Abstract
The caligid copepod Caligus lichiae Brian, 1906 is redescribed based on new material collected from the type-host, Lichia amia (Linnaeus), and from a second carangid, Seriola dumerili (Risso), both caught in the Gulf of Iskenderun, Turkey. Key diagnostic characters of both sexes are reported, supported by drawings and scanning electron microscopy images. Despite the commercial importance of its type-host, L. amia, C. lichiae has not been reported since its original description. After detailed comparison with recent descriptions of Caligus aesopus Wilson C. B., 1921, commonly found on S. dumerili, we recognise these two species as conspecific and propose to relegate C. aesopus Wilson C. B., 1921 to a junior subjective synonym of C. lichiae Brian, 1906. Caligus lichiae is a member of the C. confusus group of species and an identification key to species in this group is provided.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Carangidae Rafinesque is a commercially important and diverse family of marine fishes which includes the jacks, trevallies (crevalles), amberjacks, pompanos, scads, kingfish, pilotfish and runners. Carangids commonly serve as hosts to parasitic copepods particularly to species belonging to the genus Caligus O. F. Müller, 1785 (Caligidae). To our knowledge, 47 species of Caligus (18.4% of the 266 valid species in the genus) have been reported from carangid fishes (Table 1). In the Mediterranean, the Carangidae is represented by 20 species (Froese & Pauly, 2018) but only six have been reported as hosts of parasitic copepods (Table 2). Globally, 26.6% of carangid species are known to be infected by species of Caligus (Table 1), so it is surprising that only leerfish Lichia amia (Linnaeus) among the 20 carangids known from the Mediterranean has been recorded as host to a Caligus. Lichia amia is the type-host of C. lichiae Brian, 1906 and the type-localities were given by Brian (1906) as Genoa and the Island of Elba (Italy), but C. lichiae has never been reported since its original discovery.
In this study, we present the redescription of C. lichiae based on newly collected material from the gill cavity of L. amia and also from the mouth cavity and gill filaments of Seriola dumerili (Risso), both caught in the Gulf of Iskenderun, Turkey. Attempts were made to locate the type-material of C. lichiae. The location of the type-material of C. lichiae is given as unknown by Parker et al. (1968), but the material reported as C. curtus (Müller, 1785) by Brian (1898), which is effectively the type-material of C. lichiae, is listed as deposited in the collections of the Museo di Zoologia e Anatomia Comparata della Royale Università di Genova by Margolis et al. (1975: 8). Brian (1906: 36) stated “In my preceding publications I have wrongly referred to (Caligus curtus) some specimens of Caligus removed from Lichia amia Linnaeus specimens that here, later, I considered as a new species and described with the name of C. lichiae.”. Our attempts to locate this material were unsuccessful. However, Brian’s (1906) description is reasonably detailed and reveals many clues as to the identity of his species. The newly collected material of Caligus from these two Mediterranean carangids (L. amia and S. dumerili), was identified by reference to the original description of C. lichiae by Brian (1906).
The second host recorded here, S. dumerili, is also known as host of C. aesopus Wilson C. B., 1921 and C. confusus Pillai, 1961 (see Ho & Lin, 2001; Choe & Kim, 2010; Walter & Boxshall, 2018). Caligus aesopus was originally described by Wilson (1921) based on the material collected from “probably Seriola peruana” from the Juan Fernandez Islands in the East Pacific. Recent redescriptions of C. aesopus were presented in Ho & Lin (2004), Choe & Kim (2010), and Boxshall (2018). In addition, Boxshall (2018) established a new species group within the genus Caligus, namely the C. confusus-group which comprises 20 species including C. lichiae and C. aesopus. These species are: C. abigailae Boxshall, 2018; C. aesopus; C. alepicolus Boxshall, 2018; C. bicycletus Heegaard, 1945; C. brevicaudus Pillai, 1963; C. chorinemi Krøyer, 1863; C. confusus; C. cordyla Pillai, 1963; C. equulae Ho & Lin, 2003; C. kurochkini Kazachenko, 1975; C. lichiae; C. lunatus Wilson C. B., 1924; C. parapetalopsis Hameed & Pillai, 1973; C. platurus Kirtisinghe, 1964; C. randalli Lewis, 1964; C. seriolicolus Boxshall, 2018; C. spinosus Yamaguti, 1939; C. tenax Heller, 1865; and C. zylanica Hameed & Pillai, 1986. Caligus regalis was erroneously listed by Boxshall (2018) as a member of the C. confusus-group. It does not exhibit the diagnostic features of the C. confusus-group, and according to Cressey & Cressey (1980), it is a close relative of C. coryphaenae. All members of the C. confusus-group are characterised by a suite of character states including: (i) antenna with typically spatulate posterior process on proximal segment; (ii) postantennal process with small accessory tine; (iii) maxillule with accessory tine on posterior maxillulary process; (iv) leg 3 with raised cuticular rib (often with bifid tip) and circular array of large denticles on apron; (v) first exopodal segment of leg 3 with large, recurved, hook-like outer margin spine; (vi) leg 4 with 3-segmented exopod armed with I, I, III spines. A further four species, C. clavatus Kirtisinghe, 1964, C. fortis Kabata, 1965, C. inopinatus Kabata, 1994 and C. isonyx Steenstrup & Lütken, 1861, share most but not all of these diagnostic features (Boxshall, 2018) and can be considered as affiliated to the group. The newly collected material of C. lichiae was compared with these species, as well as with all species in and affiliated with the C. confusus group, and a key to species is provided.
Materials and methods
Specimens of Caligus lichiae Brian, 1906 were collected from the gill cavity of the leerfish, Lichia amia (Linnaeus) and from the mouth cavity and gill filaments of the greater amberjack, Seriola dumerili (Risso) caught in north-eastern Mediterranean waters off the Turkish coast. Fishes were caught by rod and line, and were examined for presence of parasitic copepods. Copepods removed from infected fish were immediately preserved in 70% ethanol. Specimens were cleared in lactic acid for 2 h prior to examination using a Nikon SMZ 800N dissecting microscope and an Olympus BX51 compound microscope. Specimens were mounted as temporary preparations in a drop of lactic acid in the well of a cavity slide. Measurements were made using an ocular micrometer and drawings were made with the aid of a drawing tube. All measurements are given in millimetres unless otherwise stated, and are presented as the range followed by the mean in parentheses. The scientific and common names of fishes follow Froese & Pauly (2018) and the morphological terminology for the copepods follows Boxshall (1990) and Huys & Boxshall (1991). The protocols for preparing crustaceans for scanning electron microscopy (SEM) outlined by Felgenhauer (1987) were followed. Ethanol-fixed specimens were hydrated to distilled water and post-fixed in 1–2% osmium tetroxide (OsO4) in buffer for 2 h, washed in distilled water, dehydrated through graded acetone series, critical point dried using liquid carbon dioxide as the exchange medium, mounted on aluminum stubs, and sputter-coated with platinum. Coated specimens were examined on a Zeiss Supra 55 (FE-SEM, Germany) field emission scanning electron microscope at 1–3 kV.
Family Caligidae Burmeister, 1835
Genus Caligus O.F. Müller, 1785
Caligus lichiae Brian, 1906
Host: Lichia amia (Linnaeus) (n = 1; total body length 52 cm; caught on 12.viii.2016); Seriola dumerili (Risso) (n = 19; total body length range 43–57 cm; caught on 16.viii.2016).
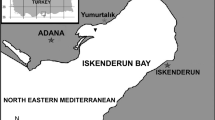
Locality: North-eastern Mediterranean waters off Yumurtalık, Gulf of Iskenderun, Turkey; depth range: 50–60 m.
Prevalence: 57.89% (11 fish infected out of 19 examined S. dumerili);
Voucher material: A total of 17 specimens: 1 ovigerous female (CUMAP-COP/2017-4) collected from the gill cavity of L. amia, 7 ovigerous females (CUMAP-COP/2017-5) and 9 males (CUMAP-COP/2017-6) collected from the mouth cavity and gill filaments of S. dumerili; fixed in ethanol and stored in the collections of the Aquatic Parasitology Museum at the Faculty of Fisheries, University of Çukurova, Adana-Turkey. Three voucher specimens: 2 female and 1 male (NHMUK 2018.191-193) were also deposited in the collections of the Natural History Museum London.
Description (Figs. 1–10)
Adult female [Based on 8 specimens). Body caligiform, comprising cephalothorax incorporating first to third pedigerous somites, free fourth pedigerous somite, genital complex and 1-segmented abdomen (Fig. 1A). Body length 4.2–6.1 (5.8, n = 8) excluding caudal setae. Dorsal cephalothoracic shield circular, slightly wider than long, 1.9–2.7 × 2.0–2.9 (2.5 × 2.6), length of cephalothorax about 43% of total body length, posterior end of lateral zones slightly angular. Thoracic zone of shield 0.09–1.09 × 1.3–1.41 (1.05 × 1.38), comprising c.42% of cephalothorax length, and with concave postero-lateral margin around deeply incised posterior sinus (Figs. 1A, 2A), surrounded with prominent membrane (Fig. 2B, C). Fourth pedigerous somite fused with genital complex, wider than long, 0.49–0.6 × 0.71–0.81 (0.55 × 0.77). Genital complex (Fig. 1A) longer than wide, 1.69–1.83 × 1.31–1.44 (1.77 × 1.38); with rounded anterior corners, slightly convex sides and with lobate posterolateral corners ornamented with patch of spinules (Fig. 2D, E); mid-half of postero-ventral margin of genital complex comprising 2 adjacent flaps (Fig. 1A) covering egg sac attachment area; outer flap larger than inner. Abdomen (Fig. 1A) 1-segmented; longer than wide 0.88–0.97 × 0.57–0.66 (0.94 × 0.61), posterior third of abdomen narrower than anterior part, entire abdomen about 55% of length of genital complex. Combined length of genital complex and abdomen (excluding caudal rami) approximately 1.05 times longer than cephalothorax, and about 46% of total body length. Caudal ramus wider than long, 0.03–0.07 × 0.09–0.13 (0.05 × 0.11), armed with 6 pinnate setae, length of caudal ramus about 5% of length of abdomen.
Antennule (Figs. 1B, 3A) 2-segmented; proximal segment bearing 27 setae; slender distal segment with 11 naked setae plus two aesthetascs; distal segment elongate, about 1.6 times longer than proximal segment. Antenna (Figs. 1C, 3A) uniramous, 3-segmented; proximal segment small and with small, rounded posterior process; middle segment subrectangular, armed with small adhesion pad on dorsal surface; distal segment forming long, weakly curved claw; bearing large, spine-like seta proximally (Figs. 1C, 3A, B) and slender distal seta (Figs. 1C,3A). Postantennal process (Figs. 1D,3C) weakly curved, ornamented with 2 multisensillate papillae; similar papilla with 2 sensillae located on body surface adjacent to process. Proximal part bearing additional small, subtriangular inner process. Convex anterior margin of postantennal process with rounded protrusion.
Maxillule (Figs. 1E, 3D) comprising anterior papilla bearing 3 unequal setae; subcircular process present on adjacent anterior sclerite and projecting over base of process; and posterior blunt tipped dentiform process bearing shorter, medial tine. Mouth tube (Figs. 1F, 3D) with convex lateral margins, enclosing paired mandibles, each armed with 12 teeth distally.
Maxilla (Fig. 1G) 2-segmented, brachiform; proximal segment (lacertus) unarmed; slender distal segment (brachium) bearing long subterminal hyaline membrane (flabellum) on outer margin plus short canna and long, curved calamus ornamented with spirally arranged strips of serrated membrane (Fig. 4A, B). Maxilliped (Figs. 1H, 4C) comprising robust proximal segment (corpus) bearing subtriangular, tapering myxal process (Fig. 4C, white arrow) slightly directed to proximal part of corpus, dorsal surface of corpus ornamented with patches of corrugated pads distally (Fig. 4C arrowheads, D, E), and distal subchela representing fused endopodal segments plus claw; subchela armed with small seta at base of claw (Fig. 1H). Sternal furca (Figs. 5A, 6A) with small box and slightly divergent tines with rounded tips; tines with large marginal flanges (Fig. 6B).
Caligus lichiae Brian, 1906. Female. A, Flabellum (arrowhead) and tip of maxilla; B, Spirally twisted membrane on tip of calamus of maxilla; C, Posteriorly-directed myxal process (arrow) and patches of corrugations (arrowheads) on maxilliped; D, Outer distal patch of corrugations on dorsal surface of maxilliped corpus; E, Inner medio-distal patch of corrugations on dorsal surface of maxilliped corpus. Scale-bars: A, 30 µm; B–D, 10 µm; C, 50 µm; E, 20 µm
Caligus lichiae Brian, 1906. Female. A, Sternal furca; B, Ventral surface of sympod ornamented with patch of spinules and blunt dentiform process close to intercoxal sclerite; C, Pecten-like process near small spinule at outer distal corner of first exopodal segment and distal exopodal segment of leg 1; D, Exopodal segments of leg 2; E, Ventral surface of coxa of leg 2 ornamented with patch of large spinules near two sensillae (arrowhead) and endopod of leg 2. Scale-bars: A–C, E, 100 µm; D, 200 µm
Caligus lichiae Brian, 1906. Female. A, Sternal furca; B, Flange surrounding tines of sternal furca; C, Swimming leg 1; D, Ventral surface of sympod bearing a blunt dentiform process (arrow) and patch of spinules (inset, closer view of spinules); E, Terminal elements and digitiform projection on distal exopodal segment of leg 1. Scale-bars: A, C, 50 µm; B, 15 µm; D, E, 10 µm; D, inset, 1 µm
Swimming leg 1 (Fig. 6C) biramous, with 2-segmented exopod and unsegmented vestigial endopod. Sympod (Fig. 5B) armed with lateral plumose seta and inner seta. Ventral surface of sympod ornamented with patch of spinules (Fig. 6D, inset) and bearing small, blunt dentiform process close to intercoxal sclerite (Figs. 5B6D arrow). Endopod (Fig. 5B) relatively long, unsegmented and carrying two fused minute elements apically. First exopodal segment ornamented with row of setules along free posterior margin and bearing small, triangular pecten-like process plus small spinule at outer distal corner (Fig. 5C). Distal exopodal segment (Fig. 5C) with 4 terminal elements; outermost element (spine 1) finely serrated along inner margin and with pecten at base; middle 2 elements (spines 2 and 3) unequally long, with fine serrations along inner margin; each with accessory process and pecten at base; short, digitiform projection present at base of middle 2 spines (Fig. 6E arrow); innermost element (seta 4) simple, longer than other three spines (Fig. 5C). Free posterior margin of distal exopodal segment bearing three plumose setae (Fig. 5C).
Leg 2 biramous with 3-segmented rami. First two exopodal segments (Figs. 5D, 7A) with pinnate seta on inner margin and long oblique spine at outer distal corner, reflexed over surface of segment. Third exopodal segment with 3 outer spines, and 5 pinnate setae (Figs. 5D, 7A). Ventral surface of coxa ornamented with patch of large spinules and two sensillae (Figs. 5 E, 7A arrow, 7B). Endopod (Fig. 5E) 3-segmented; first endopodal segment distinctly expanded laterally (Fig. 7A), carrying setules on proximal part and spinules on distal part of outer margin, plus inner pinnate seta; second endopodal segment with 2 pinnate setae and ornamented with dense spinules on outer margin; third segment with 6 pinnate setae.
Caligus lichiae Brian, 1906. Female. A, General view of leg 2 and patch of large spinules (arrow); B, Sensillae near raised patch of large spinules on coxa of leg 2; C, Paired patches of large, sclerotised knobs (arrows) located on raised cuticular swelling on inner ventral surface of leg 3 apron and rib-like outgrowth (arrowheads) with angled spatulate tip; D, Detail of patch of large, sclerotised knobs on raised cuticular swelling. Scale-bars: A, 50 µm; B, 5 µm; C, 100 µm; D, 30 µm
Leg 3 with coxa and basis fused with intercoxal sclerite to form flattened apron-like sympod, ornamented with extended strips of hyaline membrane along lateral and free posterior margins (Fig. 8A, B), with rows of spinules on mid-ventral surface (Fig. 8A); with patch of large sclerotised, knobs (n = 14–19) located on raised cuticular swelling (Fig. 7C arrows, D) on inner ventral surface. Longitudinal ridge marking plane of fusion of protopod and intercoxal sclerite extending from anterior to posterior, and forming raised rib-like outgrowth with angled spatulate tip (Fig. 7C arrowheads). Exopod (Fig. 8B) 3-segmented, with large, recurved outer spine; large hyaline flap present along concave margin of spine. Second exopodal segment with outer spine and inner plumose seta. Third segment with 3 outer spines and 4 short pinnate setae. Endopod 2-segmented; first segment forming flap-like velum closing off space between rami, and armed with with long inner pinnate seta; second with 6 pinnate setae, ornamented with rows of long setules along outer margin.
Leg 4 uniramous with 3-segmented exopod (Fig. 8C, 9A); first segment with 1 distal spine about extending just over 80% of distance along margin of second exopodal segment; second segment with 1 distal spine extending beyond base of outermost spine on distal margin of third exopodal segment; third segment with 3 apical spines along oblique distal margin, inner spine longest, outer spine slightly shorter than middle spine, each spine surrounded with hyaline membrane, and with pecten at base.
Caligus lichiae Brian, 1906. A, Female, exopod of leg 4; B, Male, patch of spinules on posterolateral corner of genital complex; C, Male, claw of antenna ornamented with longitudinal ridges (arrowhead); D, Male, maxillule bearing corrugated pad, small medial tine (white arrowhead) and small knob (black arrowhead). Scale-bars: A, 50; B, 5 µm; C, 30 µm; D, 20 µm
Spine (Roman numerals) and seta (Arabic numerals) formula of legs 1–4 as follows:
Exopod | Endopod | |
|---|---|---|
P1 | I-0; III, 1, 3 | vestigial |
P2 | I-1; I-1; II, I, 5 | 0-1; 0-2; 6 |
P3 | I-0; I-1; III, 4 | 0-1; 6 |
P4 | I-0; I, III | absent |
Leg 5 (Fig. 8D) located at posterolateral corner of genital complex, represented by 2 papillae; anterior (outer protopodal) papilla bearing single plumose seta; posterior (exopodal) papilla bearing 3 plumose setae.
Adult male [Based on four specimens]. Body 3.21–3.63 (3.41 mm, n = 9) long excluding caudal setae. Dorsal cephalothoracic shield slightly wider than long, 1.62–1.91 × 1.68–1.96 (1.82 × 1.89), excluding marginal membranes (Fig. 10A). Free thoracic zone of shield wider than long, 0.75–0.9 × 0.09–1.1 (0.86 × 1.03); about 47% length of cephalothorax. Fourth pedigerous somite wider than long 0.25–0.42 × 0.4–0.51 (0.37 × 0.46), indistinctly divided from genital complex. Genital complex subtriangular; 0.46–0.74 × 0.38–0.6 (0.68 × 0.51), with narrow anterior part and slightly convex lateral margins; posterolateral corners ornamented with patch of spinules (Figs. 9B, 10A). Length of genital complex about 37% of cephalothorax. Abdomen (Fig. 10A) 1-segmented; subrectangular, longer than wide, 0.33–0.54 × 0.27–0.42 (0.48 × 0.37); entire abdomen about 71% of length of genital complex; combined length of entire abdomen and genital complex about 64% of cephalothorax length. Caudal ramus slightly longer than wide, 0.06–0.16 × 0.05–0.13 (0.1 × 0.08), armed with 6 pinnate setae; about 21% of abdomen length. Antenna (Figs. 9C, 10B) 3-segmented; proximal segment with 2 corrugated adhesion pads; middle segment largest, with corrugated pads on medial surface; distal segment of antenna forming strongly curved, striated claw (Fig. 10C arrowhead), armed with 2 slender basal setae. Postantennal process (Fig. 10C) more acutely curved than that of female, carrying 2 papillae each bisensillate; similar bisensillate papilla located on body surface adjacent to process: proximal part bearing small, subtriangular inner process: convex margin of postantennal process with rounded protrusion. Maxillule (Figs. 9D, 10D) with dense corrugated pad, medial tine and small knob distally on posterior process (Fig. 9D): anterior papilla with 3 unequal setae as in female. Sternal furca (Fig. 10E) with square box and more divergent tines than that of female, tines extending slightly beyond anterior margin of intercoxal sclerite of leg 1. Maxilliped (Fig. 10F) with massive corpus carrying 3 conspicuous triangular process along myxal margin plus laterally-directed process located proximally on posterior surface; subchela armed with long seta at base of claw; claw ornamented with minute spinules distally. Legs 1–4 as in female. Leg 5 (Fig. 10G) comprising 2 papillae located on posterolateral margins of genital complex, outer (protopodal) papilla with 1 seta, inner (exopodal) papilla with 3 plumose setae. Leg 6 (Fig. 10G) represented by single papilla carrying 3 pinnate setae; inner seta longest.
Remarks
Caligus lichiae was described over a century ago by Brian (1906) but has not been recorded since, even though its host, L. amia is distributed along the eastern Atlantic seaboard from the Bay of Biscay in the north to South Africa in the south, including the whole Mediterranean Basin through to the western Black Sea, and round into the Western Indian Ocean as far as the Bay of Maputo in Mozambique (Froese & Pauly, 2018). This suggests that C. lichiae is either extremely rare or has been confused with another species. The collection of the new female specimen from the type-host and the discovery that it was identical with the material from S. dumerili caught at the same locality, led us to explore the possibility of confusion with another species.
Seriola dumerili is known to be a common host of Caligus aesopus which was originally described by Wilson (1921) based on eleven females collected from a large scombrid (probably Seriola peruana Steindachner, as mentioned by Wilson) from off Juan Fernandez (Masatierra), Chile. However, the original description was limited and included illustrations of female habitus, antenna, sternal furca, and legs 1, 3 and 4 only. Eighteen years later, Yamaguti (1939) described C. spinosus Yamaguti, 1939, from Japanese yellowtail, Seriola quinqueradiata Temminck & Schlegel which closely resembles C. aesopus. The similarity between these two species of Caligus has caused confusion resulting in numerous misidentifications. Recent detailed redescriptions of C. aesopus and C. spinosus (Choe & Kim, 2010) revealed that material previously identified as C. spinosus from Japanese waters by Shiino (1960), was in fact C. aesopus. In addition, Indian reports of C. spinosus (Pillai, 1963; Prabha & Pillai, 1983) were also re-identified as C. aesopus, whereas the other reports of C. aesopus from Chile, New Zealand, South Africa, Taiwan and Korea were correctly identified (Hewitt, 1963; Fernandez & Villalba 1986; Grobler, 2004; Ho & Lin, 2007; Lin & Ho, 2007; Choe & Kim, 2010).
Caligus spinosus was relegated to synonymy with C. aesopus by Fernandez & Villalba (1986) and this was followed by Lin & Ho (2007). However, this synonymy was rejected by Choe & Kim (2010) who treated the two species as distinct and valid. During the period from 1986 to 2007, while these two species were regarded as synonyms, records of C. spinosus and/or C. aesopus that are unaccompanied by a description, might refer to either species.
The general body morphology and the key diagnostic characters of the newly collected Mediterranean specimens from both L. amia and S. dumerili are in accord with those of the recently redescribed material of C. aesopus from Taiwan and Korea (Lin & Ho, 2007; Choe & Kim, 2010). In particular, the small, blunt process (Figs. 5B, 6D), described as “small tubercle” by Choe & Kim (2010), on ventral surface of the sympod of leg 1 and the presence of large sclerotised knobs located on a raised cuticular swelling (Fig. 7C arrows, D) on inner ventral surface of the apron of leg 3 are the two major key diagnostic characters of female C. aesopus which we observed in our material. In addition, the male maxilliped has a laterally-directed process located proximally on the posterior surface of the corpus, a feature present in C. aesopus and in our Turkish material.
The discovery of this Caligus on the type-host of C. lichiae now raises the question of whether C. lichiae and C. aesopus are synonymous. Unfortunately, the only description available for C. lichiae is the original (Brian, 1906) which was relatively detailed for the early 20th Century, but lacks the detail we would expect from a modern description. Brian’s description reveals that C. lichiae exhibits all the features of a member of the C. confusus-group, as enumerated by Boxshall (2018) including: the presence of accessory processes on the postantennal process and posterior process of the maxillule, the elongate endopod of leg 1, the presence of accessory processes on spines 2 and 3 of leg 1, the strongly recurved spine on the first exopodal segment of leg 3 plus the raised rib and rosette of strong denticles on the apron of the same leg, and finally, the 3 segmented exopod of leg 4 armed with 1, 1, III spines. No member of the C. confusus-group other than C. lichiae has ever been reported from the Mediterranean.
Distinguishing between species of Caligus typically involves detailed comparisons of, for example, the relative lengths of setal elements. Unfortunately, we consider that Brian’s description does not provide accurate information on such a fine scale: for example, it shows the middle spine of the three distal spines on the apical segment of leg 4 as the longest. We know of no species of Caligus where the middle spine is the longest. Similarly, it shows all three posterior margin setae on the distal exopodal segment of leg 1 as similar in length, but the outermost seta is always shorter than the other two. Another inaccuracy evident in Brian’s depiction is the male abdomen, which he shows as having a deeply incised anal somite, which is not a feature of any member of the Caligidae. Brian (1906) shows the male maxilliped as possessing processes on the myxal surface plus a laterally-directed process proximally on the posterior surface of the corpus. This proximal process is a distinctive characteristic and is shared only with C. aesopus. A small rounded knob is present in a similar position in the male of C. spinosus (see Choe & Kim, 2010) but it is relatively inconspicuous. Caligus randalli has a similar laterally-directed process on the female maxilliped but the male is unknown. These species can be distinguished using the key provided below. On the basis of the generally very close correspondence between the description of C. lichiae by Brian (1906) and the redescription of C. aesopus by Choe & Kim (2010) and the possession of the characteristic posterior surface process on the male maxilliped, we propose to relegate C. aesopus to a junior subjective synonym of C. lichiae.
Minor differences observed on the presently reported C. lichiae are as follows: the corpus of the male maxilliped has three triangular processes along the myxal margin (Fig. 10F) whereas four are shown in Lin & Ho (2007) and in Choe & Kim (2010). However, the triangular shapes of the three myxal processes in the Turkish material are all very similar to those illustrated by Lin & Ho (2007; figure 3c). The fourth process is minute and located proximally on the myxal margin but it was not present in our material. The Korean material has four rounded (vs triangular) processes on the myxal surface of the corpus of the male maxilliped (Choe & Kim, 2010; figure 6f). In the Turkish material, the male maxillule has a tiny knob on posterior end of the corrugated pad on the posterior process (Fig. 9D, black arrowhead): this has not been noted in previous descriptions, but may have been overlooked. Finally, the raised array of denticles on the ventral surface of the apron of leg 3 comprised 14–19 denticles in the Turkish material, compared to 10 in the material from Taiwan (Lin & Ho, 2007) and 11 to 14 in the material from Korea (Choe & Kim, 2010). We regard these fine-scale differences as representing geographical variation.
The core species and the four affiliated members of the C. confusus-group can be identified with the aid of the following key:
-
1a
Posterolateral corners of female genital complex produced into paired expansions enclosing, laterally, proximal half of abdomen; abdomen about 1.7 times longer than broad, and with markedly convex lateral margins ……… C. alepicolus Boxshall, 2018
-
1b
These characters not combined ……… 2
-
2a
Posterolateral corners of female genital complex produced into paired lobes extending about to middle of abdomen or beyond; first abdominal somite laterally expanded and with rounded posterolateral lobes extending along sides of much narrower anal somite ..……… 3
-
2b
Posterolateral corners of female genital complex rounded or with slight posterolateral lobes, not reaching middle of abdomen ……… 4
-
3a
Genital complex much narrower than dorsal cephalothoracic shield; posterolateral lobes on genital complex subtriangular, tapering towards tip ……… C. seriolicolus Boxshall, 2018
-
3b
Genital complex about as wide as dorsal cephalothoracic shield; posterolateral lobes on genital complex flattened and with broadly rounded tip ……………………………… C. parapetalopsis Hameed & Pillai, 1973
-
4a
Anterior quarter of genital complex forming narrow waist-like region; abdomen dorso-ventrally flattened, more than 75% width of genital complex ……… C. kurochkini Kazachenko, 1975
-
4b
These characters not combined ……… 5
-
5a
Abdomen distinctly 2-segmented, anterior somite dorsoventrally flattened and 79% of width of genital complex, anal somite narrow ……… C. constrictus Heller, 1865
-
5b
Abdomen 1-segmented or comprising 2 somites of similar width ……… 6
-
6a
Abdomen wider than long or with length:width ratio about equal ……… 7
-
6b
Abdomen distinctly longer than wide ……… 13
-
7a
Genital complex about 1.36 times wider than long; width about equal to width of dorsal cephalothoracic shield ……… C. bicycletus Heegaard, 1945
-
7b
Genital complex varying from longer than wide to just (less than 1.1 times) wider than long; genital complex distinctly narrower than dorsal cephalothoracic shield ……… 8
-
8a
Genital complex with short waist-like region anteriorly, and with small rounded posterolateral lobes; abdomen about as long as wide; posterior process of maxillule trifid ……… C. confusus Pillai, 1961
-
8b
These characters not combined; maxillule simple or bifid ……… 9
-
9a
Abdomen extremely short, about one tenth length of genital complex………C. equulae Ho & Lin, 2003
-
9b
Length of abdomen between 20–70% length of genital complex ……… 10
-
10a
Abdomen broad (about as long as wide) and about 70% length of genital complex………C. fortis Kabata, 1965
-
10b
Abdomen wider than long and comprising less than 40% of genital complex ……… 11
-
11a
Dorsal cephalothoracic shield 1.2 to 1.5 times wider than genital complex; genital complex wider than long or about as long as wide; abdomen less than half width of genital complex ……… 12
-
11b
Dorsal cephalothoracic shield about 2.0 times wider than genital complex; genital complex about 1.2–1.3 times longer than wide; abdomen more than half width of genital complex ……… C. brevicaudus Pillai, 1963
-
12a
Genital complex wider than long; abdomen unsegmented with evenly convex lateral margins; postantennal process simple ……… C. platurus Kirtisinghe, 1964
-
12b
Genital complex about as long as wide; abdomen indistinctly subdivided and widest anteriorly at junction with genital complex; postantennal process bifid ………………………… C. zylanica Hameed & Pillai, 1986
-
13a
Abdomen more than 3 times longer than wide and almost as long as genital complex; postantennal process and maxillule simple ……… C. clavatus Kirtisinghe, 1964
-
13b
These characters not combined ……… 14
-
14a
Abdomen about 70% of length of genital complex ……… 15
-
14b
Abdomen at most 50% of length of genital complex ……… 17
-
15a
Abdomen slender with parallel sides, more than 2.5 times longer than wide ……… C. chorinemi Krøyer, 1863
-
15b
Abdomen with convex or tapering lateral margins, less than 2 times longer than wide ……… 16
-
16a
Abdomen with evenly convex lateral margins, about 1.6 times longer than wide ……… C. randalli Lewis, A. G., 1964
-
16b
Abdomen widest anteriorly at junction with genital complex tapering back towards anal somite; about 1.75 times longer than maximum width ……… C. isonyx Steenstrup & Lütken, 1861/ C. lunatus Wilson C. B., 1924
-
17a
Genital complex bottle-shaped, narrow anteriorly and increasing in width posteriorly; posterolateral corners of complex rounded ……… 18
-
17b
Genital complex with parallel to slightly convex lateral margins; posterolateral corner of complex angular or slightly flared ……… 19
-
18a
Abdomen about 1.5 times longer than wide and about 35% length of genital complex ……… C. inopinatus Kabata, 1994
-
18b
Abdomen about 2 times longer than wide and about 45% length of genital complex ……… C. abigailae Boxshall, 2018
-
19a
Genital complex about 2.7 times longer than abdomen; abdomen about 1.1 times longer than wide ……… C. cordyla Pillai, 1963
-
19b
Genital complex at most 2.2 times longer than abdomen; abdomen about 1.3–1.5 times longer than wide ……… 20
-
20a
Abdomen with evenly convex lateral margins showing no trace of segmentation ……… C. spinosus Yamaguti, 1939
-
20b
Abdomen with broad anterior part and narrow posterior part ……… 21
-
21a
Posterolateral corners of genital complex projecting laterally; female maxilliped with small myxal process ……… C. lichiae Brian, 1906
-
21b
Posterolateral corners of genital complex female maxilliped lacking process ……… C. tenax Heller, 1865
Caligus isonyx and C. lunatus are very similar in body proportions of the female and cannot be readily separated on the evidence available. It is possible that they are synonymous and this problem will be addressed elsewhere.
References
Baeza-Kuroki, H., & Castro-Romero, R. (1982). Tres especies de Caligidae, nuevas para la fauna chilena (Copepoda, Siphonostomatoida). Noticiario Mensual del Museo Nacional de Historia Natural, Santiago, Chile, 25, 3–7.
Boxshall, G. A. (1974). Lepeophtheirus pectoralis (O.F. Müller); a description, a review and some comparisons with the genus Caligus Müller, 1785. Journal of Natural History, 8, 445–468.
Boxshall, G. A. (1990). The skeletomusculature of siphonostomatoid copepods, with an analysis of adaptive radiation in structure of the oral cone. Philosophical Transactions of the Royal Society, London, Series B, 328, 167–212.
Boxshall, G. A. (2018). The sea lice (Copepoda: Caligidae) of Moreton Bay (Queensland, Australia) with descriptions of thirteen new species. Zootaxa, 4398, 1–172.
Boxshall, G. A., & El-Rashidy, H. H. (2009). A review of the Caligus productus species group, with the description of a new species, new synonymies and supplementary descriptions. Zootaxa, 2271, 1–26.
Brian, A. (1898). Catalogo di copepodi parassiti dei Pesci della Liguria. Bollettino dei Musei e Laboratorii di Zoologia e Anatomia Comparata della Royale Università di Genova, 61, 1–27.
Brian, A. (1906) Copepodi parassiti dei Pesci d’Italia. Genova: Tipo-Litografico R. Istituto Sordomuti, 187 pp.
Capart, A. (1959). Copépodes parasites. Résultats Scientifiques de lʼExpédition Océanographique Belge dans les Eaux Côtieres Africaines de lʼAtlantique Sud (1948-1949). Institut Royale des Sciences Naturelles de Belgique, 3, 55–126.
Causey, D. L. (1955). Parasitic Copepoda from Gulf of Mexico fish. Occasional Papers of the Marine Laboratory of Louisiana State University, 9, 1–19.
Choe, M. K., & Kim, I. H. (2010). Redescriptions of two morphologically confusing sea lice Caligus aesopus Wilson, 1921 and C. spinosus Yamaguti, 1939 (Copepoda: Siphonostomatoida: Caligidae) parasitic on amberjacks (Seriola spp.) from Korea. Zootaxa, 2483, 23–34.
Cordeiro, A. S., & Luque, J. L. (2004). Community ecology of the metazoan parasites of Atlantic moonfish Selene setapinnis (Osteichthyes: Carangidae) from the coastal zone of the State of Rio de Janeiro, Brazil. Brazilian Journal of Biology, 64, 399–406.
Cressey, R. F., & Cressey, H. B. (1980). Parasitic copepods of mackerel and tuna-like fishes (Scombridae) of the world. Smithsonian Contributions to Zoology, 311, 1–186.
Cressey, R. F. (1991). Parasitic copepods from the Gulf of Mexico and Caribbean Sea, III: Caligus. Smithsonian Contributions to Zoology, 497, 1–53.
Dippenaar, S. M. (2005). Reported siphonostomatoid copepods parasitic on marine fishes of southern Africa. Crustaceana, 77, 1281–1328.
Felgenhauer, B. (1987). Techniques for preparing crustaceans for scanning electron microscopy. Journal of Crustacean Biology, 7, 71–76.
Fernández, J., & Villalba, C. (1986). Contribución al conocimiento del género Caligus Müller, 1785 (Copepoda: Siphonostomatoida) en Chile. Gayana Zoología, 50, 37–62.
Froese, R., & Pauly, D. (Eds) (2018). FishBase. World Wide Web electronic publication. Retrieved June, 2018 from, http://www.fishbase.org.
Grobler, N. J. (2004). A review of the genus Caligus (Copepoda: Caligidae) from South Africa. Dissertation, University of the Free State Bloemfontein, South Africa.
Grobler, N. J., Van As, J. G., & Olivier, P. A. S. (2003). Additional morphological information on two species of Caligus (Copepoda: Caligidae) parasitic on South African marine and estuarine fish. African Zoology, 38, 139–143.
Hameed, M. S., & Pillai, N. K. S. (1986). A new species of Caligus (Copepoda: Caligidae) from Kerala. Indian Journal of Fisheries, 33, 487–492.
Hewitt, G. C. (1963). Some New Zealand parasitic Copepoda of the family Caligidae. Transactions of the Royal Society of New Zealand, 4, 61–115.
Hewitt, G. C., & Hine, P. M. (1972). Checklist of parasites of New Zealand fishes and of their hosts. New Zealand Journal of Marine and Freshwater Research, 6, 69–114.
Hirunraks, W., Sittiphuprasert, U., & Sontirat, S. (1977). A checklist of parasitic copepods from marine fishes of Thailand and the adjacent areas including their hosts. In: A checklist of cartilagenous fishes (subclass Selachii) found in Thai-waters and the adjacent areas. Notes of the Faculty of Fisheries, Kasetsart University, Bangkok, 8, 6–9.
Ho, J.-S., & Lin, C.-L. (2001). Parapetalus occidentalis Wilson (Copepoda, Caligidae) parasitic on both wild and farmed cobia (Rachycentron canadum) in Taiwan. Journal of the Fisheries Society of Taiwan, 28, 305–316.
Ho, J.-S., & Lin, C.-L. (2003). Solution to the taxonomic confusion surrounding Caligus epinepheli Yamaguti, a caligid copepod (Siphonostomatoida) parasitic on marine fishes. Zoological Studies, 42, 268–283.
Ho, J.-S., & Lin, C.-L. (2004). Sea Lice of Taiwan (Copepoda: Siphonostomatoida: Caligidae). Keelung: The Sueichan Press, 388 pp.
Ho, J.-S., & Lin, C.-L. (2007). Three species of Caligus Müller, 1785 (Copepoda, Siphonostomatoida, Caligidae) parasitic on Caranx spp. (Teleostei: Carangidae) off Taiwan. Systematic Parasitology, 68, 33–43.
Ho, J.-S., Nagasawa, K., Kim, I. H., & Ogawa, K. (2001). Occurrence of Caligus lalandei Barnard, 1948 (Copepoda, Siphonostomatoida) on amberjacks (Seriola spp.) in the western North Pacific. Zoological Science, 18, 423–431.
Huys, R., & Boxshall, G. A. (1991). Copepod Evolution. London: The Ray Society.
Johnson, S. C., Treasurer, J. W., Bravo, S., Nagasawa, K., & Kabata, Z. (2004). A review of the impact of parasitic copepods on marine aquaculture. Zoological Studies, 43, 229–243.
Kabata, Z. (1965). Copepoda parasitic on Australian fishes. IV. Genus Caligus (Caligidae). Annals and Magazine of Natural History, 13, 109–126.
Kazachenko, V. N. (1975). A new species of the genus Caligus Müller, 1785 (Copepoda Parasitica, Caligidae) from Usacaranx georgianus (Cuvier et Valenciennes) of the Great Australian Bight. Parazitologiya, 9, 425–431.
Kensley, B., & Grindley, J. R. (1973). South African parasitic Copepoda. Annals of the South African Museum, 62, 69–130.
Kirtisinghe, P. (1964). A review of the parasitic copepods of fish recorded from Ceylon, with descriptions of additional forms. Bulletin Fisheries Research Station, Department of Fisheries, Ceylon, 17, 45–132.
Lagarde, P. G. (1991). Crustáceos parásitos en peces marinos de la zona central de Venezuela. Boletín del Instituto Oceanográfico de Venezuela, Universidad de Oriente, 28, 135–144.
Lewis, A. G. (1967). Copepod crustaceans parasitic on teleost fishes of the Hawaiian Islands. Proceedings of the United States National Museum, 121, 1–204.
Lewis, A. G. (1968). Copepod crustaceans parasitic on fishes of Eniwetok Atoll. Proceedings of the United States National Museum, 125, 1–78.
Lin, C.-L., & Ho, J.-S. (2003). Two species of rare sea lice (Copepoda, Caligidae) parasitic on marine fishes of Taiwan. Journal of the Fisheries Society of Taiwan, 29, 313–332.
Lin, C.-L., & Ho, J.-S. (2007). Six species of Sea Lice (Copepoda, Caligidae) new to Taiwan. Journal of the Fisheries Society of Taiwan, 34, 41–67.
Luque, J. L., & Cezar, A. D. (2004). Metazoários ectoparasitos do pampo-galhudo, Trachinotus goodei Jordan & Evermann, 1896 (Osteichthyes: Carangidae), do litoral do Estado do Rio de Janeiro, Brasil. Acta Scientiarum Biological Sciences, 26, 19–24.
MacKenzie, K., Campbell, N., Mattiucci, S., Ramos, P., Pereira, A., & Abaunza, P. (2004). A checklist of the protozoan and metazoan parasites reported from the Atlantic horse mackerel, Trachurus trachurus (L.). Bulletin of the European Association of Fish Pathologists, 24, 180–184.
Margolis, L., Kabata, Z., & Parker, R. R. (1975). Catalogue and synopsis of Caligus, a genus of Copepoda (Crustacea) parasitic on fishes. Journal of the Fisheries Research Board of Canada, 192, 1–117.
Marques, E. (1965). Copépodes parasitas de peixes marinhos de S. Tomé. Garcia de Orta, Lisbon, 13, 185–192.
Morales-Serna, F. N., Hernández-Inda, Z. L., Gómez, S., & Pérez-Ponce de León, G. (2013). Redescription of Caligus serratus Shiino, 1965 (Copepoda: Caligidae) parasitic on eleven fish species from Chamela Bay in the Mexican Pacific. Acta Parasitologica, 58, 367–375.
Oldewage, W. H., & van As, J. G. (1989). Occurrence and distribution of Caligus (Copepoda: Siphonostomatoida) in African coastal waters. Revue de Zoologie Africaine, 103, 91–98.
Palm, H.W., Klimpel, S., & Bucher, C. (1999). Checklist of metazoan fish parasites of German coastal waters. Berichte aus dem Institut für Meereskunde an der Christian-Albrechts-Universität Kiel, 307, 148 pp.
Parker, R. R., Kabata, Z., Margolis, L., & Dean, M. D. (1968). A review and description of Caligus curtus Müller, 1785 (Caligidae: Copepoda), type species of its genus. Journal of the Fisheries Research Board of Canada, 25, 1923–1969.
Pearse, A. S. (1952). Parasitic Crustacea from the Texas coast. Publications of the Institute of Marine Science, University of Texas, 2, 5–42.
Pillai, N. K. (1963). Copepods parasitic on south Indian fishes - family Caligidae. Journal of the Marine Biological Association of India, 5, 68–96.
Pillai, N. K. (1967). Copepods parasitic on Indian marine fishes. A review. Proceedings of Symposium on Crustacea, Cochin, 5, 1556–1680.
Prabha, C., & Pillai, N. K. (1983). Additions to the copepods parasitic on the marine fishes of India. 4. On twenty-six species of Caligids. Records of the Zoological Survey of India Occasional Paper, 46, 1–49.
Raibaut, A., Combes, C., & Benoit, F. (1998). Analysis of the parasitic copepod species richness among Mediterranean fish. In: Dahms, H. U., Glatzel, T., Hirche, H. J., Schiel, S., & Schminke, H. K. (Eds). Proceedings of the 6th International Conference on Copepoda, Journal of Marine Systems, 15, 185–206.
Rangnekar, M. P. (1956). Parasitic copepods from the marine fishes of Bombay. Journal of the University of Bombay, 24, 42–65.
Rangnekar, M. P. (1959). Parasitic copepods from fishes of the western coast of India with description of one new and redescription of four known species. Journal of the University of Bombay, 28, 43–58.
Rohde, K. (1980). Comparative studies on microhabitat utilization by ectoparasites of some marine fishes from the North Sea and Papua New Guinea. Zoologischer Anzeiger, 204, 27–63.
Sharp, N. J., Poortenaar, C. W., Diggles, B. K., & Willis, T. J. (2003). Metazoan parasites of yellowtail kingfish, Seriola lalandi lalandi in New Zealand: Prevalence, intensity, and site preference. New Zealand Journal of Marine and Freshwater Research, 37, 273–282.
Shiino, S. M. (1960). Copepods parasitic on the fishes collected on the coast of Province Shima, Japan. Report of the Faculty of Fisheries, Prefectural University of Mie, 3, 471–500.
Shiino, S. M. (1965). Parasitic copepods of the eastern Pacific fishes. 5. Caligus. Report of the Faculty of Fisheries, Prefectural University of Mie, 5, 391–420.
Song, D. X., & Chen, G. X. (1976). Some parasitic copepods from marine fishes of China. Acta Zoologica Sinica, 22, 406–424.
Takemoto, R. M., & Luque, J. L. (2002). Parasitic copepods on Oligoplites spp. (Osteichthyes, Carangidae) from the Brazilian coastal zone, with the redescription of Tuxophorus caligodes Wilson, 1908 (Siphonostomatoida, Tuxophoridae). Acta Scientiarum, 24, 481–487.
Venmathi Maran, B. A., Seng, L. T., Ohtsuka, S., & Nagasawa, K. (2009). Records of Caligus (Crustacea: Copepoda: Caligidae) from marine fish cultured in floating cages in Malaysia with a redescription of the male of Caligus longipedis Bassett-Smith, 1898. Zoological Studies, 48, 797–807.
Walter, T. C., & Boxshall, G. A. (2018). World of Copepods database. Accessed through: World Register of Marine Species. Retrieved July 9, 2018, from http://marinespecies.org/aphia.php?p=taxdetails&id=135765.
Wilson, C. B. (1921). Report on the parasitic Copepoda collected during the survey of the Juan Fernandez Islands, 1916–1917. Natural History of the Juan Fernandez & Easter Islands, 3, 69–74.
Yamaguti, S. (1936) Parasitic copepods from fishes of Japan. Part 2. Caligoida I, Kyoto Imperial University (published by the author), 1–22.
Yamaguti, S. (1939). Parasitic copepods from fishes of Japan. Part 5. Caligoida, III. Volume. Jubilare pro Prof. Sadao Yoshida, 2, 443–487.
Acknowledgements
We would also like to thank Associate Professor Dr Kasım Ocakoglu, former head of the Advanced Technologies Research & Application Center (MEITAM) of the University of Mersin, Turkey, and Professor Süphan Karaytuğ and Ms Seher Kuru from Mersin University for their administrative and technical support during the SEM.
Funding
This research was funded by the Çukurova University academic research projects unit (Project No. FBA-2017-7490)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection Arthropoda.
Rights and permissions
About this article
Cite this article
Özak, A.A., Sakarya, Y., Yanar, A. et al. The re-discovery of Caligus lichiae Brian, 1906 (Copepoda: Caligidae) parasitic on two carangid fishes in the Mediterranean Sea, and the recognition of Caligus aesopus Wilson C. B., 1921 as a junior subjective synonym. Syst Parasitol 96, 207–232 (2019). https://doi.org/10.1007/s11230-019-09841-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11230-019-09841-3