Abstract
The objective of the present study was to determine the diet composition and feeding habits of the crocodile shark, Pseudocarcharias kamoharai, in Ecuadorian waters, Southeast Pacific Ocean, at a general level, as well as by sex, year, and sexual maturity stage. A total of 401 individuals with their respective stomachs were sampled in the Santa Rosa fishery port, Ecuador; 200 were females and 201 males, with a total length (TL) between 50 to 117 cm and 72.5 to 100 cm, respectively. The number of stomach contents analyzed in this study was sufficient to describe the diet of P. kamoharai, as the asymptote was reached at 100 digestive tracts. A total of 24 prey species were identified, out of which 16 were cephalopods and eight bony fishes. The main identified prey species were the cockeyed squid, Histioteuthis heteropsis, the sharpear enope squid, Ancistrocheirus lesueurii, the purpleback flying squid, Sthenoteuthis oualaniensis, the Jumbo flying squid, Dosidicus gigas, and the neon flying squid, Ommastrephes bartramii. Pseudocarcharias kamoharai was considered a specialist predator that preferred five prey species (Bi = 0.09), and differences were observed by sex, years, and maturity stages. The trophic overlap was high in both females and males (Cλ = 0.98) between the years 2012–2013 and 2017–2018 (Cλ = 0.91), as well as between immature and mature individuals (Cλ = 0.95). The trophic level was Tlk = 4.37, which indicates that this species is a quaternary consumer or a tertiary carnivore.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The crocodile shark, Pseudocarcharias kamoharai (Matsubara 1936), is the only member of the family Pseudocarhariidae (order Lamniformes). It has a wide distribution in tropical and subtropical waters of the Pacific, Atlantic, and Indian Oceans (Compagno et al. 2005). Pseudocarcharias kamoharai is considered a small species (122 cm total length) with an occurrence that varies from sporadic to locally abundant. Although it uses oceanic environments, it can also be found in coastal areas; vertical distribution is epipelagic and mesopelagic, inhabiting from the surface up to depths of 590 m (Ebert 2016). This species shows a diel pattern of vertical migration toward the surface at night and away from it during daylight hours (Last and Stevens 1994; Martin 2003).
The feeding habits of P. kamoharai are scarcely known. General anatomical features consist of long and flexible teeth and strong and large jaws, in which its vigorous activity allows the capture of moderately large oceanic prey. Its jaws can protrude a considerable distance from its head. The large eyes of the crocodile shark, equipped with a reflective green or yellow and lacking an expanded iris, suggest that it is a nocturnal hunter that relies on sight to pick out the silhouettes or bioluminescence of its prey (Martin 2003). This shark is a carnivorous species that feeds on luminous fish (Gonostomatidae), lanternfish (Myctophidae), cranch squid (Cranchiidae), clubhook squid (Onychoteutidae), scaled squids (Pholidoteuthidae), sharpear enope squid (Ancistrocheiridae), and small shrimp (Compagno 2001).
The crocodile shark is globally captured as a bycatch from pelagic longline industrial swordfish, Xiphias gladius, and tuna fisheries (family Scombridae) (Hazin et al. 1990). It is also caught with other fishing gears such as gillnets (Compagno 2001). Globally, the total catches for 2007 were 14 t (FAO 2021). In Ecuador, this species has a targeted fishery, and according to Martínez-Ortiz and García-Domínguez (2013), it is captured both artisanal and industrially for local consumption. Moreover, the kind of fishing gears employed are shallow longlines and gillnets, as well as trawl nets. Despite its commercial importance, there are no current statistics regarding landings. A total of 37,748 t of chondrichthyans, including more than 30 species of sharks and rays, were landed during September 2007–December 2011 (Subsecretaría de Recursos Pesqueros 2013), out of which 0.73 t were P. kamoharai. It is important to mention that there are no records of the species since then, and there has been a high local consumption in Salinas (Santa Elena) during the last decade.
This species has a relatively rapid growth rate (k = 0.14–0.18/year), an early sexual maturation (5 years in females and 3–4 years in males), an unknown gestation period, and it presents small litters (4 pups per litter) (Fujita 1981; White 2007; Oliveira et al. 2010; Dai et al. 2012; Lessa et al. 2015; Kindong et al. 2020; Wu et al. 2020); thus, its fecundity is considered low when compared with other sharks. Most Chondrichthyan populations can only resist moderate levels of mortality before their stock is exhausted and collapsed (Camhi et al. 1998; Musick 1999; Cortés 2000), which results in a faster decline compared to other bony fishes (Sminkey and Musick 1995, 1996). Overfishing of oceanic sharks is the primary cause of population declines and increased extinction risk. Since 1970, the global abundance of oceanic sharks and rays has declined by 71%, owing to an 18-fold increase in relative fishing pressure (Pacoureau et al. 2021). Furthermore, the absence of landing records and the lack of biological and ecological studies prevent the knowledge of its current population status both locally and globally. Even though the International Union for the Conservation of Nature (IUCN) has classified this species as the least concern (Kyne et al. 2019), other authors have cataloged it as highly susceptible and vulnerable to fishing due to its low biological productivity (Murua et al. 2018).
Some of the most important biological and ecological aspects of any species are feeding, age and growth, reproductive biology, population dynamics, demography, genetics, and fisheries. Regarding trophic ecology, diet and feeding habit analyses are relevant to evaluate the ecological role and the position of a species in the trophic web (Allan and Castillo 2007). The stomach content analysis of fish provides information concerning the feeding patterns and the quantitative evaluation of the trophic habits; it is an important aspect of the management of fisheries. The diet of fish represents the integration of the most relevant ecological aspects, such as behavior, condition, habitat use, energy consumption, and intraspecific and interspecific interactions (Sagar et al. 2018). Dietary information provides support for the management of aquatic resources, mainly in aquaculture and conservation (Saikia 2015). There is scarce information on the trophic ecology of P. kamoharai, although other aspects have been studied in more detail, such as its distribution, age and growth, reproductive biology, and genetics.
The objective of the present study was to describe the diet composition and feeding habits of P. kamoharai in the Ecuadorian Pacific Ocean, along with its relationship with sex, years, and maturity stages for the estimation of the trophic niche, overlap, and level.
Methods
Sampling
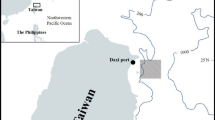
The fishing port of Santa Rosa is an urban parish in Salinas, located in the Santa Elena Province, Ecuador (02° 12′ 56″ S, 80° 57′ 26″ W). The economy of the community in this area depends on fishing activity (Fig. 1). It is important to consider that there are only two seasons in Ecuador: rainy season (December–May) and dry season (June–November).
The Santa Rosa fishing camp was visited for two years (June 2012–May 2013 and June 2017–2018) to collect samples of P. kamoharai landings from artisanal fisheries. The total length (TL) was recorded in cm, as well as the sex of the individuals. The criteria from Conrath (2005) and Oliveira et al. (2010) were considered to evaluate the maturity stages of each organism. Sexual maturity was identified based on the following criteria: In males, the size and the condition of the clasper were evaluated (rotation, calcification, rhipidion aperture, and semen presence); while in females, the form of the cloaca (open or close) was observed to identify evidence of reproductive activity. Moreover, the mating marks and embryos were noted; in these cases, the sex, TL, and weight were annotated.
The stomach was obtained from each shark, and its gastric filling state was estimated using the reference values of Stillwell and Kohler (1982), classifying them as follows: 0 (empty), 1 (25% full), 2 (50% full), 3 (75% full), and 4 (100% full). Posteriorly, the stomach contents were placed in plastic bags and ice for their transportation to the laboratory, where they were frozen.
Laboratory work
Samples were thawed to separate the prey species found in the stomachs by taxonomic groups; they were also counted, measured (cm), and weighed (g). They were grouped according to their digestive degree according to Olson and Galván-Magaña (2002): state 1 (individuals that present complete morphological characteristics, which make it easily identifiable); state 2 (individuals without skin or eyes and exposed muscles); state 3 (individuals without a head, some body parts present and axial skeleton); and state 4 (otoliths, skeletons, and squid beaks). The identification of prey with a minimum state of digestion (state 1) was based on fish identification guides (Fischer et al. 1995; Jiménez and Beárez 2004; Robertson and Allen 2015). The axial skeleton and otoliths were used for those with an advanced state of digestion (states 3 and 4). For the vertebrae count, the works of Clothier (1950) and Clothier and Baxter (1969) were used, while for otoliths, the works of García-Godos (2001), Muñoz (2012), and Vinueza (2015) were used. The identification guides of Wolff (1982, 1984), Clarke (1986), Ingrid et al. (1971), and Xavier and Cherel (2009) were used to identify cephalopods. The rostrum length of the upper and/or inferior jaw of cephalopod beaks were recorded in order to carry out a retro-calculation of the weights, according to Wolff (1982) and Clarke (1986).
It is worth mentioning that, in the case of fishes with an advanced digestive state, the otoliths and skeletons were identified to know if they belonged to the same or different individual of one species for its respective counting. The same process was carried out for different fish species. When fragments of fishes were found (e.g., part of a head, fish eyes, skin, muscle tissue, an incomplete skeleton, only vertebrae, or an incomplete otolith), they were classified as fish remains and they were counted. Likewise, in the case of cephalopods with an advanced digestive state, the upper and lower beaks were identified to investigate if they belonged to the same or different individual of one species for its respective counting. The same process was carried out for the different cephalopod species. When fragments of squids and octopus were found (e.g., part of a mantle, part of the tentacles, cephalopod eyes, skin, muscle tissue, pens, or worn beaks), they were classified as cephalopod remains and they were counted. Lastly, we did not find detritus in any of the stomachs.
Data analysis
A parametric Kruskal–Wallis (H) test was applied for the comparison of sizes since data on length did not have a normal distribution, and it presented heterogeneity of variances both by sexes and time (Daniel 1991, Celis-De la Rosa and Labrada-Martagón 2014). Regarding the temporal analysis, females and males were grouped independently of their sex based on their sizes.
The cumulative prey curve was applied to determine whether a sufficient number of stomachs had been examined to precisely describe the diet of P. kamoharai (Hoffman 1978; Ferry and Cailliet 1996; Cortés 1997; Jiménez-Valverde and Hortal 2003). It consists in plotting the number of stomachs with food on the horizontal axis versus the accumulated diversity of the consumed prey on the vertical axis to obtain the cumulative curve and the number of tracts where the asymptote will be reached, which indicates the minimum sample size. Moreover, the coefficient of variation for each digestive tract was obtained and graphed as a secondary axis; the latter coefficient was obtained as the relationship between the standard deviation and the average diversity. For this purpose, the number of stomachs was estimated with the software EstimateS V. 8.0 (Colwell 2019), in which the number of analyzed stomachs was subjected to 100 permutations to eliminate bias with an α = 0.05. The variation of the respective number of selected stomachs was 0.05.
The ecological indices used were the numeric methods (% Ni) (Hyslop 1980) of frequency of occurrence (% FOi) and weight (% Wi) (Peláez 1997). The contribution importance to the diet of P. kamoharai of each species was evaluated through the Prey-Specific Index of Relative Importance (% PSIRI) (Brown et al. 2012):
where % PNi is the prey-specific abundance and % PWi is the prey-specific weight. These parameters were obtained based on the models:
Additionally, the arrangement of the taxonomic order of the species consumed by the crocodile shark was carried out based on Young et al. (2019) for cephalopods and Van der Laan et al. (2021) for bony fishes.
The amplitude of the predator’s diet was calculated through the Levin’s Index (Bi) (Krebs 1985):
where Pij is the proportion of prey j in the predator’s diet i and n is the number of prey species. The values of this index range from 0 to 1, where values < 0.6 indicate that the diet is dominated by few prey species, and thus, it would be a specialist predator; while results > 0.6 indicate generalist predators (Labropoulou and Eleftheriou 1997). Furthermore, sharks can also be classified as selective and opportunistic predators. Selective predators are those that use resources (habitats and food) out of proportion or in different proportions to their availability; while opportunistic predators use resources in the same proportion as their availability (Johnson 1980; Buskirk and Millspaugh 2006). Additionally, Munroe et al. (2014) suggest the use of patterns and defining behaviors when studying shark resources, given it is more appropriate to measure and refer to a species’ degree of specialization, rather than categorizing a species as a generalist or specialist. Sharks can be categorized with a low or high degree of specialization. In this context, a shark species with a low degree of specialization would feed on a diverse array of prey such as ctenophores, sea anemones, nematodes, crustaceans, mollusks, squids, elasmobranchs, teleostean fishes, sea turtles, sea birds, and marine mammals. Conversely, shark species with a high degree of specialization would feed on a small array of prey such as zooplankton, as well as fish in the case of certain species of sharks; in other cases, cephalopods and fish; crustaceans, cephalopods, and fish; only crustaceans; only cephalopods; only teleostean fishes, etc.
The trophic overlap analysis between sexes, sexual maturity, and annual season was carried out through the Morisita-Horn Index (Cλ) (Morisita 1959; Horn 1966; Smith and Zaret 1982):
where Cλ is the Morisita and Horn Index between the sex of x and the sex of y; Pxi is the prey proportion i of the total prey consumed by the sex of the predator x; Pyi is the prey proportion i of the total prey consumed by the sex of the predator y. According to Langton (1982), values from 0 to 0.29 indicate a low overlap; from 0.30 to 0.59, a medium overlap; and from 0.60 to 1, a high overlap. Additionally, the diet composition was compared with each category based on a one-way analysis of similarities (ANOSIM) through the Bray–Curtis Index (R), where values close to 0 indicate that there are no differences between groups or confronted categories; while values close to 1 suggest that there is a difference between groups or that they are completely separated (Clarke 1993). The program Past V. 4.01 was used to calculate this index (Hammer 2020).
The trophic level (Tlk) was obtained through the equation proposed by Cortés (1999):
where Tlk is the trophic level of the predatory species, n is the number of prey species, Pjx is the relative proportion of prey that compose the diet of the consumer, and Tlj is the trophic level of prey. The trophic levels of the consumed prey employed in the Tlk estimations of P. kamoharai were obtained from Cortés (1999), Froese and Pauly (2021), and Palomares and Pauly (2021).
Results
Characteristics of the sample
A total of 401 sharks were analyzed, out of which 200 were females (49.9%) and 201 were males (50.1%). Females measured between 50 cm TL and 117 cm TL (mean \(\overline{x }\) = 102.5 SD ± 9.2) and males between 72.5 cm TL and 110 cm TL (\(\overline{x }\) = 94.2 ± 7.1). Females were bigger than males, and significant differences were observed between sizes by sex (H = 123.6; p < 0.05). Regarding sample times, the sharks from the first period of the study were slightly bigger than in the second, and temporal significant differences were observed (H = 132.1; p < 0.05).
Out of the 401 analyzed stomachs, 149 contained food (37.2%) and 252 were completely empty (62.8%). More than half of the stomachs had a gastric filling state of 0 (n = 260, 64.8%), followed by state 1 (n = 129, 32.2%), state 2 (n = 7, 1.7%), state 4 (n = 3, 0.7%), and state 3 (n = 2, 0.5%). The number of prey that conformed the diet (n = 318) where in a completely digested state, or in state 4 (n = 294, 90%); followed by an advanced digestive state, or state 3 (n = 18, 6%); then a state of digestion 1 (n = 5, 3%); and finally a state 2 (n = 1, 1%). The number of stomachs with analyzed content was sufficient to characterize the general dietary spectrum of P. kamoharai since the asymptote was reached in 100 digestive tracts. The variability of the prey items decreased sharply when stomach 36 was reached (Fig. 2).
General cumulative curve of the prey species consumed by Pseudocarcharias kamoharai in Santa Rosa, Province of Santa Elena, Ecuador. Graph plot depicting Shannon–Weiner diversity index values and coefficients of variation against the numbers of stomachs analyzed. Arrow indicates the number of stomachs where the curve got the asymptote
Food composition
A total of 24 prey species or food components were found, of which 16 were cephalopods and eight were bony fishes. The diet of P. kamoharai was composed of 318 individuals with a total weight of 27,549.8 g. The most abundant food components were three: the cockeyed squid, Histioteuthis heteropsis (% PSIRI = 27.6), the sharpear enope squid, Ancistrocheirus lesueurii (% PSIRI = 27.4), and the purpleback squid, Sthenoteuthis oualaniensis (% PSIRI = 63.7) (Table 1).
Concerning feeding by sex, females presented 21 prey species distributed in 200 organisms with a weight of 16,346.2 g. The most abundant food components were three: A. lesueurii (% PSIRI = 28.6), H. heteropsis (% PSIRI = 25.0), and S. oualaniensis (% PSIRI = 10.8). The male’s diet was composed of 17 species represented by 118 specimens and weight of 11,203.6 g. The most abundant food components were three: H. heteropsis (% PSIRI = 31.4), A. lesueurii (% PSIRI = 25.5), and S. oualaniensis (% PSIRI = 13.4) (Fig. 3). There were no significant differences between the diet composition of P. kamoharai by sexes (H = 0.07; p > 0.05).
The crocodile sharks obtained during 2012–2013 fed on 14 species (n = 109 specimens) with a total weight of 14,611.8 g. The most abundant food components were four: H. heteropsis (% PSIRI = 27.0), A. lesueurii (% PSIRI = 21.1), S. oualaniensis (% PSIRI = 20.3), and the neon flying squid, O. bartramii (% PSIRI = 10.3). The sharks from 2017 to 2018 had a diet composed of 19 species represented by 209 organisms and a weight of 12,938 g. The most abundant species were three: A. lesueurii (% PSIRI = 30.9), H. heteropsis (% PSIRI = 30.0), and the Jumbo flying squid, Dosidicus gigas, (% PSIRI = 14.9) (Fig. 4). No significant differences were observed between the diet composition of P. kamoharai by years (H = 0.00; p > 0.05).
Immature sharks had a diet composed of seven species distributed in 18 individuals with a weight of 1864.7 g. The most abundant species were two: H. heteropsis (% PSIRI = 46.6) and A. lesueurii (% PSIRI = 25.0). The diet of mature sharks was composed of 21 species represented by 300 specimens and a total weight of 25,685.1 g. The most abundant food components were three: A. lesueurii (% PSIRI = 27.6), H. heteropsis (% PSIRI = 26.2), and S. oualaniensis (% PSIRI = 12.7) (Fig. 5). Significant differences were registered between the diet composition of P. kamoharai by maturity stages (H = 6.12; p < 0.05).
Feeding habits
Amplitude of the trophic niche
Pseudocarcharias kamoharai was a specialist predator that preferred between three and five species of the total 24 (Bi = 0.09). This behavior was similar to the rest of the analyzed categories. Males (Bi = 0.18) presented twice the value compared to females (Bi = 0.09). The sharks from the first year (Bi = 0.27) had a triple value compared to the second (Bi = 0.08). Similarly, immature organisms (Bi = 0.38) registered a value three times higher than mature individuals (Bi = 0.10 for mature). In addition, this specialist predator is selective because it fed on its prey in different proportions based on their availability; it also showed a high degree of specialization due to its preference for squids and teleostean fishes.
Trophic overlap
The trophic overlap was high both in females and males (Cλ = 0.98), as well as for the years 2012–2013 and 2017–2018 (Cλ = 0.91) and immature and mature individuals (Cλ = 0.95). Therefore, there was no feeding segregation based on sexes, years, nor maturity stages. This feeding pattern in each category was confirmed by the Bray–Curtis Index for the prey that compose the diets, which confirmed the trophic overlap between sexes (R = 0.03; p = 0.02), years (R = 0.08; p = 0.01), and maturity stages (R = 0.06; p = 0.29).
Trophic level
The trophic level estimated was Tlk = 4.37, which indicated that P. kamoharai is a top predator, either a quaternary consumer or a tertiary carnivore. The trophic levels by sexes, years, and maturity stages were similar and ranged between Tlk = 4.37 and 4.38.
Discussion
Food composition
The present study demonstrated that the diet of P. kamoharai is mainly composed of cephalopods, which were more abundant than bony fishes. The squids A. lesueurii, H. heteropsis, S. oualaniensis, D. gigas, and O. bartramii were the most abundant species in its trophic spectrum. Cephalopods are crucial in the diets of other top predators that inhabit the same area, e.g., Alopias pelagicus had preferences for O. bartramii, D. gigas, and S. oualaniensis (Calle-Morán and Galván-Magaña 2020); Sphyrna zygaena preferred D. gigas, S. oualaniensis, A. lesueurii, and the dart squid L. diomedeae as its main prey (Estupiñán-Montaño et al. 2019); the diet of P. glauca was dominated by A. lesueurii, H. dofleini, and D. gigas (Loor-Andrade et al. 2017); the shortfin mako shark Isurus oxyrinchus mainly consumed A. lesueuri, D. gigas, and the diamond squid Thysanoteuthis rhombus (Rosas-Luis et al. 2016). Cephalopods are an abundant and ecologically important group within marine ecosystems, including the Ecuadorian Pacific; they are one of the major food sources for top predators such as sharks and large pelagic fish, i.e., Xiphiidae and Istiophoridae (Rosas-Luis et al. 2015).
Feeding habits
The crocodile shark is an active oceanic and coastal swimmer; it is considered epipelagic and mesopelagic and inhabits from the surface down to 590 m of depth (Ebert 2016). This allows the shark to access prey from different ecosystems, both neritic as well as oceanic, which is why its trophic spectrum varies. Jereb and Roper (2010) sustain that the squids A. lesueurii, H. heteropsis, S. oualaninesis, D. gigas, and O. bartramii, among others, are oceanic, epipelagic, mesopelagic, and abyssopelagic species that carry out considerable horizontal and vertical migrations from the surface down to 1,000 m deep. Their average depth during the day ranges from 200 and 400 m, while their range during the night is between the surface and 200 m deep. Squid are active and fast hunters that feed during the night near the surface (down to 70 m) on fish such as sardines, tuna, hake, and others. According to their morphological characteristics and behavior, as well as the theory of optimal foraging, it is possible that P. kamoharai feeds on such species during the night, taking advantage of the arrival of squid shoal to the surface or close to it to ingest their prey. Thus, it would employ less energy in the search and persecution of these cephalopods, and it would obtain more energy at a lower cost.
The Changos octopus, Octopus mimus, prefers habitats from the surface down to 30 m of depth. This species lives in rocky reefs, and it is common in intertidal reefs, where it looks for shelter in the cracks and below the rocks. It feeds on grapsid crabs and bivalve mollusks (Jereb et al. 2014), and P. kamoharai benefits from this coastal habitat to feed on this species of the octopus when it comes out to eat.
The frigate tuna, Auxis thazard brachydorax, the South Pacific hake, Merluccius gayi peruanus, and the pilot fish, Naucrates ductor, are coastal and oceanic, except for the last species which is completely oceanic; also, they are epipelagic, and M. gayi peruanus is also mesopelagic; they are distributed from the surface down to 500 m of depth (Robertson and Allen 2015). Pseudocarcharias kamoharai would take advantage of the horizontal movements of bony fishes to coastal and oceanic areas to consume them. However, the blue-barred parrotfish Scarus ghobban is a coastal and epipelagic species that inhabits shallow reefs and adjacent areas with sandy and rocky bottoms and feeds on detritus and benthic algae (Robertson and Allen 2015). The crocodile shark would exploit this type of habitat to feed on this species, as well as on O. mimus.
Based on the feeding behavior of P. kamoharai, as well as its specialist and selective high degree of specialization that prefers a reduced number of prey species within its trophic spectrum, it is suggested that this shark would be exploiting a common feeding zone. Independently from sex, time, and maturity stage, it benefits from these zones with high availability of prey, which allows a selection based on its energy requirements. Furthermore, this topic is of great significance because in the past, sharks have been depicted as mobile generalists with wide, sometimes global ranges, that feed opportunistically rather than forage for specific prey items (Wetherbee et al. 1990). Based on the number of prey found in the stomach analysis of the sharks, different proportions of cephalopods and teleostean fishes were consumed by sexes combined, in females and males, immatures and matures, and by year. This elucidates how the predator and its prey are distributed in the environment. There is no method to analyze the exact number of available prey items in their ecosystems, but it is possible to know the number of prey consumed by this predator due to their trophic spectrum.
The trophic level of this species suggests that it is a top predator, either a quaternary consumer or a tertiary carnivore. These values were similar to those from the common thresher Alopias vulpinus (Tlk = 4.20, Cortés 1999), P. glauca (Tlk = 4.20, Córdova-Zavaleta et al. 2018), I. oxyrinchus (Tlk = 4.23, Rosas-Luis et al. 2016), and the white shark Carcharodon carcharias (Tlk = 4.50, Cortés 1999), while A. pelagicus obtained higher values (Tlk = 5.00, Calle-Morán and Galván-Magaña 2020). The latter is in accordance with Cortés (1999), which sustains sharks are a group of tertiary consumers with trophic levels higher than four, and that they occupy similar trophic positions than marine mammals but higher than birds.
Conclusions
The present study provided relevant information regarding the diet and feeding habits of P. kamoharai on a regional and global scale due to the lack of information on the trophic ecology of this species. This predator demonstrated a preference between three and five prey species from a diet constituted by 24 dietary components, of which 16 were cephalopods and eight were bony fishes. It is a specialist predator, with a trophic level of a quaternary consumer or a tertiary carnivore. A high overlap was observed between sexes, years, and maturity stages. This study on the feeding behavior of the crocodile shark and the species that compose its diet, that is, those that coexist in its habitat, is relevant given it allows us to understand the trophic relationships between the predator and its prey, as well as the flow of matter and energy that characterizes an ecosystem. This is the first study that provides information regarding the ecological importance of the crocodile shark in the marine ecosystems of the Pacific Ocean, given they act as natural regulators of the population density of their prey.
Data availability
Not applicable.
Code availability
Not applicable.
References
Allan JD, Castillo MM (2007) Stream ecology: structure and function of running waters, 2nd edn. Springer, New York
Brown SC, Bizzarro JJ, Cailliet GM, Ebert DA (2012) Breaking with tradition: redefining measures for diet description with a case study of the Aleutian skate Bathyraja aleutica (Gilbert 1896). Environ Biol Fishes 95:3–20
Buskirk SW, Millspaugh JJ (2006) Metrics for studies of resource selection. J Wildl Manage 70:358–366
Calle-Morán MD, Galván-Magaña F (2020) Diet composition and feeding habits of the pelagic thresher shark Alopias pelagicus in Eastern Central Pacific Ocean, Ecuadorian waters. J Mar Biol Assoc UK 100(5):837–845
Camhi M, Fowler S, Musick J, Bräutigam A, Fordham S (1998) Sharks and their relatives: ecology and conservation. Occasional Paper of the IUCN Species Survival Commission Occas. Pap. No. 20. IUCN, Oxford
Celis-De la Rosa AJ, Labrada Martagón V (2014) Bioestadística. 3° edición. Editorial El Manual Moderno, Ciudad de México
Clarke KR (1993) Non-parametric multivariate analysis of changes in community structure. Aust J Ecol 18(1):117–143
Clarke M (1986) A handbook for the identification of cephalopod beaks. Clarendon Press, Oxford
Clothier C (1950) A key to some southern California fishes based on vertebral characters. Fishery Bulletin no. 79. Bureau of Marine Fisheries, California
Clothier C, Baxter J (1969) Vertebral characters of some Californian fishes with notes on other Eastern Pacific species. The Resources Agency, California
Colwell RK (2019): Estatistical estimation of species richness and shared species from samples EstimateS V. 9.1.0 University of Connecticut, Storrs, USA. URL http://viceroy.eeb.uconn.edu/estimates/ Accessed 2 June 2021
Conrath CL (2005) Reproductive biology. In: Musick JA. Bonfil R (eds). Management techniques for elasmobranch fisheries. FAO Fisheries Technical Paper. No. 474. FAO, Rome, pp 133–164
Córdova-Zavaleta F, Mendo J, Briones-Hernández SA, Acuña-Perales N, González-Pestana A, Alfaro-Shigueto J, Mangel JC (2018) Food habits of the blue shark, Prionace glauca (Linnaeus, 1758), in waters off northern Peru. Fish Bull 116(3–4):310–322
Cortés E (1997) A critical review of methods of studying fish feeding based on analysis of stomach contents: application to elasmobranch fishes. Can J Fish Aquat Sci 54(3):726–738
Cortés E (1999) Standardized diet composition and trophic level in sharks. ICES J Mar Sci 56(5):707–717
Cortés E (2000) Life history patterns and correlations in sharks. Rev Fish Sci 8(4):299–344
Compagno LJV (2001) Sharks of the world. Bullhead, mackerel and carpet sharks (Heterodontiformes, Lamniformes and Orectolobiformes). An annotated and illustrated catalogue of the shark species known to date. FAO Species Catalogue for Fisheries Purposes. Vol. 2. No. 1. FAO, Rome
Compagno LJV, Dando M, Fowler SL (2005) A field guide to the sharks of the World. Collins, London
Dai XK, Zhu JF, Chen XJ, Xu LX, Chen Y (2012) Biological observations on the crocodile shark Pseudocarcharias kamoharai. J Fish Biol 8(5):1207–1212
Daniel WW (1991) Bioestadística, base para el análisis de las Ciencias de la Salud. 4 edición. Editorial Limusa, Ciudad de México
Ebert DA (2016) Deep–sea cartilaginous fishes of the southeastern Pacific Ocean. FAO Species Catalogue for Fishery Purposes No. 10. FAO, Rome
Estupiñán-Montaño C, Cedeño-Figueroa L, Estupiñán-Ortiz JF, Galván-Magaña F, Sandoval-Londoño A, Castañeda-Suárez D, Polo-Silva CJ (2019) Feeding habits and trophic level of the smooth hammerhead shark, Sphyrna zygaena (Carcharhiniformes: Sphyrnidae), off Ecuador. J Mar Biol Assoc UK 99(3):673–680
FAO (2021) Pseudocarcharias kamoharai (Matsubara, 1936). https://www.fao.org/fishery/en/aqspecies/14647. Accessed 15 February 2021
Ferry LA, Cailliet GM (1996) Sample size and data analysis: are we characterizing and comparing diet properly? In: MacKinlay D, Shearer K (eds) Feeding Ecology and Nutrition in Fish, International Congress of the Biology of Fishes. American Fisheries Society, Bethesda, pp 71–80
Fischer W, Krupp F, Schneider W, Sommer C, Carpenter K, Niem V (1995) Guía FAO para la identificación de especies para los fines de la pesca. Pacífico centro oriental. Vol. 2–3. Vertebrados. partes 1 y 2. FAO, Roma
Froese R, Pauly D (2021) FishBase. www.fishbase.org. version. Accessed 10 April 2021
Fujita K (1981) Oviphagous embryos of the pseudocarchariid shark. Pseudocarcharias kamoharai from the central Pacific. Jap J Icthyol 28(1):37–44
García-Godos I (2001) Patrones morfológicos del otolito sagitta de algunos peces óseos del mar peruano. Vol. 20 (1–2). Instituto del Mar de Perú (IMARPE), Callao
Hammer O (2020) Paleontological statistics, PAST, Version 4.01. University of Oslo, Oslo
Hazin FHV, Couto AA, Kihara K, Otsuka K, Ishino M (1990) Distribution and abundance of pelagic sharks in the South-Western Equatorial Atlantic. J Tokyo Univ Fish 77:51–64
Hoffman M (1978) The use of Pielou’s method to determine simple size in food studies. In: Fish Food Habits Studies. Proc. 2nd. Pac. NW Technical Workshop. University of Washington, Seattle, pp 56–61
Horn HS (1966) Measurement of overlap in comparative ecological studies. Am Nat 100(914):419–424
Hyslop E (1980) Stomach contents analysis, a review of methods and their application. J Fish Biol 17(4):411–429
Ingrid L, Iverson K, Pinkas L (1971) A pictorial guide to beaks of certain Eastern Pacific cephalopods. Fish Bull 152:7–35
Jereb P, Roper CFE (2010) Cephalopods of the world. An annotated and illustrated catalogue of cephalopod species known to date. Myopsid and Oegopsid Squids. FAO Species Catalogue for Fishery Purposes. Vol. 2, No. 4. FAO, Rome
Jereb P, Roper CFE, Norman MD, Finn JK (2014) Cephalopods of the world. An annotated and illustrated catalogue of cephalopod species known to date. Octopods and Vampire Squids. FAO Species Catalogue for Fishery Purposes. Vol. 3, No. 4. FAO, Rome
Jiménez P, Béarez P (2004) Peces marinos del Ecuador continental. Tomo 2. SIMBIOE/NAZCA/IFEA, Quito
Jiménez-Valverde A, Hortal J (2003) Las curvas de acumulación de especies y la necesidad de evaluar la calidad de los inventarios biológicos. Rev Iber Aracnol 8:151–161
Johnson DH (1980) The comparison of usage and availability measurements for evaluating resource preference. Ecology 61:65–71
Kindong R, Wang H, Wu F, Dai X, Tian S (2020) Age, growth and sexual maturity of the crocodile shark Pseudocarcharias kamoharai from the eastern Atlantic Ocean. Front Mar Sci 7:586024. https://doi.org/10.3389/fmars.2020.586024
Krebs CJ (1985) Ecología: estudio de la distribución y la abundancia (2° ed). Editorial Harla, Ciudad de México
Kyne PM, Romanov E, Barreto R, Carlson J, Fernando D, Fordham S, Francis MP, Jabado RW, Liu KM, Marshall A, Pacoureau N, Sherley RB (2019) Pseudocarcharias kamoharai. The IUCN Red List of Threatened Species. https://doi.org/10.2305/IUCN.UK.2019-1.RLTS.T39337A171964644.en.Accessed3January2021
Labropoulou M, Eleftheriou A (1997) The foraging ecology of two pairs of congeneric demersal fish species: importance of morphological characteristics in prey selection. J Fish Biol 50(2):324–340
Langton RW (1982) Diet overlap between Atlantic cod, Gadus morhua, silver hake, Merluccius bilinearis, and fifteen other northwest Atlantic finfish. Fish Bull 80(4):745–759
Last PR, Stevens JD (1994) Sharks and Rays of Australia. CSIRO, Hobart
Lessa R, Andrade HA, De Lima KL, Santana FM (2015) Age and growth of the midwater crocodile shark Pseudocarcharias kamoharai. J Fish Biol 89(1):1–15
Loor-Andrade P, Pincay-Espinoza J, Rosas-Luis R (2017) Diet of the blue shark Prionace glauca in the Ecuadorian Pacific Ocean during the years 2013 to 2015. J Appl Ichthyol 33(8):558–562
Martin RA (2003) Biology of the crocodile shark. ReefQuest Centre for Shark Research. http://www.elasmo-research.org/education/shark_profiles/pseudocarcharias.htm Accessed 8 December 2021
Martínez-Ortiz J, García-Domínguez M (2013) Guía de campo: condrictios del Ecuador. Quimeras, tiburones y rayas. Ministerio de Agricultura, Ganadería, Acuacultura y Pesca (MAGAP). Viceministerio de Acuicultura y Pesca (VMAP)/ Subsecretaría de Recursos Pesqueros (SRP), Manta
Matsubara K (1936) A new carcharoid shark found in Japan. Zoological Magazine 48(7):380–382
Morisita M (1959) Measuring interspecific association and similarity between communities. Mem Facy Sci Kyushu Univ Ser E Bio 3:65–80
Munroe SEM, Simpfendorfer CA, Heupel MR (2014) Defining shark ecological specialisation: concepts, context, and examples. Rev Fish Biol Fisheries 24:317–331
Muñoz H (2012) Características de los otolitos sagitales de peces pelágicos pequeños de interés comercial en las costas de Santa Elena, Pacífico ecuatoriano, julio 2011-enero 2012. Dissertation, Universidad Península de Santa Elena
Murua H, Santiago J, Coelho R, Zudaire I, Neves C, Rosa D, Zudaire I, Semba Y, Geng Z, Bach P, Arrizabalaga H, Bach P, Baez JC, Ramos ML, Zhu JF, Ruiz J (2018) Updated ecological risk assessment (ERA) for shark species caught in fisheries managed by the Indian Ocean Tuna Commission (IOTC). Conference: 21st Scientific Committee IOTC. IOTC, Seychelles
Musick JA (1999) Ecology and conservation of long-lived marine animals. In: Musick JA (ed) Life in the slow lane: ecology and conservation of long-lived marine animals. Am Fish Soc Symp 23 pp 1–10
Oliveira P, Hazin FHV, Carvalho F, Rego M, Coelho R, Piercy A, Burgess G (2010) Reproductive biology of the crocodile shark Pseudocarcharias kamoharai. J Fish Biol 76(7):1655–1670
Olson J, Galván-Magaña F (2002) Food habits and consumption rates of dolphinfish (Coryphaena hippurus) in the Eastern Pacific Ocean. Fish Bull 100(2):279–298
Pacoureau N, Rigby CL, Kyne PM, Sherley RB, Winker H, Carlson JK, Fordham SV, Barreto R, Fernando D, Francis MP et al (2021) Half a century of global decline in oceanic sharks and rays. Nature 589:567–571
Palomares MLD, Pauly D (2021) SeaLifeBase. www.sealifebase.org. Accessed 5 May 2021
Peláez M (1997) Hábitos alimenticios de la cabrilla sardinera Mycteroperca rosacea Streets. (Pisces: Serranidae) en la Bahía de La Paz, BCS y las zonas adyacentes. Dissertation, Universidad Autónoma de Baja California Sur
Robertson DR, Allen GR (2015) Peces costeros del Pacífico oriental tropical: sistema de información en línea. Versión 2.0. Instituto Smithsoniano de Investigaciones Tropicales. https://biogeodb.stri.si.edu/sftep/es/pages Accessed 10 March 2020
Rosas-Luis R, Loor-Andrade P, Carrera-Fernández M, Pincay-Espinoza JE, Vinces-Ortega C, Chompoy-Salazar L (2015) Cephalopod species in the diet of large pelagic fish (sharks and billfishes) in Ecuadorian waters. Fish Res 173(2):159–168
Rosas-Luis R, Pincay-Espinoza JE, Loor-Andrade P, Carrera-Fernández M (2016) Trophic ecology of the shortfin mako Isurus oxyrinchus (Lamniformes: Lamnidae) in the Eastern Pacific Ocean. In: Kovács A, Nagy P (eds) Advances in Marine Biology, vol 1. Nova Science Publishers, New York, pp 147–182
Sagar MV, Gop AM, Nair RJ (2018) Stomach content analysis techniques in fishes. In: Nair RJ (ed) Recent advances in fishery biology techniques for biodiversity evaluation and conservation. Indian Agricultural Statistics Research Institute, New Delhi, pp 104–115
Saikia SK (2015) Food and feeding of fishes. What do we need to know? Transylv Rev Syst Ecol Res 17(1):71–84
Sminkey TR (1995) Musick JA (1995) Age and growth of the sandbar shark, Carcharhinus plumbeus, before and after population depletion. Copeia 4:871–883
Sminkey TR, Musick JA (1996) Demographic analysis of the sandbar shark, Carcharhinus plumbeus, in the western North Atlantic. Fish Bull 94(2):341–347
Smith P, Zaret M (1982) Bias in estimating niche overlap. Ecology 63(5):1248–1253
Subsecretaría de Recursos Pesqueros (2013) Peces pelágicos, grandes y tiburones. MAGAP/. VAP/SRP. http://tiburon.viceministerioap.gob.ec/tiburon-ecuador/estadisticas-globales-por-mes-todos-los-puertos-264.html. Accessed 20 June 2014
Stillwell C, Kohler N (1982) Food, feeding habits, and estimates of daily ration of the shortfin mako (Isurus oxyrinchus) in the Northwest Atlantic. Can J Fish Aquat Sci 39(3):407–414
Van der Laan R, Fricke R, Eschmeyer WN (2021) Eschmeyer’s catalog of fishes: classification. http://www.calacademy.org/scientists/catalog-of-fishes-classification/. Accessed 4 august 2021
Vinueza E (2015) Catálogo de otolitos sagitta de especies comerciales en los cantones de Sucre y San Vicente, Manabí, Pacífico ecuatoriano. Dissertation, Pontificia Universidad Católica del Ecuador Sede Manabí
Wetherbee BM, Gruber SH, Cortes E (1990) Diet, feeding habits, digestion and consumption in sharks with special reference to the lemon shark Negaprion brevirostris. In: Pratt HL Jr, Gruber SH, Taniuchi T (eds) Elasmobranchs as living resources: advances in the biology, ecology, systematics, and the status of the fisheries, NOAA Technical Report NMFS 90, Proceedings of the Second United States-Japan Workshop 9–14 December 1987. U.S. Department of Commerce, Hawaii, pp 29–48
White WT (2007) Biological observations on lamnoid sharks (Lamniformes) caught by fisheries in eastern Indonesia. J Mar Biol Assoc UK 87(3):781–788
Wolff G (1982) A beak key for eight Eastern Tropical Pacific cephalopod species, with relationship between their beak dimension and size. Fish Bull 80(2):357–370
Wolff G (1984) Identification and estimation of size from beaks of eighteen species of cephalopods from the Pacific Ocean. NOAA Technical Report NMFS no. 17. NOAA, USA
Wu F, Kindong R, Dai X, Sarr O, Zhu J, Tian S, Li Y, Nsangue BTN (2020) Aspects of the reproductive biology of two pelagic sharks in the eastern Atlantic Ocean. J Fish Biol 97(6):1651–1661
Young RE, Vecchione M, Mangold KM (2019) Cephalopoda, Cuvier 1797, octopods, squids, nautiluses. http://tolweb.org/Cephalopoda/19386/2019.03.26. Accessed 5 March 2021
Acknowledgements
The authors thank the Facultad de Ciencias Naturales of the Universidad de Guayaquil and the Escuela de Biología of the Pontificia Universidad Católica del Ecuador Sede Manabí for the use of their laboratories for the analysis of the samples during this study. Also, we want to thank Ofelia Escobar Sánchez for helping in the construction of the cumulative curve of prey species and for her advice on the trophic ecology of fishes.
Author information
Authors and Affiliations
Contributions
Marcos D. Calle-Morán: conceptualization, methodology, software, validation, formal analysis, investigation, resources, data curation, writing – original draft, writing – review and editing, visualization, supervision; Ana R. Hernández-Téllez: conceptualization, methodology, software, validation, formal analysis, investigation, resources, writing – original draft, writing – review and editing, visualization, supervision; Evelyn R. Tibán-Vivar: conceptualization, methodology, software, validation, formal analysis, investigation, resources, data curation, writing – original draft, writing – review and editing, visualization, supervision; Yari E. Intriago-Vera: conceptualization, methodology, software, validation, formal analysis, investigation, resources, data curation, writing – original draft, writing – review and editing, visualization, supervision; Israel G. Del Valle-Coello: conceptualization, methodology, software, validation, formal analysis, investigation, resources, data curation, writing – original draft, writing – review and editing, visualization, supervision; Beatriz C. Loor-Jama: conceptualization, methodology, software, validation, formal analysis, investigation, resources, data curation, writing – original draft, writing – review and editing, visualization, supervision; Ángel R. Ganchozo-López: conceptualization, methodology, software, validation, formal analysis, investigation, resources, data curation, writing – original draft, writing – review and editing, visualization, supervision.
Corresponding author
Ethics declarations
Ethics approval
All experiments have been conducted as per the guidelines of the Institutional Animal Ethics Committee from the Universidad de Guayaquil and Pontificia Universidad Católica del Ecuador Sede Manabí. However, the unregulated shark species used in this study were collected for commercial sale as food. Therefore, the use of this species in research does not require ethical clearance.
Consent to participate
Not applicable.
Consent for publication
All authors agreed with the publication of this research.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Calle-Morán, M.D., Hernández-Téllez, A.R., Tibán-Vivar, E.R. et al. Diet composition and feeding habits of the crocodile shark, Pseudocarcharias kamoharai. Environ Biol Fish 105, 685–697 (2022). https://doi.org/10.1007/s10641-022-01277-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-022-01277-x