Abstract
Industrial and urban waste is drained and dumped directly into Algiers Bay without any prior treatment. Increasingly. this waste has damaged the marine environment. The main objective was to evaluate the degree of contamination by trace metals in the continuous monitoring of the marine environment. The study was carried out in two different phases on three marine sediment cores from Algiers Bay. In the first phase. the identification of the mineral phases was carried out by XRD and the chemical composition of the sediment by XRF, and the determination of the organic matter rate and the carbonate rate. In a second phase, the study focused on the determination of lead 210 on the two cores (A and B) and the contents of 20 trace elements (Sc, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, As, Sr, Ag, Cd, Sn, Sb, Cs, Ba, Pb, Th, and U). The trace elements were determined by ICP-MS. The enrichment factor EF and the correlation matrix between the trace metals were studied. The results show that the predominant mineralogical phases are carbonates, followed by silica and clays (illite, kaolinite, etc.). An enrichment in organic matter is observed in the deep layers of the cores. The trace metal contents show quite heterogeneous and variable distributions from one element to another and from one core to another. The first few centimetres of the sediment core are marked by a moderate to high enrichment in EF of the metallic elements. Very strong positive correlations are observed in the three cores such as V-Ni, Pb–Zn, and V-Fe.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Mediterranean Sea is semi-enclosed. Exposure to urban and coastal pollution makes it vulnerable to environmental change. UNEP has defined the Mediterranean Sea as one of the five regions globally with the most serious environmental problems (Boudouresque 1996). The untreated urban and industrial waste into the environment generates increased anthropogenic coastal inputs from industrialising riparian countries (Bachari Houma 2009; Salomon 2003; Houma et al. 2004; Lambert and Penot 1981). The problem is compounded when these discharges are laden with non-biodegradable and/or toxic pollutants, such as trace metals. Trace metals are considered the most dangerous harmful elements for humans and the environment (Issabayeva et al. 2008).
Trace metals are considered one of the major anthropogenic contaminants in coastal and marine environments worldwide (Ruilian et al. 2008). Many anthropogenic activities in coastal areas have a negative impact on the local environment, as they release trace metals through point sources such as industrial activities that discharge them into wastewater, thus contaminating coastal areas, and also through non-point sources via wind and/or alluvial processes (Chester 1990; Lu et al. 2014; El Houssainy et al. 2020). Metal levels in aquatic ecosystems have increased dramatically due to anthropogenic activities’ input (Thevenon et al. 2011). Due to their toxicity, persistence, and bioaccumulation characteristics (DeForest et al. 2007), trace metals introduced into estuarine and coastal areas pose a serious threat to human health, living organisms, and natural ecosystems (Zaidi et al. 2014; Qian et al. 2015). These non-biodegradable trace elements are ultimately deposited on the seabed through complex physical and chemical adsorption mechanisms that depend on the nature of the sediment matrix and the properties of the adsorbed metals. More than 90% of the trace metal load in aquatic systems is associated with suspended particles and sediments (Calmano et al. 1993; Gómez-Parra et al. 2000; Amin et al. 2009), making sediments an ultimate transporter and sink of contaminants (Pekey 2006; Pradhap et al. 2017), reflecting the history of pollution (Singh et al. 2005). These sediments also play an important role in the remobilisation of contaminants in aquatic systems under favourable conditions. Interactions between water and sediment play a major role in determining the pollution pattern of aquatic systems (Zarei et al. 2014). Sediments are considered to be an indicator of pollution memory. They are a witness of the degree of pollution (Lesouef et al. 1979). They provide a record of water inputs to the catchment (Mwamburi 2003).
Like all countries bordering the Mediterranean Sea, Algeria has a sensitive coastline; Algiers Bay, a region located in the centre of this coastline, receives a significant pollution load every day. Wastewater discharged into the rivers is responsible for various organic and inorganic chemical contaminants (Boulahdid et al. 2003). The absence of water pre-treatment systems aggravates this situation. Metal contamination is one of the most threatening aspects of pollution in this marine area (Yoshida et al. 2005). Studying the distribution of metals in sediments adjacent to urbanised and agricultural areas can provide evidence of anthropogenic impact on ecosystems and thus help assess the risks associated with human discharges. This work aims to evaluate the degree of inorganic contamination by trace metals in the context of marine environmental monitoring. It is part of the COMETALG-Mermex cooperation project between the European Centre for Research and Teaching in Environmental Geosciences (CEREGE, Marseille, France) and the National Higher School of Marine Sciences and Coastal Management (ENSSMAL, Algiers, Algeria).
Study area
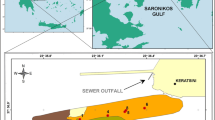
The Algiers Bay is located in the central part of the Algerian coast, east of the Mitidja plain. It has a semi-circular shape with an approximate surface of 180 km2. It is bounded to the west by Pointe Pescade (Rais Hamidou), to the east by Cap Matifou (Bordj El-Bahri), and to the north by the Mediterranean Sea (Maouche 1987; Bachari Houma 2009; Bachari et al. 2011) (Fig. 1). The Algiers Bay is a softsubsidencezone. It undergoes constant sedimentation whose elements come from the reliefs of the Blidean Atlas. At the end of the Pliocene and Quaternary, two aquifer reservoirs were identified. These are the Astian formed by limestones and sandstones of continental origin and the alluvial Quaternary, mainly pebbles and gravels (Djoudar and Toubal 2008). The total rainfall data obtained from the ANRH (Agence Nationale des Ressources Hydrauliques) for the period (1951–2010) show that the flood period in this region extends from October to February with average rainfall between 72 and 114.01 mm. Floods mark this period, and the most catastrophic ones were recorded in October 1957 (total rainfall = 423.6 mm) and in November 2001 (total rainfall = 203 mm), where the cumulative rainfall was 190 mm for the period from 9 to 11 November (Yahiaoui 2012).
The Algiers Bay is 35 km long and includes 11 coastal municipalities (Rabehi et al. 2018), and more than 4.3 million people (950 inhabitants km2) live in the Algiers agglomeration (Soualili et al. 2008). Algiers has transformed itself from an airy capital into a highly urbanised metropolis where the urbanisation rate reached 94.30% in 2008 (ONS 2008). In addition, 1000 companies are active in metallurgy, construction materials, petrochemicals, pharmaceuticals, mechanical, electrical, and electronic industries, and food and paper production (Larid 2003). The majority of these units are concentrated in the industrial zones, mainly the port of Algiers, Rouiba, and Réghaia (Bachari Houma 2009). In the Algiers region, industrial and urban discharges are drained by the main rivers (river El Hamiz and river El Harrach) into Algiers Bay. Wastewater is treated at 8%and discharged directly into Algiers Bay ((PAC) Programme d’Aménagement Côtie 2005). It contains organic matter, suspended solids, trace metals, detergents, lubricating oils, etc. (Houma et al. 2004). These particles precipitate in the sediments of the bay. According to Guendouzi et al. (2015), the sediment is subject to anthropogenic disturbances of different origins.
Materials and methods
Three sediment cores were taken in the Algiers Bay using a “multi-corer” type box corer. During a campaign conducted by ENSSMAL from 14 to 19/03/09, these samples were taken on board the oceanographic vessel “MS Benyahia”. The cores are cut at thicknesses of 0.5 cm for the 0–2 cm layer, 1 cm for the 2–14 cm layer, and 2 cm for the 14–20 cm layer to understand the variation in metal concentration better. Depending on the depth, the samples were kept frozen at − 18 °C on board the ship; freezing essentially preserves the chemical properties of the samples to avoid contamination with the surrounding environment and loss of volatile elements (Charlou and Joanny (1983)) (Table 1, Fig. 1). The sampling points were chosen near the port of Algiers (core A) and the river El Harrach (core C), which cross a large part of the agglomeration of Algiers, but especially the largest industrial zone in Algeria: the industrial zone of Oued Smar and the open sea (core B).
The samples were treated by CHRIST-type freeze-drying for 48 h under vacuum (10–1 bar) and at very low temperature (− 50 °C to − 60 °C), crushed to powder within a porcelain mortar. The samples were subjected to several analytical techniques at the European Centre for Research and Teaching in Environmental Geosciences (CEREGE) in Aix-en-Provence: identification of the mineralogical composition of the samples, particularly the clay minerals, using the oriented aggregate method. This method is based on the preparation of three tests (Normal without treatment, heating to 490 °C, Ethylene–Glycol). The determination of the mineralogical phases was carried out by the diffractometer (X’Pert Pro. PANalytical) equipped with a cobalt anticathode X-ray tube. XRD (X-ray diffraction) is a non-destructive technique commonly used to identify mineral species in a solid matrix (Agostini 2006; Hubert 2008; Cazalet 2012). For the chemical composition of major elements, we used the portable XRF of the type Tracer IV SD manufactured by Burker, which uses a physical property of matter, X-ray fluorescence (Bouzonville et al. 2008). It allowed the sample to be analysed directly in its solid form without going through the processing and mineralisation stages (Bougherara 2011). The quantification of total carbonates (CaCO3) was carried out by Bernard calcimetry according to the NF X 31–105 standard. Direct measurement of organic matter in the sediment of each sample was carried out by the loss on ignition method (Wang et al. 1996; Wang et al. 2011). The samples were tempered in a muffle furnace at 550 °C for 12 h. The formula to calculate the organic matter content is as follows:
OM%: percentage of organic matter. P0: initial weight. P1: weight after drying at 105 °C. P2: weight after calcination at 550 °C.
The determination of trace metals was carried out by ICP-MS (Pekin Elmer, model NexION 300). The digestion takes place in an ultra-wave microwave oven. Three millilitres of 69% nitric acid (HNO3) and 2 ml of 37% hydrochloric acid (HCl) were added to 0.04 g of sediment and left to stand in the cold in a Teflon tube. At the same time, the microwave bath was prepared and then 150 μl of hydrofluoric acid. Then, 150 μl of hydrofluoric acid is added. After 1 h and 30 min of digestion, we proceeded to the analysis of metallic elements. This technique is based on the ionising properties of the inductively coupled plasma (ICP) source. ICP-MS allows detection limits in the ppb (μg/L) or ppt (ng/L) range (Cazalet 2012; Achard 2013). The elements analysed are Sc, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, As, Sr, Ag, Cd, Sn, Sb, Cs, Ba, Pb, Th, and U. The results of the analysis of the 20 trace metals on the IAEA-433 standard sample show that about 91% of the metals confirm the accuracy of this analytical method (Table 2).
The enrichment factor calculation (EF) makes it possible to discriminate between anthropogenic inputs and natural sources and define the contamination’s intensity, based on the standardisation of the data (Meybeck et al. 2007; Radakovitch et al. 2008; Emmanuelle 2010). For the standardisation, we used a conservative element representative of the clay fraction, Scandium (Emmanuelle 2010). The calculation compares the metal content measured in the sample [Cx] with the local geochemical background content of the same element [Cn] RM. Natural background levels can be established from measurements in the sediment background outside of anthropogenic influence. Scandium (Sc) was chosen as the stationary reference element for this calculation. The absence of scandium in most industrial processes eliminates the problem of its introduction into the environment in an enriched state or significant quantities (Yusof and Wood 1993) and its combined structure in clay minerals and micas. This makes it a good indicator of clay (Dias and Prudêncio 2008). Scandium is mainly of natural origin and almost entirely located in the lithic fraction (Buat-Menard and Chesselet 1979). Therefore, it is a more suitable lithic and conservative element for standardising chemical data than other elements.
EF, enrichment factor; [Cx], concentration of metal studied; [Cn], scandium concentration;S, sample; RM, reference materials.
Enrichment factors can be grouped into five classes (Tessier et al. 2011):
-
EF < 2: no or low enrichment;
-
2 < EF < 5: moderate enrichment;
-
5 < EF < 20: significant enrichment;
-
20 < EF < 40: very strong enrichment.
For the dating of 210Pb, the results were processed using the CRS (constant rate of supply) model proposed by Appleby and Oldfield (1978). 210Pb was measured indirectly using its daughter element, 209Po. Two hundred fifty milligrams of sediment was weighed into a Teflon beaker. One hundred microlitres of the 209Po solution was added. The sample was subjected to an attack by strong acids (3 ml nitric acid (HNO3 70%), 3 ml hydrofluoric acid (HF 48%), 3 ml hydrochloric acid (HCl 36%)); each time acid was added. It was heated to 60 °C and evaporated at 90 °C. Two hundred fifty millilitres of 0.3 N hydrochloric acid was added and heated to 90 °C with stirring. Then, the temperature was lowered to 60 °C, and 100 mg of ascorbic acid was added.
At the same time, the silver discs were placed in HNO3 to clean them. The acrylic varnish was applied to the numbered side of the discs and left to dry. Fixing the 209 Po solution to the disc took 4 h, and the discs were removed, rinsed with Milli-Q water, and left to dry. Counting was performed at CEREGE in ORTEC counting chambers. The α spectrometer was used, and the measured activity (in Bq.kg−1) was brought back to the sampling date.
The statistical software XLSTAT Version 2016.02.28451 for Windows was used for the Pearson correlation matrix analysis for the 20 trace metals studied in the sediments of the three Algiers Bay cores and for the multivariate analysis (component analysis, PCA).
Results
Determination of mineral phases by XRD, chemical composition by XRF, and carbonates
The results indicate that the mineralogical composition is relatively homogeneous in the samples of the three cores. They were identified by XRD and confirmed by XRF and Bernard calcimetry, where carbonates dominate, followed by silica. The distribution of carbonate contents is homogeneous with the following means [core A (24.91 ± 3.08%), core B (30.09 ± 3.77%), and core C (28.4 ± 4.27%)]. The CaCO3 values of the cores are similar to the CaO values determined by XRF, confirming that the CaO is in the form of calcite (Fig. 2a). The CaO identified by XRF is mainly bound to carbonates and sulphates (calcite (CaCO3) (d = 3.03 Å). dolomite (CaMg (CO3)2) and gypsum (a doubly hydrated calcium sulphate (CaSO4 2 H2O) (d = 7.63 Å)) and accounts for [13–20%] of the total. Silica (quartz (SiO2) (d = 3.34 Å, 2.6 Å)) which is about 40% in all three cores enters the formation of clays.
Examination of diffractograms obtained from oriented preparations shows that the mineralogical composition of the total AT clay ([44–60%] of the sediment) is almost identical along with all three cores and consists mainly of illite (K, H3O) (Al, Mg. Fe)2 (Si, Al) 4O10 [(OH) 2, (H2O); interbedded illite/smectite (10 Å) and illite/chlorite (14 Å) clays, kaolinite (Al4Si4O10 (OH)8) (d = 7.15 Å) and vermiculite (Mg, Ca)0.7 (Mg, Fe, Al)6 (Al, Si) 8O22 (OH) 4.8H2O (d = 3.6 Å), Al2O3 [10–17%] and K2O ≈ of 2.7% confirm the dominance of illite, which is a clay mineral (most typical of marine environments). Silica (SiO2) and alumina (Al2O3) indicate the high proportion of aluminosilicates and the low proportion of quartz. If the SiO2/Al2O3 ratio is between 2.4 and 3.3, this confirms the dominance of aluminosilicates (Brosse 1982). In our case, the ratio values are between 2.7 and 3.4 (Table 3, Fig. 3). Iron (Fe2O3) indicates the presence of haematite with values increasing slightly towards the bottom of the three cores [6. 1–7. 3%].
Organic matter
The sediments of all three cores are rich in 8 [cores: A (9.06 ± 2.24%); B (11.19 ± 3.97%); and C (5.67 ± 2.93%)]. The organic matter content fluctuates considerably with depth in all three cores (Fig. 2b). These fluctuations are related to the degradation of organic matter and terrigenous and marine inputs. In the marine environment, sediments rich in organic matter can be explained by the following: preservation under anoxic conditions in a static situation or by high primary productivity of the system. The sediments of the three cores are relatively rich in organic matter, particularly core B. The sources of organic matter at the sampling points are essential: the contributions of the rivers, in particular the El Harrach river, which also includes wastewater, and the contributions of urban and industrial waste discharged into the port and transferred into the Algiers Bay. According to Bakalem (2008), the highest values are found in the sediments of the central and western sector near the port of Algiers and the lowest in the sediments of the eastern sector, notably near the river Hamiz whose flow is regulated by a dam. The sediments are rich in organic matter in the central sector and the sector near the port of Algiers. This is mainly due to the El Harrach river inputs in the first sector and the port area and domestic wastewater discharges in the second sector.
Lead 210 content
To carry out the geochronological dating of the horizons of the two cores A and B, we used the theoretical CRS (Constant Rate of Supply) model (Appleby and Oldfield 1978), which assumes that variations in 210Pb deposition are negligible over the last 150 years (the interval during which sediments can be dated by the 210Pb method). This method assumes a resolution in the very fine data with a measurement of 210Pb activity for all depths to calculate the different terms of this equation. Furthermore, it does not assume a constant sedimentation rate. However, the gamma-ray spectrometer values showed that the levels of ‘excess Pb-210’ radioactivity in the deposits in Core A are within the upper limits of the values measured in Cores A and B. They range from 151 ± 9 mBq/g at the surface to 30 ± 5 mBq/g at the bottom of core A and from 114 ± 3 mBq/g in the middle of the core to 38 ± 3 mBq/g at the bottom of core B. It can be said that the measured values of ‘excess Pb-210’ radioactivity in both cores reflect the presence of bioturbation and/or homogenisation effects that mark the first few centimetres of the surface down to the (10.5 cm) horizon for core A and down to (12.5 cm) for core B. The resolution of the ‘excess Pb-210’ measurements does not allow the different depths of the two cores to be dated, but it does allow an estimate of the rate of sediment accumulation (Fig. 4). However, the rate of sediment accumulation and sedimentation is estimated in the deep part of both cores where there is no mixing:
-
1.
For core B, from 12.5 cm to the bottom, the sediment accumulation rate is estimated to be about 0.21 g/cm2/year with a sedimentation rate of about 0.11 cm/year.
-
2.
For the 9.5 cm core A at the bottom, there is a low accumulation rate of about 0.07 g/cm2/year and a sedimentation rate of about 0.05 cm/year.
Trace metals
The ICP-MS results of the three cores allow to establish vertical distribution profiles for each metal analysed (Sc, V, Cr, Mn, Fe, Co, Ni, Cu, Zn, As, Sr, Ag, Cd, Sn, Sb, Cs, Ba, Pb, Th, and U) (Table 4, Fig. 5). The local reference background values reported in Table 4 were derived from three sediment cores taken in Algiers Bay. The proposed local background values average 9 samples taken from these cores between 20 and 34 cm depth. The average levels of trace metals in the local background sediment are referred to as “background”, the concentration of metals in pristine sediment unaltered by human activity, and “enrichment” is the extent present-day sediment metal concentrations exceed pre-anthropogenic levels (Birch. 2017). Local background reference values were used to assess the enrichment of marine sediment by anthropogenic metals.
The analysis of the vertical profiles of the different metallic elements in the sedimentary cores shows rather heterogeneous and variable distributions from one element to another and from one core to another. In general, the metallic elements studied are present in concentrations that can be considered usual. However, special cases of certain elements and relatively high concentrations with pollution and/or geochemical anomalies are noted and discussed below. The sediments of the three cores are characterised by the following:
-
Fairly stable vertical profiles for the following elements: Cd, U, Sb Sn, As, Sc, Co, Mn, Sr, Ba,V, Cr, Zn. This distribution stability is observed for Sn in core B and Co, As, Cu, Ni, Mn, Sr, Ba, V, Cr, Zn, Ag in cores A and C. This behaviour may provide information on the sedimentary regime, which may be quite stable with similar and/or identical origins of sediments and elements. These profiles, which do not show significant fluctuations and low to moderate metal concentrations, may provide information on a much more natural than the anthropogenic origin of the metals.
-
Lead has a particular distribution. Its concentration increases progressively from the base of the core towards the surface, where it is found multiplied by a factor of about 3 in core A and by a factor of about 2 in core B. This enrichment can be directly related to anthropogenic pressure from road and sea traffic, where tetraethyl lead is indexed. In core C, this element and the other show an anomaly in the superficial part of the core: between 2 and 6 cm depth. This anomaly can only be anthropogenic.
EF and correlation matrix
Enrichment factor (EF)
Core A (Table 5): it is observed that the elements V, Cr, Mn, Sc, Co, Ni, Cu, As, Sr, Cd, Sb, Cs, Ba, U, and Sn follow the same evolution in time with values that do not exceed 2. This puts them within the range of natural variability. except at depth 8 cm, where there is slight contamination, which does not exceed value 3. Higher values of Ag, Pb, and Zn (1.38 < EF (Ag) < 5.19; 2.11 < EF (Pb) < 3.65; 1.36 < EF (Zn) < 4.96) are identified at the surface of core [0–8 cm]. In this section, continuous moderate enrichment is observed for zinc Zn, lead Pb; after the 11 cm depth. These metals fall within the range of natural variability. Core B shows moderate to heavy contamination in the core’s upper [0–11 cm]. In this section, trace metal EFs with values above 5 are as follows: (3.54 < EF (Zn) < 5.66; 3.45 < EF (Cd) < 6.87; 3.52 < EF (Pb) < 5.69; 4.29 < EF (U) < 6.94; 3.26 < EF (Sn) < 5.48; 2.29 < EF (Sb) < 7.26) (Fig. 6). For all metallic elements, enrichment factor values decrease after the 11 cm depth to reach a value of about 1. In core C, three groups of trace metals are observed according to the enrichment factor; the first group contains V, Cr, Mn, Sc, Co, Ni, Cu, Sr, Ba, and Th. It is characterised by a moderate enrichment that does not exceed 4 in the upper part [0–3 cm] of the core. For the rest of the core, the values are low, indicating little or no enrichment except at depth 10 cm, where there is moderate enrichment of all 10 elements. The second group includes the elements (As, Cd, Sn, Cs, Pb), with moderate enrichment in the 0–10 cm of the core. Significant enrichment is observed for the third group (1.37 < EF (Zn) < 5.71; 2.44 < EF (Ag) < 7.3; 1.54 < EF (Sb) < 5.28) in the same section of core from 0 to 10 cm. After the 10 cm depth and up to 34 cm depth, enrichment factors are low for all trace metals except for Cu, Zn, As, Ag, Sb, and Ba, where a moderate enrichment is observed at 18 cm depth, implying a moderate input from the same source and in the same period.
Vertical EF profiles for trace metals in the three sedimentary cores (A, B, and C) from Algiers Bay. Vertical red lines represent the limit of EF scales: EF < 2, no or low enrichment; 2 < EF < 5, moderate enrichment; 5 < EF < 20, significant enrichment; 20 < EF < 40, very strong enrichment (Tessier et al. 2011)
Statistical analysis
Correlation analysis was carried out to test the relationship between trace metals, based on Pearson’s statistical analysis of element. The results from all three cores show that for the whole data set, the majority of metals are significatively and positively correlated with each other (p < 0.05), except Sc, Sb, and MO% (Table 5). Manganese Mn and iron Fe are positively correlated with most elements. Ni and Co have significant correlations (r ˂ 0.9) with Fe indicating the likely adsorption of these metals onto Fe oxyhydroxides. Similar sources and similar behaviour can explain the strong positive correlations between trace metals during their transport (Jamshidi and Bastami. 2016).
These significant correlations in sediment cores A and C reflect similar levels of contamination and/or discharges from the same pollution sources (Inal et al. 2018). The sampling points for both cores are located near the coast of the bay and the mouth of the El Harrach River, where there is continuous discharge of urban and industrial discharges (from industrial areas). The organic matter in core B, which is higher than in the other two cores, has non-significant correlations with all metals, which contradicts the affinity of trace metals to be fixed by adsorption to the surface of the organic matter. By examining the MO% and EF profiles of metals in core B (Figs. 4 and 6), it can be seen that the enrichment of MO% in the sediment is marked after the depth of 12 cm. Conversely, the enrichment of trace metals is located in the surface layer of the core between 0 and 12 cm. After this depth, all metals enter the range of natural enrichment except uranium which displays moderate to significant EF; after 20 cm, the enrichment is natural.
Significant correlations (p < 0.05) are presented in bold.
The results of the PCA are presented in Fig. 7; two principal components are extracted. which explain 66.66% of the total variance. The first factor (PC1) explains 48.78% of the total variance and contains positive loading of V, Cr, Mn, Fe, Co, Ni, Cu, Zn, As, Sr, Ag, Cd, Sn, Cs, Th, and U and negative loading of MO%. The second factor (PC2) accounts for 17.16% of the total variance (Fig. 7a). The fact that the factors are different means that two different contributions (natural and anthropogenic contribution) are involved in determining trace element concentrations in marine sediments (Rahman et al. 2014), the PCA results of the 20 metal elements and samples from the three sediment cores (A, B, C) showed three significant groupings (Fig. 7b): the first contains Th, Cs, Cr, Ag, Sn, Sb, and Cu correlated with the surface samples from core C; the second contains Mn, Co, Fe, Ni, Sr, Cd, V, As, Ba, U, and Cr correlated with the rest of the samples from core C and the samples from core A. The third grouping contains only the B core samples which correlated with organic matter.
Discussion
This study presents a general overview of the nature of the sediments in the three cores. We observe that the mineralogical composition is relatively homogeneous in the samples of the three cores that can represent the study area. This mineralogical composition was identified by XRD and confirmed by XRF and Bernard calcimeter where carbonates dominate, followed by silica. The carbonate input can be of detrital origins such as fluvial input or biogenic origin from planktonic and/or benthic organisms (Schneider et al. 2000). Silica is involved in clays, representing 40–60% of the total sediment.
These results are consistent with those reported by Beladel et al. (2013). The authors indicated the dominance of illite. It is a clay mineral and the most typical of marine environments. It is often found associated with other clay minerals (Lamouri 2008), forming interbedded clays of the illite/smectite and illite/chlorite type; their proportion varies with depth in the three cores. The dominance of illite is linked to dominant mechanical erosion conditions, and therefore (Le Gouche 2017), the presence of kaolinite is related to the alteration of an acid rock such as granite and/or to the good drainage and leaching of the El-Harrach and El-Hamiz rivers. Gypsum is present in the surface samples of all three cores and due to the increase in salinity with depth, leading to gypsum precipitation (Hoareau 2009).
The sediments of the three cores are rich in organic matter; the organic matter content fluctuates considerably at depth in the three cores. These fluctuations are linked to the phenomenon of organic matter degradation and terrigenous and marine inputs. The richness of organic matter in marine sediments can be explained by the following: preservation under anoxic conditions in a static situation or by high primary productivity in an adynamic system (Pedersen and Calvert 1990; 1991). In addition, the level of organic matter increases with the volume of the fine fraction present in the sediment (Thamban et al. 1997; Berthois et al. 1968).
The high organic matter contents used in this study are located in core B in the sedimentary layer (12–32 cm). Conversely, the enrichment of trace metals is located in the surface layer of the core between 0 and 12 cm. After this depth, all metals enter the natural enrichment range except uranium, which is moderate to significant EF. Moreover, after 20 cm, the enrichment of U becomes natural. The enrichment of the sediment of core B by MO% is related to the sampling point located off the bay where the fine fraction is above 95% (Baba-Ahmed et al. 2018), which gives it an adsorption capacity for organic matter (Bergamaschi et al. 1997). The sedimentation rate is a major factor in accumulation, especially for preserving organic matter (Bohacs et al. 2005). Sedimentation rate and anoxia seem to be key parameters in preserving organic matter. Indeed, anoxia and a very low sedimentation rate allow the concentration of organic matter (Le Gouche 2017). The low sedimentation rate is linked to the absence of detrital inputs, allowing the concentration of organic matter (Wignall and Hallam 1991).
The Pb-210 curves produce the calculation of the sedimentation rate in both cores A and B in the sediments, which allow two episodes of radioactive decay to be distinguished. The change in sediment is very particular in both cores. In the upper part, highly mixed sediments are observed. In the deeper part, more stable sediments are observed, where low sedimentation rates could be calculated (core A: from 10. 5 cm at the bottom, sediment accumulation rate 0.07 g/cm2/year, sedimentation rate 0.05 cm/year; core B: 12.5 cm at the bottom, sediment accumulation rate 0.21 g/cm2/year, sedimentation rate 0.11 cm/year). These rates are respectively related to the maximum organic matter content (16.4%; 17.06%) in both cores. The results from core B are close to the study carried out by Arabi et al. (2006) on the sedimentation rate of 137Cs in the sediments of Algiers Bay. The authors found a sedimentation rate (0.133 cm/yr) and an accumulation rate of 0.335 g/cm/yr, which seems to be slightly high. According to Trentesaux (2005), 210Pb calculates the indicated sedimentation rates while 137Cs highlight anthropogenic events (Table 6).
*(core B from 12.5 cm to the bottom and core A from 9.5 cm to the bottom).
The sedimentation rate is related to many parameters (bioturbation, resuspension by bottom currents, and anthropogenic alterations) (Monna et al. 1997). The more the environment is subject to one of these parameters, the higher the sedimentation rate. This is the case of Kaštela Bay, Croatia (Mikelić et al. 2017), which records the higher sedimentation rates (Table 6). Matsumoto and Togashi 1980) described core sediments as undisturbed sediments from Funka Bay (Japan). Their sedimentation rates are similar to those of Algiers Bay. Sagami Bay (Japan) records a high sedimentation rate in the continental slope domain (Kato et al. 2003) with that of the Alboran Sea. Miralles (2004) explains this by the strong vertical flows present in the area. The cores are taken from the western part of the Alboran Sea. The metal element profiles of the three sediment cores resulting from the ICP-MS analysis confirm the results for 210 Pb, where two layers are observed. The upper sedimentary layer is very mixed. Two hypotheses can explain this phenomenon:
-
1.
The centre and east of the bay have been subjected to the most significant human pressures (Belhai-Benazzouz and Djelal 2010) due to the development work carried out on the bay’s coastline, in particular, the construction of the eastern motorway along the shoreline of the river El Harrach-Port of Algiers sector. The three communes (Hussein Dey, Mohammadia, Bordj El Kiffan) to the east of the bay are characterised by high socio-economic vulnerability (urbanisation, high demography, road network density) (Rabehi et al. 2018). During construction, the main disturbances are the resuspension of fine sediments, the increase in water turbidity, and the input and sedimentation of solid materials (Bakalem 2008).
-
2.
Several rivers flow into the Algiers Bay, the most important of which are river El Harrach, river El Hamiz, and Oued Koriche. They are subject to a series of natural hazards that have recently been the cause of major catastrophes. Floods, as was dramatically the case in Bab el Oued in 2001 (Rabehi et al. 2018; Djellouli and Saci 2003), devastating earthquakes affected Boumerdes in 2003 (Yelles-Chaouche et al. 2009). The Algerian coasts are areas of high seismicity due to their location near the Sahel. This high seismic activity generates a latent tsunami risk (Maouche et al. 2009; Larara et al. 2012). This risk probability of a few decades to 100 years, based on known historical events (Egis Eau/IAU-IDF/BRGM 2013). These natural effects have caused a sudden discharge of terrigenous materials towards the Algiers bay through the three rivers. The most important is the river El Harrach whose watershed covers an area of about 1250 km2. It extends mainly to the wilayas of Algiers and Blida. It touches in the South the wilaya of Medea and the North-East a small part of the wilaya of Boumerdes, including 4 industrial zones and ten commercial activity zones (Louati 2015). The 100-year floods of the El Harrach and El Hamiz rivers affect large areas, partly urbanised with sensitive zones, such as business zones, facilities, and dense urbanisation zones. The often recent urbanisation of these areas reinforces their vulnerability. The sedimentary contributions of these rivers, which emerge on the coast, are likely to bring materials to the shoreline during flood periods (Egis Eau/IAU-IDF/ BRGM 2013).
Fluctuations in trace metal profiles are observed above 10 cm for core A. Conversely, disturbances in metal content are found below 10 cm in core B. These fluctuations in trace metal profiles correlate with the enrichment factor results. It is noted that the slight fluctuations in trace metal profiles in core A are related to low enrichments due to natural inputs, and even the elements Th, Cs, Sc, and Fe, which show profiles with fairly large fluctuations, are within the range of natural enrichments due to fluvial inputs but of different origin and this is explained by the very low correlation between these elements, except between Cs and Fe, the latter showing a strong positive correlation with the metals: As, Cd, V, Cr, Ni, Co, and Sr indicating the probable adsorption of these metals on Fe oxyhydroxides. The elements Zn, Pb, and Ag show moderate enrichment on the 0–8 cm core surface, with strong pairwise correlations: Pb/Cu, Pb/Zn, and Pb/Sn indicating a common origin.
The B core sediments are characterised by moderate to high contamination for all trace metals. These EFs exceed the value of 7 in the case of Sb in the range 0–11 cm. This antimony (Sb) originates from air emissions from road traffic (Weckwerth 2001) and/or is used as a hardener in Pb-Sb alloys in lead battery plates, the chemical industry, the manufacture of printing type. The metal is also used in soldering and the preparation of other alloys to colour certain glasses, etc. (Bernatchez 2008). Although core B shows a more stable distribution of metal contents in this part than in the rest of the core, below this depth, the EF values of the metal elements indicate natural enrichment. The correlation matrix of these metals shows non-significant correlations of these metals, which means that these metals are not controlled by a single factor (Suresh 2011), and that they have different behaviour and origin, except for the pairs Co/Mn, Fe/Mn, Pb/Zn, Ni/V, and Cd/Ni where the positive correlations are strong.
Two peaks characterise core C in the concentration of certain metals: the first at the 6 cm depth and the second at the 16 cm depth of the core. The distribution of the elements C, Zn, Pb, Cu, and Sn is linked to the strong positive correlation of these metals with Cs, Sn, Th, U, Ag, Sb, and Cd. In the sediment core, these correlations exceed 0.80, moderate to significant enrichment is observed in the first 3 cm of the core with values exceeding EF = 8.5 in the case of uranium (U). This enrichment is also observed at the 10 cm depth of the sediment core for all metals, and with an EF exceeding 8 for U, at a depth of 18 cm, a moderate enrichment is noted for Cu, Zn, As, Ag, Sb, Ba, and U (EF = 5.17).
A very strong correlation of the Pb/Zn couple is observed in the three cores with significant enrichment of these two elements, indicating the same continuous and important source of pollution of these metals (Kükrer et al. 2014). According to Atroune and Boutaleb (2012), the association of these two trace elements is due to the metallurgical factories installed along the rivers El Harrach and Oued Smar, which will generate significant contamination by these trace metals (Chouikhi et al. 1988; Boudjellal et al. 1993; Maouche 1987) (Table 7) with a relatively high contamination rate in the Algiers Bay. In general, the PCA analysis indicates a correlation of trace metals by the sediments of cores A and C, which confirms that the origin and concentration of these trace metals show and are related to anthropogenic inputs. The wastewater resulting from human activities in the Algiers region arrives directly or indirectly in the marine environment.
Overall, the trace metal contents in the three cores compared to previous work in the Algiers Bay (Table 8) show that the values in our study are higher, particularly in core C, which is located near the river El Harrach. The latter contains the highest concentrations of heavy metals due to the presence of a highly industrialised area characterised by the presence of iron, paper and pulp factories, antifouling paints, chlorine and soda factories, chemical industries, textile industries, metal processing, food processing, beverage manufacturing and bottling, tanneries, oil and soap factories (Table 7). The high lead values in the work of Atroune and Boutaleb (2012) were recorded near the port of Algiers. This is due to discharges from domestic and industrial tributaries into the ports and port activities. The cores of the latter are taken far from the port of Algiers. According to Bachouche et al. (2017), the high Cu content was measured in the inner part of Algiers Bay and could be related to the intensive use of Cu in antifouling paint products. According to a previous study (Yoshida et al. 2005), high levels of Zn and Mn were recorded in the surface sediments of river El Harrach.
Comparison of the metal values in this study with those of other works around the world revealed that most metal elements show enrichment in this study compared to the studies displayed in Table 9, except the following: Mn (511.3–694.4 µg/g) and Co (13.9–18.1 µg/g) in the Yangtze River estuary, China. This is due to more than 6 million tons/day of industrial and domestic wastewater discharge into the Yangtze estuary and a population density (more than 600 inhabitants/km2) (Chen et al. 2004), Cadmium (0.12–6.6 µg/g) in St Helena Bay. South Africa is due to the intensity of urbanisation (Monteiro and Roychoudhury 2005). Sn is a trace pollutant (01–11.01 µg/g) in the bay, and in particular at the edge of the Fort de France urban area, but Sr levels (682–932 µg/g). Although they are very high compared to our study, they are within the range of natural phenomena, certain clays of hydrothermal origin being particularly rich in this element (Pons et al.1988). A remarkable enrichment of metals [Pb (32.6–818.3), Ni (40.8–95.2), Cd (0.19–4.08)] can be observed in the Seybouse Annaba river, which is due to the El-Hadjar steel complex. The latter discharges effluents and industrial wastewater without any treatment (Belabed et al. 2017). Near this river are the gulfs of Annaba, where the study made by Ouali et al. (2018) on trace metals (Zn, Pb, Cu, and Cd) in sediments shows a similarity of the average contents of these metals with one of our studies, which is drained by the river Seybouse towards the gulf of Annaba.
Conclusion
The distribution and dominance of clay minerals are controlled by continuous terrigenous inputs and coastal processes such as waves. The CaCO3 values of the cores are similar to the CaO values determined by XRF, which confirms that the CaO is in the form of calcite. The organic matter richness of the core sediments is due to several factors: the dominance of the fine fraction in the sediment, the anoxia, and the low sedimentation rate is related to the absence of detrital inputs and the discharge of domestic and industrial wastewater drained by the El Harrach River and to the activities of the port area. The non-significant correlation of MO% with metals shows two sediment layers in core B or the surface layer (0–12 cm) rich in trace metals with EF > 5, the bottom layer rich in MO% and EF < 2. The geochemical behaviour of trace metals is related to the origin of the sediments and trace elements and the sediment regime of the study area. The latter is subjected to several factors such as the great development works on the coast of the Bay of Algiers and the catchment area of the river El Harrach. Another factor is the natural phenomena of great extent, which caused a brutal discharge of terrigenous materials towards the bay of Algiers, particularly the floods of Bab El Oued 2001 and the earthquake of Boumerdes 2003. The fluctuations in the distribution of trace metals and their enrichment are partly natural, but the anthropic signature is present. The anomalies observed for some elements (Pb, Cd, U, Zn, Ag, and Sb) can only be anthropogenic, but the anthropogenic signature is present.
References
Achard R (2013) Dynamics of inorganic contaminants in dredged sediments; specific role of organic matter. Dissertation.University of Sud Toulon Var. France, p 183
Agostini F(2006) Inerting and valorisation of marine dredged sediments. Dissertation. University of Sciences and Techniques of Lille, France, p 212
Appleby PG, Oldfield F (1978) The calculation of lead-210 dates assuming a constant rate of supply of unsupported 210Pb to the sediment. CATENA 5(1):1–8
Arabi M. Benamar MA. Toumert I et al (2006) Evaluation of the sedimentation rate by 137Cs and heavy metals in a column of sediment sampled at the El-Kittani site (Algiers Bay ). 2 nd National Conference on Radiation and its Applications. Algiers. 210–213p
Atroune F, Boutaleb A (2012) Heavy metals in the surface sediments of the Algiers Bay: influence of the el Harrach on their distribution. Bulletin du service géologique national 23(2):169
Amin B, Ismail A, Arshad A, Yap CK, Kamarudin MS (2009) Anthropogenic impacts on heavy metal concentrations in the coastal sediments of Dumai. Indonesia 148(1–4):291–305. https://doi.org/10.1007/s10661-008-0159-z
Bachari N, Abdellaoui A, Houma F (2011) Modeling of multisource data for the study of the pollution of the Algiers Bay based on a geographic information system. Mediterranean Coastal and Maritime Conference Edition 2. Tangier. Morocco
Bachari Houma F (2009) Modeling and mapping of marine pollution and bathymetry from satellite imagery.Dissertation.University Val de Marne Paris XII. France
Bakalem A (2008) Contribution to the study of benthic populations of the Algerian continental shelf. Dissertation . University of Science and Technology Houari Boumediene. Algeria
Bachouche S, Houma F, Gomiero A et al (2017) Distribution and evaluation of the environmental risks of heavy metals in surface sediments and red mullet (Mullus barbatus) of Algiers and BouIsmail bay (Algeria). Approximately Model Assess. https://doi.org/10.1007/s10666-017-9550-x
Belabed BE (2010) Heavy metal pollution in the Annaba region (Doctoral dissertation. University of Annaba-Badji Mokhtar)
Beladel B, Chohra M, Nacer DE et al (2013) Distribution of the rare earth metals concentrations in the sediments of Algiers bay and surkouf area. Annals Sci Technol 5(2):127–135
Belabed BE, Meddour A, Samraoui B, Chenchouni H (2017) Modeling seasonal and spatial contamination of surface waters and upper sediments with trace metal elements across industrialised urban areas of the Seybouse watershed in North Africa. Environ Monit Assess 189(6):265. https://doi.org/10.1007/s10661-017-5968-5
Belhai-Benazzouz A, Djelal N (2010) Land as a vector of urban sprawl in Algiers. In Symposium: Identity and territorial competitiveness. ASRDLF-AISRE, Italie, pp 135–145
Benamar MA, Toumert I, Tobbeche S, Tchantchane A, Chalabi A (1999) Assessment of the state of pollution by heavy metals in the surficial sediments of Algiers Bay. 50(5). 975–980. https://doi.org/10.1016/s0969-8043(98)00111-0
Bergamaschi BA, Tsamakis E, Keil RG et al (1997) The effect of grain size and surface area on organic matter lignin and carbohydrate concentration and molecular compositions in Peru Margin sediments. Geochimica and Cosmochimica Acta. 61(6):1247–1260
Bernatchez MN (2008) Deposit and mobility of antimony in sediments of Canadian shield lakes. Dissertation. University of Quebec, Canada, p 116
Berthois L, Crosnier A, Le Calvez Y (1968) Contribution à l'étude sédimentologique du plateau continental dans la baie de Biafra. Cahiers, O.R.S.T.O.M., série Océanographie 6:55–86
Birch GF (2017) Determination of sediment metal background concentrations and enrichment in marine environments – a critical review. Sci Total Environ 580:813–831. https://doi.org/10.1016/j.scitotenv.2016.12.028
Bohacs KM, Grawbowski GJ, Carroll AR et al (2005) Production, destruction, and dilution - the many paths to source-rock development. in nb harris. (ed.). The deposition of organic-carbon-rich sediments; models. mechanisms. and consequences: SEPM Special Publication 82 61–101
Boudjellal B, Sellali B, Benoud D, Mallem MT (1993) Heavy metals in the surface sediment of the Algiers Bay. In: “Water circulation and pollution in the maghrebian coasts of the mediterranean sea”. Proc. of the INOC workshop on SOSMED Projet. Rabat (Morocco), 153-156
Boudouresque CF (1996) Human impact and conservation of the marine environment in the Mediterranean. GIS Posidonie publ. 2nd ed. Marseille, France, pp 1–243
Bougherara M (2011) Study of the geochemical background of the Tlemcen region Using the XRF technique. Dissertation. University of Tlemcen, Algeria, p 77. https://dspace.univ-tlemcen.dz/handle/112/2336
Boulahdid M, Eddalia N, Boudjellal B, Azzouz M (2003) The waters of the Algiers Bay. Some physicochemical and environmental aspects. Annals of the National Agronomic Institute - El Harrach 24(1 and 2): 39–54
Bouzonville A, Colin A et al (2008) Analysis rapid analysis of metals and other minerals in polluted solid media (waste. soil) using non-destructive X-ray fluorescence field methods. RECORD Final Report. p 44
Brosse E (1982) Contribution to the mineralogy and geochemistry of deep pelagic sediments: comparison of the “blacks-shales” of the Cretaceous in the North Central Atlantic and of the Malm and Cretaceous deposits in Briançonnais. Dissertation. Ecole Nationale Supérieure des Mines de Paris, France, p 476
Buat-Menard P, Chesselet R (1979) Variable influence of the atmospheric flux on the trace metal chemistry of oceanic suspended matter. Earth Planet Sci Lett 42:3999411
Calmano W, Hong J, Forstner U (1993) Binding and mobilisation of heavy metals in contaminated sediments affected by pH and redox potential. Water Sci Technol 28:223e235
Cazalet LM (2012) Physico-chemical characterisation of a marine sediment treated with hydraulic binders - evaluation of the potential mobility of inorganic pollutants. Dissertation. Natl Inst Appl Sci Lyon, France, p 267
Charlou J, Joanny M (1983) Dosage du mercure et d’autres me´taux (Pb, Zn, Cu, Cd, Co, Mn) dans les se´diments marins par absorption atomique. In Aminot A, Chaussepied M (eds) Manuel des Analyses Chimiques en Milieu Marin. CNEXO, Brest, pp 285–295
Chen ZY, Saito Y, Kanai Y et al (2004) Low concentration of heavy metals in the Yangtze estuarine sediments. China: a diluting setting. Is Coast Shelf Sci 60:91–100
Chester R (1990) Marine geochemistry. The Academic Division of Unwin Hyman Ltd. https://doi.org/10.1007/978-94-010-9488-7
Chouikhi A, Sellali B, Azzouz M (1988) Heavy metal concentrations contained on the sediment’s surface of Algiers bay Rapp. P. V. Réun. CIESM. 31(2):161
DeForest D, Brix K, Adams W (2007) Assessing metal bioaccumulation in aquatic environments: the inverse relationship between bioaccumulation factors. trophic transfer factors and exposure concentration. Aquat Toxicol 84:236–246
Dias MI, Prudêncio MI (2008).On the importance of using scandium to normalise geochemical data preceding multivariate analyses applied to archaeometric pottery studies. Microchem J 88: 136–141
Djellouli Y, Saci A (2003) Natural disaster: the devastating floods of November 9 and 10. 2001 in Bab el Oued (Algiers. Algeria). Int Assoc Climatol 15:241–242
Djoudar Hallal D, Toubal AC (2008) Current state of the marine invasion in the Algiers Bay. 13th IWRA World Water Congress (http://www.iwra.org/congress/2008/index.phppage=proceedings&abstract_id=142). Accessed 08/11/2008
El Houssainy A, Abi-Ghanem C, Huy DD, Mahfouz C, Omanović D, Khalaf G, Mounier S, Garnier vol, (2020) Distribution and diagenesis of trace metals in marine sediments of a coastal Mediterranean area: St Georges Bay (Lebanon). Marine Pollut Bull 155:111066. https://doi.org/10.1016/j.marpolbul.2020.111066
Egis Eau / IAU-IDF / BRGM (2013) Study on the vulnerability and adaptation of the Wilaya of Algiers to climate change and natural risks. Final report. 278p
Emmanuelle F (2010) Determination of historical levels of radionuclides and metallic trace elements from sedimentary recordings within the alluvial margins of the downstream Rhône and the Têt. Final report DEI / SESURE N° 2010–02. IRSN
Guendouzi Y, Ghalmi R, Boudjellal M, El morhit M, (2015) Impact of metals traces on the ecosystem at Posidonia oceanica in the Algiers Bay. J Mater about Sci 6(4):918–923
Heimbürger LE, Cossa D, Thibodeau B, Khripounoff A, Mas V, Chiffoleau JF, Migon C (2012) Natural and anthropogenic trace metals in sediments of the Ligurian Sea (Northwestern Mediterranean). Chem Geol 291:141–151
Hoareau G (2009) Sulphate/carbonate relationships during diagenesis of marine sediments: sedimentological study of the Eocene delta of Sobrarbe (Spain) and thermodynamic modeling of ODP / IODP data. Mineralogy. Paul Sabatier University - Toulouse III, French
Houma F, Boulahdid M, Belkessa R, Khouider A (2004) Development of a methodology to characterise and determine the pollution of seawater by hydrocarbons using satellite images. Report of the 37th CIESM Barcelona Congress, p 37
Hubert F (2008) Modeling of diffractograms of clay minerals in complex assemblages in two soils of temperate climates. Mineralogical and pedological implications. Dissertation. University of Poitiers, France, p 23
Issabayeva G, Aroua M, Sulaiman N (2008) Continuous adsorption of lead ions in a column packed with palm shell activated carbon. J Hazard Mater 155(1–2):109–113
Inal A, Boulahdid M, Angelleti B, Radakovitch O (2018) Levels and ecological risk assessment of heavy metals in surface sediments of fishing grounds along Algerian coast. Mar Pollut Bull 136:322–333
Jamshidi S, Bastami K (2016) Metal contamination and its ecological risk assessment in the surface sediments of Anzali wetland Caspian Sea. Mar Pollut Bull 113(1–2):559–565. https://doi.org/10.1016/j.marpolbul.2016.08.049
Kato Y, Kitazato H et al (2003) 210Pb and 137Cs in sediments from Sagami Bay. Japan: sedimentation rates and inventorie. Progress in Oceanography. https://doi.org/10.1016/S0079-6611(03)00052-1
Kükrer S, Şeker S, et al (2014) Ecological risk assessment of heavy metals in surface sediments of northern littoral zone of Lake Çıldır. Ardahan. Turkey. Environ Monit Assess. https://doi.org/10.1007/s10661-014-3662-4
Lambert Castel F, Penot M (1981) Actions of Amoco Cadiz oils on the growth and certain aspects of the metabolism of a phytoplankton alga Pavlova lutheri (DROPP) Green. In Biochemical indices and marine environments. Proceedings of the GABIM conference. 14. CNEXO. Brest. 411–422 p
Lamouri B (2008) Geological. geochemical and mineralogical study of the attapulgite occurrence in the rhoufi area (W. Batna) (Doctoral dissertation. Annaba)
Larara M, Nedjari A, Maouche S, Benhamouche A, Meghraoui M (2012) Algerian coastline and tsunami risks. Bulletin Du Service Géologique National 23(3):241–251
Larid M (2003)Sustainability analysis within the framework of the “Algerian Coastal Zone” CAP (Algeria). Report of the first stage. p 36
Lu X, Zhang Y, Liu H, Xing M, Shao X, Zhao F, Li X, Liu Q, Yu D, Yuan X, Yuan M (2014) Influence of early diagenesis on the vertical distribution of metal forms in sediments of Bohai Bay. China Mar Pollut Bull 88:155–161. https://doi.org/10.1016/j.marpolbul.2014.09.011
Le Gouche C (2017) Sedimentation of organic matter in deep basins: case of the Arctic Basin in the Eocene. Dissertation.University of Rennes 1, France, p 86
Lesouef A, Belamie R, Montiel A (1979) An indicator of pollution with memory. Analysis of metals in river sediments. J Fr Hydrol 10(3):165–172
Louati B (2015) Study of industrial depollution of the watershed of the El Harrach river. Dissertation.University Badji Mokhtar of Annaba, Algeria, p 208
Mikelić IL, Oreščanin V, Škaro K (2017) Variation of sedimentation rate in the semi-enclosed bay determined by 137Cs distribution in sediment (Kaštela Bay, Croatia). J Environ Radioact 166:112–125
Maouche S (1987) Hydrosedimentary mechanisms in the Algiers Bay (Algeria): sedimentological. geochemical and statistical treatment approach. Dissertation. University of Perpignan. France
Maouche S, Morhange C,Meghraoui M (2009) A large accumulation of rocks on the Algerian coast testifies to a tsunami in the western Mediterranean. Marine Geology. Volume 262. Numbers 1–4. July 1. p96–104
Matmed A (2004) Contribution to the study of heavy metals and carbon in the deep sediment of Algiers and Jijel. These Magister en Environnement Littoral et Resources Vivantes. Université Badji-Mokhtar, Annaba, p 77p
Matsumoto E, Togashi S (1980) Sedimentation rate in Funka Bay. Hokkaido. J Oceanogr Soc Jpn 35:261–267. https://doi.org/10.1007/BF02108931
Meybeck M, Lestel L, Bonté P et al (2007) Historical perspective of heavy metals contamination (Cd. Cr. Cu. Hg. Pb. Zn) in the Seine River basin (France) following a DPSIR approach (1950–2005). Sci Total Approx 375 (1–3): 204–231
Miralles J (2004) Coupled study of radionuclides and stable lead isotopes in the Western Mediterranean. Dissertation. University P. Cezanne of Aix-Marseille III, France, p 1439
Monna F, Lancelot J et al (1997) Sedimentation rate in the Thau basin (France) according to geochronological. geochemical and stratigraphical data. Oceanol Acta 20(4):627–638. Open Access version https://archimer.ifremer.fr/doc/00093/20422/
Monteiro PMS, Roychoudhury AN (2005) Spatial characteristics of sediment trace metals in an eastern boundary upwelling retention area (St. Helena Bay. South Africa): a hydrodynamic – biological pump hypothesis. Estuar Coast Shelf Sci 65:123–134
Mwamburi J (2003) Variations in trace elements in bottom sediments of major rivers in Lake Victoria’s Basin. Kenya. Lakes Reserv Res Manag 8:5–13. https://doi.org/10.1046/j.1440-1770.2003.00212.x
ONS (National Statistics Office - ALGIERS) (2008) Urban framework RGPH 2008/the main results of the exhaustive exploitation. National Statistics Office. - Algiers: ONS. 2011. - (Coll. Statist. N ° 163: Série S) ISSN: 1111 – 5114
Ouali N, Belabed BE, Chenchouni H (2018) Modelling environment contamination with heavy metals in flathead grey mullet Mugil cephalus and upper sediments from north African coasts of the Mediterranean Sea. Sci Total Environ 639:156–174. https://doi.org/10.1016/j.scitotenv.2018.04.377
(PAC) Programme d’Aménagement Côtie (2005) “Algerian coastal zone”. Activity: protection of sensitive natural marine sites in the Cap Djinet sector in Mont Chenoua. Pilot actions. action plan and recommendations. UNEP / MAP. RAC / PAP. MATE
Pedersen TF, Calvert SE (1990) Anoxia vs productivity: what controls the formation of carbon-rich organic sediments and sedimentary rocks? AAPG Bulletin 74(4):454–466. https://doi.org/10.1306/0C9B232B-1710-11D7-8645000102C1865D
Pedersen TF, Calvert SE (1991) Anoxia vs productivity: what controls the formation of carbon-rich organic sediments and sedimentary rocks ?: answer. Bulletin of the AAPG. https://doi.org/10.1306/0C9B282B-1710-11D7-8645000102C1865D
Pekey H (2006) Heavy metal pollution assessment in sediments of Izmit Bay. Turkey Environ Monitor Assess 123:219–231
Pons JC, Parra M, Julius C (1988) Heavy metal content of fine sediments in the bay of Fort-de-France. Martinique. Lesser French Antilles. Oceanologica Acta. . VOL.11-N ° 1. p 47–54
Pradhap D, Srinivasalu S, Silva JD, Parthasarathy P, Krishnakumar S, Saravanan P (2017) Trace element accumulation and depositional environment of surface sediments off rameshwaram Gulf of Mannar. India Front Cur Trends Engg Tech 2(1):8–18
Qian Y, Zhang WYu, Feng L, H, (2015) Metal pollution in coastal sediments. Curr Pollut Reports 1:203–219. https://doi.org/10.1007/s40726-015-0018-9
Rabehi W, Guerfi M, Mahi H (2018) “Cartography of the vulnerability of municipalities in the Algiers Bay”. Mediterranean [Online]. Urban spaces. https://journals.openedition.org/mediterranee/8625. Accessed 21 May 2022
Radakovitch O, Roussiez V, Ollivier P et al (2008) Input of particulate heavy metal from rivers and associated sedimentary deposit on the Gulf of Lion continental shelf. Estuar Coast Shelf Sci 77:285–295
Rahman MS, Saha N, Molla AH, Al-Reza SM (2014) Assessment ofAnthropogenic influence on heavy metals contamination in theaquatic ecosystem components: water. sediment. and fish. Soil Sed Contam 23:353–373
Ruilian Y, Xing Y, Yuanhui Z, Gongren H, XianglinT, (2008) Heavy metal pollution in intertidal sediments from Quanzhou Bay. China J Environ Sci 20:664–669
Salomon JN (2003) Danger of pollution !. Collection “Scieteren”. University Press of Bordeaux. France. 170 P
Sanchez-Cabeza JA, Masqué P, Ani-Ragolta I, Merino J, Frignani M, Alvisi F, Palanques A, Puig P (1999) Sediment accumulation rates in the southern Barcelona continental margin (NW Mediterranean Sea) derived from 210Pb and 137Cs chronology. . 44(1–3). 313–332. https://doi.org/10.1016/s0079-6611(99)00031-2
Schneider R, Schulz H, Hensen C (2000) Marine carbonates: their formation and destruction. In: Eds HD, Schulz M, Zabel and, (eds) Marine Geochemistry. Springer-Verlag, Berlin Heidelberg New York, pp 283–308
Singh KP, Mohan D, Singh VK, Malik A (2005) Studies on distribution and fractionation of heavy metals in Gomti river sediments - a tributary of the Ganges. India J Hydrol 312:14–27. https://doi.org/10.1016/j.jhydrol.2005.01.021
Soualili D, Dubois P, Gosselin, P et al,(2008) Assessment of seawater pollution by heavy metals in the neighborhood of Algiers: use of the sea urchin. Paracentrotus lividus. as a bioindicator. - ICES J Mar Sci, 65: 132–139
Suresh KP (2011) An overview of randomisation techniques: an unbiased assessment of outcome in clinical research. J Hum Reprod Sci. https://www.jhrsonline.org/text.asp?2011/4/1/8/82352
Tessier E, Garnier C, Mullot JU, Lenoble V, Arnaud M, Raynaud M, Mounier S (2011) Study of the spatial and historical distribution of sediment inorganic contamination in the Toulon Bay (France). Mar Pollut Bull 62(10):2075–2086
Thamban M, Purnachandra Rao V, Raju SV (1997) Controls on organic carbon distribution in sediments from the eastern Arabian Sea Margin. Geo-Mar Lett 17:220–227
Thevenon F, Guedron S, Chiaradia M, Loizeau JL, Pote J (2011) (Pre-) historic changes in natural and anthropogenic heavy metals deposition inferred from two contrasting Swiss Alpine lakes. Quat Sci Rev 30:224–233
Trentesaux A (2005) Recording of pleistocene sedimentary processes examples of detrital platforms and deep basins North Sea and China Seas. Dissertation. University of Science and Technology of Lille, France, p 83
Wang X, Smethurst P, Herbert A (1996) Relationships between three measures of organic matter or carbon in soils of eucalypt plantations in Tasmania. Aust J Soil Res 34:545–553
Wang Q, Li Y, Wang Y (2011) Optimising the weight loss-on-ignition methodology to quantify organic and carbonate carbon of sediments from various sources. Environ Monit Assess 174:241–257
Weckwerth G (2001) Verification of traffic emitted aerosol components in the ambient air of Cologne (Germany). Atmos Environ 35(32):5525–5536
Wignall PB, Hallam A (1991) Biofacies. stribution and stratigraphic dideposition models of British terrestrial Jurassic black schists. Geol Soc. https://doi.org/10.1144/GSL.SP.1991.058.01.19
Yahiaoui A (2012) Torrential floods mapping of vulnerable areas in northern Algeria (Case of river Mekerra. Wilaya of Sidi Bel Abbès). Dissertation. Ecole Nationale Polytechnique. 21–22p
Yelles-Chaouche A, Roger J, Deverchere J et al (2009) The 1856 Tsunami of Djidjelli (Eastern Algeria): seismotectonics. Modeling and hazard implications for the algerian coast. Pure Appl Geophys 166:283–300
Yoshida M, Moali M, Houas O, Lakhdari M, Nechaoui L, Guerrida D, Chatal A, Oussalem S, Makour F, Khelifi F, Laleg A (2005) Environmental Pollution in Oued El Harrach area, Alger. A preliminary report on mercury and heavy metals contaminations. Compte-Rendu du Séminaire sur les «Pollution et Protection de l’Environnement en Algérie», Alger, 5 et 6 avril 2005, p 19–37, ONEDD et JICA
Yoshida M, Moali M, Houas O, Lakhdari M (2007) Concentration of the potentially toxic element in sediments in the Algiers Bay. Report of the Algeria-Japan Joint Workshops and Seminar on Quality Standards and Environmental Protection Efforts 2007. Algiers. 96–106
Yusof AM, Wood AKH (1993) Environmental assessment of coastal sediments by the elemental ratioing technique. Journal of Radioanalytical and Nuclear Chemistry. Articles 167:341–351. https://doi.org/10.1007/BF02037192
Zaidi A, Wani PA, Khan MS (2014) Toxicity of heavy metals to legumes and bioremediation. Toxicity of Heavy Metals to Legumes and Bioremediation. Springer, Vienna. Austria, pp 29–44
Zarei I, Pourkhabbaz A, Khuzestani RB (2014) An assessment of metal contamination risk in sediments of Hara Biosphere Reserve southern Iran with a focus on application of pollution indicators. Environ Monitor Assess 186(10):6047–6060. https://doi.org/10.1007/s10661-014-3839-x
Acknowledgements
This work was part of the COMETALG-Mermex cooperation project between the European Centre for Research and Teaching in Environmental Geosciences (CEREGE. Marseille. France) and the National Higher School of Marine Sciences and Coastal Management (ENSSMAL. Algiers. Algeria). The study is dedicated to our friends and colleagues to ENSSMAL and CEREGE for their help, respectively, during the preliminary phase of samples treatment and trace metals analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by Haroun Chenchouni.
This paper was selected from the 3rd Conference of the Arabian Journal of Geosciences (CAJG), Tunisia 2020.
Rights and permissions
About this article
Cite this article
Athmani, H., Mostefa, B., Radakovitch, O. et al. Trace metal contamination in sediment cores from Algiers Bay (Algeria). Arab J Geosci 15, 1107 (2022). https://doi.org/10.1007/s12517-022-10218-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-022-10218-y