Abstract
A total of 52 cephalopod beaks were found in the stomachs and intestines of 17 out of 54 green turtles, Chelonia mydas, stranded on the Uruguayan coast between 2009 and 2013 (frequency of occurrence = 31.5 %). Upper and lower beaks were assigned to at least six Oegopsid species of four different genera, Chiroteuthis, Histioteuthis, Onykia (= Moroteuthis) and Mastigoteuthis. Although the presence of cephalopods in the diet of green turtles has been reported previously, it has been quoted as a sporadic or less important diet category. Our findings suggest that this intake of cephalopods by juvenile green turtles during their oceanic stages may be more common in the Southwestern Atlantic Ocean waters than previously thought. According to our records, two of the six Oegopsid squid species found would have a more extended distribution than previously reported, ranging from circumpolar sub-Antarctic areas to the Brazil-Malvinas confluence zone. We highlight the potential of diet analysis of pelagic predators as a tool to enhance our knowledge of the diversity and distribution of poorly known cephalopod species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oegopsid squids are widely distributed in all oceans and seas of the world, inhabiting virtually all depths and areas of the oceans, from surface to deep waters of ca. 4,000 m (Collins and Rodhouse 2006; Hoving et al. 2014). Many Oegopsid species undergo significant diel vertical migrations, occurring in deep waters during the day and then ascending towards surface waters (0–200 m) at night. In the past few years there has been increasing interest in South Atlantic cephalopods, given the role they play in the food webs in the area (Rodhouse et al. 1992; 1994; Rodhouse and Nigmatullin 1996; Okutani 1994). This order includes the ommastrephid squid Illex argentinus and the loliginid squid Loligo gahi, which are targeted by a multi-national fishing fleet in the Malvinas Islands region, where these species are particularly abundant (e.g. Csirke 1987; Hatfield and Rodhouse 1991). Despite this, the Southern Ocean Oegopsid fauna is poorly known (Xavier et al. 1999; Collins and Rodhouse 2006; Rodhouse et al. 2014). Most of the species are poorly represented in global databases, and it is still critical to determine most of their distributions (Rodhouse and White 1995; Cherel et al. 2004; Xavier et al. 2011).
There is, however, substantial potential for deriving data on the ecology and distribution of cephalopod species using diet analysis of their predators; especially for those species that are not subject to commercial fishing or are difficult to sample (Cherel and Weimerskirch 1995, 1999; Croxall and Prince 1996; Rodhouse and Nigmatullin 1996; Xavier et al. 2002, 2006; Field et al. 2007). Such potential is enhanced by the increasing knowledge on the morphology of cephalopod beaks (chitinous hard structures that resist predator digestion) allowing identification to the species level of most of the accumulated items found in predators’ stomachs (Clarke 1986; Imber 1992; Cherel et al. 2004; Xavier et al. 2005; Morais et al. 2012).
The green turtle, Chelonia mydas, an endangered species (International Union for Conservation of Nature and Natural Resources 2013), seems to be included among the squid’s predators in various different areas (Parker et al. 2011; Seminoff et al. 2002; Morais et al. 2012). This species is distributed worldwide, inhabiting continental shelves, bays, lagoons, and estuaries in the temperate, subtropical and tropical waters of the Atlantic, Pacific, and Indian Oceans (Hirth 1997). In the Southwestern Atlantic (SWA) waters, juvenile green turtles occur in neritic waters along the continent and around oceanic islands (Marcovaldi and Marcovaldi 1999; Domingo et al. 2006). In the Uruguayan coast, juveniles of this species occur year round (López-Mendilaharsu et al. 2006; Vélez-Rubio et al. 2013) along the saline gradient defined by the confluence of the freshwater discharged by the second largest South Atlantic estuary, the Río de la Plata estuary, and oceanic water masses. Important foraging and development areas for immature green turtles are located along the coast, with the Marine Protected Area of Cerro Verde, Punta del Diablo, the Marine protected Area of Cabo Polonio (Rocha) and Bajos del Solís (Canelones-Maldonado) being the most relevant areas for the species in the region (López-Mendilaharsu et al. 2006; Vélez-Rubio et al. 2013). Immature green turtles occurring in these areas recruit mainly from the nesting populations from Ascension Island (UK) but also from Trinidad Island (Brazil), Aves Island (Venezuela), Surinam and Guinea Bissau (Africa) (Caraccio 2008).
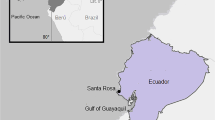
Although green turtles are the only marine turtles considered to be herbivorous throughout most of their life (Bjorndal 1997), they are known to undergo an ontogenetic shift in their diet, from opportunistic during the earlier oceanic phase of their lives (Boyle and Limpus 2008) to nearly complete herbivorous once they settle in coastal habitats (Chaloupka and Limpus 2001; Arthur and Balazs 2008), upon reaching carapace lengths of 25–35 cm (Bjorndal et al. 1990; Limpus et al. 1994; Reich et al. 2007; Cardona et al. 2009) and an age of 3–6 years (Zug and Glor 1999; Zug et al. 2001; Balazs and Chaloupka 2004; Chaloupka et al. 2004; Reich et al. 2007; Cardona et al. 2009). However, the sizes at which this habitat shift occurs may vary among the different green turtle populations. In the SWA (including South Brazil, Uruguay and North Argentina), when they recruit to neritic areas after the oceanic stage they perform seasonal latitudinal migrations along these areas over the years (north of 40°S; López-Mendilaharsu et al. 2006; Martinez-Souza et al. 2012; González Carman et al. 2012) (Fig. 1).
Overview of neritic-benthic (red) and oceanic-pelagic (blue) putative feeding areas for juvenile green turtles (Chelonia mydas) in the Southwestern Atlantic (SWA), based on satellite telemetry data from nine individuals provided by González Carman et al. (2013). Black points indicate the stranding locations of studied turtles along the Uruguayan Coast in the present study
Studies based on esophageal lavage and stomach contents of dead animals in other regions suggest that the diet of neritic-stage juvenile green turtles is composed almost exclusively of seagrasses and macroalgae, with diet composition driven by the relative availability and quality of these food types (e.g., Brand et al. 1999a; Read and Limpus 2002; López-Mendilaharsu et al. 2008; Carrión-Cortez et al. 2010; Guebert-Bartholo et al. 2011; Reisser et al. 2013). However, a persistent omnivorism of green turtles is present in some areas, as in North West African waters (Cardona et al. 2009), the eastern Mediterranean (Godley et al. 1998), Japan (Hatase et al. 2006), western Colombia (Amorocho and Reina 2007) and probably in other regions (Seminoff et al. 2002; Ferreira et al. 2006; Russell et al. 2011; Nagoaka et al. 2012). Furthermore, during their juvenile oceanic stage, cephalopods may constitute a complementary food resource to the normal diet of cnidarians, gastropods and crustaceans (Boyle and Limpus 2008; Parker et al. 2011). Indeed, pelagic squids have been reported in the green turtles diet in other areas (e.g. Morais et al. 2012; Parker et al. 2011; Seminoff et al. 2002). Consequently, green turtles could function as “biological samplers” of pelagic cephalopods and other animals living in epipelagic waters.
Under such a context, the present paper aims (1) to improve knowledge on the ecology and distribution of Oegopsid squids in the SWA, and (2) to evaluate the importance of cephalopods in the diet of green turtles in Uruguayan waters.
Materials and methods
The Uruguayan coast is part of a complex hydrological system that is comprised of the frontal zone of the Rio de la Plata estuary (RP) and the Atlantic Ocean, presenting a strong along-shore salinity gradient (Ortega and Martínez 2007; Campos et al. 2008; Horta and Defeo 2012) (Fig. 1). The waters of this country have been described as part of a feeding ground for three marine turtles species, with records of two other species (Vélez-Rubio et al. 2013). One of the former three is the green turtle, considered in the present study. The gut contents analyzed here were collected from 2009 to 2013 from green turtles stranded along the southern and western Uruguayan coast (Fig. 1). Stranded turtles were found during beach surveys conducted by technicians of the Marine Turtle Stranding Network run by the local NGO Karumbe (Vélez-Rubio et al. 2013).
The curved carapace length, notch to tip (CCLn-t) of each green turtle was measured using a flexible tape (error ± 0.1 cm). Digestive tract contents were collected separately (esophagus, stomach and intestines) and fixed in 4 % formaldehyde. Diet items (both animal and vegetable) were identified to the lowest taxonomic category, depending on decomposition state. Cephalopod beaks were found in gut contents. Almost all cephalopod beaks (82 % of the total) were identified at least to the genus level, based on beak morphology and morphometrics. No other cephalopod remains but the beaks were found. Beak identification was made by identification keys available in the scientific literature (Clarke 1986; Xavier and Cherel 2009). Broken beaks were excluded from the analyses. Lower rostral length (LRL) of lower beaks was measured with a vernier calliper, following Clarke (1986). Depending on the color and transparency of the wing beaks and the bottom of the lateral wall, we distinguished between fresh (transparent wing), recently consumed beaks (within few days) or old beaks (darkened wing), those that had potentially stayed in the turtles for a longer time (weeks/months). The beak color can also be used to determine the development status of the squids [i.e., juvenile (mostly transparent beaks), sub-adults (partially darkened) or adults (wings already darkened)]. The digesta retention time (DRT) of particulate matter for green turtles is almost 6–13 days (Brand et al. 1999b). Therefore, it is likely that the beaks probably were attached to and remained in the gut of the green turtles for longer than this.
Voucher material is deposited at the Museo Nacional de Historia Natural (Montevideo, Uruguay) and Cavanilles Institute of Biodiversity and Evolutionary Biology (Universidad de Valencia, Spain). Statistical analyses were conducted in R 2.11.1 (R Development Core Team 2008). The relationships between green turtle sizes and the presence/absence of cephalopods beaks were tested using simple linear regression models followed by an analysis of variance. Values are presented as mean ± SD, and statistical significance on differences was assumed at p < 0.05. In all cases, normality of residuals was assessed using the Shapiro–Wilk test.
Results
A total of 52 cephalopod beaks were found in the stomachs and intestines of 17 out of the 54 green turtle individuals analyzed [frequency of occurrence (FO), 31.5 %]. Cephalopod beaks found were assigned to six different species (see below). Based on the CCL of the green turtles studied, all the turtles measured were of juvenile size (Vélez-Rubio et al. 2013 and the references therein), (mean CCLn-t ± SD = 40.0 ± 7.0 cm; range, 29.8-62.0 cm). Turtles with cephalopod beaks in their guts (CCLn-t = 36.8 ± 7.8 cm; range, 29.8–62.0; N = 17) seem to be smaller (F 1, 45 = 4.567; p < 0.05) than those without beaks (CCLn-t = 41.4 ± 6.3 cm; range, 32.9-61.0; N = 37) (Fig. 2). Gut contents of the 54 turtles analyzed included 20 macroalgae species, 7 animal taxa and marine debris. A comprehensive study of the diet of these turtles is out of the scope of the present paper, although detailed studies are ongoing. The presence of cephalopod beaks in the gut contents was associated in all cases with the presence of floating marine debris (soft and hard plastics, foam, rope, etc.) and other floating dietary items including Sargassum spp., pelagic molluscs (such as Janthina sp.) and gelatinous zooplankton (such as pyrosomids and salps). All the turtles containing cephalopod beaks were found stranded in the oceanic influence zone of the Uruguayan coast (Fig. 1) during the warmer months (January to May).
The majority of beaks found (82 % of the total) were in relatively good condition and could be measured. All the beaks present were in stomachs and intestines. No beaks were detected in the esophagus. According to their state of conservation, all the beaks found were classified as ‘old’, probably having been consumed several months before the stranding, and remained attached in the gut of the turtles. The beaks seemed to correspond to sub-adult and adult cephalopod specimens (Fig. 3). The dominant cephalopod species in terms of occurrence were no. 1 with 22 beaks (FO = 52.9 %), and no. 2 with 13 beaks (FO = 25 %), whereas species 4, 5 and 6 were represented by one specimen each (Table 1). A description of morphological features of the beaks that lead to specific determination is provided in the next section, following Clarke (1986) and Xavier and Cherel (2009).
Cephalopod species
Species 1
Examined material: 22 complete lower beaks (Fig. 3a): size (mean LRL ± SD) = 3.64 ± 0.47 mm; range, 3.08-4.56 mm. Description: thin in profile, with a triangular form, a thick fold running to the middle of the posterior edge of the lateral wall. Crest similar in size or slightly smaller than the hood. Shoulder tooth absent. Obtuse jaw angle hidden in profile by the wing fold, a weakly marked angle point. Hood lying close to the crest. Assigned to Chiroteuthis veranyi (Ferussac 1835).
Species 2
Examined material: 13 complete lower beaks (Fig. 3b): size (mean LRL ± SD) = 4.04 ± 0.74 mm; range, 3.17-5.59 mm. Description: rather square in profile, a deep notch in the back of the hood. Crest equal or slightly larger than the hood. A well-developed ridge runs to the free corner of the lateral wall. Low wing fold and a shoulder tooth. Assigned to Histioteuthis bonnellii corpuscula (Clarke 1980).
Species 3
Examined material: two complete lower beaks: Size (mean LRL ± SD) = 4.52 ± 0.40 mm. Description: rather square in profile, a deep notch in the back of the hood, and a ridge running to the free corner of the lateral wall. Tends to be deeper than longer, with a very obvious ridge (also called a keel) under the hood. Assigned to Histioteuthis arcturi (Robson 1948) [also known as Histioteuthis A5 (Clarke 1986)].
Species 4
Examined material: one complete lower beak (Fig. 3c): size (mean LRL) = 2.79 mm. Description: rather square in profile, a shallow notch in the back of the hood, a weakly developed ridge that becomes a slight fold running to the free corner of the lateral wall, and a high wing fold. Assigned to Histioteuthis atlantica (Hoyle 1885).
Species 5
Examined material: one complete lower beak (Fig. 3d): size (mean LRL) = 3.43 mm. Description: a broad notch in the back of the hood, a well defined fold running to below the midpoint of the posterior edge of the lateral wall, and a shoulder tooth. Assigned to Mastigoteuthis psychrophila (Nesis 1977).
Species 6
Examined material: one complete lower beak (Fig. 3e): size (mean LRL) = 3.75 mm. Description: a long step below the jaw angle extends past the lower edge of the darkened part of the lateral wall. A fold runs to a position about halfway between the crest and the free corner of the lateral wall. Assigned to Moroteuthis sp. B (Imber) (Xavier and Cherel 2009), now the genus Moroteuthis is considered as Onykia (Tsuchiya and Okutani 1991; Wakabayashi et al. 2007).
Discussion
The present paper includes the first report of cephalopods in the diet of green turtles in Uruguayan waters. The studied turtles ingested several different squid species: beaks were assigned to six Oegopsid species from four genera: Chiroteuthis, Histioteuthis, Onykia (= Moroteuthis), and Mastigoteuthis. This finding suggests that cephalopod ingestion by juvenile green turtles during their oceanic stages does not seem to be a rare event in this part of the SWA waters. To date, only four Oegopsid species, Onychoteuthis banksii, Illex argentinus, Ommastrephes bartramii and Histioteuthis macrohista, have been reported in Uruguayan waters (Scarabino 2003; Ayçaguer and Nieddu 2014). None of them were detected in the green turtles examined in the present paper. If squid species found in the analyzed green turtles were ingested in Uruguayan waters, our findings should add at least other six species to the Oegopsid fauna reported in the Brazil-Malvinas Confluence Zone. However, we cannot ensure that since cephalopod beaks are composed of chitin, which is almost indigestible to stomach acids, in contrast to the soft tissues that are rapidly digested. Indeed, beaks may accumulate and be attached for months or years into the stomachs or intestines of marine vertebrates (Hernández-García 1995; Xavier et al. 2005; Morais et al. 2012). The pointed and complex shape of the beaks may help in such retention in the intestines. Hence it is possible that some cephalopods detected in the present study would have been ingested elsewhere out of Uruguayan waters.
Available knowledge on the distribution of juvenile green turtles in the SWA suggests that the distribution limit for this species is most likely located around 40° S (see González Carman et al. 2013 and references therein). These authors stressed that, overall, green turtles expended ca. 38 % of their time foraging in the Río de la Plata and deep-water areas. Based on this evidence, we are confident that cephalopod ingestion occurs north of this latitude (Fig. 1). The beaks found were determined as ‘old’ (see the ‘Materials and methods’ section), hence they probably were ingested and accumulated in the turtles’ digestive tracts during their oceanic stage, remaining in the gut after they reached the neritic zones. This inference is supported by the fact that beaks were found predominantly in smaller turtles (Fig. 2), and by the darker color of the beaks found (Fig. 3).
The squid species found in the diet of green turtles form Uruguayan waters are frequent in the diet of pelagic predators such as albacore (Salman and Karakulak 2009), swordfish (Hernández-García 1995), blue and short-fin mako sharks (Vaske-junior and Rincon-Filho 1998), petrels (Cherel and Klages 1998), albatrosses (Croxall and Prince 1994), and porpoises (Ohizumi et al. 2003). However, it is still unclear how non-expert divers such as small juvenile green turtles, among other predators, may predate over fast-swimming squid species close to surface waters (see Xavier et al. 2013). The families Chiroteuthidae and Histioteuthidae are comprised of oceanic medium-depth to deep-water gelatinous squids (Young and Vecchione 2007). Both have ammonia-mediated fluctuation mechanisms (Voight et al. 1994), apparently undergoing ontogenetic and diel vertical migrations in the water column of offshore habitats (Roper and Young 1974), but little is known about this vertical distribution of these species. Since the presence of cephalopod beaks was always associated with the presence of floating marine debris and other floating dietary items, and juvenile green turtles do not dive to more than 30 m depth (Blumenthal et al. 2010), we suggest that all squid species were ingested in waters close to the surface. Since certain squids present high levels of ammonia in their tissues (e.g., Histioteuthidae, Voight et al. 1994), these organisms may float after death (Lu and Williams 1994). Thus, green turtles analyszd could have been scavenging these floating dead squids. In addition, individuals of Histioteuthis are a relatively common by-catch in deep-water trawling operations in the southeast and south Brazil, and could be discarded in open waters (Perez et al. 2004; Morais et al. 2012), where small pelagic juvenile green turtles can feed on them.
Concerning the distribution of the cephalopod species found in the present study, C. veranyi is distributed throughout most of the Atlantic and the southern subtropical areas of the Indian and Pacific Oceans (Nesis 1987). This species is known to be distributed also in Antarctic and sub-Antarctic waters (Rodhouse and Lu 1998; Xavier et al. 2002). H. atlantica has a circumglobal distribution in southern waters. H. bonnellii corpuscula is a small species and is widely but unevenly distributed in the Atlantic. It is absent from northern subtropical and western tropical waters and probably other areas, but can be found in a narrow band of subtropical waters that includes areas off Argentina, South Africa and the region between Australia and New Zealand (Voss et al. 1998). The distribution of H. arcturi extends throughout the tropical and subtropical Atlantic between about 40°N and 30°S (Voss et al. 1998). These three species present records within the Brazil-Malvinas confluence zone. However, M. psychrophila presents a circum-Antarctic distribution occurring both north and south of the Antarctic Convergence (Nesis 1977). This species is pelagic, apparently occupying deep water, but no conclusive information is available about its vertical distribution. We could not reach species level for the Moroteuthis (= Onykia) species, although it is very likely that the species corresponds to the poorly known Moroteuthis sp. B (Imber) (Xavier and Cherel 2009). The distribution of this species include subtropical and sub-Antarctic waters, but no record of this species seems to exist north of ca. 46° S (Bolstad 2010). The present findings suggest changes in the distribution of these species. Our results suggest an extension of the Atlantic distribution of at least two of these species [M. psychrophila and Moroteuthis sp. B (Imber)] well north of the Antarctic Convergence to the region of the Subtropical Front.
Our results also provide new insight into Oegopsid squid distribution in the SWA area. The presence of cephalopod beaks in the digestive tracts of green turtles stranded on Uruguayan coasts seems to be increasing in recent years. No beaks were found in digestive tracts prior to 2009, although gut contents of stranded turtles are being revised since 2000 (Karumbe, unpublished data). Under a climate change scenario, sea surface temperature (SST) anomalies at the SWA, showing increasing trends and a progressive dominance of temperature positive anomalies for the period 1950–2000, have been observed (Wainer and Venegas 2002). This is associated with variability in the intensity of the Malvinas western boundary current and in the position of the Brazil–Malvinas confluence zone. Anomalous advection of cold waters northward and warm waters southward, resulting from changes in Malvinas current intensity, seem to be responsible for these temperature anomalies in the subtropical SWA (Wainer and Venegas 2002). This variability in the location of the Brazil–Malvinas confluence zone may increase the occurrence of Patagonian and/or circumpolar and subtropical cephalopods in waters north of Uruguay, thus increasing the likelihood of them being ingested by green turtles foraging there.
References
Amorocho D, Reina RD (2007) Feeding ecology of the East Pacific green sea turtle Chelonia mydas agassizii at Gorgona National Park, Colombia. Endanger Species Res 3:43–51
Arthur KE, Balazs GH (2008) A comparison of immature green turtle (Chelonia mydas) diets among seven sites in the Main Hawaiian Islands. Pac Sci 62:205–217
Ayçaguer CB, Nieddu MA (2014) (In press) New records of Histioteuthis macrohista N. Voss, 1969 (Cephalopoda: Teuthida) from the South Western Atlantic. Comunicaciones de la Sociedad Malacológica del Uruguay
Balazs GH, Chaloupka M (2004) Spatial and temporal variability in somatic growth of green sea turtles (Chelonia mydas) resident in the Hawaiian Archipelago. Mar Biol 145:1043–1059. doi:10.1007/s00227-004-1387-6
Bjorndal K (1997) Foraging ecology and nutrition of the sea turtles. In: Lutz P, Music J (eds) The biology of sea turtles. CRC Press, New York, pp 199–231
Bjorndal KA, Bolten AB, Moore JE (1990) Digestive fermentation in herbivores: effect of food particle size. Physiol Zool 63:710–721
Blumenthal JM, Austin TJ, Bothwell JB, Broderick AC et al (2010) Life in (and out of) the lagoon: fine-scale movements of green turtles tracked using time-depth recorders. Aquat Biol 9:113–121
Bolstad KSR (2010) Systematics of the Onychoteuthidae Gray, 1847 (Cephalopoda: Oegopsida). Zootaxa 2696:1–186
Boyle MC, Limpus CJ (2008) The stomach contents of post-hatchling green and loggerhead sea turtles in the South-west Pacific: an insight into habitat association. Mar Biol 155:233–241
Brand SJ, Lanyon JM, Limpus CJ (1999a) Diet selection by immature green turtles, Chelonia mydas, in subtropical Moreton Bay, south-east Queensland. Aust J Zool 47:181–191
Brand SJ, Lanyon JM, Limpus CJ (1999b) Digesta composition and retention times in wild immature green turtles, Chelonia mydas: a preliminary investigation. Mar Freshwat Res 50:145–147
Campos EJD, Piola AR, Matano RP, Miller JL (2008) PLATA: a synoptic characterization of the southwest Atlantic shelf under influence of the Plata River and Patos Lagoon outflows. Cont Shelf Res 28:1551–1555
Caraccio MN (2008) Análisis de la composición genética de Chelonia mydas (tortuga verde) en el área de alimentación y desarrollo de Uruguay. Tesis de Maestría del Programa de Desarrollo de las Ciencias Básicas (PEDECIBA), Área Biología-Subárea Genética, Facultad de Ciencias, Universidad de la República, Montevideo
Cardona L, Pazos L, Aguilar L (2009) Delayed ontogenic dietary shift and high levels of omnivory in green turtles (Chelonia mydas) from the NW coast of Africa. Mar Biol 156:1487–1495
Carrión-Cortez JA, Zárate P, Sminoff JA (2010) Feeding ecology of the green sea turtle (Chelonia mydas) in the Galapagos Islands. J Mar Biol Assoc UK 90:1005–1013. doi:10.1017/S0025315410000226
Chaloupka M, Limpus CJ (2001) Trends in the abundance of sea turtles resident in southern Great Barrier Reef waters. Biol Conserv 102:235–249
Chaloupka M, Limpus C, Miller J (2004) Green turtle somatic growth dynamics in a spatially disjunct Great Barrier reef metapopulation. Coral Reefs 23:325–335. doi:10.1007/s00338-004-0387-9
Cherel Y, Klages N (1998) A review of the food of albatrosses. In: Robertson G, Gales R (eds) Albatross biology and conservation. Surrey Beatty & Sons, Chipping Norton, pp 113–136
Cherel Y, Weimerskirch H (1995) Seabirds as indicators of marine resources: black-browed albatrosses feeding on ommastrephid squids in Kerguelen waters. Mar Ecol Prog Ser 129:295–300
Cherel Y, Weimerskirch H (1999) Spawning cycle of onychoteuthid squids in the southern Indian Ocean: new information from seabird predators. Mar Ecol Prog Ser 188:93–104. doi:10.3354/meps188093
Cherel Y, Duhamel G, Gasco N (2004) Cephalopod fauna of subantarctic islands: new information from predators. Mar Ecol Prog Ser 266:143–156
Clarke MR (1986) A handbook for the identification of cephalopod beaks. Claredon Press, Oxford, 273 pp
Collins MA, Rodhouse PGK (2006) Southern ocean cephalopods. Adv Mar Biol 50:191–265
Croxall JP, Prince PA (1994) Dead or alive, night or day: how do albatrosses catch squid? Antarct Sci 6:155–162
Croxall JP, Prince PA (1996) Cephalopods as prey: seabirds. Philos Trans R Soc B 351:1023–1043
Csirke J (1987) The Patagonian fishery resources and the offshore fisheries in the Southwest Atlantic. Fish Tech Pap 286. FAO, Rome, p 75
Domingo A, Bugoni L, Prosdocimi L, et al (2006) The impact generated by fisheries on Sea Turtles in the Southwestern Atlantic. Tech. Rep. WWF Programa Marino para Latinoamérica y el Caribe, San José, Costa Rica: 71 pp
Ferreira B, Garcia M, Jupp BP, Al-Kryumi A (2006) Diet of the green turtle (Chelonia mydas) at Ra’s Hadd, Sultanate of Oman. Chelon Conserv Biol:141–145
Field I, Bradshaw C, Van Den Hoff J, Burton H, Hindell M (2007) Age-related shifts in the diet composition of southern elephant seals expand overall foraging niche. Mar Biol 150:1441–1452
Godley BJ, Thompson DR, Waldron S, Furness RW (1998) The trophic status of marine turtles as determined by stable isotope analysis. Mar Ecol Prog Ser 166:277–284
González Carman V, Falabella V, Maxwell S, Albareda D, Campagna C, Mianzan H (2012) Revisiting the ontogenetic shift paradigm: the case of juvenile green turtles in the SW Atlantic. J Exp Mar Biol Ecol 429:64–72. doi:10.1016/j.jembe.2012.06.007
González Carman V, Botto F, Gaitán E, Albareda D, Campagna C, Mianzan H (2013) A jellyfish diet for the herbivorous green turtle Chelonia mydas in the temperate SW Atlantic. Mar Biol. doi:10.1007/s00227-013-2339-9
Guebert-Bartholo FM, Barletta M, Costa MF, Monteiro-Filho ELA (2011) Using gut contents to assess foraging patterns of juvenile green turtles Chelonia mydas in the Paranaguá Estuary, Brazil. Endanger Species Res 13:131–143
Hatase H, Sato K, Yamaguchi M, Takahashi K, Tsukamoto K (2006) Individual variation in feeding habitat use by adult female green sea turtles (Chelonia mydas): are they obligately neritic herbivores? Oecologia 149:52–64
Hatfield EMC, Rodhouse PG (1991) Biology and fishery of the Patagonian squid Loligo gahi (d’Orbigny, 1835): a review of current knowledge. J Ceph Biol 2:41–79
Hernández-García V (1995) The diet of the swordfish Xiphias gladius Linnaeus, 1758, in the central east Atlantic, with emphasis on the role of cepahlopods. Fish Bull 93:403–411
Hirth HF (1997) Synopsis of the biological data on the green turtle, Chelonia mydas (Linnaeus 1758). United States Fish and Wildlife Service Biological Report 97–1:120 pp
Horta S, Defeo O (2012) The spatial dynamics of the white mouth croaker artisanal fishery in Uruguay and interdependencies with the industrial fleet. Fish Res 125–126(1):21–128. doi:10.1016/ j.fishres.2012.02.007
Hoving HJT, Perez JAA, Bolstad K, Braid H, Evans AB, Fuchs D, Judkins H, Kelly JT, Marian JEAR, Nakajima R, Piatkowski U, Reid A, Vecchione M, Xavier JC (2014) The study of deep-sea cephalopods. Adv Mar Biol (in press)
Imber MJ (1992) Cephalopods eaten by wandering albatrosses (Diomedea exulans L.) breeding at six circumpolar localities. J R Soc N Z 22:243–263
International Union for Conservation of Nature and Natural Resources (2013) Red list of threatened species, version 2013.2. www.iucnredlist.org
Limpus CJ, Couper PJ, Read MA (1994) The green turtle, Chelonia mydas, in Queensland: Population structure in a warm temperate feeding area. Mem Queensland Mus 35:139
López-Mendilaharsu M, Estrades A, Caraccio M et al (2006) Biología y ecología de las tortugas marinas en la zona costera uruguaya. In: Menafra R, Rodríguez-Gallego L, Scarabino F, Conde D (eds) Bases para la Conservación y el Manejo de la Costa Uruguaya. VIDA SILVESTRE, Montevideo, pp 247–258
López-Mendilaharsu M, Gardner S, Riosmena-Rodríguez R, Seminoff JA (2008) Diet selection by immature green turtles (Chelonia mydas) at Bahía Magdalena foraging ground in the Pacific Coast of the Baja California Peninsula, México. J Mar Biol Assoc UK 88:1–7
Lu CC, Williams R (1994) Contribution to the biology of squid in the Prydz Bay region, Antarctica. Antarct Sci 6:223–229
Marcovaldi M, Marcovaldi G (1999) Marine turtles of Brazil: the history and structure of Projeto TAMAR-IBAMA. Biol Conserv 91:35–41
Martinez-Souza G, Vélez-Rubio GM, Techera B, Russomagno M, Berrondo L, Kinas P (2012) Cerro Verde, Uruguay: a year-round feeding area for juveniles green turtles? In: Jones TT, Wallace BP (eds) Proceedings of the 31st symposium on sea turtle biology and conservation. International Sea Turtle Society, NOAA Techincal Memorandum NOAA NMFS-SEFSC, pp 148–149
Morais RA, Longo GO, Yoshida ETE, Stahelin GD, Horta PA (2012) Cephalopod ingestion by juvenile green sea turtles (Chelonia mydas): Predatory or scavenging behavior? Herpetol Rev 43:47–50
Nagoaka S, Martins A, Santos R et al (2012) Diet of juvenile green turtles (Chelonia mydas) associating with artisanal fishing traps in a subtropical estuary in Brazil. Mar Biol 159:573–589
Nesis KN (1987) Cephalopods of the world. T.F.H. Publications, Neptune City
Ohizumi H, Kuramochi T, Kubodera T, Yoshioka M, Miyazaki N (2003) Feeding habits of Dall’s porpoises (Phocoenoides dalli) in the subarctic North Pacific and the Bering Sea basin and the impact of predation on mesopelagic micronekton. Deep Sea Res (I Oceanogr Res Pap) 50:593–610
Okutani T (1994) The importance of the Southern Ocean cephalopod fauna. Antarct Sci 6:2
Ortega L, Martínez A (2007) Multi annual and seasonal variability of water masses and fronts over the Uruguayan shelf. J Coast Res 23:618–629. doi:10.2112/04-0221.1
Parker DM, Dutton PH, Balazs GH (2011) Oceanic diet and distribution of haplotypes for the green turtle, Chelonia mydas, in the central North Pacific. Pac Sci 65(4):419–431. doi:10.2984/65.4.419
Perez JAA, Martins RS, Santos RA (2004) Cefalópodes capturados pela pesca commercial de talude no sudeste e sul do Brasil. Not Téc FACIMAR 8:65–74
R Development Team (2008) R statistcs. Available at: http://www.r-project.org/
Read MA, Limpus CJ (2002) The green turtle, Chelonia mydas, in Queensland: feeding ecology of immature turtles in Moreton Bay, southeastern Queensland. Mem Queensland Mus 48:207–214
Reich KJ, Bjorndal KA, Bolten AB (2007) The ‘lost years’ of green turtles: using stable isotopes to study cryptic life-stages. Biol Lett 3:712–714
Reisser J, Proietti M, Sazima I, Kinas P, Horta P, Secchi E (2013) Feeding ecology of the green turtle (Chelonia mydas) at rocky reefs in western South Atlantic. Mar Biol 160:3169–3179
Rodhouse PG, Lu CC (1998) Chiroteuthis veranyi from the Atlantic sector of the Southern Ocean (Cephalopoda: Chiroteuthidae). S Afr J Mar Sci 20:311–322. doi:10.2989/025776198784126593
Rodhouse PG, Nigmatullin ChM (1996) Role as consumers. Phil Trans R Soc Lond B 351. doi: 10.1098/rstb.1996.0090
Rodhouse PG, White MG (1995) Cephalopods occupy the ecological niche of epipelagic fish in the Antarctic Polar frontal zone. Biol Bull 189:77–80
Rodhouse PG, Arnbom T, Fedak MA, Yeatman J, Murray AWA (1992) Cephalopod prey of the southern elephant seal, Mirounga leonina L. Can J Zool 70:1007–1015
Rodhouse PG, Robinson K, Gajdatsy SB, Daly HL, Ashmore MJS (1994) Growth, age structure and environmental history in the cephalopod Martialia hyadesi (Teuthoidea: Ommastrephidae) at the Antarctic Polar Frontal Zone and on the Patagonian Shelf Edge. Antarct Sci 6(2):259–267
Rodhouse PG, Xavier JC, Griffiths H (2014) Southern Ocean squid. In: De Broyer C, Koubbi P, Griffiths HJ, Raymond B, Udekem d’Acoz C d’, Van de Putte AP, Danis B, David B, Grant S, Gutt J, Held C, Hosie G, Huettmann F, Post A, Ropert-Coudert Y (eds) Biogeographic Atlas of the Southern Ocean. Scientific Committee on Antarctic Research, Cambridge, 400 pp
Roper CFE, Young RE (1974) Vertical distribution of pelagic cephalopods. Smithson Contrib Zool 209:1–59
Russell DJ, Hargrove S, Balazs G (2011) Marine sponges, other animal food, and nonfood items found in digestive tracts of the herbivorous marine turtle Chelonia mydas in Hawai’i. Pac Sci 65(3):375–381
Salman A, Karakulak FS (2009) Cephalopods in the diet of albacore, Thunnus alalunga, from the eastern Mediterranean. J Mar Biol Assoc UK 89:635–640
Scarabino F (2003) Lista sistemática de los Cephalopoda vivientes de Uruguay. Comunicaciones de la Sociedad Malacológica del Uruguay 8(78–79):197–202
Seminoff JA, Resendiz A, Nichols WJ (2002) Diet of East Pacific green turtles (Chelonia mydas) in the central Gulf of California, Mexico. J Herpetol 36:447–453
Tsuchiya K, Okutani T (1991) Growth stages of Moroteuthis robusta (Verrill, 1881) with the re-evaluation of the genus. Bull Mar Sci 49:137–147
Vaske-junior T, Rincon-Filho G (1998) Conteúdo estomacal dos tubarões azul (Prinace glauca) e anequim (Isurus oxyrinchus) em águas oceânicas no sul do Brasil. Rev Bras Biol 58(3):445–452
Vélez-Rubio GM, Estrades A, Fallabrino A, Tomás J (2013) Marine turtle threats in Uruguayan waters: insights from 12 years of stranding data. Mar Biol 160:2797–2811
Voight JR, Portner HO, O’Dor RK (1994) A review of ammonia-mediated bouyancy in squids (Cephalopoda: Teuthoidea). Mar Freshw Behav Physiol 25:193–203
Voss NA, Nesis KN, Rodhouse PG (1998) The Cephalopod family Histioteuthidae (Oegopsida): systematics, biology, and biogeography. In: Voss NA, Vecchione M, Toll RB, Sweeney MJ (eds) Systematics and Biogeography of Cephalopods. Smithsonian Contributions to Zoology, Washington, pp 293–372
Wainer I, Venegas SA (2002) South Atlantic multidecadal variability in the climate system model. J Clim 15:1408–1420
Wakabayashi T, Kubodera T, Sakai M, Ichii T, Chow S (2007) Molecular evidence for synonymy of the genera Moroteuthis and Onykia and identification of their paralarvae from northern Hawaiian waters. J Mar Biol Assoc UK 87:959–965
Xavier JC, Cherel Y (2009) Cephalopod Beak Guide for the Southern Ocean. British Antarctic Survey, Cambridge, 129 pp
Xavier JC, Rodhouse PG, Trathan PN, Wood AG (1999) A Geographical Information System (GIS) atlas of cephalopod distribution in the Southern Ocean. Antarct Sci 11:61–62
Xavier JC, Rodhouse PG, Purves MG, Daw TM, Arata J, Pilling GM (2002) Distribution of cephalopods recorded in the diet of Patagonian toothfish (Dissostichus eleginoides) around South Georgia. Polar Biol 25:323–330
Xavier JC, Croxall JP, Cresswell KA (2005) Boluses: a simple, cost-effective diet method to assess the cephalopod prey of albatrosses? Auk 122:1182–1190
Xavier JC, Geraint GA, Croxall JP (2006) Determining large scale distribution of pelagic cephalopods, fish and crustaceans in the South Atlantic from wandering albatross (Diomedea exulans) foraging data. Ecography 29:260–272
Xavier JC, Phillips RA, Cherel Y (2011) Cephalopods in marine predator diet assessments: why identifying upper and lower beaks is important. ICES J Mar Sci 68(9):1857–1864. doi:10.1093/icesjms/fsr103
Xavier JC, Cherel Y, Roberts J, Piatkowski U (2013) How do cephalopods become available to seabirds: can fish gut contents from tuna fishing vessels be a major food source of deep-dwelling cephalopods? ICES J Mar Sci 70:46–49
Young RE, Vecchione M (2007) Cephalopoda Cuvier 1797. Octopods, squids, nautiluses, etc. The Tree of Life Web Project, version 10. http://tolweb.org/Cephalopoda/19386/2012.11.10
Zug GR, Glor RF (1999) Estimates of age and growth in a population of green seaturtles Chelonia mydas from the Indian River lagoon system, Florida: a skeletochronological analysis. Can J Zool 76:1497–1506. doi:10.1139/cjz-76-8-1497
Zug GR, Balazs GH, Wetherall JA, Parker DM, Murakawa SKK (2001) Age and growth of Hawaiian green seaturtles (Chelonia mydas): an analysis based on skeletochronology. Fish Bull 100:117–127
Acknowledgments
We would like to thank the volunteers and Karumbe members, specially Andrés Estrades, Alejandro Fallabrino, Virginia Ferrando, Daniel González, Luciana Alonso, Virginia Borrat, and Natalia Teryda, for their collaboration in the study and green turtle diet analyses. We also thank the support of the Marine Zoology Unit of the Cavanilles Institute of Biodiversity and Evolutionary Biology (University of Valencia, Spain), specially Ohiana Revuelta, Natalia Fraija and Ana Born. The authors are really grateful to all the persons and institutions that collaborated in the Uruguay marine turtle stranding network: local fishermen, government institutions (DINARA and DINAMA), naval prefectures, lifeguard service, rangers, civil organizations (particularly SOCOBIOMA), citizens and tourist. We also acknowledge the financial support from IFAW and Rufford Small Grants to GVR and Agencia Nacional de Investigación e Innovación (ANII) to A.C. J.T. is supported by projects CGL2011-30413 of the Spanish Ministry of Economy and Competitiveness and Prometeo (UV-CI-12-151) of the Generalitat Valenciana. This research was conducted under license (No. 200/04, 073/08 and 323/11) from the Fauna Department-Ministry of Cattle, Agriculture and Fishing of Uruguay.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vélez-Rubio, G.M., Tomás, J., Míguez-Lozano, R. et al. New insights in Southwestern Atlantic Ocean Oegopsid squid distribution based on juvenile green turtle (Chelonia mydas) diet analysis. Mar Biodiv 45, 701–709 (2015). https://doi.org/10.1007/s12526-014-0272-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12526-014-0272-x