Abstract
The biology and systematics of the squid genus Asperoteuthis Nesis, 1980, are poorly known. Although there have been four named and five described species in this genus, it now appears that there are only three valid species: A. acanthoderma (Lu, 1977), A. mangoldae Young, Vecchione & Roper, 2007a, and A. lui Salcedo-Vargas, 1999. Using a combination of mitochondrial DNA sequences (cytochrome c oxidase subunit I [COI], 16S rRNA, and 12S rRNA) and morphology, A. nesisi Arkhipkin & Laptikhovsky, 2008, and Clarke’s (1980) ‘?Mastigoteuthis A’ both appear to be junior synonyms of A. lui. The most distinctive feature of this species is the aboral tentacle club photophore distribution, which is chiral, with more photophores dorsally (∼11–16) than ventrally (∼9–12). Genetically, there is low intraspecific variation within A. lui and higher interspecific variation between this species and other chiroteuthids. Previously only known from the type description, A. lui now appears to have a circumpolar distribution in the Southern Ocean and is the most commonly encountered Asperoteuthis species in the diet of marine predators.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Asperoteuthis Nesis, 1980, is an enigmatic genus of poorly known meso-bathypelagic squids in the family Chiroteuthidae Gray, 1849. This genus is characterized by the presence of a tail structure, the absence of arm photophores, and unique tentacle clubs, each of which lacks suckers on the proximal half and is surrounded by a wide protective membrane (Young and Roper 2015). The exact use of asperoteuthid tentacles remains unknown and there is currently no data available on the feeding ecology of Asperoteuthis.
The type species Asperoteuthis was originally described as ‘Chiroteuthis’ acanthoderma Lu, 1977. Nesis (1980) established a new genus for this species after examining additional specimens of A. acanthoderma. However, he incorrectly synonymized A. acanthoderma with ‘Chiroteuthis’ famelica Berry, 1909 (now Echinoteuthis famelica, see Joubin 1933; Braid et al. 2014), which was poorly known at the time, because of similarities with Berry’s description (e.g., skin tubercles and lanceolate fin), despite previous publications that supported the validity of this species (e.g., Roper and Young 1975; Young 1978). Young (1991) supported the validity of E. famelica and its place in the family Mastigoteuthidae, and suggested that the type species for the genus Asperoteuthis should be ‘Chiroteuthis’ acanthoderma. Young et al. (2007a) officially fixed ‘C.’ acanthoderma as the type species for Asperoteuthis.
There are currently four named species in this genus. Asperoteuthis acanthoderma was described from two immature individuals collected in the Celebes Sea in the Western Pacific Ocean (Lu 1977). This species has also been reported from the Northwestern Pacific Ocean near Okinawa (Tsuchiya and Okutani 1993), the Eastern Pacific near Hawaii (Young and Roper 2010), the North Atlantic Ocean in the Gulf of Mexico (Judkins et al. 2009), Straits of Florida (Judkins et al. 2009), the Caribbean (Judkins et al. 2009), and the Indian Ocean off western Australia (Judkins et al. 2009). Asperoteuthis lui Salcedo-Vargas, 1999, was described from a single specimen from Cook Strait in New Zealand waters. Asperoteuthis mangoldae Young, Vecchione & Roper, 2007a, was described from specimens captured in Hawaiian waters. Most recently, Asperoteuthis nesisi Arkhipkin & Laptikhovsky, 2008, was described from a single specimen that was captured around the Falkland Islands in the Southern Ocean. A fifth species was reported by Clarke (1980) as ‘?Mastigoteuthis A’, but is now believed to belong in Asperoteuthis rather than in the family Mastigoteuthidae Verrill, 1881 (Arkhipkin and Laptikhovsky 2008).
The purpose of this paper is to review the systematics of A. lui, which was originally described from a single, partial specimen that was from the stomach contents of a ling (Genypterus blacodes), and was damaged due to digestion (Salcedo-Vargas 1999). A redescription was possible because of additional, recently collected, material. The status of the species described in the genus Asperoteuthis is assessed using a combination of mitochondrial genes (cytochrome c oxidase subunit I [COI], 16S rRNA, and 12S rRNA) and morphology.
Methods
Genetic analysis
DNA was extracted from eight specimens. Frozen tissue was available for four specimens (one whole individual, three tentacles only), which were identified by tentacle-club morphology as A. lui (Table 1). Tissue fixed in 80 % ethanol was available for the other four specimens (one whole individual without tentacles, three that were beaks and buccal mass only), which were identified as ‘?Mastigoteuthis A’ based on beak morphology (by Darren Stevens) (Table 1). Additional sequences were obtained from GenBank for the type specimen of A. nesisi and the outgroup species A. mangoldae, which was chosen based on the results from Braid et al. (2016). Their study showed some support for a close relationship between A. lui and A. mangoldae. This outgroup was included to show the relationship between A. lui, A. nesisi, and ‘?Mastigoteuthis A’. Asperoteuthis acanthoderma was not included in this phylogeny because it does not form a monophyletic clade with these species (Braid et al. 2016), but comparative sequences are available for this species in the Barcode of Life Data System (BOLD; CHSQX002-16, CHSQX001-16) and GenBank (KX783171, KX783229, KX783197, KX783172, KX783230, KX783198, and KT326921).
DNA was extracted using EconoSpin columns with QIAGEN reagents following protocols for the DNeasy Blood & Tissue Kit (QIAGEN). Three mitochondrial gene regions (COI, 16S rRNA, and 12S rRNA) were amplified and sequenced following protocols and using primers in Braid et al. (2014). Bidirectional sequencing reactions were performed by Macrogen (Korea) using the same primers used for the polymerase chain reaction (PCR). Sequences were assembled into contigs and edited using Sequencher v.4.9 (Gene Codes) and then uploaded to the BOLD (Ratnasingham and Hebert 2007) public project titled ‘Resolving the taxonomic status of Asperoteuthis lui’ (project code: ALUI) and subsequently submitted to GenBank (Table 1). Sequences were checked for potential contamination using the Basic Local Alignment Search Tool (BLAST) through GenBank.
Sequences were aligned separately for each gene using the Multiple Alignment using Fast Fourier Transform (MAFFT) online server (Katoh and Standley 2013), then concatenated in SequenceMatrix 1.8 (Vaidya et al. 2011). The concatenated alignment was analyzed in PartitionFinder 1.1.1 (Lanfear et al. 2012) to determine optimal partitioning and models using all substitution models included under the Bayesian Information Criterion (BIC). Each gene, as well as all codon positions for COI, were searched separately for a maximum of five partitions. The model TrN was chosen for both 16S rRNA and 12S rRNA. Different models were chosen for each codon position of COI as TrNef, F81, and HKY, respectively. A maximum-likelihood combined phylogeny with 1000 bootstrap replicates was created in GARLI 2.0.1 (Zwickl 2006). A consensus tree was created in Geneious 7.1.7 (Biomatters, Auckland, New Zealand) and branches were collapsed when bootstrap support values were less than 50. The final phylogeny was visualized in FigTree 1.4.0 (Rambaut 2012). The Barcode Index Number (BIN) system was also used to test the species boundaries of A. lui, A. nesisi, and ‘?Mastigoteuthis A’ (Ratnasingham and Hebert 2013). Intra- and interspecific distances for each gene region (COI, 16S rRNA, and 12S rRNA) were calculated using MEGA 6.06 (Tamura et al. 2013) using the Tamura–Nei (Tamura and Nei 1993) model with gamma correction.
Morphological analysis
Original type descriptions for all asperoteuthid species were reviewed. The type specimen for A. lui from the National Museum of New Zealand Te Papa Tongarewa (NMNZ) was examined, and photographs of the type specimen for A. nesisi from the British Museum of Natural History (BMNH) were observed. All specimens that were used for DNA sequencing were examined. A specimen of A. mangoldae was examined from the Field Museum of Natural History (FMNH). The entire national collections of asperoteuthid specimens were loaned and examined from the NMNZ and the National Institute of Water and Atmospheric Research Ltd. (NIWA) in Wellington. Additional non-morphological abbreviations used are: MNHN—Muséum National d’Histoire Naturelle; RV—research vessel; Stn—station; USNM—U.S. National Museum of Natural History, USA.
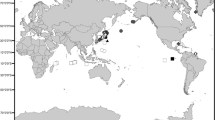
Distribution maps of Asperoteuthis species (Fig. 1) and A. lui specimens, found in New Zealand waters (Fig. 2), were created with ArcGIS 10.2 (Environmental Systems Research Institute [ESRI], Redlands, CA). The distribution for A. acanthoderma is based on specimen records from the Smithsonian National Museum of Natural History (USNM 1111098, USNM 1179399, USNM 1179402, USNM 1179422, USNM 1179632, and USNM 1179696) and MNHN (MNHN-IM-2002-2266), and localities from Lu (1977), Nesis (1980), Tsuchiya and Okutani (1993), Judkins et al. (2009), Young and Roper (2010), and Braid et al. (2016). The distribution for A. mangoldae is based on Young et al. (2011). The locality for ‘?Mastigoteuthis A’ (Clarke 1980) and the type localities for A. nesisi (BMNH 20070615) and A. lui (NMNZ M.143859) are indicated.
Collection data for some specimens were not available (ex-gut-content material). Collection dates are listed as dd/mm/yyyy. Specimens are listed by order of decreasing latitude, and secondarily by lower rostral length (LRL). Specimens were sexed when a viscera was present, while badly damaged specimens or beak-only specimens where sex could not be determined were designated ‘sex indet.’. Measurements were taken from the most complete side of the specimen and ranges are given in the format of lowest value (X), mean (Y), and largest value (Z) in the format of X–Y–Z; when the range was less than 5 % ML, only the mean is provided. Measurements of damaged features are indicated by an asterisk (*). Morphological examinations focused on both internal (beak, palps, and radula) and external anatomy following Braid and Bolstad (2015).
The species description was made in accordance with the guidelines provided by Roper and Voss (1983), with some modification (see Braid and Bolstad 2015). Beaks were described following Clarke (1986) and drawn using a camera lucida. For scanning electron microscopy (SEM), specimens were critical-point dried at the University of Auckland, then platinum plated and imaged at the Auckland University of Technology. Sucker descriptions were based on Salcedo-Vargas (1995), with some modifications (see Braid and Bolstad 2015). Palatine teeth on the lateral buccal palps are described following Bolstad (2010). Radular tooth descriptions followed Bolstad (2010), with some modifications (see Braid and Bolstad 2015).
Specimen measurements used in the text and tables include the following: ML—dorsal mantle length (measured to the end of the fin); MW—mantle width; FL—fin length; FW—fin width; HL—head length (measured from anterior tip of nuchal cartilage to separation of Arms I); HW—head width; ED—eye diameter; AL—arm length (arms measured from most-proximal sucker to arm tip); TnL—tentacle length; CL—tentacle club length; LRL—lower rostral length; URL—upper rostral length.
Results
Genetic analysis
Bidirectional sequences were successfully recovered from all eight individuals for COI, 16S rRNA, and 12S rRNA. COI sequences were all 658 bp and did not contain stop codons or indels. The 16S rRNA sequences were 517 bp and the 12S rRNA sequences were 403 bp. All COI sequences for A. nesisi, A. lui, and ‘?Mastigoteuthis A’ were assigned to the same BIN (BOLD: AAJ9359) and these sequences also formed a single clade on the combined maximum-likelihood phylogeny that was distinct from A. mangoldae (Fig. 3). Within the sequences used in this study for A. nesisi, A. lui, and ‘?Mastigoteuthis A?’: COI showed an average divergence of 0.5 %, with a minimum of 0 %, and a maximum of 1.4 %; 16S rRNA showed no variation; and 12S rRNA showed an average divergence of 0.1 %, with a minimum of 0 %, and a maximum of 0.3 %. For the divergence between this clade and A. mangoldae: COI had an average interspecific divergence of 19.4 %, with a minimum of 19.0 %, and a maximum of 20.9 %; 16S rRNA showed a divergence of 5.4 %; and 12SrRNA showed an average divergence of 7.3 %, with a maximum of 7.6 %, and a minimum of 7.2 %.
Systematics
Genus Asperoteuthis Nesis, 1980
Asperoteuthis Nesis, 1980: 613. Type species Chiroteuthis acanthoderma Lu, 1977, by subsequent designation of Young et al. (2007a:357).
Diagnosis: Mantle length at maturity 100 mm to >780 mm. Fins circular to oval in outline when considered together; fin length ∼40–65 % ML; tail structure present. Funnel-locking cartilage inverted Y-shaped groove, comma shaped, or ear shaped, with weak tragus, antitragus variably present; funnel pocket absent; buccal formula DDVV. Mantle-locking cartilage inverted Y-shaped, crescent, or approximately oval. Arm suckers arranged in two distinct series, with sharp or blunt teeth; arm length approximately subequal (∼50–115 % ML). Tentacular suckers present only on distal portion of club; trabeculate protective membrane present, expanded on proximal half of club; terminal-club photophore present, aboral club with small embedded photophores near lateral edges, photophores present on tentacular stalk. Photophore present on ventral surface of eye. Integumental photophores absent from mantle, fins, head, and arms.
Remarks: This diagnosis is based on descriptions of A. acanthoderma (Lu 1977), A. nesisi (Arkhipkin and Laptikhovsky 2008), A. mangoldae (Young et al. 2007a), and A. lui (Salcedo-Vargas 1999). Additional information was taken from Young et al. (2011) and the present findings.
Asperoteuthis lui Salcedo-Vargas, 1999 (Tables 2 and 3, Figs. 3, 4, 5, 6, 7, 8 and 9)
‘?Mastigoteuthis A’ Clarke, 1980: 191–194, figs. 155, 156; Clarke (1986): 160, 161, fig. 83.
Asperoteuthis lui Salcedo-Vargas, 1999: 48, 49, fig. 1.
Asperoteuthis nesisi Arkhipkin & Laptikhovsky, 2008: 203–205, figs. 1, 2.
Type material examined: NMNZ M.143859, A. lui holotype, sex indet., head only, LRL 6.62 mm, no locality data, Cook Strait, Genypterus blacodes stomach content. Photographs of BMNH 20070615, A. nesisi holotype, ♀, ML 363 mm, 53°44’S, 58°46’W, 913 m, RV Dorada, pelagic trawl fishing near-bottom, 20/07/05, Stn 2132, cruise ZDLH1-07-2005, ‘Asperoteuthis nesisi’.
Additional local material examined (10 specimens): NIWA 95041, ♀, beak only, LRL 7.34 mm, 39.40°S, 178.32°E, 998 m, RV Tangaroa, 26/03/2010, Stn TAN1003/64; NIWA 93270, sex indet., beak only, LRL 6.70 mm, 39.95°S, 178.28°E, 1285 m, RV Tangaroa, 26/03/2010, Stn TAN1003/65; NIWA 95040, sex indet., ML 345 mm, LRL 7.70 mm, 42.35°S, 174.20°E, 1226 m, RV Tangaroa, 01/04/2010, Stn TAN1003/119; NIWA 96168, sex indet., tentacle only, CL 138 mm, 42.74°S, 178.07°E, 42.75°S, 178.05°E, 868–822 m, RV Tangaroa, 16/11/2011, TAN1116/115; NIWA 96166, sex indet., tentacle only, CL 144 mm, 42.74°S, 178.07°E, 42.75°S, 178.05°E, 868–822 m, RV Tangaroa, 16/11/2011, TAN1116/115; NIWA 97258, sex indet., tentacle only, CL 170 mm, 42.77°S, 175.48°E, 886–889 m, RV Tangaroa, 20/01/2014, TAN1401/102; NIWA 95039, sex indet., beak only, LRL 5.16 mm, 42.82°S, 179.87°E, 42.83°S, 179.83°E, 960–962 m, RV Tangaroa, 16/06/2010, Stn TAN1008/04; NIWA 93268, ♀, fins missing, CL 79 mm, LRL 5.43 mm, 43.79°S, 174.54°W, 810–811 m, RV Tangaroa, 11/01/2014, Stn TAN1401/56; unaccessioned, ♀, ML 315 mm, LRL 6.51 mm, no locality data, New Zealand; NMNZ M.302215, sex indet., head only, LRL 3.26 mm, no locality data, Cook Strait, Genypterus blacodes stomach content.
Comparative material examined: Asperoteuthis mangoldae, FMNH 278099, ♂, ML 144* mm, 21.33°N, 158.33°W, 21.58°N, 158.58°W, 975–1040 m RV New Horizon, 04/07/1996, Stn 962-sta#76, mother tucker trawl. Asperoteuthis acanthoderma, unaccessioned, sex indet., ML 1030* mm, Indian Ocean, no locality data.
Distribution: Circumpolar distribution in the Southern Ocean; New Zealand in Cook Strait and the Chatham Rise; South Atlantic around the Falkland Islands.
Diagnosis: Fins circular in outline when considered together, length ∼64 % ML, width 52–60–68 % ML; circular skin depressions present. Single elongate photophore on ventral surface of eye. Funnel-locking cartilage ear shaped, weak tragus, strong antitragus; mantle-locking cartilage approximately oval. Largest suckers of all located mid Arms II and III (∼75 % arm width); arm suckers with 7–11 blunt, rectangular or sharp, conical teeth. Tentacles with ∼120–160 suckers with 5–7 sharp, conical teeth; aboral tentacle-club surface midline with five smaller proximal and two larger distal photophores; small embedded photophores near lateral edges bilaterally asymmetrical with more photophores dorsally (∼11–16) than ventrally (∼9–12); trabeculae present on protective membrane, trabeculae on distal portion of expanded proximal membrane fused to form a solid muscular area.
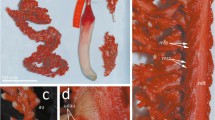
Description: Mantle cone shaped anteriorly, with mantle cavity terminating approximately one third of FL from anterior of fins (thereafter gladius and surrounding musculature continue as narrow cylinder), widest (∼28 % ML) at anterior margin; dorsal anterior mantle margin triangular with point produced over nuchal-locking cartilage. Fins circular in outline when considered together, length ∼64 % ML, width 52–60–68 % ML; anterior lobes absent; tail structure missing due to damage from all specimens examined. Integumental photophores absent from mantle, fins, head, and arms; skin tubercles not observed (skin always damaged); circular skin depressions present on dorsal and ventral surface of fins, and all external surfaces of mantle, head, and funnel (absent from collar).
Head narrowly conical, length 24–32–39 % ML, widest posteriorly (width at midline ∼11 % ML). Olfactory papilla cylindrical. Eye diameter ∼8 % ML. Single elongate photophore on ventral surface of eye (Fig. 4d–f). Funnel widely conical with recurved end, width ∼13 % ML, length ∼13 % ML; aperture posterior to eyeball; funnel pocket absent. Funnel-locking cartilage ear shaped (Fig. 4a), ∼5 % ML; anterior groove concave due to strong antitragus; weak tragus along inner/medial margin; nearly straight along outer/lateral margin. Mantle-locking cartilage approximately oval (Fig. 4b), ∼4 % ML; posteriorly undercut.
Asperoteuthis lui. a–c NIWA 93268, ♀, LRL 5.43 mm; d–f NMNZ M.143859, sex indet., LRL 6.62 mm. a Right funnel-locking cartilage; b right mantle-locking cartilage; c nuchal-locking cartilage; d left eye photophore, anterior view; e left eye photophore, lateral view; f left eye photophore, ventral view. Scale bars: a, b = 1 mm; c = 5 mm; d–f = 10 mm
Arm formula IV ≥ III ≥ II > I; arm length 83–99–108 % ML (Table 2); arms of approximately subequal thickness, with Arms IV thickest and Arms I thinnest; oral faces of arms bordered by membranes, trabeculae absent; aboral keels present on Arms I–III; expanded lateral membrane present on Arms IV. Each arm with ∼122–206 suckers in two series; largest suckers of all located on Arms II (∼75 % arm width) at about row 18–21 (∼30–40 % arm length) and Arms III (∼75 % arm width) at about row 18–23 (∼30–40 % arm length).
Arm-sucker infundibular rings (Fig. 5) proximally adentate, distally with ∼7–11 blunt, rectangular or sharp, conical teeth. Polygonal processes on oral surface of sucker papillated ring often damaged, in ∼2–4 concentric rings; distally, central and intermediate rings with ovate, porous pegs; proximally, central and intermediate rings nearly flat or slightly raised proximally, peripheral ring with flat rectangular or ovate processes.
Tentacle length ∼570 % ML, club length ∼6 % TnL (∼40 % ML), sucker-covered surface ∼75 % club length; proximal protective membrane ∼25 % of club length, widest portion of club surface ∼30 % maximum membrane width; stalk width at base of club ∼70 % club surface width, mid-stalk width ∼40 ∼ % club surface width. Distal club with ∼120–160 suckers in four series (Fig. 6). Proximal rounded expanded portion of protective membrane trabeculate, distally fused to form a solid muscular area; distal non-expanded protective membrane trabeculate; medial aboral club with proximal series of ∼6–8 smaller photophores, medium-sized photophore distally, large club-tip photophore; lateral aboral club with more ventral photophores (∼11–16) than dorsal (∼9–12); tentacle-stalk photophores alternating between larger (∼75–100 % stalk width) and smaller (∼25 % stalk width) along length of stalk, most distal ∼5 % stalk length with only smaller photophores, which decrease in size distally. Sucker infundibular rings (Fig. 7d–m) proximally adentate, distally with ∼5–7 sharp, conical teeth; proximal polygonal processes in papillated ring flat, often irregularly shaped varying from ovate to spindle shaped, in ∼4–6 concentric rings; distal polygonal processes approximately circular to rectangular, slightly elevated proximally, in ∼3 rings.
Asperoteuthis lui. a–c NIWA 93268, ♀, LRL 5.43 mm; d–m NIWA 97258, sex indet., tentacle only, CL 170 mm. a Radula; b radula margin; c palatine palp; d most-proximal tentacle sucker; e outer tentacle sucker close to base; f inner sucker 25 % from most-proximal sucker; g outer sucker 25 % from most-proximal sucker; h inner sucker 50 % from most-proximal sucker; i outer sucker 50 % from most-proximal sucker; j inner sucker 75 % from most-proximal sucker; k outer sucker 75 % from most-proximal sucker; l inner sucker tentacle-tip sucker; m outer sucker tentacle-tip sucker. Scale bars = 500 μm
Lower beak, lateral profile (Fig. 8a, d, g, j, m): lower rostral length ∼43 % wing length, rostral edge with strong curve, rostral tip without hook, rostral tip behind leading edge of wing by 20–28–35 % baseline; wing angle slightly obtuse (nearly right angle), jaw angle obscured by prominent wing fold, shoulder groove present; height 79–87–92 % baseline; hood close to crest, hood length ∼64 % crest length, crest length 54–57–63 % of baseline, visible portion of crest nearly straight; broad lateral-wall fold extending to posterior edge of lateral wall; no or slight notch in lateral wall. Lateral oblique view (Fig. 8b, e, h, k, n) with wing narrowest level with jaw angle, 48–58–68 % of greatest width. Ventral view with broad notch in hood, free corners well separated. Wings remain entirely clear at LRL 3.26 mm, anterior edge of wing below shoulder clear at LRL 5.43 mm, anterior and posterior edge of wing remains clear through at least LRL 7.70 mm.
Asperoteuthis lui beaks. a–c NMNZ M.302215, sex indet., head only, LRL 3.26 mm; d–f NIWA 93268, ♀, LRL 5.43 mm; g–i NMNZ M.143859, sex indet., LRL 6.62 mm; j–l NIWA 95041, ♀, beak only, LRL 7.34 mm; m–o NIWA 95040, sex indet., beak only, LRL 7.70 mm. a, d, g, j, m Lower beaks in lateral profile view; b, e, h, k, n lower beaks in lateral oblique view; c, f, i, l, o upper beaks. Scale bars = 5 mm
Upper beak, lateral profile (Fig. 8c, f, i, l, o): upper rostral length ∼32 % hood length; hood length ∼67 % beak length; hood height ∼39 % beak width. Lateral-wall fold absent; shoulder produced into point or smooth curve, shoulder step 6–43–92 % URL; jaw edge slightly curved; jaw angle nearly right angle.
Radula (Fig. 7a) with tricuspid rachidian, base width ∼60 % height, proximal margin of base rectangular, with broad, sharp triangular mesocone and small, sharp lateral cusps, slightly laterally directed, their height ∼45 % mesocone height. First lateral tooth strongly bicuspid; inner cusp broad, triangular, slightly curved towards rachidian, its height ∼100 % that of overall rachidian; outer cusp sharply pointed, medially directed, its height ∼60 % that of inner cusp. Second lateral tooth simple, curved slightly towards rachidian, ∼125 % rachidian height. Marginal tooth simple, straight, ∼145 % height of rachidian. Marginal plate absent (Fig. 7b). Palatine palp (Fig. 7c) with ∼70 narrow, flat teeth, each ∼15–45 % rachidian height, evenly distributed over palp.
Epidermis damaged on all examined material. Translucent white when fresh, yellow when preserved; purple and red chromatophores dense and evenly distributed on all exterior surfaces, absent from internal mantle.
Taxonomic remarks
Additional, recently collected specimens made this review possible. The most complete specimen available for genetic analysis was in good condition, except that it lacked fins and eyes (NIWA 93268; Fig. 9). The tentacles (Fig. 6) and beaks (Fig. 8d–f) of this specimen are morphologically consistent with those of the holotype for A. lui (Fig. 8g–i). Some of the specimens examined herein consisted of only beaks (with buccal masses, which allowed for genetic analysis) or tentacles; these specimens were used to examine variation in beak morphology (Fig. 8) and tentacle-club photophore patterns, respectively. It appears that the tentacle-club photophores show some intraspecific variation, but a greater sample size will be needed to determine if this is related to sex, growth, or locality.
The original description of A. lui was based on a single, incomplete specimen that was taken from the stomach of a ling (Genypterus blacodes) (Salcedo-Vargas 1999). The specimen only consists of a head and arm crown, including two slightly damaged eyes and one tentacle (Salcedo-Vargas 1999). Unfortunately, the sucker rings from the tentacle club and arms were degraded due to digestion. The only images in the type description were of the eye photophore and the tentacle club and stalk (Salcedo-Vargas 1999). There are some inconsistencies with the type description for A. lui and the observations on that specimen made herein. The description of A. lui stated that the suckers on mid-Arms II and III were enlarged (Salcedo-Vargas 1999); however, they appear to be the largest suckers on the animal, rather than enlarged suckers. The eyeball photophore was described as a patch (Salcedo-Vargas 1999), but the examination herein found that it is elongate (Fig. 4d–f). Salcedo-Vargas (1999) stated that there was no tentacle-club-tip photophore; however, there is a photophore located near the tentacle-club tip (Fig. 6b, d), which is a characteristic of this genus. Two important features that were overlooked in this description were the small embedded photophores on the lateral edges of the aboral surface of the club (Fig. 6b, d) and the beak morphology (Fig. 8).
The characters that Arkhipkin and Laptikhovsky (2008) used to distinguish A. nesisi from other species of Asperoteuthis were based on features that were missing from the A. lui holotype (mantle musculature, fin shape, mantle skin texture, funnel-locking cartilage morphology, and arm-sucker dentition) or not included in the original description of A. lui (arm sucker count and beak morphology). The specimens identified herein as A. lui have morphological characters that are consistent with those of A. nesisi, with some exceptions due to damage or size. The eye photophore was described as a single longitudinal photophore on the ventral surface of the eye (Arkhipkin and Laptikhovsky 2008), which is consistent with the present findings (Fig. 4d–f). The arm suckers described for A. nesisi had 12–14 sharp triangular teeth (Arkhipkin and Laptikhovsky 2008), while the specimen examined herein had 7–11 blunt, rectangular or sharp, conical teeth (Fig. 5); this difference is likely related to size, because the specimen examined in the present study was smaller. Arkhipkin and Laptikhovsky (2008) reported small cartilaginous tubercles on the skin of the head and mantle, which were not observed herein, likely due to the damaged skin on all specimens examined herein. Arkhipkin and Laptikhovsky (2008) suggested that tentacles may be absent in maturing specimens; however, this seems unlikely because the holotype for A. lui, which has similar arm lengths and head width, has a tentacle attached (CL 138 mm) and larger tentacles have been found (NIWA 97258, CL 170 mm).
Arkhipkin and Laptikhovsky (2008) proposed that, due to a similar appearance, A. nesisi was probably synonymous with Clarke’s (1980) ‘?Mastigoteuthis A’, which is supported by the results of the present study. There are few differences between Clarke’s (1980) description and the specimens examined herein. Clarke’s (1980) description is consistent with A. lui based on the characteristic gelatinous tissue that overlies the posterior portion of the mantle, the acutely pointed dorsal mantle, the long and narrow head, radula morphology, funnel- and mantle-locking-cartilage morphology, eye photophore shape, beak morphology, and general appearance. However, Clarke (1980) described a small muscular pad on each side of the posterior end of the mantle in both of the specimens he examined, which was not observed herein or reported by Arkhipkin and Laptikhovsky (2008). It is possible that this was either an artefact due to damage caused by being eaten and partially digested, or possibly a character that is only associated with males (the specimens examined in this study were female or sex indet.). No swimming membranes were described on the arms, but these are easily damaged.
The taxonomic placement of ‘?Mastigoteuthis A’ remained unclear for some time. Nesis (1987) suggested that his new species, ‘Chiroteuthis’ n. sp. Nesis, 1974, from the South Atlantic was synonymous with ‘?Mastigoteuthis A’ and placed this species in a new unnamed genus, n. gen. B. However, there are several morphological differences that distinguish Nesis’s (1974) ‘Chiroteuthis’ n. sp. from Asperoteuthis: six series of suckers on the developing club; photophore present on the ink sac; Arms IV longer than other arms; and funnel-locking cartilage with rounded antitragus and without tragus (Nesis 1974). Because of these differences, the species reported by Nesis (1974) is now considered to belong in a new genus in the family Chiroteuthidae (Nesis and Nikitina 1999).
There are many similarities between tentacle suckers of A. lui and those of the chiroteuthid genus ‘New Genus C’ (Young and Roper 2000a). The tentacle suckers for both have approximately the same number of conical teeth and are surrounded by a wide papillated ring (Young and Roper 2000b). The only known specimen found to represent this new genus was a badly damaged brachial crown from Antarctic waters (Young and Roper 2000a), which is within the distribution for A. lui. It is possible that the ‘New Genus C’ is actually A. lui. Previously, this specimen could not be compared to A. lui because the present study is the first report to describe the tentacle suckers in A. lui.
A comparison of characters found in A. lui, A. mangoldae, and A. acanthoderma is summarized in Table 3 and mean indices are found in Table 2. Asperoteuthis lui is distinguished from both of these species by: (1) an elongate ventral eye photophore; (2) a medial row of photophores on the aboral tentacle club; (3) chiral photophores on the lateral margins of the aboral tentacle club with more ventral photophores than dorsal; (4) ear-shaped funnel-locking cartilage with a weak tragus; (5) approximately oval mantle-locking cartilage; (6) tentacle suckers with 5–7 sharp, conical teeth; (7) more than twice as many tentacle-club suckers (∼120–160); (8) circular skin depressions; (9) a relatively wide mantle; (10) a relatively longer club; and (11) a lack of trabeculae in the distal portion of the proximal, expanded region of the tentacle-club protective membrane. Asperoteuthis lui and A. mangoldae both have approximately circular fins, while A. acanthoderma has an oval fin, but the fin of A. lui is relatively larger. Asperoteuthis acanthoderma is additionally distinguished from the other two species by a larger club-tip photophore, inverted Y-shaped funnel- and mantle-locking cartilages, less teeth on arm and tentacle suckers, and a larger size at maturity (Lu 1977). Asperoteuthis acanthoderma and A. mangoldae both have an oval ventral-eye photophore and lack circular skin depressions. Asperoteuthis acanthoderma has the longest arms relative to mantle length, A. mangoldae has the shortest arms, and A. lui arms are intermediate in length.
There are many commonalities in the aboral tentacle - club photophores in the genus Asperoteuthis. The tentacle-club structure of all three species shares a lack of suckers in the proximal region of the tentacle, but the structure of the tentacles of A. acanthoderma is much more similar to A. mangoldae in terms of trabeculae distribution, sucker count, club length index, and photophores. Photophores in the lateral margins of the tentacle club have been reported for A. mangoldae (Young et al. 2007a), A. acanthoderma (Lu 1977), and were found herein for A. lui. However, unlike the other species in this genus, A. lui photophores are chiral, which is a type of asymmetry in which a structure cannot be superimposed on its mirror image. In A. lui, the left and right tentacle clubs have an asymmetric distribution of photophores and the clubs are mirror images of each other—more photophores are present along the ventral margin (11–16) than along the dorsal margin (9–12). Although chirality is common in squid tentacles, the small embedded aboral photophores are symmetrical in A. acanthoderma (Young and Roper 2010) and A. mangoldae (Young et al. 2007a). In addition, bilaterally asymmetric photophore patterns are found in other squid; for example, the photophore patterns of histioteuthids (Young and Vecchione 2003). Asperoteuthis lui has a series of medial club photophores with approximately five to eight smaller proximal photophores and two larger photophores distally, while A. mangoldae and A. acanthoderma only have a single medial club-tip photophore.
Discussion
The taxonomic study of chiroteuthids is especially difficult because specimens are not frequently caught and are almost always damaged by capture. In addition, species in this family are especially delicate, and nearly all Asperoteuthis specimens lose their tail structure and tentacles during capture, which are important morphological features for their identification. Genetic analyses, such as DNA barcoding (Hebert et al. 2003), can be used for species identification of even badly damaged (St-Onge et al. 2008) and juvenile specimens (Victor et al. 2009). The DNA barcode, along with 16S rRNA and 12S rRNA, has been helpful in species delimitation and the recognition of new species in the chiroteuthid families (Young et al. 2008; Braid et al. 2014, 2016). Herein, the DNA barcode, 16S rRNA, and 12S rRNA, in conjunction with a morphological analysis, have been used to determine that A. ‘nesisi’ and ‘?Mastigoteuthis A’ are junior synonyms of A. lui.
Asperoteuthis ‘nesisi’ and A. lui were both described from single, damaged specimens that were unfortunately missing different morphological features: A. lui had a tentacle present, but the arm and tentacle suckers were damaged, and it lacked a mantle (Salcedo-Vargas 1999), while A. ‘nesisi’ was intact other than the missing tentacles, tail, and damaged fin (Arkhipkin and Laptikhovsky 2008). Because the description for A. lui primarily focused on the tentacle-club morphology, the relationship between A. ‘nesisi’ and A. lui was not clear when A.. ‘nesisi’ was described. The type description for A. ‘nesisi’ included GenBank accession numbers for three mitochondrial genes from the holotype (Arkhipkin and Laptikhovsky 2008). There were no DNA sequences associated with the A. lui holotype because this specimen was formalin-fixed and described before DNA sequences were regularly included with species descriptions.
Sequences for COI, 16S rRNA, and 12S rRNA from the holotype of A. ‘nesisi’ were compared with specimens collected in New Zealand waters with tentacle - club morphology consistent with A. lui and beaks with morphology consistent with ‘?Mastigoteuthis A’, and they all formed a single cluster on the maximum-likelihood phylogeny, with very little variation within that clade, and a large gap between that clade and A. mangoldae (Fig. 3). These three taxa were also assigned the same BIN (Fig. 3), and there is a high concordance between BINs and species (Ratnasingham and Hebert 2013). BINs have been successfully used to delimitate species in other species in the Chiroteuthidae (Braid et al. 2016) and in the closely related squid family Mastigoteuthidae (Braid et al. 2014).
The relationship between the three valid species in this genus remains unclear. Arkhipkin and Laptikhovsky (2008) suggested that, based on morphology, A. acanthoderma and A. ‘nesisi’ have a closer relationship to each other than to A. mangoldae. Herein, it appears that there are more similarities in the tentacle-club morphology of A. acanthoderma and A. mangoldae than with A. lui. However, Braid et al. (2016) found that the genus Asperoteuthis was polyphyletic, and their Bayesian phylogeny showed some support for a sister relationship between A. lui and A. mangoldae. Their results suggested the possibility that there may be additional species of Asperoteuthis that have not been sequenced yet, or that this genus needs to be reassessed.
There are very few records of specimens identified as Asperoteuthis in the stomach contents of predators. However, they have been reported from the gut contents of sperm whales (Physeter macrocephalus) (Gómez-Villota 2007), ling (Genypterus blacodes) (Salcedo-Vargas 1999), and blue sharks (Prionace glauca) (Kubodera et al. 2007). It was only recently recognized that Clarke’s (1980) ‘?Mastigoteuthis A’ is an Asperoteuthis species (Arkhipkin and Laptikhovsky 2008; Young 2015); therefore, this species has been previously incorrectly attributed to the family Mastigoteuthidae in dietary analyses. Even recent ecological studies continue to apply the original incorrect mastigoteuthid classification (e.g., Alvito et al. 2015; Bloom 2012; Guerreiro et al. 2015). Beaks identified as ‘?Mastigoteuthis A’ have been reported from the stomach contents of southern bottlenose whales (Hyperoodon planifrons) (Clarke and Goodall 1994), sperm whales (P. macrocephalus) (Clarke 1980; Pascoe et al. 1990), grey-headed albatrosses (Thalassarche chrysostoma) (Cherel et al. 2002; Richoux et al. 2010; Alvito et al. 2015), wandering albatross (Diomedea exulans) (Guerreiro et al. 2015; Rodhouse et al. 1987; Xavier et al. 2011), white-chinned petrels (Procellaria aequinoctialis) (Bloom 2012), Antarctic fur seals (Arctocephalus gazelle) (Lea et al. 2002), Patagonian toothfish (Dissostichus eleginoides) (Cherel et al. 2004), and porbeagles (Lamna nasus) (Cherel and Duhamel 2004). Herein, ‘?Mastigoteuthis A’ has been recognized as a junior synonym of A. lui. Therefore, the previous importance of ‘?Mastigoteuthis A’ in the diet of marine predators must be transferred to A. lui. The role of Asperoteuthis in the feeding ecology of other species has been dramatically underestimated.
Using a combination of morphology and mitochondrial genes, the identity of A. lui, A. nesisi, and ‘?Mastigoteuthis A’ has been resolved. This demonstrates the importance of including genetic sequences with species descriptions whenever possible. The ecological importance of Asperoteuthis in the diet of marine mammals, birds, and fish has been underestimated because the identity of ‘?Mastigoteuthis A’ remained unknown until recently. It appears that the biodiversity of the genus Asperoteuthis has been overestimated, with a previous estimate of five species (Young and Roper 2015). Currently, only three valid species are recognized in this genus: A. acanthoderma, A. mangoldae, and A. lui. This study highlights the significance of critical taxonomic revisions as additional material for poorly known species becomes available. Accurate species identification is fundamental for all biological research, with ramifications for predator-prey relationships and conservation.
References
Alvito PM, Rosa R, Phillips RA, Cherel Y, Ceia F, Guerreiro M, Seco J, Baeta A, Vieira RP, Xavier J (2015) Cephalopods in the diet of nonbreeding black-browed and grey-headed albatrosses from South Georgia. Polar Biol 38(5):631–641. doi:10.1007/s00300-014-1626-3
Arkhipkin AI, Laptikhovsky VV (2008) Discovery of the fourth species of the enigmatic chiroteuthid squid Asperoteuthis (Cephalopoda: Oegopsida) and extension of the range of the genus to the South Atlantic. J Molluscan Stud 74(3):203–207. doi:10.1093/mollus/eyn007
Berry SS (1909) Diagnoses of new cephalopods from the Hawaiian Islands. Proc US Natl Mus 37:407–419. doi:10.5479/si.00963801.37-1713.407
Bloom S-A (2012) Feeding ecology of white-chinned petrels: diet and their diving patterns around South Georgia. Dissertation, Universidade de Coimbra
Bolstad KSR (2010) Systematics of the Onychoteuthidae Gray, 1847 (Cephalopoda: Oegopsida). Zootaxa 9626:1–186
Braid HE, Kubodera T, Bolstad KSR (2016) One step closer to understanding the chiroteuthid families in the Pacific Ocean. Mar Biodiv
Braid HE, Bolstad KSR (2015) Systematics of the Mastigoteuthidae Verrill, 1881 (Cephalopoda: Oegopsida) from New Zealand waters. N Z J Zool 42(3):187–256. doi:10.1080/03014223.2015.1063516
Braid HE, McBride PD, Bolstad KS (2014) Molecular phylogenetic analysis of the squid family Mastigoteuthidae (Mollusca, Cephalopoda) based on three mitochondrial genes. Hydrobiologia 725(1):145–164. doi:10.1007/s10750-013-1775-3
Cherel Y, Duhamel G (2004) Antarctic jaws: cephalopod prey of sharks in Kerguelen waters. Deep Sea Res I 51:17–31. doi:10.1016/j.dsr.2003.09.009
Cherel Y, Weimerskirch H, Trouvé C (2002) Dietary evidence for spatial foraging segregation in sympatric albatrosses (Diomedea spp.) rearing chicks at Iles Nuageuses, Kerguelen. Mar Biol 141:1117–1129. doi:10.1007/s00227-002-0907-5
Cherel Y, Duhamel G, Gasco N (2004) Cephalopod fauna of subantarctic islands: new information from predators. Mar Ecol Prog Ser 266:143–156. doi:10.3354/meps266143
Clarke MR (1980) Cephalopoda in the diet of sperm whales of the southern hemisphere and their bearing on sperm whale biology. Discov Rep 37:1–324
Clarke MR (1986) A handbook for the identification of cephalopod beaks. Clarendon Press, Oxford
Clarke M, Goodall N (1994) Cephalopods in the diets of three odontocete cetacean species stranded at Tierra del Fuego, Globicephala melaena (Traill, 1809), Hyperoodon planifrons Flower, 1882 and Cephalorhynchus commersonii (Lacepede, 1804). Antarct Sci 6(2):149–154. doi:10.1017/s0954102094000234
Gómez-Villota F (2007) Sperm whale diet in New Zealand. Dissertation, Auckland University of Technology
Gray JE (1849) Catalogue of the Mollusca in the collection of the British Museum. Part I. Cephalopoda Antepedia. Printed by order of the Trustees, London. doi:10.5962/bhl.title.21153
Guerreiro M, Phillips RA, Cherel Y, Ceia FR, Alvito P, Rosa R, Xavier JC (2015) Habitat and trophic ecology of Southern Ocean cephalopods from stable isotope analyses. Mar Ecol Prog Ser 530:119–134. doi:10.3354/meps11266
Hebert PDN, Cywinska A, Ball SL, deWaard JR (2003) Biological identifications through DNA barcodes. Proc R Soc Lond B Biol 270:313–321. doi:10.1098/rspb.2002.2218
Joubin L (1933) Notes préliminaires sur les Céphalopodes des croisières du Dana (1921–1922), 4e Partie. Ann Inst Oceanogr 13:1–49
Judkins H, Ingrao DA, Roper CF (2009) First records of Asperoteuthis acanthoderma (Lu, 1977) (Cephalopoda: Oegopsida: Chiroteuthidae), from the North Atlantic Ocean, Straits of Florida. Proc Biol Soc Wash 122(2):162–170. doi:10.2988/08-30.1
Katoh K, Standley DM (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol 30:772–780. doi:10.1093/molbev/mst010
Kubodera T, Watanabe H, Ichii T (2007) Feeding habits of the blue shark, Prionace glauca, and salmon shark, Lamna ditropis, in the transition region of the Western North Pacific. Rev Fish Biol Fish 17:111–124. doi:10.1007/s11160-006-9020-z
Lanfear R, Calcott B, Ho SYW, Guindon S (2012) PartitionFinder: combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol Biol Evol 29:1695–1701. doi:10.1093/molbev/mss020
Lea M-A, Cherel Y, Guinet C, Nichols PD (2002) Antarctic fur seals foraging in the Polar Frontal Zone: inter-annual shifts in diet as shown from fecal and fatty acid analyses. Mar Ecol Prog Ser 245:281–297. doi:10.3354/meps245281
Lu CC (1977) A new species of squid Chiroteuthis acanthoderma, from the Southwest Pacific (Cephalopoda, Chiroteuthidae). Steenstrupia 4:179–188
Nesis KN (1974) The oceanic cephalopods of the South-Western Atlantic. Trudy Inst Oceanol Acad Sci USSR 98:51–75
Nesis KN (1980) Taxonomic position of Chiroteuthis famelica Berry (Cephalopoda, Oegopsida). Byull Moskovsk Obshch Isp Prir Otd Biol 85:59–66
Nesis KN (1987) Cephalopods of the world (English translation). Tropical Fish Hobbyist (T.F.H.) publications, Neptune City
Nesis KN, Nikitina I (1999) New Genus B. Available online at: http://tolweb.org/New_Genus_B/19460. Accessed 11 April 2016
Pascoe PL, Mickiewicz MC, Castello HP (1990) Cephalopod remains from the stomach of a sperm whale stranded off Patagonia. Mar Biol 104:1–4. doi:10.1007/bf01313150
Rambaut A (2012) FigTree version 1.4.0. Home page at: http://tree.bio.ed.ac.uk/software/figtree
Ratnasingham S, Hebert PDN (2007) BOLD: The Barcode of Life Data System (http://www.barcodinglife.org). Mol Ecol Notes 7:355–364. doi:10.1111/j.1471-8286.2007.01678.x
Ratnasingham S, Hebert PDN (2013) A DNA-based registry for all animal species: the barcode index number (BIN) system. PLoS One 8(7), e66213. doi:10.1371/journal.pone.0066213
Richoux NB, Jaquemet S, Bonnevie BT, Cherel Y, McQuaid CD (2010) Trophic ecology of Grey-headed albatrosses from Marion Island, Southern Ocean: insights from stomach contents and diet tracers. Mar Biol 157:1755–1766. doi:10.1007/s00227-010-1448-y
Rodhouse PG, Clarke MR, Murray AWA (1987) Cephalopod prey of the wandering albatross Diomedea exulans. Mar Biol 96:1–10. doi:10.1007/bf00394833
Roper CFE, Voss GL (1983) Guidelines for taxonomic descriptions of cephalopod species. Mem Natl Mus Victoria 44:49–63
Roper CFE, Young RE (1975) Vertical distribution of pelagic cephalopods. Smith Contrib Zool 209:1–51. doi:10.5479/si.00810282.209
Salcedo-Vargas MA (1995) Systematic value of the ultrastructure of the sucker surface in the squid family Mastigoteuthidae (Mollusca: Cephalopoda). Contrib Zool 65:65–77
Salcedo-Vargas MA (1999) An asperoteuthid squid (Mollusca: Cephalopoda: Chiroteuthidae) from New Zealand misidentified as Architeuthis. Zoosyst Evol 75:47–49. doi:10.1002/mmnz.19990750106
St-Onge M, LaRue B, Charpentier G (2008) A molecular revision of the taxonomic status of mermithid parasites of black flies from Quebec (Canada). J Invertebr Pathol 98:299–306. doi:10.1016/j.jip.2008.04.001
Tamura K, Nei M (1993) Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol 10(3):512–526
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30(12):2725–2729. doi:10.1093/molbev/mst197
Tsuchiya K, Okutani T (1993) Rare and interesting squids in Japan, recent occurrences of big squids from Okinawa. Venus 52:299–311
Vaidya G, Lohman DJ, Meier R (2011) SequenceMatrix: concatenation software for the fast assembly of multi-gene datasets with character set and codon information. Cladistics 27:171–180. doi:10.1111/j.1096-0031.2010.00329.x
Verrill AE (1881) Report on the cephalopods and on some additional species dredged by the U.S. Fish Commission Steamer ‘Fish-hawk’, during the season of 1880. Bull Mus Comp Zool 8:99–116
Victor BC, Hanner R, Shivji M, Hyde J, Caldow C (2009) Identification of the larval and juvenile stages of the Cubera Snapper, Lutjanus cyanopterus, using DNA barcoding. Zootaxa 2215:24–36
Xavier JC, Phillips RA, Cherel Y (2011) Cephalopods in marine predator diet assessments: why identifying upper and lower beaks is important. ICES J Mar Sci 68(9):1857–1864. doi:10.1093/icesjms/fsr103
Young RE (1978) Vertical distribution and photosensitive vesicles of pelagic cephalopods from Hawaiian waters. Fish Bull 76:583–615
Young RE (1991) Chiroteuthid and related paralarvae from Hawaiian waters. Bull Mar Sci 49:162–185
Young RE (2015) Clarke’s ?Mastigoteuthis A. Available online at: http://tolweb.org/notes/?note_id=5668. Accessed 11 April 2016
Young RE, Roper CFE (2000a) New Genus C. Available online at: http://tolweb.org/New_Genus_C/19459. Accessed 1 June 2016
Young RE, Roper CFE (2000b) New Genus C: Scanning electron micrographs of suckers. Available online at: http://tolweb.org/accessory/New_Genus_C_Suckers?acc_id=753. Accessed 1 June 2016
Young RE, Roper CFE (2010) Asperoteuthis acanthoderma (Lu, 1977). Available online at: http://tolweb.org/Asperoteuthis_acanthoderma/19466. Accessed 11 April 2016
Young RE, Roper CFE (2015) Asperoteuthis Nesis, 1980. Available online at: http://tolweb.org/Asperoteuthis/19461. Accessed 11 April 2016
Young RE, Vecchione M (2003) Histioteuthidae: Photophore Patterns. Available online at: http://tolweb.org/accessory/Histioteuthidae:_Photophore_Patterns?acc_id=1180. Accessed 9 April 2016
Young RE, Vecchione M, Roper CFE (2007a) A new genus and three new species of decapodiform cephalopods (Mollusca: Cephalopoda). Rev Fish Biol Fish 17:353–365. doi:10.1007/s11160-007-9044-z
Young RE, Vecchione M, Roper CFE (2007b) Asperoteuthis mangoldae: description continued. Available online at: http://tolweb.org/notes/?note_id=2788. Accessed 11 April 2016
Young RE, Lindgren A, Vecchione M (2008) Mastigoteuthis microlucens, a new species of the squid family Mastigoteuthidae (Mollusca: Cephalopoda). Proc Biol Soc Wash 121:276–282. doi:10.2988/07-40.1
Young RE, Vecchione M, Roper CFE (2011) Asperoteuthis mangoldae Young, Vecchione and Roper, 2007. Available online at: http://tolweb.org/Asperoteuthis_mangoldae/19467. Accessed 11 April 2016
Zwickl DJ (2006) Genetic algorithm approaches for the phylogenetic analysis of large biological sequence datasets under the maximum likelihood criterion. Dissertation, The University of Texas
Acknowledgments
I am grateful to the Royal Society of New Zealand’s Hutton Fund, which supported the genetic analysis for this study. I would like to thank the Museum of New Zealand Te Papa Tongarewa, particularly Bruce Marshall, and the National Institute of Water and Atmospheric Research Ltd. (NIWA), especially Darren Stevens for help with specimen collection, beak identification, access to his beak collection, and photographs of Asperoteuthis lui. Many thanks go to the scientists and crew of the RV Tangaroa for always checking carefully for cephalopods and especially Ian “Hearn” Smith for finding the most complete A. lui specimen available for genetic analysis. Thanks to Adrian Turner at the Auckland University for assistance with critical-point drying, and Patrick Conor at AUT for platinum plating and imaging assistance. Many thanks go to Jesse Kelly for insightful comments and encouragement, Kat Bolstad for her endless patience, and to Steve O’Shea for the most intact A. lui specimen available from New Zealand waters for morphological examination.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Vecchione
Rights and permissions
About this article
Cite this article
Braid, H.E. Resolving the taxonomic status of Asperoteuthis lui Salcedo-Vargas, 1999 (Cephalopoda, Chiroteuthidae) using integrative taxonomy. Mar Biodiv 47, 621–635 (2017). https://doi.org/10.1007/s12526-016-0547-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12526-016-0547-5