuration. In addition to this major feature, the new species has the following character states: (i) distal exopodal segment of leg 1 bearing three plumose setae posteriorly plus four distal elements with spine 1 naked, spines 2 and 3 each with an accessory process, spines 1 to 3 of similar length, and seta 4 about twice the length of the spines; (ii) the maxilla has spinules on its posterodistal margin; (iii) antenna with posterior process on proximal segment; (iv) a large, prominent cuticular swelling is present near the basal segment of the female antenna; (v) the female has a 1-segmented abdomen; and (vi) the male has a 2-segmented abdomen; (vii) the male maxilliped has a large myxal process opposing the tip of the subchela. Six of the seven character states are also shared with the 44 members of the C. macarovi group (see Boxshall 2018). The new species has a female genital complex that is about 1.2 times longer than wide and 4.2 times longer than the abdomen. In addition, the female abdomen is about 1.1 times longer than wide. During comparisons of the body proportions of the new species with those of the 44 species listed in the C. macarovi group (Table 5 in Boxshall 2018), we took into account that the length to width ratio of the genital complex may vary according to the reproductive state of the individual female (Parker et al. 1968; Boxshall 1974). Therefore, we allowed for variability of about 10% to eliminate species with a female genital complex that is markedly wider than long (length:width ratio ≤ 1.0:1) and species where it is markedly longer than wide (L:W ratio ≥ 1.3:1). Only 20 species share a genital complex with a L:W ratio between 1.0:1 and 1.3:1. Among these 20 species, only two, Caligus kuwaitensis Kabata & Tareen, 1984 and C. klawei Shiino, 1959, have a genital complex that is more than 4 times longer than the abdomen as in the new species. However, in C. kuwaitensis, the genital complex is 7.9 longer than the abdomen (vs. 4.2 in the new species) and the abdomen is wider than long (ratio of 0.6:1) instead of longer than wide (1.2:1) as in the new species. Caligus kuwaitensis further differs from the new species in having an inwardly curved, tapering, and sharply pointed posterior process on the basal segment of the female antenna (vs. straight, not tapering, and with rounded tip); a subrectangular female genital complex (vs. subcircular); a sternal furca with tapering tines (vs. slightly expanded, incurved tip); inner of the middle two spines (spine 3) on distal exopodal segment of leg 1 slightly longer than the length of the outer spine (spine 2) (vs. equal) and the accessory process on spine 3 relatively longer than the accessory process on spine 2 (1.3:1.0) (vs 1.0:1.0); the spine on the second exopodal segment of leg 2 is as long as the spine on the first exopodal segment and extends beyond the posterior margin of the third segment (vs. shorter than the spine on the first segment and not extending beyond the margin of the third segment); and a 1-segmented abdomen in the male (vs. 2-segmented abdomen).
Similar to C. klawei Shiino, 1959, a large, prominent cuticular swelling is also present near the basal segment of the female antenna of the presently reported new species. However, Caligus klawei differs from the new species in having a rectangular cephalothorax (vs. trapezoidal cephalothorax with slightly convex lateral margins); a combined length of the genital complex and abdomen that is about 1.27 times longer than the length of cephalothorax (vs. about 1.04 times longer than the cephalothorax); a genital complex 1.28 times wider than the width of cephalothorax (vs. the cephalothorax 1.5 times wider than the genital complex); anterior part of the abdomen as long as the posterior part (vs. anterior part distinctly wider than the posterior half); a recurved post-antennal process (vs. almost straight); an outwardly curved, tapering, and sharply pointed posterior process on the basal segment of the female antenna (vs. straight, not tapering, and with rounded tip); distinctly divergent tines on the sternal furca (vs. slightly divergent); two setae at the base of the terminal claw on the female maxilliped (vs. one); sympod of leg 1 without ornamentations (vs. ornamented with patch of spinules); accessory process on spines 2 and 3 of leg 1 distinctly shorter than the spines 2 and 3 (vs. longer than the spines 2 and 3); outer proximal margin of inner coxal seta of leg 2 rounded, and carrying dense setules (vs. straight and bearing fine setules); a distal spine on the first exopodal segment of leg 4 that is as long as the outer margin of the second exopodal segment (vs. about two-thirds); subequal inner and middle apical spines on the second exopodal segment of leg 4 (vs. unequal).
The morphology of C. adanensis also reveals close similarities with Caligus wilsoni Delamare Deboutteville and Nuñes-Ruivo, 1958 and Caligus hyporhamphi Boxshall, 2018. However, C. adanensis can be distinguished from these two congeners by the differences observed in some of the characters presented in Table 2.
These detailed comparisons justify the establishment of a new species to accommodate the specimens collected from Belone belone in the eastern Mediterranean waters, off the Turkish coast.
Discussion
Eight species of parasitic copepods have been reported from two of the three species belonging to the genus Belone Cuvier, 1816 and four of these eight copepods are members of Caligus (Table 3). Caligus adanensis sp. nov. is, therefore, the fifth species of Caligus reported from Belone belone and the 45th member of the C. macarovi group. Of the four species of Caligus previously reported from Belone, C. belones Krøyer, 1863 and C. elongatus von Nordmann, 1832 were reported from the Mediterranean Sea. Caligus belones was reported on B. belone from the Mediterranean coast of France (Delamare Deboutteville and Nuñes-Ruivo 1958). There are few records of C. elongatus from the Mediterranean Sea. Krøyer (1863a, b) reported C. elongatus (as C. trachypteri) from Trachipterus sp. caught off Sicily. Brian (1908) reported C. elongatus (as C. rapax) from pelagic pipefish, Syngnathus phlegon Risso, 1827 and ocean sunfish, Mola mola (Linnaeus, 1758) (as Orthagoriscus mola) in the Ligurian Sea, and subsequently reported it from broadnosed pipefish, Syngnathus typhle Linnaeus, 1758 (as Siphonostoma rotundatum) off Corsica (Brian 1912). A heavy infestation of C. elongatus on cultured European seabass, Dicentrarchus labrax (Linnaeus, 1758), was recently reported from Alexandria, Egypt (Elgendy et al. 2015). However, D. labrax is the type host of C. minimus and Elgendy et al. (2015) stated that they followed Venmathi Maran et al. (2009) for the identification of the Caligus species they had collected, yet there is no description of C. elongatus in Venmathi Maran et al. (2009). We consider, therefore, that the report of C. elongatus on D. labrax from Alexandria, Egypt, requires confirmation as it is probably a misidentification.
Cressey and Collette (1970), in their global survey of copepods parasitic on needlefishes, examined 2720 individual fish and collected 3863 copepods. In addition to the widespread caligid Caligodes laciniatus (Krøyer, 1863), their survey recorded seven species of Caligus, only three of which were identified: C. belones, C. malabaricus Pillai, 1961, and C. tylosuri (Rangnekar, 1956). The other four species were not described or named but were referred to as species A to D. One of these, species B (Cressey and Collette 1970: figures 124–127), exhibits several similarities with the new species, e.g., the segmentation and setation of leg 4, and the ornamentation of the endopod of leg 2, but differs in the shape of the female abdomen and in the length of the tines of the sternal furca. Given that Cressey and Collette (1970) found four rare species that they were unable to identify, the discovery reported here of a new species of Caligus on a well-known needlefish (B. belone) in well-studied Mediterranean waters indicates that a much greater sampling effort is necessary to reveal the true diversity of these sea lice.
References
Boxshall GA (1974) Lepeophtheirus pectoralis (O.F. Müller); a description, a review and some comparisons with the genus Caligus Müller, 1785. J Nat Hist 8:445–468. https://doi.org/10.1080/00222937400770381
Boxshall GA (1990) The skeletomusculature of siphonostomatoid copepods, with an analysis of adaptive radiation in structure of the oral cone. Philos Trans R Soc Lond Ser B Biol Sci 328:167–212. https://doi.org/10.1098/rstb.1990.0113
Boxshall GA (2008) A new genus of sea louse (Copepoda: Siphonostomatoida: Caligidae) parasitic on the bluespine unicornfish (Naso unicornis). Folia Parasitol 55:231–240
Boxshall GA (2018) The sea lice (Copepoda: Caligidae) of Moreton Bay (Queensland, Australia), with descriptions of thirteen new species. Zootaxa 4398(1):172. https://doi.org/10.11646/zootaxa.4398.1.1
Boxshall GA, El-Rashidy HH (2009) A review of the Caligus productus species group, with the description of a new species, new synonymies and supplementary descriptions. Zootaxa 2271:1–26
Boxshall GA, Gurney AR (1980) Descriptions of two new and one poorly known species of the genus Caligus Müller, 1785 (Copepoda: Siphonostomatoida). Bull Br Mus Nat Hist Zool 39:161–178
Boxshall GA, Justine JL (2005) A new genus of parasitic copepod (Siphonostomatoida: Caligidae) from the razorback scabbardfish, Assurger anzac (Trichiuridae) off New Caledonia. Folia Parasitol 52:349–358
Brian A (1908) La presenza del Caligus rapax (Copepoda parassita) nel Mediterraneo. Bol Naturalista 28:96–98
Brian A (1912) Copépodes parasites des poissons et des échinides provenant des campagnes scientifiques de S.A.S. le Prince Albert Ier de Monaco (1866−1910). Résultats des Campagnes Scientifiques du Prince Albert Ier de Monaco 38:1–58. https://doi.org/10.4236/ojms.2015.53027
Brian A (1939) Copépodes parasites recueillis par M. E. Dartevelle a l’embochure du fleuve Congo. Rev Zool Bot Afr 32:176–198
Burmeister H (1833) Chalimus. Mémoires des Curieux de la Nature de Bonn 17
Burmeister H (1835) Beschreibung einiger neuen oder weniger bekannten Schmarotzerkrebse, nebst allgemeinen Betrachtungen über die Gruppe, welcher sie angehören. Nova Acta Physico-Medica Academiae Caesareae Leopoldino-Carolinae Naturae Curiosorum (Acta der Kaiserlichen Leopoldinisch-Carolinischen Deutschen Akademie der Naturforscher), Halle 17:269–336
Châari M, Feki M, Neifar L (2015) Metazoan parasites of the Mediterranean garfish Belone belone gracilis (Teleostei: Belonidae) as a tool for stock discrimination. OJMS 5:324–334. https://doi.org/10.4236/ojms.2015.53027
Claus C (1875) Neue Beiträge zur Kenntnis parasitischer Copepoden nebst Bemerkungen über das System derselben. Z Wiss Zool 25:327–360
Çoker T, Akyol O, Bilge G (2013) Determination of batch fecundity of garfish, Belone belone, in the northern Aegean Sea. J Black Sea/Medit Environ 19:385–392
Collette BB, Parin NV (1970) Needlefishes (Belonidae) of the eastern Atlantic Ocean. Atl Rep 11:7–60
Cressey RF (1967) Caritus, a new genus of caligoid copepod, with a key to the genera of Caliginae. Proc US Natl Mus 123:1–8
Cressey RF (1990) Belizia brevicauda, a new genus and species of caligid copepod from the western Caribbean Sea. Syst Parasitol 15:151–154
Cressey RF (1991) Parasitic copepods from the Gulf of Mexico and Caribbean Sea, III: Caligus. Smithson Contrib Zool 49:1–53
Cressey RF, Collette BB (1970) Copepods and needlefishes: a study in host-parasite relationships. Fish Bull 68:347–432
Cuvier G (1816) Le Règne Animal distribué d’après son organisation pour servir de base à l’histoire naturelle des animaux et d’introduction à l’anatomie comparée. Les reptiles, les poissons, les mollusques et les annélides. A Paris: Chez Déterville 2:1–540
Delamare Deboutteville C, Nuñes-Ruivo L (1958) Copépodes parasites des poisons Méditerranéens. Vie Milieu 9:215–234
Dojiri M, Cressey RF (1991) Arrama, new genus (Siphonostomatoida: Caligidae), with two new species, copepods parasitic on Australian fishes. J Crustacean Biol 11:594–606
Dorman JA (1991) Investigations into the biology of the garfish, Belone belone (L.) in Swedish waters. J Fish Biol 39:59–69. https://doi.org/10.1111/j.1095-8649.1991.tb04341
Dorman JA, Holmes JMC (1991) Crustacean ectoparasites of the garfish Belone belone (L.) from Courtmacsherry Bay, Co. Cork. Ir Nat J 23:419–423
Elgendy MY, Abdelsalam M, Moustafa M, Kenawy AM, Seida A (2015) Caligus elongatus and Photobacterium damselae subsp piscicida concomitant infections affecting broodstock European seabass, Dicentrarchus labrax, with special reference to histopathological responses. J Aquac Res Dev 6:1–7. https://doi.org/10.4172/2155-9546.1000346
Froese R, Pauly D (eds) (2018) FishBase. World Wide Web electronic publication. Available at: www.fishbase.org, version (06/2018)
Günther A (1866) Catalogue of the fishes of the British Museum. Catalogue of the Physostomi, containing the families Salmonidae, Percopsidae, Galaxidae, Mormyridiae, Gymnarchidae, Esocidae, Umbridae, Scombresocidae, Cyprinodontidae, in the collection of the British Museum. Taylor and Francis, London, p 368
Heller C (1865) Crustaceen. In: Reise der Osterreichischen Fregatte Novara um die Erde in den Jahren 1857, 1858, 1859, vol 2. Zoologische Theil, pp 1–280
Holmes JMC (1998) A checklist of the Siphonostomatoida (Crustacea: Copepoda) of Ireland. Bull Irish Biogeogr Soc 22:194–228
Huys R, Boxshall GA (1991) Copepod evolution. The Ray Society, London, p 468
Johnson SC, Treasurer JW, Bravo S, Nagasawa K, Kabata Z (2004) A review of the impact of parasitic copepods on marine aquaculture. Zool Stud 43:229–243
Kabata Z, Tareen IU (1984) Description of Caligus kuwaitensis n. sp. (Copepoda: Siphonostomatoida) with comments on Caligus antenuatus Boxshall and Gurney, 1980. Syst Parasitol 6:57–62
Krøyer H (1837) Om Snyltekrebsene, isaer med Hensyn til den danske Fauna. Naturh Tidsskr 1:605–628
Krøyer H (1846) Danmarks Fiske. Copenhagen (Kjobenhavn) 3:1–704
Krøyer H (1863a) Bidrag til kundskab om Snyltekrebsene. Naturh Tidsskr 2:75–426
Krøyer H (1863b) Bidrag til Kundskab om Snyltekrebsene. Naturhist Tidsskr 3:75–320
Linnaeus C (1758) Systema Naturae per regna tria naturae, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Editio decima, reformata. Laurentius Salvius: Holmiae. ii, 824 pp.
Linnaeus C (1760) Systema natvrae per regna tria natvrae, secvndvm classes, ordines, genera, species, cvm characteribvs, differentiis, synonymis, locis. Tomvs I. Praefactvs est Ioannes Ioachimvs Langivs. Ad editionem decimam reformatam Holmiensem [1–8]: 1–824
Milne Edwards H (1840) Histoire naturelle des crustacés; comprenant l’anatomie, la physiologie et la classification de ces animaux, Paris, vol 3, pp 1–638
Müller OF (1785) Entomostraca, seu Insecta testacea, quae in aquis Daniae et Norvegiae resperit. XI. Caligus, Leipzig, pp 128–134
Özdikmen H (2008) Nomenclatural changes for nine crustacean genera (Crustacea: Copepoda). Mun Ent Zool 3:265–274
Papoutsoglou SE (1976) Metazoan parasites of fishes from Sacronicos Gulf Athens-Greece. Thalassografica 1:62–102
Parker R, Kabata Z, Margolis L, Dean MD (1968) A review and description of Caligus curtus Müller, 1785 (Caligidae: Copepoda), type species of its genus. J Fish Res Board Can 25:1923–1969
Pillai NK (1962) A revision of the genera Parapetalus Steenstrup and Lütken and Pseudopetalus nov. Crustaceana 3:285–303
Prabha C, Pillai NK (1979) Pseudoechetus fimbriatus gen. et sp. nov., a caligid copepod from Kerala coastal waters. Parasitology 79:425–429
Prabha C, Pillai NK (1983) Additions to the copepods parasitic on marine fishes of India. 1. On twelve species of caligids. Rec Zool Surv India 46:1–49
Raibaut A, Combes C, Benoit F (1998) Analysis of the parasitic copepod species richness among Mediterranean fish. J Mar Syst 15:185–206
Rangnekar MP (1956) Parasitic copepods from the marine fishes of Bombay. JUB 24:42–65
Rangnekar MP (1958) Mappates plataxus gen. et sp. nov., a copepod parasitic on the fish Platax teira (Forsk.), vol 53. Rec Indian, Calcutta, pp 303–308
Risso A (1827) Histoire naturelle des principales productions de l’Europe Méridionale et particulièrement de celles des environs de Nice et des Alpes Maritimes, vol 3. Levrault, Paris, p 480
Rodrigues AMV, Özak AA, Silva LMH, Boxshall GA (2018) Caligus mulli n. sp. (Copepoda:Caligidae) parasitic on two Mullid fishes from the eastern Mediterranean and adjacent Atlantic waters. Parasitol Res 117:3843–3850. https://doi.org/10.1007/s00436-018-6090-5
Rosenthal H, Fonds M (1973) Biological observations during rearing experiments with the garfish Belone belone. Mar Biol 21:203–218
Sever TM, Bayhan B, Bilge G, Taşkavak E (2009) Diet composition of Belone belone (Linnaeus, 1761) (Pisces: Belonidae) in the Aegean Sea. J Appl Ichthyol 25:702–706. https://doi.org/10.1439/0426.2009.01368.x
Shen CJ (1957) Parasitic copepods from fishes of China. Part II. Caligoida, Caligidae (1). Acta Zool Sin 9:351–378
Shiino SM (1957) Copepods parasitic on Japanese fishes, 16. Bomolochidae and Taeniacanthidae. Rep Fac Fish Pref Univ Mie 2:411–428
Shiino SM (1959) Ostpazifische parasitierende Copepoden. (Eastern Pacific parasitic copepods). Rep Fac Fish Pref Univ Mie 3:267–333
Stebbing TRR (1900) On Crustacea brought by Dr. Wiley from the South Seas. In: Zoological results based on material from New Britain, New Guinae, Loyalty Islands and elsewhere, collected during the years 1875, 1896, and 1897, vol 5. pp 605–690
Steenstrup JJS, Lütken CF (1861) Bidrag til kundskab om det aabne havs snyltekrebs og lernæer samt om nogle andre nye eller hidtil kun ufuldstændigt kjendte parasitiske copepoder. K dansk Vidensk Selsk Skr Naturhistorisk og Mathematisk Afd 5:341–432
Thomsen R (1949) Copépodos parásitos de los peces marinos del Uruguay. Com Zool Mus Montevideo 3:1–41
Van Beneden PJ (1892) Quelques nou veaux caligidaes de la côte d’Afrique et etd l’archipel des Açores. Bull Acad Belg 24:241–262
Venmathi Maran BA, Leong TS, Susumu O, Kazuya N (2009) Records of Caligus (Crustacea : Copepoda : Caligidae) from marine fish cultured in floating cages in Malaysia with a redescription of the male of Caligus longipedis Bassett-Smith, 1898. Zool Stud 48:797–807
Von NA (1832) Mikrographische Beiträge zur Naturgeschichte der Wirbellosen Thiere. Heft 2, I–XVIII. G. Reimer, Berlin, pp 1–150
Westernhagen H (1974) Incubation of garpike eggs (Belone belone Linné) under controlled temperature and salinity conditions. J Mar Biol Assoc UK 54:625–634
Wilson CB (1908) North American parasitic copepods: new genera and species of Caliginae. Proc US Natl Mus 33:593–627
Wilson CB (1911) North American parasitic copepods. Descriptions of new genera and species. Proc US Natl Mus 39:625–634
Yamaguti S (1936) Parasitic copepods from fishes of Japan. Part 2. Caligoida, I. Kyoto Imperial University. Published by author, pp 1–22
Zorica B, Čikeš Keč V (2013) Age, growth and mortality of the garfish, Belone belone (L. 1761) in the Adriatic Sea. J Mar Biol Assoc UK 93:365–372. https://doi.org/10.1017/S002531541200149X
Zorica B, Sinovčić G, Čikeš Keč V (2011) The reproductive cycle, size at maturity and fecundity of garfish (Belone belone, L. 1761) in the eastern Adriatic Sea. Helgol Mar Res 65:435–444. https://doi.org/10.1007/s10152-010-0233-0
Acknowledgements
We would like to thank Assoc. Prof. Caner Enver Özyurt, Res. Ass. Volkan Barış Kıyağa and Dr. Surhan Tabakoğlu from the Cukurova University, Adana, Turkey, for their help during sampling of the garfish, Belone belone.
Funding
This research was funded by the Cukurova University academic research projects unit (Project No. FBA-2017-7490).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Sampling and field studies
All necessary permits for sampling and observational field studies have been obtained by the authors from the competent authorities and are mentioned in the acknowledgements, if applicable. The study is compliant with CBD and Nagoya protocols.
Data availability
All data generated or analysed during this study are included in this published article.
Additional information
Communicated by L. Menzel
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This publication is registered in ZooBank under urn:lsid:zoobank.org:pub:BBF8EA18-8513-4F79-A596-70F0FC1E94B2 Caligus adanensis sp. nov. is registered in ZooBank under urn:lsid:zoobank.org:act:5E339123-0764-44FE-A6EF-DE1AEAF92FB1
Rights and permissions
About this article
Cite this article
Özak, A.A., Sakarya, Y. & Boxshall, G.A. Caligus adanensis sp. nov. (Copepoda: Caligidae Burmeister, 1835) parasitic on garfish, Belone belone (Linnaeus, 1760), from the eastern Mediterranean Sea, off the Turkish coast. Mar Biodiv 49, 1877–1890 (2019). https://doi.org/10.1007/s12526-019-00949-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12526-019-00949-9