Abstract
Çarşamba plain located in the north-east of Turkey is a region where agricultural and industrial activities have strongly developed in recent years. In this region, excessive use of pesticides and fertilizers in agricultural areas and waste discharges from industrial zones can lead to the pollution of groundwater by heavy metal(loid)s. The purpose of the present study is to assess heavy metal(loid)s pollution in groundwater of Çarşamba costal aquifer using pollution indices and multivariate statistical methods. For this study, 33 groundwater samples were taken in this region and for these samples’ physical parameters and Pb, Cu, Al, Zn, Cr, Mn, Fe, Ni, Co, As, Mo, Se, B, Ti, V, and Ba values were determined. The analysis results showed that the Al, Mn, Se, Cr, and Ni values in some wells exceeded the permitted limit of WHO and Turkish Standards. The HPI and HEI values of most water samples fall in low pollution water class. Multivariate statistical methods revealed a significant anthropogenic influence in the accumulation of metal(loid)s in groundwater. The decreasing order of heavy metal(loid)s in most water samples is Al > Mn > Zn > Se > Ti > Ba > Cr > Ni > As > V > Cu > Mo > Se > Co > Pb > B > Cd. Five principal components that explain 80.44% of the total variance have been formed. The component 1 includes Al, Pb, Co, Ti, Cu, Ni, Zn, and V while the second component is associated with EC, Se, and B. The component 3 includes pH, Ba, and Mn; component 4 represents As and Mo; and finally component 5 includes only Cr.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Groundwater is in general an excellent source of potable water for consumption and irrigation. The natural filter made up of geological materials most often produces high-quality water with low levels of microorganisms and other suspended elements. The groundwater quality can however be altered when undesirable or even toxic substances come into contact with the aquifer (Ahoussi et al. 2010). The chemical fertilizers used in agricultural activities as well as wastewater from industrial and urban origins present a risk for groundwater quality. Among the wide variety of pollutants affecting the quality of groundwater, heavy metals are the most worrying due to their high toxicity even at low concentrations (Hooda and Alloway 1998). Excessive heavy metal contamination of soils causes metals entrance in the food chain through plants and water, posing a possible risk for human health (Yaylalı-Abanuz 2019). One of the major properties of heavy metals and that sets them apart from other toxic contaminants is that they are not biodegradable in the environment (Sougo et al. 2005). Among the metals some such as copper, iron, manganese, nickel, and zinc are indispensable in animals and plants while many others such as chromium, cadmium, and lead have no importance (Kar et al. 2008). Monitoring of the concentration of heavy metals especially in groundwater is important as it can help remedy health problems particularly waterborne diseases (Kwaya et al. 2019). Several methods for assessing the quality of groundwater are used to characterize the sources of contamination and the associated parameters. The determination of pollution indices is one of the most efficient methods used to assess the pollution of surface and groundwater by heavy metals and metalloids. Among these methods, the heavy metal pollution index (HPI) calculation is one of the most important methods used to assess surface and groundwater quality based on the concentrations of metals that they contain (Arslan et al. 2017; Abou Zakhem and Hafez 2015). The use of heavy metal pollution index method in the spatial distribution study of metals in water can be useful in determining of water quality tendencies. It can also provide information that can help resource management and regulatory agencies to assess alternatives and to make necessary decisions (Milivojević et al. 2016). In addition to this method, the use of the contamination index (Cd) and heavy metal evaluation index (HEI) methods can also help to assess the recent level of contamination in water (Sobhanardakani et al. 2017). Many research have been realized through the world to assess the heavy metal pollution in surface and groundwater. The work carried out in India by Kumar et al (2020) to assess the intensity of pollution in Marar industrial area based on the physicochemical parameters and heavy metals analysis results of water samples highlighted that most of water samples exceeded the desirable limit of Al and Mn. The HEI, HPI, and Cd values of water samples of this area showed that most of water samples belong to low and medium pollution classes. In addition, their study demonstrated that the Fe, Ni, Cr, Pb, Cu, and Mn are the prominent contributors for the three water pollution indices (HPI, HEI, and Cd). In Bangladesh, Rahman et al. (2020) using the water pollution indices approach assessed the heavy metal pollution in the groundwater of the Meghna Ghat industrial area. According to the results of their study, the Cr, Cd, and Pb mean values of water samples exceeded the drinking water standards set by Bangladesh. The water quality index (WQI) values indicated that almost all water samples from this area are polluted. In addition, the heavy metal pollution index (HPI), the heavy metal evaluation index (HEI), and the degree contamination (DC) values indicated that some water samples were subjected to high level of pollution, therefore are unsuitable for drinking. The work realized in Nigeria by Adeyemi and Ojekunle (2021) to assess the concentration and health risk of industrial heavy metal pollution in groundwater of Ogun state highlighted that the metals concentration in groundwater samples was in the order Pb > Fe > Ni > Cr > Cd > Cu > Mn > Zn and Pb. In addition, the Cd, Cr, and Ni can be considered as a carcinogenic metal in groundwater samples of this area. The total hazard index (HI) of the metals in the groundwater samples of this region presented a high risk across the different age groups and the health risk index value indicated major adverse effects on human health. In Saudi Arabia, the work carried out by Alfaifi et al. (2021) to evaluate the heavy metal contamination and groundwater quality along the Red Sea Coast indicated that maximum As, Mn, Cr, Ni, Se, and Zn values of water samples exceeded the permissible limits for drinking water. The calculated pollution indices values of samples indicated that most of groundwater samples of this region are unsuitable for agricultural and domestic purposes. In addition, the polluted water samples were dispersed mostly in the western part along the Red Sea coast.
In Turkey among the sources of water pollution in most coastal areas, heavy metals are one of the most worrying factors which mainly threaten surface and groundwater resources. In these areas, unfavorable factors such as urbanization and industrial organizations, wild storage areas, and discharges of untreated liquid waste into basins cause rapid pollution of surface and groundwater. Due to soil fertility in coastal areas, intense agricultural activities take place and in case of excessive use of fertilizers such as phosphorus fertilizers and metal-containing pesticides in agricultural areas, groundwater can easily be polluted by heavy metals. The work carried out by Arslan and Avşar (2020) in Köyceğiz-Dalyan coastal lagoon watershed to assess surface and groundwater heavy metals pollution highlighted high values of Pb, As, and Fe in most water samples collected in this area. In addition, almost all water samples from this region have HPI value higher than 100 which is the critical pollution index level. In Ispala district, Varol and Tokatlı (2021) carried out research to assess the level of toxic metal (loid)s in drinking water by using the pollution indices and to estimate health risks that these metals represent for children and adults. According to the results of their study, the Cd, Ni, Cu, Zn, Mn, Pb, As, and Cr values of water samples collected during the both seasons did not exceed the drinking water limit values. These low toxic metals content in the water of this region may be due mainly to a high clay content and a low rate of soil infiltration in the study area. However, the concentrations of these toxic metals measured during the wet season are higher than those measured during the dry season. In addition, the calculated heavy metal pollution indices values indicated that all water samples from this area were subjected to low level of pollution, therefore are suitable for drinking. In this region, it was also found that the low concentrations of toxic metals in drinking water present no health risk to the local residents. The work realized in the district of Eskipazar by Keskin (2010) to assess the nitrate and heavy metal pollution in agricultural area demonstrated that water samples from wells drilled in clastic levels present a high level of pollution of NO3 and metals such as Na, B, Pb, Hg, and Se. It was also revealed that in this region, the main causes of NO3 and metals pollution in groundwater are fertilizers and pesticides used in agricultural activities. In the Amik plain, Ağca et al. (2014) carried out research to assess the ammonium, nitrate, phosphate, and heavy metal pollution in groundwater. According to the results of their study, the metals concentration in groundwater samples were in the order Fe > Mn > Ni > Cr > Cu > Zn > Co > Cd > Pb. In addition, the Cd values of all water samples and the NO3 concentration of some samples exceeded the drinking water limit values. Agricultural activities are considered as the main sources of nitrogen and some heavy metal pollution in this region.
Çarşamba aquifer is one of the most important coastal aquifers in Turkey. In this region where intense agricultural and industrial activities take place, excessive use of fertilizers and pesticides in agricultural areas and waste discharges from industrial areas can easily lead the pollution of surface and groundwater by various pollutants such as heavy metal. In this region, the work carried out by Sağlam et al. (2011) to determine contents of heavy metal and physico-chemical properties of soil showed that due to the natural and anthropogenic influences, 77.6% of soils samples exceeded the limit level. In addition, their study demonstrated a slight increase of Cd, Cu, and Zn contents of soils due to excessive phosphorus fertilization and field traffic in this region (Saglam et al. 2011). The work realized by Arslan and Turan (2015) revealed that the main groundwater pollutants in Çarşamba coastal aquifer are iron, manganese, aluminum, arsenic, and cadmium while boron and zinc can be considered as potential pollutants. Their study slighted in addition that most of groundwater samples collected in this area were polluted due to the high levels of iron, manganese, and arsenic.
The purpose of this study is to assess the sources and the concentrations of heavy metals in Çarşamba costal aquifer by using the pollution indices and multivariate statistical methods. This study therefore made it possible to highlight the influence of industrial and agricultural activities on the groundwater quality of Çarşamba coastal aquifer. It also allowed to highlight the main pollutants as well as their spatial distribution in the study area. Thanks to the present study, regional and national authorities could take the necessary measures to combat water pollution through programs for the prevention and monitoring of pollution by toxic metals in groundwater of Çarşamba plain.
Study area
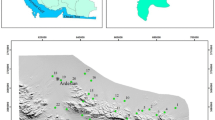
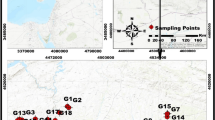
The study area is located on the left bank of the Yeşilırmak river and the western part of the Çarşamba plain, lying between the latitudes 41° 11′ 30′′ and 41° 16′ 30′′ and the longitudes 36° 22′ 00′′ and 36° 36′ 00′′; the plain of Çarşamba is situated in the Middle of Black Sea Region (Fig. 1). The climate of this region is humid and the average annual precipitation varies between 600 and 700 mm. The rainiest period is from October to the end of December. The average annual temperature varies between 15 and 17 °C. The hottest months are July and August while January and February are the coldest.
Geology and hydrogeology
There are three formation types in the study area, which are the Tertiary age Tekkeköy formation, Eocene age Sarıyurt formation, and Quaternary age Alluvium formation (Fig. 2). The Sarıyurt formation consists of sandstones, siltstones, marls, and conglomerates. Its upper part is composed of interstratified siltstones, sandstones, and marls while an alternation with sandstones is observed in the middle part. In the lower part of this formation, conglomerates and sandstones are observed. The Tekkeköy Formation consists of sandstones, mudstones, basalts, marls, and tuffites alternation and agglomerates. The Quaternary Alluvium which covers almost the entire study area consists of sands, gravels, clays, sandstones, muds, and silts (Yoldaş et al. 1985). The geology of the study area indicates that only the Quaternary Alluvium can be considered as important groundwater reservoirs. The volcanic rocks of the Çarşamba plain are geological formations which have the characteristics of the secondary aquifer after the alluvial deposits in terms of groundwater. In these formations, the weathered basalts and in particular the interconnected cracks and the fracture systems developed in the agglomerates levels facilitate transportation and accumulation of groundwater. In terms of hydrogeological characteristics, the Çarşamba plain can be divided into two parts: the left bank and the right bank of the Yeşilırmak river. The left bank is generally composed of unconfined and semi-confined aquifers while on the right bank semi-confined and confined aquifers are the most frequent. On the left bank of Yeşilırmak river where the study area is located, the aquifer begins generally at a depth varying between 4 and 40 m and its thickness values ranged from 1 and 20 m. The majority of aquifers in the left bank of Yeşilırmak river consists of various layers of gravels, clayey-sands, clayey gravels, and silty sands within the Quaternary Alluvium (DSI 1993).
Materials and methods
For this purpose, in July 2019, 33 groundwater samples were taken in the study area and for these samples the physical parameters (TDS, pH, and EC) and heavy metal(loid)s pollutants (As, Pb, Se, Cu, Al, Ti, Zn, Co, Cr, Mn, Ba, Fe, Ni, Mo, B, and V) values were determined. The global coordinates of all the sampling points were taken and recorded with a GPS device. The analysis of the physical parameters of groundwater samples was determined using a portable multimeter Hach-HQ40d. The heavy metal(loid)s analysis was carried out at the General Directorate of State Hydraulic Works Technical Research and Quality Control Department (TAKK) by using inductively coupled plasma-mass spectrometer (ICP-MS) method according to the United States Environmental Protection Agency (US EPA Method 200.8) standard method.
Heavy metal pollution and heavy metal evaluation indices were calculated for to evaluate the degree of contamination of groundwater in the study area. The determination of HPI is one of the effective techniques used to assess surface and groundwater quality with respect to heavy metals. This method was first established by Mohan et al. (1996) and consists in allocating a rating or a weight (Wi) for each designated pollution parameters according to its importance in terms of water quality. The rating value varies between 0 and 1 depending on the importance of each considered metal. This value is inversely proportional to the suggested standard (Si) value for each parameter (Horton 1965; Mohan et al. 1996). In this study, Ni, Zn, Mn, Pb, Se, Cr, Cu, As, B, Mo, Cd, and Al concentrations were used to determine the heavy metal pollution index values. Cobalt, barium, and vanadium for which there are no defined limit values were not included in the HPI calculation. The heavy metal pollution index can be determined by using the following Eq. 1:
where Wi represents the unit weightage of each metal, n the total number of considered metals, and Qi represents the sub-index of each metal. The unit weightage value of each considered heavy metal can be calculated by using the following Eq. 2:
where Si represents the permissible value of each metal and K the constant of proportionality.
The sub-index value of each metal was determined by using the following equation:
where Mi represents the detected value of each metal in µg/L, Si the recommended value, and Ii the ideal value of ith metal. The critical pollution index value for drinking water is 100. In this study, the recommended value of each parameter was taken from Turkish Standards and the international WHO (World Health Organization) guidelines. The ideal value of each heavy metal is considered as equal to zero.
Another method used to evaluate the quality of water in relation with metals concentrations is the determination of heavy metal evaluation index value (Sobhanardakani 2016). The heavy metal evaluation index can be determined by using the following Eq. 4:
where Hc and Hmac represent the measured value and the maximum admissible concentration of the ith parameter, respectively.
The classification of water samples according to pH and heavy metals values was carried out by using the method developed by Ficklin et al. (1992) and modified by Caboi et al. (1999). In general, cationic species are less mobile when the pH of the environment is high and are more mobile when the pH value is low. Since most metals form a + 2 charge, their movement and bioavailability are improved under low pH conditions (Smith 2007). Using Ficlin-Caboi method, the water class is determined by plotting the samples on a diagram on which the pH values are placed on the horizontal axis and the metal load values on the vertical axis. The metal load of each water sample is determined by summing the concentrations of the different metals that it contains. This method classified groundwater samples into 12 classes including ultra-acid ultra-metal class, high acid ultra-metal class, acid ultra-metal class, near neutral ultra-acid class, high acid high metal class, acid high metal class, near neutral high metal class, high acid extreme metal class, near neutral extreme metal class, high acid low metal class, acid low metal class, and near neutral low metal class. The Ficklin-Caboi diagram is a simple and efficient method of classifying water samples based on the differences in the sum of the base metal concentrations that they contain (Manoj et al. 2012).
Multivariate analysis methods, such as principal component analysis (PCA), hierarchical cluster analysis (CA), and correlation analysis methods were used to investigate the sources of heavy metal(loid)s of Çarşamba coastal aquifer. Correlation analysis method was utilized to determine the relationships existing between heavy metal(loid)s of groundwater in the study area. When the correlation coefficient is close to 0, there is no relationship between the variables. When this value is greater than 0.7, there is a strong correlation between the variables and when this value varies between 0.5 and 0.7, there is then a moderately correlation between the variables. A very high value (r = + 1 or − 1) of the correlation coefficient reflects a strong relationship between the variables (Kumar et al. 2006). The principal component analysis method is one of the most commonly used statistical analysis methods. This method is used to measure and explain the relationships between many variables (Çakır 1994). The main objective of the principal component analysis method is to reduce the large number of variables under a smaller number of components that can be easily interpreted. The Kaiser-Mayer-Olkin coefficient (KMO) and the Bartlett’s test are performed in this study to determine whether the collected data are suitable for principal component analysis. The KMO measure is an index value that tests the relevance of samples for principal component factor analysis by comparing the observed correlation coefficients with the significance of the partial correlation coefficients. When this value is less than 0.50, the data are not accepted for principal component analysis (Kellekçi and Berköz 2010). Bartlett’s test for homogeneity of variances is used to check whether the variances are equal for all samples. This test is used so to verify whether the equality of variance assumption is true before running the statistical test. In order for the data to be compatible with the principal component analysis, Bartlett test must be at the level of significance with p values less than 0.05. The factor analysis was made by using varimax rotation that is a method allowing to minimize the number of parameters with a high loading on each factor, thus simplifying the interpretation of the principal component analysis results (Boateng et al. 2015).
Cluster analysis is a multivariate statistical method that is used to classify objects into group based on their similarity. The use of this method in geology and hydrogeology is recent. This method is therefore commonly used to analyze the hydrochemical data of water and complements the principal component analysis method. Cluster analysis is a powerful tool for the analysis of water chemical data given the complexity of hydrochemical systems and the difficulties encountered in their interpretation (Soro et al. 2019). In this study, hierarchical cluster analysis was carried out to determine the relationship between heavy metal(loid)s and between groundwater samples. The Ward method that uses the analysis of variance approach to evaluate the distances between clusters was applied. This method also has the advantage of minimizing the sum of the squares of any two groups that can be formed at each step (Ward 1963). Q-mode CA was carried out to classify groundwater samples on the basis of their similarities while R-mode analysis was realized to determine the association of different heavy metal and physical parameters of groundwater samples.
In this study, multivariate statistical analyzes were performed using the SPSS software (version 23.0 for Windows) and all maps were made by using ArcGIS software. The spatial distribution maps of heavy metal concentrations, heavy metal pollution index, and heavy metal evaluation index were produced by using inverse distance weighted (IDW) interpolation method. This method is one of the efficient and most popular methods adopted by geoscientists and geographers that used to estimate unknown depth data from known measured depths (Maleika 2020). IDW method approximates the values corresponding to the points where sampling was not executed using a linear combination of values corresponding to the points where the sampling was performed weighted by an inverse function of the distance between the point of interest and the sampled points (Keshavarzi and Sarmadian 2012; Arslan and Turan 2015). This method has a significant advantage when the distribution of estimated parameters is not a normal distribution (Chen and Liu 2012).
Results
Heavy metal pollution and spatial distribution in groundwater
Table 1 presents the summary of descriptive statistical results of heavy metal(loid)s of groundwater samples collected in the study area. The decreasing order of heavy metal(loid)s in most water samples is Al > Mn > Zn > Se > Ti > Ba > Cr > Ni > As > V > Cu > Mo > Se > Co > Pb > B > Cd. The Cr concentrations of groundwater samples vary between 0 and 286.54 µg/L, Mo values between 0 and 6.13 µg/L, Se values between 0 and 10.29 µg/L, Mn values between 2.85 and 1469.28 µg/L, As values between 0 and 113.61 µg/L, and Ti values ranged from 98 to 159.1 µg/L. The spatial distribution of Cr in the study area indicated that the high concentrations of Cr occupy the eastern part (Fig. 3a) while those of Mo were detected in the western part and southeast of the study area (Fig. 3f). The spatial variation map of Se showed that high concentrations of Se were observed in wells located near the center and the south part of the study area (Fig. 3e). The highest value of Mn was measured in well number 10 situated in the southwest of the study area while the lowest value was detected in well number 2 located in the northwestern part. The distribution map of Mo concentrations indicated that high values of Mo occupy the west part of study area while the low values were observed in wells situated in the western and northern parts (Fig. 3c). The high As concentrations were detected in wells located in the east and southeast of the study area (Fig. 3d) while those of Ti were observed in the northeast, the east, and the southeastern parts of the study area (Fig. 3b). The Co values of groundwater samples ranged from 0 to 8.22 µg/L, V values between 0 and 47.63 µg/L, Al values ranged from 38.94 to 11,014.83 µg/L, Ba values ranged from 0.63 and 69.98 µg/L, Cu values vary between 0 and 16.89 µg/L, and Zn concentrations are between 0 and 346.21 µg/L. The spatial distribution maps of Co, V, Cu, Al, and Zn showed that the high concentrations of Co, Cu, and Al occupy the southeast and the northeast part of the study area (Fig. 4a, c, f) while those of V and Zn are located in the northeast, the southeast and the west part of the study area (Fig. 4b, d). The high concentrations of Ba were observed in the eastern part of the study area (Fig. 4e). The Ni values of water samples are between 2.23 and 63.61 µg/L, B values are between 0.14 and 0.67 µg/L, and Pb values ranged between 0 and 2.80 µg/L. The spatial variation maps of Ni and Pb indicated that high concentrations of these metals occupy the northeast and southeast of the study area (Fig. 5a, c). The distribution map of B concentrations given in Fig. 5b showed that in the study area the high values occupy the east, west, and the center part of the study area.
Heavy metal(loids) pollution indices and Ficklin-Caboi diagram
The calculated HPI and HEI values of each groundwater samples are given in Table 2. The HPI values of samples ranged from 3.12 to 325.09 with an average of 75.32. The highest value of HPI was observed in well number 24 while the lowest value was detected in well number 2 of the study area. The mean value of HPI determined using the mean concentrations of the various heavy metals considered in this study is 75.32, therefore lower than the critical value of 100 suggested by Prasad and Sangita (2008) (Table 2). The computed heavy metal evaluation index values (HEI) ranged from 0.51 and 90.81 with an average of 14.92 (Table 2). The highest value of HEI were observed in well number 25 located in the southeast of the study area while the lowest value was detected in well number 2 situated in the northwestern part (Fig. 6).
According to Ficklin-Caboi diagram, the classification of groundwater samples collected from the study area are presented in Fig. 7. According to this diagram, 42.42% of groundwater samples falls within the near neutral high metal class and 57.58% within the near neutral metal class. The highest values of metal load were observed in wells number 25, 28, and 29 located in the eastern part of the study area while the lower values were detected in wells number 7 and 15 situated respectively in the western and southern parts of study area (Table 3).
Multivariate statistical analyses
Correlation analysis
The correlation coefficient matrix for heavy metal(loid)s and physical parameters of groundwater samples are presented in Table 4. Heavy metals which have the same source and similar geochemical behavior exhibit strong correlations between them (Lu et al. 2010; Boateng et al. 2015). The Ni values of water samples exhibited a strong correlation with Ti and moderate correlation with Al, Cr, and Co. The B values indicated a moderate correlation with Se, As, and EC. Pb showed a moderate correlation with Ti, Al, Cr, Co, and V. There is no significant correlation between Mo values and the other heavy metals in the study area. Se presented a moderate correlation with B, As, and EC. The Mn values are only correlated with Ba. As concentrations showed a moderate correlation with B and Se. Ti exhibited a strong correlation with Ni, Al, and Cr; and a moderate correlation with Pb and Co. The Al values indicated a moderate correlation with Ni, Pb, V, and Cu; and strong correlation with Cr and Co. Cr concentrations revealed a moderate correlation with Ni, Pb, Ba, and Cu; and strong correlation with As, Al, and Co. Co values indicated a moderate correlation with Ni, Pb, Ti, and V; and strong correlation with Al and Cr. The concentrations of Ba showed a moderate correlation with Mn and Cr. The V values presented a moderate correlation with Pb, Al, Co, and Cu. Moderate correlations were observed between Cu-Al, Cu-Cr, and Cu-V. The pH and EC values revealed no significant correlation with all metal(loid)s.
Principal components analysis and hierarchical cluster analysis
In this study, principal component analysis and hierarchical cluster analysis methods have been used both to identify the sources of heavy metals pollution of groundwater in the study area and to visualize the relations between these heavy metals. The heavy metals and physical parameters of groundwater used for the principal component analysis and hierarchical analysis were As, Pb, Se, Cu, Al, Ti, Zn, Co, Cr, Mn, Ba, Fe, Ni, Mo, B and V, EC, and pH. The results of the KMO and Bartlett tests applied to the heavy metals and physical parameters of the groundwater samples are given in Table 5. This table showed that the KMO value of the data used for principal component analysis is 0.588. The significance value for Bartlett’s test is 0; therefore, the data are suitable for principal component analysis. The values of the total variance explained, the eigenvalues, the cumulative % of variance, and the rotated factor loading for each principal component were summarized in Table 6. The spatial distribution of principal components is given in Fig. 7.
According to the principal component analysis results, five principal factors with eigenvalues greater than one have been formed. These five factors alone explain 80.44% of the total variance. The first factor represents 35.097% of variance, factor 2 accounts 18.26%, factor 3 accounts 10.93%, factor 4 accounts 9.496%, and factor 5 represents 6.657% of the total variance (Table 6). These five principal components can help to identify the main sources of heavy metals of groundwater in the study area.
The R-mode cluster analysis result which was realized in this study to determine the association of heavy metals and physical parameters based on their similarities and sources is given in Fig. 10a. According to the result of this analyze, three large groups of variables were formed. The first group includes aluminum, cobalt, lead, nickel, titanium, manganese and barium; the second group consists of vanadium, copper, zinc, chromium, and pH; and the third group includes selenium, electro-conductivity, boron, molybdenum, and arsenic.
The Q-mode cluster analysis result that was realized in this study to classify the groundwater samples on the basis of their similarities is given in Fig. 10b. The average concentrations of heavy metals (loids) in each group were given in Table 7. According to Fig. 10b, five large groups of groundwater samples were formed in the study area. The first group includes 20 groundwater samples and represents 60.60% of samples; the second group characterizes water samples number 17, 27, 18, and 11 and concerns 12.12% of samples; the third group is associated with groundwater samples number 23 and 33 and represents 6.06% of samples; the fourth group is associated with wells number 13 and 24 and represents 6.06% of samples; and finally the fifth group consists of water samples number 28, 29, 10, and 25 and represents 12.12% of samples.
Discussions
This study which focuses on the pollution of groundwater of Çarşamba costal aquifer highlighted the pollution by toxic metals of some wells in the study area. According to Cr concentrations of groundwater samples, excepted water sample from well number 33, all samples have Cr value below the limit value recommended by Turkish Standards and WHO. Similar results were observed in the work carried out by Ağca et al. (2014) on groundwater from the Amik plain where the Cr concentrations in most water samples were below the acceptable limit defined by the WHO. Consumption of water containing high concentration of Cr can cause in human health problems such as respiratory cancers, infertility, and developmental disorder in children (Shams et al. 2020). The main sources of Cr in the study area are waste discharges from industrials areas. According to Se values of groundwater samples, excepted groundwater sample from well number 13, all water samples fall within the WHO guideline and Turkish Standards of 10 µg/L. Chronic exposure to high levels of Se can cause selenosis, which is a disease that can lead skin rash, gastrointestinal problems, hair loss, neurological damage, fingernail brittleness, and even cirrhosis of the liver (Goldhaber 2003; Bailey 2017). The main source of the high concentrations of Se in the study area is fertilizers and pesticides used in agricultural zones. In term of the Mn concentrations of groundwater samples, it appears that most samples have Mn values that exceeded the permissible limit value defined by World Health Organization guidelines and Turkish Standards. Similar results were observed in the work carried out by Arslan and Turan (2015) where high concentrations of Mn in the groundwater of Çarşamba coastal plain were detected. Exposure to a high concentration of Mn in drinking water can cause in adult neurological problems and diseases like manganism, a Parkinson-like disorder (Homoncik et al. 2010). Therefore, water samples with Mn values that exceeded the permissible limit are not recommended for drinking. The main sources of Mn in the study area are industrial discharges and urban activities. According to As values of groundwater samples, it is observed that excepted water samples from wells number 24, 25, 26, and 28 where very high values were observed, all water samples fall within the WHO guideline and Turkish Standards of 10 µg/L. The consumption of water with a high content of arsenic can cause in human diseases such as diseases of the blood vessels of the legs and feet, the skin disorders, skin cancers, internal cancers (lung, bladder, and kidney), increased blood pressure, reproductive disorders, and possibly diabetes (Shankar and Shanker 2014; WHO 2011). Therefore, groundwater from wells number 24, 25, 26, and 28 are polluted and non-recommended for drinking. The main sources of arsenic in the study areas are discharge wastes from industrial area and pesticides used in the agricultural zones. Around 66.66% of groundwater samples collected in the study area have Ti values more than the average Ti value of normal water. The mean concentration of Ti in normal groundwater is 3 µg/L (Şahıncı 1993). The highs concentrations of Ti are mainly linked to wastes from industrial activities that take place in the study area. WHO guidelines and Turkish Standards have not included Ti in drinking water standards. The V and Co values in almost groundwater samples of study area are very low. These low levels of vanadium observed in most water samples indicated that the main origins of this element in the study area are geogenic. However, fertilizers used in agricultural areas can be considered as the second origin of vanadium in groundwater. The concentrations of Co in the study area are linked to wastes from industrial activities and pesticides used in agricultural areas. There is no limit value of Co and V defined by WHO and the Turkish standards for drinking water. Regarding the Al concentrations, it is observed that most of the groundwater samples of the study area present high Al concentrations. Therefore, groundwater of this region can be considered as polluted and non-recommended for drinking and irrigation purpose. These high concentrations of Al in the groundwater of the study area were also observed in the work carried out by Arslan and Turan (2015). The main sources of Al in the study area are waste discharges from industrial activities. According to the World Health Organization guidelines and Turkish Standards, only 54.55% of samples have Al values below the acceptable limit concentration of 200 µg/L. Several epidemiological studies showed the link between high levels of aluminum in drinking water and serious diseases such as Alzheimer disease (Momodu and Anyakora 2010). The Cu and Ba concentrations in all groundwater samples of study area are not significant. It is estimated that the mainly sources of Cu in the study area are pesticides and fertilizers that are used in agricultural area while wastes discharge from the industrial zones are considered as the main source of Ba. The copper is a common constituent of pesticides and fertilizers as well as insecticides (Huang and Jin 2008; Nathalia et al. 2021). According to the World Health Organization guidelines, the Cu and Ba values in all samples are below the acceptable limit of 2000 µg/L and 700 µg/L, respectively. The Ni concentrations in most groundwater samples are not significant. However, water samples collected in wells number 24, 25, 28, 29, and 33 have Ni values which exceeded the acceptable limit of 20 µg/L recommended by World Health Organization guidelines and Turkish Standards. These wells in which high concentrations of Ni were observed are not recommended for drinking and irrigation purposes. The work carried out by Saglam et al. (2011) revealed high concentrations in the soil of the study area. It supposed that the high Ni contents would be linked to the original material (volcanic) of the soils formed from alluvial deposits comprising a large amount of nickel and an industrial effect on part of the study area (Saglam et al. 2011). High exposure to nickel can cause for human toxic effects such as allergic reactions, contact dermatitis, nephrotoxic effects, and embryo toxic effects (Das and Dhundasi 2008). The Zn, Pb, B, and Mo values observed in all groundwater samples are below the permissible limit concentration defined by World Health Organization guidelines and Turkish Standards. Industrial discharge wastes can be considered as the main source of Ni, Zn, Pb, and Mo pollutions in the study area. However, in addition to industrial wastes, fertilizers and pesticides used in agricultural areas can also be the sources of Mo in Çarşamba coastal plain.
The HPI values of some groundwater samples of study area exceeded the critical pollution index (100) value. Similar results have been observed by Sirajudeen et al. (2015) in groundwater of Fathima Nagar of India and by Chiamsathit et al. (2020) in groundwater of hillside area of Kalasin in Thailand. The HPI values of groundwater samples can be classified into 3 categories by using a multiple of HPI mean value (Prasanna et al. 2012). Based on the HPI values of groundwater samples of study area, a new classification was proposed by using the calculated mean HPI value. Based on this new classification, water samples with HPI values less than 75 belong to the low pollution class, those that HPI values are between 75 and 150 belong the medium pollution class, and finally water samples with HPI values higher than 150 belong to the high pollution class. According to this classification, 66.66% of the groundwater samples fall in low pollution water class, 21.22% in medium pollution water class, and 12.12% of samples fall within the high pollution water class (Table 3). The spatial variation map of the HPI showed that the high values were focused in the eastern and southeastern part of the study area while the low concentrations extend from the center to the entire western part (Fig. 6a). Edet and Offiong (2002) classified the HEI values of water samples into 3 level class by using the multiple of calculated HEI mean value. Based on this approach, the different levels of contamination of groundwater samples were classified in 3 classes that are the low pollution water class (HEI < 15), the medium pollution water class (HEI between 15 and 30), and the high pollution water class (HEI > 30). According to this new classification, 72.73% of water samples of study area fall within the low pollution water class, 15.15% within the medium pollution water class, and 12.12% fall within the high pollution water class (Table 3). The spatial distribution map of heavy metal evaluation index showed that the high values were concentrated in the eastern and southeastern part of the study area while the low values occupy most of the study area (Fig. 6b).
The principal components analysis results revealed that the factor 1 which represents 35.097% of total variance is principally linked with strong positive loading of Al (0.926), Pb (0.905), Co (0.875), and Ti (0.844) and moderate loading of Cu (0.772) and Ni (0.638) and weak loading of Zn (0.596) and V (0.594) (Table 6). The association of Al, Pb, Co, Ti, Cu, Ni, Zn, and V indicated that in the study area these heavy metals have common origin. It is believed that these heavy metals are mainly linked to agricultural activities and waste from industrial activities that take place in Çarşamba plain. The work carried out by Saglam et al. (2011) in the Çarşamba region and that carried out by Ozyazıcı et al. (2017) throughout the Black Sea region revealed high concentrations of Co, Ni, Zn, and Cu in the soil (Fig. 8). According to the results of their works, these high concentrations may be due to excessive P-fertilization and field traffic. Kumar et al. (2020) showed that industrial activities contribute also to the accumulation of Cu and Ni in agricultural soil. The score plot of samples (Fig. 9) indicated that the water samples which are more influenced by factor 1 are samples number 25, 28, and 29. The factor 2 which represents 18.26% of total variance is linked with strong positive loading of EC (0.958) and Se (0.954); and moderate loading of B (0.792). In the study area, the main source of B and Se are fertilizers and pesticides used in the study area. The score plot of the water samples (Fig. 9) showed that the samples which are more influenced by factor 2 are groundwater samples number 13, 28, and 22. The factor 3 that represents 10.93% of total variance is connected with strong negative loading of pH (− 0.867) and weak loading of Ba (0.649) and Mn (0.62). The strong negative loading of pH associated with weak loading of Ba and Mn indicated that acidic conditions favor the release of B and Mn into groundwater. The factor 3 represents then the effect of pH on the dissolution and mobility of Ba and Mn in the study area. The score plot of the water samples (Fig. 9) indicated that the samples that are more influenced by factor 3 are groundwater samples number 17, 18, and 25. High Ba and Mn levels observed in some water samples were mainly caused by industrial waste and agricultural activities. The factor 4 which represents 9.496% of total variance is linked with strong positive loading of As (0.828) and moderate positive loading of Mo (0.736). The score plot of groundwater samples (Fig. 9) shows that water samples that are more influenced by factor 4 are wells number 24 and 8. The source of the accumulation of Mo and As in the study area are anthropogenic. Anthropogenic source of these metals includes waste discharges from industrial areas and fertilizers and pesticides that are used in agricultural zone. The work carried out by Kumbur et al. (2008) demonstrated that in agricultural areas fertilizers, insecticides, herbicides, and pesticides are the mainly sources of Mo. Industrial activities such as energy production and waste incineration can be considered as the principal origins of As concentration in the study zone (Li and Zhang 2010; Arslan and Turan 2015). The factor 5 that represents 6.657% of total variance is only associated with strong positive loading of Cr (0.927). Cr concentration in groundwater is a good indicator of paint and metal industrial waste in the study area (Arslan and Turan 2015). The score plot of the water samples (Fig. 9) indicated that the samples which are more influenced by factor 5 is groundwater sample number 33.
Cluster analysis dendrogram in R-Mode indicated that excepted Mn and Ba, all elements of group 1 belong to the first factor obtained from the principal component analysis method. The spatial distributions maps of heavy metals obtained using the IDW method revealed that the high values of heavy metal(loid)s observed in this first group are concentrated in the eastern part of the study area. The dendrogram presented in Fig. 10a showed that the first group can be subdivided into 3 subgroups. The first subgroup is mainly associated with Al, Co, and Pb; the second subgroup is linked with Ni and Ti; and the third subgroup consists of Mn and Ba. These subgroups confirm the result obtained in the correlation analysis where significant correlations were observed between Al-Co-Pb, Ni–Ti, and Mn-Ba. The second group of elements can be subdivided into 2 subgroups. The first subgroup of this second group includes V, Cu, and Zn and the second subgroup consists of Cr and pH. The first subgroup which includes V, Cu, and Zn confirms the results obtained in correlation analysis where significant correlations were observed between V-Cu and Zn-Cu. The second subgroup which contains only Cr as a heavy metal confirms the results observed in factor 5 of the principal component analysis method where Cr is not associated with any other metal in the study area. The third group of elements obtained in R-mode cluster analysis can be subdivided in 2 subgroups. The first subgroup of this third group is associated with Se, EC, and B; and the second subgroups includes Mo and As. The first subgroup which includes Se, EC, and B is identical to the second factor obtained in the principal component analysis method where the association of Se, EC, and B were observed. The second subgroup that is linked with Mo and As is identical to the fourth factor of principal component analysis where the association of Mo and As were observed. Therefore, the first and second subgroup of the third group confirms the results obtained in principal component analysis.
The first group of samples obtained in Q-mode cluster analysis revealed that the average Se value of 16.35 µg observed in this group is higher than that observed in the other groups. The mean values of Al, Mn, Ti, and Ba in this group are 707.54 µg/L, 212.54 µg/L, 50.69 µg/L, and 14.36 µg/L, respectively. The average concentrations of the dominant metals in this first group are in the following decreasing order: Al > Mn > Zn > Se > Ti > Ba. The spatial distribution maps of heavy metal showed that most of water samples from this group are located in the less polluted zones of the study area. In the second group of elements obtained with Q-mode analysis, the average Ba concentration of 48 µg/L observed is higher than that observed in the other groups. In this group, the mean concentrations of the dominant metals are in the following decreasing order: Al > Mn > Zn > Ba > Ti > As. Most of water samples of this group are located in the eastern part of the study area. The Al, Mn, Zn, Ba, Ti, and As mean values of groundwater samples of this group are 840.71 µg/L, 603.06 µg/L, 64.56 µg/L, 48 µg/L, 20.27 µg/L, and 5.48 µg/L, respectively. The spatial distribution maps of metals indicated that the samples which make-up this second group were located in the west part of the study area and are among the least polluted samples. The third group of elements obtained with Q-mode analysis indicated that the mean Cr concentration of 143 µg/L observed in this group is higher than that observed in the other groups. The mean concentrations of the dominant metals of this group are in the following decreasing order: Al > Zn > Cr > Mn > V. The average Al, Zn, Cr, Mn, and V values of this group are 450.15 µg/L, 168.53 µg/L, 143.27 µg/L, 49.74 µg/L, and 24.62 µg/L, respectively. The fourth group of this classification indicated that the mean values of Mn observed in this group are higher than those observed in other groups. It was also observed that the well number 24 of this group is more polluted by As, Ni, and Mo than the other wells. In this group, the mean concentrations of the dominant metals are in the following decreasing order: Mn > Al > As > Ti > Ni. The average Mn, Al, As, Ti, and Ni values of this group are 241.27 µg/L, 90.75 µg/L, 59.83 µg/L, 38.01 µg/L, and 35.15 µg/L, respectively. The spatial distribution heavy metal maps indicated that these water samples which make up this fourth group are among the most polluted samples of the study area. Finally, the fifth group of this Q-mode classification indicated that the average value of Al in this group is higher than those observed in other groups. The average concentrations of the dominant metals of this group are in the following decreasing order: Al > Mn > Zn > Ti > Ba. The mean values of Al, Mn, Zn, Ti, and Ba of this group are 6940.19 µg/L, 914.47 µg/L, 175.60 µg/L, 106.99 µg/L, and 36.75 µg/L, respectively.
Conclusion
In this study carried to assess the heavy metal pollution in groundwater of Çarşamba costal aquifer, heavy metal pollution index, heavy metal evaluation index, Ficklin-Caboi diagram, and the multivariate statistical analysis methods have been used. This study also made it possible to highlight the impact of industrial and agricultural activities on the quality of groundwater in the Çarşamba plain. It will enable local, regional, and national authorities to take the necessary measures to combat water pollution through programs for the prevention and monitoring of pollution by toxic metals in groundwater of Çarşamba plain.
The decreasing order of heavy metal(loid)s in most water samples is Al > Mn > Zn > Se > Ti > Ba > Cr > Ni > As > V > Cu > Mo > Se > Co > Pb > B > Cd. The Al, Ni, Mn, Se, As, and Cr values of some wells were above the allowed limits of WHO and Turkish standard while in all groundwater samples the B, Pb, Mo, Ti, V, Co, and Zn values are below the permissible upper values. Based on the HPI values, most of water samples have HPI value less than the critical value of 100. The classification of HPI and HEI values showed that most of groundwater samples fall in low pollution water class. The spatial distribution maps of HEI and HPI values showed that the eastern part of the study area is more polluted than the other parts. It was observed that Al, Ni, Mn, Se, As, and Cr are the mainly toxic metals that contribute to water pollution in the study area. Ficklin-Caboi diagram exhibited that 42.42% of groundwater samples fall within the near neutral high metal class and 57.58% within the near neutral metal class. Spearman correlation analysis showed strong correlations between Ni and Ti, Ti and Al, Ti and Cr, Al and Cr, Al and Co, and between Co and Cr. Five principal components which represent 80.44% of the total variance have been formed. The first factor which represents 35.097% of total variance is linked with strong positive loading of Al, Pb, Co, and Ti and moderate loading for Cu and Ni and weak loading of Zn and V. The second factor that represents 18.26% of total variance relates to strong positive loading of EC and Se, and moderate loading of B. The factor 3 which represents 10.93% of total variance is mainly associated with strong negative loading of pH and weak loading of Ba and Mn. The factor 4 which represents 9.496% of total variance is mainly associated with strong positive loading of As and moderate positive loading of Mo.
References
Abou Zakhem B, Hafez R (2015) Heavy metal pollution index for groundwater quality assessment in Damascus Oasis. Syria Environ Earth Sci 73(10):6591–6600. https://doi.org/10.1007/s12665-014-3882-5
Ağca N, Karanlık S, Ödemiş B (2014) Assessment of ammonium, nitrate, phosphate, and heavy metal pollution in groundwater from Amik Plain, southern Turkey. Environ Monit Assess 186(9):5921–5934. https://doi.org/10.1007/s10661-014-3829-z
Alfaifi H, El-Sorogy AS, Qaysi S, Kahal A, Almadani S, Alshehri F, Zaidi FK (2021) Evaluation of heavy metal contamination and groundwater quality along the Red Sea coast, southern Saudi Arabia. Mar Pollut Bull 163:111975. https://doi.org/10.1016/j.marpolbul.2021.111975
Arslan Ş, Yücel Ç, Çallı SS, Çelik M (2017) Assessment of heavy metal pollution in the groundwater of the northern Develi Closed Basin, Kayseri. Turkey Bull Environ Contam Toxicol 99(2):244–252. https://doi.org/10.1007/s00128-017-2119-1
Arslan H, Turan NA (2015) Estimation of spatial distribution of heavy metals in groundwater using interpolation methods and multivariate statistical techniques; its suitability for drinking and irrigation purposes in the Middle Black Sea Region of Turkey. Environ Monit Assess 187(8):1–13. https://doi.org/10.1007/s10661-015-4725-x
Arslan Ş, Avşar Ö (2020) Assessment of heavy metal pollution in Köyceğiz-Dalyan coastal lagoon watershed (Muğla) SW Turkey. Arab J Geosci 13(15):1–11. https://doi.org/10.1007/s12517-020-05690-3
Ahoussi EK, Soro N, Kouassi AM, Soro G, Koffi YB, Zade SP (2010) Application des méthodes d’analyses statistiques multivariées à l’étude de l’origine des métaux lourds (Cu 2+, Mn 2+, Zn 2+ et Pb 2+) dans les eaux des nappes phréatiques de la ville d’Abidjan. Int J Biol Chem Sci 4(5):1753–1765. https://doi.org/10.4314/ijbcs.v4i5.65537
Adeyemi AA, Ojekunle ZO (2021) Concentrations and health risk assessment of industrial heavy metals pollution in groundwater in Ogun state. Niger Sci Afr 11:e00666. https://doi.org/10.1016/j.sciaf.2020.e00666
Bailey RT (2017) Selenium contamination, fate, and reactive transport in groundwater in relation to human health. Hydrogeol J 25(4):1191–1217. https://doi.org/10.1007/s10040-016-1506-8
Boateng TK, Opoku F, Acquaah SO, Akoto O (2015) Pollution evaluation, sources and risk assessment of heavy metals in hand-dug wells from Ejisu-Juaben Municipality. Ghana Environ Syst Res 4(1):1–12. https://doi.org/10.1186/s40068-015-0045-y
Caboi R, Cidu R, Fanfani L, Lattanzi P, Zuddas P (1999) Environmental mineralogy and geochemistry of the abandoned Pb-Zn Montevecchio-Ingurtosu mining district, Sardinia Italy. Chron Rech Min 534:21–28
Çakır F (1994) Karşılıklı Bağımlılığın Ölçüsünde Kümeleme Analizi ve Bir Uygulama. Master's thesis Marmara University. https://katalog.marmara.edu.tr/eyayin/tez/T0041974.pdf
Chen FW, Liu CW (2012) Estimation of the spatial rainfall distribution using inverse distance weighting (IDW) in the middle of Taiwan. Paddy Water Environ, 10(3):209–222. https://doi.org/10.1007/s10333-012-0319-1
Chiamsathit C, Auttamana S, Thammarakcharoen S (2020) Heavy metal pollution index for assessment of seasonal groundwater supply quality in hillside area, Kalasin. Thail Appl Water Sci 10(6):1–8. https://doi.org/10.1007/s13201-020-01230-2
DSI (1993) Çarşamba Ovası Hidrojeolojik Etüd Raporu. Samsun DSİ (in Turkish)
Edet AE, Offiong OE (2002) Evaluation of water quality pollution indices for heavy metal contamination monitoring. A study case from Akpabuyo-Odukpani area, Lower Cross River Basin (southeastern Nigeria). GeoJournal 57(4): 295–304. https://doi.org/10.1023/B:GEJO.0000007250.92458.de
Ficklin WH, Plumlee GS, Smith KS, McHugh JB (1992) Geochemical classification of mine drainages and natural drainages in mineralized areas. In: International symposium on water-rock interaction, pp 381–384
Goldhaber SB (2003) Trace element risk assessment: essentiality vs. toxicity. Regul toxicol pharmacol 38(2): 232–242. https://doi.org/10.1016/S0273-2300(02)00020-X
Homoncik SC, MacDonald AM, Heal KV, Dochartaigh BÉÓ, Ngwenya BT (2010) Manganese concentrations in Scottish groundwater. Sci Total Environ 408(12):2467–2473. https://doi.org/10.1016/j.scitotenv.2010.02.017
Horton RK (1965) An index number system for rating water quality. J Water Pollut Control Fed 37(3):300–306
Hooda PS, Alloway BJ (1998) Cadmium and lead sorption behaviour of selected English and Indian soils. Geoderma 84(1–3):121–134. https://doi.org/10.1016/S0016-7061(97)00124-9
Kar D, Sur P, Mandai SK, Saha T, Kole RK (2008) Assessment of heavy metal pollution in surface water. Int J Environ Sci Tech 5(1):119–124
Kumar M, Ramanathan AL, Rao MS, Kumar B (2006) Identification and evaluation of hydrogeochemical processes in the groundwater environment of Delhi. India Environ Geol 50(7):1025–1039. https://doi.org/10.1007/s00254-006-0275-4
Kumar S, Toppo S, Kumar A, Tewari G, Beck A, Bachan V, Singh TBN (2020) Assessment of heavy metal pollution in groundwater of an industrial area: a case study from Ramgarh, Jharkhand, India. Int J Environ Anal Chem 1–23. https://doi.org/10.1080/03067319.2020.1828391
Kwaya MY, Hamidu H, Mohammed AI, Abdulmumini YN, Adamu H, Grema HM, Kana AM (2019) Heavy metals pollution indices and multivariate statistical evaluation of groundwater quality of Maru town and environs. Journal of Mate Environ Sci 10(1):32–44
Kellekçi ÖL, Berköz L (2010) Konut ve çevresel kalite memnuniyetini yükselten faktörler. İTÜDERGİSİ/a 5(2)
Keshavarzi A, Sarmadian F (2012) Mapping of spatial distribution of soil salinity and alkalinity in a semi-arid region. Annals of Warsaw University of Life Sciences-SGGW. Land Reclamation 44(1)
Keskin TE (2010) Nitrate and heavy metal pollution resulting from agricultural activity: a case study from Eskipazar (Karabuk, Turkey). Environ Earth Sci 61(4):703–721. https://doi.org/10.1007/s12665-009-0385-x
Kumbur H, Özsöy HD, Özer Z (2008) Mersin İlinde Tarımsal Alanlarda Kullanılan Kimyasalların Su Kalitesi Üzerine Etkilerinin Belirlenmesi. Ekoloji Dergisi 17(68)
Li S, Zhang Q (2010) Spatial characterization of dissolved trace elements and heavy metals in the upper Han River (China) using multivariate statistical techniques. J Hazard Mater 176(1–3):579–588. https://doi.org/10.1016/j.jhazmat.2009.11.069
Lu X, Wang L, Li LY, Lei K, Huang L, Kang D (2010) Multivariate statistical analysis of heavy metals in street dust of Baoji, NW China. J Hazard Mater 173(1–3):744–749. https://doi.org/10.1016/j.jhazmat.2009.09.001
Maleika W (2020) Inverse distance weighting method optimization in the process of digital terrain model creation based on data collected from a multibeam echosounder. Appl Geomat 12(4):397–407. https://doi.org/10.1007/s12518-020-00307-6
Manoj K, Kumar B, Padhy PK (2012) Characterisation of metals in water and sediments of Subarnarekha River along the Projects' sites in lower basin, India. Univers J Environ Res Technol 2(5)
Milivojević J, Krstić D, Šmit B, Djekić V (2016) Assessment of heavy metal contamination and calculation of its pollution index for Uglješnica River. Serbia Bull Environ Contam Toxicol 97(5):737–742. https://doi.org/10.1007/s00128-016-1918-0
Mohan SV, Nithila P, Reddy SJ (1996) Estimation of heavy metals in drinking water and development of heavy metal pollution index. J Environ Sci Health Part A 31(2):283–289. https://doi.org/10.1080/10934529609376357
Momodu MA, Anyakora CA (2010) Heavy metal contamination of groundwater: The Surulere case study. Res J Environ Earth Sci 2(1):39–43
Nathalia D, Suresh A, Panda B (2021) Assessment of ground water pollution through metal index analysis and its source identification along the Delhi-Haryana boarder. India Arab J Geosci 14(9):1–10. https://doi.org/10.1007/s12517-021-06905-x
Prasad B, Sangita K (2008) Heavy metal pollution index of groundwater of an abandoned open cast mine filled with fly ash: a case study. Mine Water Environ 27(4):265–267. https://doi.org/10.1007/s10230-008-0050-8
Prasanna MV, Praveena SM, Chidambaram S, Nagarajan R, Elayaraja A (2012) Evaluation of water quality pollution indices for heavy metal contamination monitoring: a case study from Curtin Lake, Miri City. East Malaysia Environ Earth Sci 67(7):1987–2001. https://doi.org/10.1007/s12665-012-1639-6
Rahman MAT, Paul M, Bhoumik N, Hassan M, Alam MK, Aktar Z (2020) Heavy metal pollution assessment in the groundwater of the Meghna Ghat industrial area, Bangladesh, by using water pollution indices approach. Appl Water Sci 10(8):1–15. https://doi.org/10.1007/s13201-020-01266-4
Saglam M, Dengiz O, Kizilkaya R (2011) Application of geostatistical methods to heavy metals status in Çarsamba plain soils. Asian J Chem 23(8):3454
Sougo CA, Sylvain M, Ama WV, Kouamé A (2005) State of anthropic pollution in the estuary of Ebrié Lagoon (Cote d’Ivoire) by analysis of the metal elements traces. Eur J Sci Res 372–390
Sobhanardakani S, Taghavi L, Shahmoradi B, Jahangard A (2017) Groundwater quality assessment using the water quality pollution indices in Toyserkan Plain. Environ Health Eng Manag 4(1):21–27. https://doi.org/10.15171/EHEM.2017.04
Sirajudeen J, Arulmanikandan S, Manivel V (2015) Heavy metal pollution index of groundwater of Fathima Nagar area near Uyyakondan channel Tiruchirappalli district, Tamil Nadu, India. World J Pharm Pharm Sci (WJPPS) 4(1):967–975
Soro G, Soro TD, Adjiri OA, Soro N (2019) Application des méthodes statistiques multivariées à l’étude hydrochimique des eaux souterraines de la région des lacs (centre de la Côte d’Ivoire). Int J Biol Chem Sci 13(3):870–1889. https://doi.org/10.4314/ijbcs.v13i3.54
M Shams N Tavakkoli Nezhad A Dehghan H Alidadi M Paydar AA Mohammadi A Zarei 2020 Heavy metals exposure, carcinogenic and non-carcinogenic human health risks assessment of groundwater around mines in Joghatai Iran. Int J Environ Anal Chem 1–16 https://doi.org/10.1080/03067319.2020.1743835
Sobhanardakani S (2016) Evaluation of the water quality pollution indices for groundwater resources of Ghahavand plain, Hamadan province, western Iran. Iran J Toxicol 10(3):35–40
Smith KS (2007) Strategies to predict metal mobility in surficial mining environments. Rev Eng Geol 17:25–45
Şahinci A 1991 Doğal Suların Jeokimyası. Reform Matbaası 548, İzmir
Varol M, Tokatlı C (2021) Seasonal variations of toxic metal (loid) s in groundwater collected from an intensive agricultural area in northwestern Turkey and associated health risk assessment. Environ Res 111922. https://doi.org/10.1016/j.envres.2021.111922
Ward JH Jr (1963) Hierarchical grouping to optimize an objective function. J Am Stat Assoc 58(301):236–244
World Health Organization (2011) Guidelines for Drinking-Water Quality, vol 4. World Health Organization, WHO, Geneva
Yaylalı-Abanuz G (2019) Application of multivariate statistics in the source identification of heavy-metal pollution in roadside soils of Bursa. Turkey Arab J Geosci 12(12):1–14. https://doi.org/10.1007/s12517-019-4545-3
Yoldaş R, Balkıray K, Granit S, Korkmaz S, Didik S, Kalkan İ, Ağrıdağ DS, Besbelli B (1985) Samsun ve dolayının (Kızılırmak-Yeşilırmak arasındaki bölgenin) jeolojisi ve petrol olanaklarına ilişkin rapor, MTA Rapor No 8130. (Turkish language)
Acknowledgements
The authors gratefully acknowledge scientific research project of Karadeniz Technical University which supported this study under project Number 8168. The authors also thank the Directorate of 7th Region of State Hydraulic Works (DSİ, Samsun) which support was essential for the success of this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Responsible Editor: Broder J. Merkel
Rights and permissions
About this article
Cite this article
Hassan, A.M., Ersoy, A.F. & Turan, N.A. Assessment of heavy metal(loid)s in groundwater by multivariate statistical analysis and metals pollution indices: a case study of Çarşamba coastal aquifer, North Turkey. Arab J Geosci 14, 2658 (2021). https://doi.org/10.1007/s12517-021-09007-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-021-09007-w