Abstract
In most countries of the world, groundwater and surface water are at a serious risk of pollution due to chemicals used in agricultural activities. The present study examined whether such a risk exists in Eskipazar, Turkey and the surrounding area, which covers a surface area of 696 km2. Nitrate pollution (NO3) was observed in waters discharging from the Örencik Formation, consisting of loose conglomerate, sandstone, mudstone, siltstone, and claystone levels; from the Yörük member of the Örencik Formation consisting of limestone, from areas where the Örencik Formation and Yörük member are located together, and from alluvium. Agricultural is practiced in these areas, and the waters discharging from these formations are used as drinking water and for domestic purposes. In particular, periodically varying levels of pollutants, such as B, Pb, Hg, Se were detected in wells drilled in Örencik Formation featuring a high NO3 concentration. The concentrations of S, Cr, Mn, Fe, Cu, Zn, Ga, Br, Sr, Y, I, Ba, and U in these waters are also slightly higher than other cold waters in the study area. In addition to the NO3 pollution, high levels of Ca and SO4 pollution was observed at a well drilled in alluvium. In addition, some trace element concentrations identified in the wells drilled in the Örencik Formation were higher than the average values at geothermal and/or mineral springs in the study area. The study area has an adequate sewage system and has no sources of pollution, such as mineralization, industrial center, waste disposal area, etc. Therefore, it is believed that the main causes of NO3 and trace element pollution are fertilizers and pesticides used in agricultural activities. Water–rock interaction, usage period of fertilizers and pesticides, amount of precipitation, groundwater level, usage of elements by plants, mobility of elements, pH value of the environment, redox potential, adsorption/desorption, biochemical processes, etc. are thought to be the causes of the periodical variation of some trace element concentrations observed in these waters.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The suitability of physical, chemical and biological parameters of groundwater and surface water are highly significant in terms utilization by living organism. However, excess use of fertilizers and pesticides in agricultural activities to enhance productivity due to rapid population increase and development of technology threaten the groundwater and surface water on a large scale. Turkey, and most other countries, have soils and waters which have been polluted by fertilizers and pesticides used during agricultural activities. These waters and soils continue to be polluted, as the necessary precautions have not been taken. In this instance, it shows that there is an obvious risk for human in the future. A direct relationship was found between the levels of agricultural chemical usage and levels of groundwater and surface water pollution (Mikayilov and Acar 1998). Therefore, identifying the optimal levels of agricultural chemicals required by plants and appropriate regulation of substances with potentially harmful effects is of crucial importance.
Nitrate usually exists in surface and groundwaters organically and anthropogenically. Decomposed vegetable and animal wastes, leaching of solid wastes, domestic wastes, industrial waste waters (industries such as nitrogenous manure, nitric acid, etc.), fertilizers used in agriculture, irrigation return run-off, washing of atmospheric nitrogen by rainfall and effluent waters of waste water treatment facilities are the major sources of nitrate in surface and groundwaters (McNeely et al. 1979; Lawrance 1983; Ritter and Chirnside 1984; Hem 1985; Kaçaroğlu 1991, 1997).
Previous researchers have detected different types of pollution in groundwaters and surface water, which originate from different types of fertilizers and agricultural pesticides. Evaluation of these studies indicated that the range of chemicals in fertilizers and pesticides includes major ions, such as Na, Mg, Ca, K, Cl, SO4; nitrogen pollutants, such as NH4, NO2, NO3; and trace elements, such as As, Cd, Cr, Cu, Hg, Mo, Ni, Pb, Se, U, V, Zn, Fe, Mn, Br, Cs, Co, F, Sn, Sr, Te, Ba, B, S (Chu and Wong 1984; Wong 1990; Chen et al. 1997; Alloway 1990; Fergusson 1990; Herrero and Martin 1993; Mermut et al. 1996; Moon et al. 1999; Kaçaroğlu 1991, 1997; Alloway 1995; Kumbur et al. 2008; Sen 1996; Yüksel et al. 1997; Mikayilov and Acar 1998; Arslan and Akkaya 2001; Baba and Ayyıldız 2006; Eryurt and Sekin 2001; Böhlke 2002; Manta et al. 2002; Elhatip et al. 2003; Kara et al. 2004; Krishna and Govil 2004; McBride 2004; He et al. 2005; Fuge 2004; Atabey 2005a; Danış 2005; Atabey 2005; Cangir 2005; Mühendis and Birliği 2006; Nouri et al. 2006; Polat et al. 2007; Guo et al. 2007; Kumbur et al. 2008; http://www.gubretas.com.tr, http://www.tarimziraat.com/gubreleme; http://www.artesalimited.com, etc.).
Increased use of fertilizer to improve agricultural productivity has also affected the quality of groundwater, including nitrate pollution in groundwaters (Smith et al. 1971; Schepers et al. 1983; Houzim et al. 1986; Kaçaroğlu 1991, 1997). In a study conducted in Eskişehir Plain, Kaçaroğlu (1991, 1997) stated that some wells in the plain displayed NH3, NO2 and NO3 pollution parameters as a result of agricultural activities. Sen (1996) suggested that the nitrate pollution in Bursa Nilüfer and Ayvalı Basins probably stemmed from the use of nitrate fertilizers (Baba and Ayyıldız 2006). Chen et al. (1997) found As, Cd, Cu and Zn pollution in agricultural soils in Hong Kong due to application of pesticide, animal manure and chemical fertilizer. Mikayilov and Acar (1998) discussed the amounts of different trace elements which may be present in some fertilizers and stated that the use of fertilizers is generally excessive, leading to the pollution of ground and surface water sources, soil, agricultural products and atmosphere. Furthermore, they indicated that the major source of heavy metal pollution in soil is organic fertilizers. In their studies carried out in the northeast China, Moon et al. (1999) mentioned the presence of Cd, Pb, Ni and Cr in agricultural soils and adjacent river sediments.
During a study conducted in the Manisa region, Eryurt and Sekin (2001) found that the main source of the nitrate pollution in some wells was fertilizers and agricultural activities. Arslan and Akkaya (2001) reported that the source of high levels of nitrate pollution in groundwater in the vicinity of Urla and Menemen, located in the Aegean region, was fertilizers and pesticides used in agricultural activities (Baba and Ayyıldız 2006). Manta et al. (2002) stated that the use of garden fertilizers had an effect on Hg, Pb, Zn, Cu contents of green field and greenery park zone soils in Palermo, Italy. According to Böhlke (2002), agricultural activities have a direct or indirect effect on inorganic chemical concentrations in groundwaters, including NO3, N2, Cl, SO4, H, P, C, K, Mg, Ca, Sr, Ba, Ra and As. In studies carried out in Kayseri and its vicinity, Elhatip et al. (2003) indicated that agricultural activities are directly or indirectly effective on the concentrations of some elements in groundwater, such as NO3, N2, CI, SO4, H, K, Mg, Ca, Fe, Cu, B, Pb and Zn. They reported that direct effects include the dissolution and transport of excess quantities of fertilizers with associated materials and hydrological alterations related to irrigation and drainage. Indirect effects may include changes in water–rock reactions in soils and aquifers caused by increased concentrations of dissolved oxidants, protons, and major ions. Krishna and Govil (2004) detected Pb, Cr, Cu, Zn, Sr, V pollution in the soil of the Pali industrial area of India, due to industrialization and intense agricultural activities, and stated that increasing amount of heavy metals in the soil may result in groundwater pollution. In studies performed in the Misli Plain of Niğde, Kara et al. (2004) reported Cd and Cu deposition in the soil as a result of fertilizers and pesticides used for potato farming. In a study carried out in southwest China, Guo et al. (2007) found an increasing amount of SO4 and NO3 in groundwater and river due to seasonal use of fertilizer. Kumbur et al. (2008) suggested that Cu, Mn, Cr, Ni and Mo levels detected in stream and irrigation channel samples from agricultural areas of Mersin province originated from agricultural pesticides.
From the findings of previous studies, it is clear that excessive use of fertilizers and pesticides during agricultural activities in different countries presents a significant pollution risk to groundwater and surface water. The waters in Eskipazar and the immediate vicinity were examined to determine the existence and scale of this pollution risk. NO3 and some trace element pollutions identified in the groundwater are believed to originate from agricultural activities. The findings are in accordance with those in the literature. NO3 pollution was observed in waters discharged from slightly cemented, conglomerate, sandstone, mudstone, siltstone and claystone levels and intensive cracked, cavernous and fragile limestones on which agricultural activities are conducted. In addition, heavy metal pollution, such as B, Pb, Hg, Se and major ion pollutants, such as Na, Ca, SO4 as well as NO3 pollution were observed in wells drilled in clastic materials.
Materials and methods
The present study was conducted between July 2007 and December 2008. Data were collected from 24 springs, 5 drilled wells, 7 geothermal and/or mineral spring and 2 river measurement points within the study area. The survey examined discharge (Q), electrical conductivity (EC), pH, total dissolved solid (TDS), temperature (T) and dissolved CO2 measurements. Chemical analyses of water samples included major anion–cation, F and nitrite (NO2), nitrate (NO3), ammonium (NH4) analysis, carried out using an ion chromatography device at the Water Chemistry Laboratory, Faculty of Engineering, Department of Geological Engineering, Cumhuriyet University (Turkey). Trace element analyses were performed in the Water Chemistry and Environmental Tritium Laboratory, Hacettepe University, Turkey. HCO3 and CO3 ions were analyzed using a titration method at the Water Chemistry Laboratory, Faculty of Engineering, Department of Geological Engineering, Cumhuriyet University (Turkey). As a result of these measurements, physical property, chemical composition, nitrogen contaminants and trace element concentrations of the samples were identified. Evaluation was carried out in accordance with the Turkish Standards for Water Intended for Human Consumption (TS 266 2005), as revised 29 April 2005, and; the World Health Organization regulations (WHO 2006).
Geology
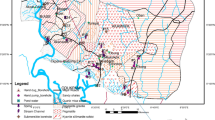
Eskipazar is located 35-km southwest of Karabük Province in the Eastern Black Sea Region of Turkey (Fig. 1). Figure 2 shows a geological–hydrogeological map of the study area and its vicinity.
In this study, the geological studies of Şaroğlu et al. (1995), Tokay (1973), Alan and Aksay (2002), Timur and Aksay (2002), Sevin and Aksay (2002) and Bilginer et al. (2002) were used (with revision in some parts) and a geological map of the study area was produced at a scale of 1:100,000. The study area is located in a region where Western Pontides and Central Sakarya come together along the Northern Anatolian Fault Zone (NAFZ). The area covers units of the Western Pontide Zone, Armutlu-Almacık-Arkotdağı Zone and Sakarya Zone. In the northern part of the NAFZ, the base of the Western Pontide is made up of Precambrian metamorphics (not outcropped in the study area) and crosscutting pre-Ordovician granitoid and volcanites (Bolu Granitoid). This base is overlain unconformably by Ereğli and Yılanlı Formations consisting of Paleozoic clastics and carbonates. On these units, the Ulus Formation composed of turbiditic clastics containing various Early Cretaceous olistoliths of the Western Pontide Zone, is unconformable, whereas the Abant Formation, which consists of Upper Campanian-Maestrichtien olistostrome and turbiditic clastics, is unconformable with the former formation. These units are covered by the Early Eocene Akveren Formation consisting of limestone and clastics; the Early Middle Eocene Kışlaköy Formation, comprising volcanic and, more commonly, clastics; the Safranbolu Formation, composed of Early Middle Eocene limestone constitutes a transitive coverage thereof. A subsection of the Sakarya Zone, in the south of the NAFZ, includes the Soğukçam Formation, consisting of Callovian-Aptian subpelagic cherty limestone and calciturbidites. This unit is conformably overlain by the Yenipazar Formation, featuring Albian-Maestrichtien turbiditic rocks. These units, located in the north and south of the NAFZ, are conformably overlain by Upper Miocene Özlü Basalt, the Pliocene Örencik Formation and Quaternary terrace, travertine and alluvium (Şaroğlu et al. 1995; Tokay 1973; Alan and Aksay 2002; Timur and Aksay 2002; Sevin and Aksay 2002 and Bilginer et al. 2002) (Fig. 2).
Within the study area, the Örencik Formation, comprises terrestrial conglomerate, sandstone, mudstone, siltstone and claystone alternations and lacustrine limestone. This formation mainly outcrops in the vicinity of Eskipazar county seat and ensures the recharge and discharge of the waters that NO3, Na and some trace element pollutants were observed. Lacustrine limestone that is intricate with the formation in some sections was differentiated as the Yörük member. There are mudstones on the formation base. The existence of mudstone levels in the unit precipitated in a fluvial environment indicates a connection with the lake (Şaroğlu et al. 1995; Sevin and Aksay 2002). The formation is slightly cemented; in general, it is horizontal or close to horizontal. The formation obtained its gravels from the Abant Formation. The ormation features type section of the well field with a thickness of 250–300 m (Şaroğlu et al. 1995) and the thickness of the Yörük member, which is primarily related with the formation, is 100–150 m. The limestone is highly jointed, brittle, decomposed and some sections are travertine-like and have karstic openings.
The Abant Formation (KTab), which is the source rock of the clastic levels of Örencik Formation, has a wide distribution in the study area and ensures the recharge and/or discharge of springs with various flow rates. The formation consists of marl, siltstone, sandstone, blocky conglomerate and pelagic limestone of different ages (Şaroğlu et al. 1995; Tokay 1973; Alan and Aksay 2002; Timur and Aksay 2002; Sevin and Aksay 2002 and Bilginer et al. 2002). Limestones feature intensive cracks and karstic openings in places. No significant NO3 or trace element pollution was observed in waters discharged from this formation.
Hydrogeology
The units indicating primary aquifer characteristics in the study area are the Abant Formation, Soğukçam Formation, Örencik Formation and alluviums. The Abant Formation consists of levels, indicating limestone and flysh characteristics; Soğukçam Formation consists of intensive fractured crystallized limestones; Örencik Formation consists of limestone and slightly cemented conglomerate, sandstone, mudstone, siltstone and claystone. The limestones of Örencik Formation are lacustrine. Limestones are transitive from place to place with clastic level and the source rock of clastic levels is Abant Formation.
Fifteen springs with flow rates between 0.5 and 305 1/s and 5 geothermal and/or mineral springs with flow rates between 0.3 and 2.5 1/s are recharged and/or discharged from Abant Formation. Two springs with flow rates between 15 and 30 1/s and 1 geothermal spring with a flow rate of 0.3 1/s are recharged and/or discharged from Soğukçam Formation. Nine springs with flow rates between 0.15 and 3 1/s and four drilled wells with approximate flow rates between 0.1 and 1.5 1/s are recharged and/or discharged from the Örencik Formation. There is one drilled well with a flow of 1.2 1/s in the alluvium. As can be seen on the map in Fig. 2, the main flow direction of the Eskipazar River draining the waters in the study area is from SW to NE, and the flow direction of its tributaries are from NW to SE in the north section and from SE to NW in the south section. River gauging was performed at two points on the Eskipazar River during the field survey and the flows were gauged to be between 247 and 2,046 1/s.
Measurements and analyses were carried out during various periods, in such a way as to cover rainy and dry periods between July 2007 and December 2008. Spring, well and river measurement points are given in the geological–hydrogeological map in Fig. 2. In the study area, Örencik Formation and the alluvium are usually observed in slightly sloping regions. Agricultural activities are carried out in the large part of these areas. The areas are approximately 90 km2 in size.
NO3 pollution
The presence of even 5–10 mg/l of nitrate in waters is an indicator that the waters are at a risk of pollution (McNeely et al. 1979; Şahinci 1991; Atabey 2005a, b). The level of nitrate in waters is of particular importance for use as drinking water. The usage of waters with a high nitrate level for drinking purpose reduces the oxygen carrying capacity of the blood and can lead to “blue disease” (methemoglobinaemia) in babies. Because babies younger than 6 months have relatively low levels of gastric acid, nitrate is reduced to nitrite, which reacts with hemoglobin in blood, forming methemoglobin. The blood loses its ability to carry oxygen and as a result breathing difficulties may be observed in babies (Lahl et al. 1983; WHO 1984b; Uslu and Türkman 1987; Kaçaroğlu 1991, 1997). Furthermore, nitrate intake may be readily converted into nitrite in the mouth or another body part where acidity is relatively low. Nitrite reacts with secondary and tertiary amines, alcohol ammonium bases and amides in an acidic environment and forms nitrosamines and nitrosamides. Recent studies have indicated that these compounds (especially dimethylnitrosamine and diethylnitrosamine) may have strong carcinogenic effects (WHO 1984b; Uslu and Türkman 1987). In addition, there are numerous findings that these compounds increase the risk of cancer in the digestive system and led to urinary tract diseases (Pontius 1993; Wasik et al. 2001; Polat et al. 2007). In addition, nitrate concentration in water above 500 mg/l causes inflammation of the bowel, digestive and urinary systems (Uslu and Türkman 1987).
In the study area, nitrate pollution is observed in the water discharging from the Örencik Formation, consisting of unconsolidated conglomerate, sandstone, mudstone, siltstone and claystone alternations; and areas where the Örencik Formation and Yörük member are tangled; where the Yörük member, comprises fractured-fissured and partly karstic limestone; and alluvium, where agricultural activities are carried out. Field measurement values and chemical analysis of geothermal and/or mineral springs, springs discharging from the Abant Formation and rivers in the study area are given in Tables 1 and 2, respectively. Field measurement values and chemical analysis of waters that NO3 pollution is observed are given in Tables 3 and 4, respectively.
As shown in Table 3, discharge of the springs and wells where nitrate pollution is observed vary between 0.15 and 3.5 l/s. EK-8, EK-10, EK-35 discharge from the Yörük member consisting of lacustrine limestone. EK-14, EK-36, EK-37, EK-44, and EK-45 discharge from the areas where the Yörük member and Örencik Formation, which comprises both limestone levels and loose conglomerate-sandstone levels, are tangled. EK-38, EK-39, EK-40, EK-43 and EK-46 discharge from the Örencik Formation consisting of conglomerate, sandstone, mudstone, siltstone and claystone. These waters are used as drinking water and domestic water. On the other hand, EK-47 is a well drilled in alluvium for drinking, domestic and irrigational purposes.
The wells located in the Örencik Formation were drilled in ancient years and there are no regular well logs. Nevertheless, in accordance with the information obtained from well owners and drilling companies, it is understood that the drilled lithologies of EK-40 (depth 37 m), EK-46 well (depth 40 m), and EK-39 well (depth 96 m) consist of an intercalation of poorly cemented coarse conglomerate, sandstone, siltstone and claystone. However, it is understood that the clayed levels at lower altitudes in EK-39 are more extensive and thicker, when compared with other wells. The fact that the flow rate of EK-40 and EK-46 wells range between 0.5 and 1.55 1/s and that the flow rate of EK-39 well is approximately 0.1 1/s (occasionally dry) supports this situation. The existence of lithologies of different thicknesses in wells that are close to each other indicates that the lithology is irregular.
NO3 pollution is highest in the wells drilled in the clastic levels of the Örencik Formation (EK-39, EK-40, EK-46), at medium levels in wells and in springs discharging from areas featuring clastic and limestone levels of the Örencik Formation and drilled wells in alluvium, and at low levels in springs of the formation discharging from mainly limestone levels (Table 4). NO3 concentration contours are given in Fig. 3. It is clear that NO3 pollution is concentrated in Eskipazar and its vicinity, where intensive agricultural activities are carried out.
Nitrate (NO3) values of the springs and wells in the study area range between 0 and 135.2 mg/l. Cezaevi Well (EK-39) with a value of 57.7 mg/l, Yorgalar Well (EK-4) with a range of 121.5–135.2 mg/l, and Kurs Well (EK-46) with a range of 115.6–130.2 mg/l, exceed the Turkish Standards for Water Intended for Human Consumption (TS 266 2005) and the World Health Organization (WHO 2006) limit value of 50 mg/l. EK-39 is a well belonging to an open prison located in the region; the water is used for drinking, domestic and irrigational purposes, sometimes directly and sometimes by being mixed with city water by the prison officials and prisoners. EK-40 is privately owned and is sometimes used for drinking and domestic purposes. EK-46 is owned by a theological study center and the water is used by course attendees and students for drinking and domestic purposes, after passing through a purification unit. As it is seen in Table 4, the purification unit is not operated systematically and the necessary controls are not performed properly. Parts, such as filters, cartridges, etc. become dirty and are not replaced on schedule. As a result, the purification system can neither purify the water sufficiently nor reduce nitrates and other ions. Thus, nitrate levels at EK-46 considerably exceed the limit stipulated in the regulations. The nitrate values of Cezaevi Well (EK-39), Bahçepınar Spring (EK-36), Bahçepınar Well (EK-37), Pazarbaşı Fountain (EK-38) and Köpekler Çay Spring (EK-10) range between 32.9 and 57.7, 30.4 and 43.4, 21.1 and 36, 29.1 and 34.8, and 7.44 and 11.2 mg/l, respectively. The nitrate values of Kölepınar Fountain (EK-45), Bahçepınar Pool Spring (EK-44), Üçevler Well (EK-47), Bahçelievler Fountain (EK-43), and Gökdere Spring (EK-8) are 32.2, 27.3, 27.3, 24.2 and 12.4 mg/l, respectively. The majority of these waters are used continuously for drinking and domestic purposes without undergoing any operation such as purification and/or chlorination.
Nitrite and nitrate are inorganic ions produced during various stages of the nitrogen cycle. Nitrate is the predominant ion in well-oxygenated water, because of the rapid oxidation of nitrite. Nitrate is used mainly in inorganic fertilizers. Therefore, its concentration in groundwater and surface water is normally low; nitrate can reach high levels as a result of leaching or runoff from agricultural land or contamination by human or animal wastes (Ekmekçi 2005). Owing to its negative charge (NO3) is not held in the soil, the majority being leached to the saturated zone (Canter 1996; Novais et al. 2008).
It was found that nitrate pollution in waters in the study area comprises NO3, and NO2 and NH3 are absent or below detectable levels. This indicates that the pollution is not actual and that the areas from which the waters recharge are oxygen rich and feature an oxidizing environment, in terms of redox potential, that is sufficient to perform transition of NH3 to NO3 (NH4 + + 2O2 = NO3 − + H2O). This indicates that the irrigation water, rain and snow water do not reach the groundwater rapidly and therefore, the permeability of recharge areas of the waters is not very high. This indicates that the levels of mudstone, siltstone, and claystone cause a reduction in the permeability of loose conglomerate and sandstone levels in the Örencik Formation.
Trace element pollution
Most fertilizers contain small amounts of trace elements. Phosphorus (P) fertilizers such as triple superphosphates and calcium/magnesium phosphate contain varying concentrations of Cd, depending on the source of phosphate rock (Mortvedt and Beaton 1995; He et al. 2005). P fertilizers are among the other sources of heavy metal input into agricultural systems. On an average, phosphate rock contains 11, 25, 188, 32, 10, and 239 mg/kg of As, Cd, Cr, Cu, Pb and Zn, respectively (Mortvedt and Beaton 1995; He et al. 2005). In the USA, the trace elements, such as Cu, Zn, B, Fe, and Mn were intentionally added to regular blend fertilizers to meet the requirement for these elements for plant growth. These fertilizers are important sources of trace elements for crops growing in soils which are severely deficient in these elements, such as sandy soils, peaty soil, and calcareous soils. Organic materials, such as farm manures, bio-solids or composts contain higher concentrations of trace elements than most agricultural soils. The use of bio-solids and composts was reported to increase total amounts of Cu, Zn, Pb, Cd, Fe, and Mn in soils (McBride 2004; He et al. 2005). The concentrations of trace metals in bio-solids/composts are given by Chaney et al. (2001), He et al. (2005) and Mikayilov and Acar (1998).
The mobility of metals depends not only on the total concentration in the soil, but also on soil properties, metal properties and environmental factors. The mobility and availability of trace elements are controlled by many chemical and biochemical processes, such as precipitation–dissolution, adsorption–desorption, complexation–dissociation, and oxidation–reduction. Not all the processes are equally important for each element, but all these processes are affected by soil pH and biological processes. Therefore, it is crucial to understand some major reactions in soils that control the release of a specific trace element in the soil and the environment to overcome problems related to deficiency and contamination of these elements (He et al. 2005). Heavy metals accumulate in soils in various forms: water soluble, exchangeable, carbonate associated, oxide associated, organic associated and residual forms. The metals present in these categories have different levels of mobility (Moore et al. 1998; Iyengar et al. 1981; Sims and Kline 1991; He et al. 2005). Water soluble and exchangeable fractions are readily released to the environment, whereas the residual fractions are immobile under natural conditions. Dowdy and Volk (1983) suggested that the movement of heavy metals in soils could occur in sandy, acid, low organic matter soil if subjected to heavy rainfall or irrigation (He et al. 2005). Increased anthropogenic inputs of heavy metals in soils have received considerable attention, because transport of the metals may result in an increased content of heavy metals in groundwater or surface water (Purves 1985; Moore et al. 1998; He et al. 2005).
Certain trace element pollutions are observed in the study area, especially in wells drilled in the clastic levels of the Örenlice Formation with the highest NO3 concentration. Trace element analysis of geothermal and/or mineral springs, springs discharging from the Abant Formation and rivers without observing NO3 pollution are given in Table 5. Trace element analyses of waters featuring NO3 pollution are given in Table 6. According to Tables 5 and 6, it can be seen that Se, Hg, Pb, Cu, Zn, Ga, Y, Pd, I, Ba, and U concentrations of EK-39 well in different periods are higher than the average values of springs discharging from the Abant Formation that provides materials to the Örencik Formation and geothermal and/or mineral springs in the study area (the reservoir rock of most geothermal and/or mineral springs is the Abant Formation). Se, Hg, Pb concentrations exceed the upper limit values within the Turkish Standards for Water Intended for Human Consumption (TS 266 2005) and World Health Organization regulations (WHO 2006). Furthermore, B, S, Cr, Mn, Fe, Br, and I concentrations in this well are higher than the concentrations in springs discharging from Abant Formation, and the B value exceeds the upper limit value stated in the World Health Organization regulations (WHO 2006).
Pb, Ga, Y, Pd, and U concentrations of well EK-40 are higher than the average values of springs and geothermal and/or mineral waters discharging from the Abant Formation at different periods, and Pb concentration in rainy periods is close to, but does not exceed the upper limits stated in Turkish Standards for Water Intended for Human Consumption (TS 266 2005) and World Health Organization Regulations (WHO 2006), Pd and U value of well EK-46 are higher than the values of other springs and geothermal waters, and the Mn value is higher than the concentrations of cold springs discharging from the Abant Formation (Tables 5, 6). Furthermore, B, S, Cr, Fe, Cu, Zn, Ga, Br, Y, I, Ba, Hg, and Pb values of other springs and wells discharging from the Örencik Formation are generally slightly higher than the concentrations of springs discharging from the Abant Formation in different periods.
Large amounts of boron (B) may result in indigestion and affect the central nervous system (Department of National Health and Welfare 1979; McNeely et al. 1979). Lead (Pb) has a considerable toxic effect. It penetrates into red blood cells in the body and emits the iron from the cell, and thus may lead to anemia due to iron deficiency (Peker 1970; Atabey 2005a, b). Furthermore, it is stated that the lead accumulated in bones dissolves over time and may lead to inflammation in kidneys and abnormalities brain and nervous system functions, and that IQ levels of children decrease due to the increase of lead in blood (Kahvecioğlu et al. 2003). Danış (2005) indicated that lead exceeding the regulatory limits may result in diseases, such as metabolic intoxication, fatigue, anemia and nervousness (Atabey 2005a, b). Excessive intakes of mercury may lead to physiological and neurological diseases, such as central nervous system failures (Appleton 2001). Increased mercury concentration causes considerable damage in the brain and kidneys and may lead to increasing blood pressure, heart attack, rashes, wound formation and damage to the eyes (Güven et al. 2004; Atabey 2005a, b). High intakes of selenium may be toxic and result in diseases such as anorexia (Güven et al. 2004; ATSDR 2003; Atabey 2005a, b).
General evaluations
Figure 4 illustrates a horizontal geologic–hydrogeologic section of the springs discharging from the Abant Formation, where no pollution is observed, and; springs and wells discharging from the Örenlice Formation, where NO3 and trace element pollution are observed.
Evaluation of the probable flow paths in Fig. 4, together with the factors given below shows that the main cause of NO3 and trace element pollution is agricultural activities. These factors are (1) in the study area, NO3 and trace element pollutions are observed in springs and wells discharging from areas of intensive agricultural activities, which utilize various types of pesticides and fertilizers (ammonium nitrate, ammonium sulfate, diammonium phosphate, urea (nitrogenous fertilizer), 20 × 20, 15 × 15 × 15, barnyard manure etc.). (2) There is no significant NO3 and trace element pollution in springs discharging from Abant Formation, which is the source rock of clastic levels of the Örenlice Formation. (3) In wells with the highest NO3 concentrations, concentrations of certain trace elements are mostly higher than in cold and geothermal and/or mineral waters within the study area (Tables 4, 5). (4) There is mudstones in the base of the Örencik Formation (Şaroğlu et al. 1995), which reduces the possibility for deeper circulating water to get mixed into the wells. (5) There are no polluting sources (waste disposal area, industrial center, mineralization, etc.) within the recharge area of waters; and the sewage system of the study area is fully operational.
In well EK-39, Na pollution was observed in addition to NO3 and trace element pollution. Na pollution in this well may be deemed to be due to the presence of higher levels of clay in EK-39 when compared with other wells, and from water–rock interaction, cation exchange with clayed levels and, to some extent, from fertilizers containing Na used in agricultural activities and the mixture of deep circulating water. In addition, the fact that Şaroğlu et al. (1995) mentioned the existence of mudstones in the base of formation hosting wells; that the discharge of the well is very low, that the well dries up periodically and the redundancy of clayed levels reduce the possibility for mixing of deep circulating water. In addition to NO3 pollution, Ca and SO4 pollutions were observed in the EK-40 well drilled in the alluvium. The fact that no units containing intensive SO4 such as gypsum were observed that no alteration that may lead to SO4 are observed within the recharge area of the well, and the fact that agricultural activities are conducted around the well suggest that this pollution may stem from agricultural activities. Unlike other wells in the vicinity of this well, fruit and vegetable farming that require high levels of irrigation are conducted, and fertilizers containing SO4, Ca and Na in agricultural activities are used. The water returning from irrigation may lead to salinization in the soil and groundwater. Consequently, it is suggested that these pollutions in the well are due to agricultural practices.
Atabey (2005a, b) reported that water with a Na level above 200 mg/l presents a serious health risk for people with hearth conditions, nephropathy and circulatory problems. High rates of NaSO4 and MgSO4 in water may have a laxative effect on humans (Atabey 2005a, b). Excessive Ca loading may result in the formation of kidney stones and bone calcification (P.M. Journal 1995; Boz 2004; Atabey 2005a, b).
Elhatip et al. (2003) reported that high nitrate content and significant mineralization in the groundwater are probably due to contamination of the recharge to the aquifer by irrigation drainage, poorly maintained sewage networks, and septic tanks (APHA, AWWA, WPCF 1989; Elhatip et al. 2003). Previous research indicates some other inorganic constituents of groundwater that are present in agricultural additives including, Cl, K, Ca, Mg, and a variety of minor elements and agricultural sources of some of these elements and can dominate natural sources locally. In addition, it has been suggested that agricultural effects on the recharge fluxes of various ions such as NO3 and H+ can cause changes in weathering rates and ion-exchange equilibrium in the subsurface, thereby indirectly altering the concentrations of other constituents in groundwater. These indirect effects have important implications for geochemical studies of water–rock interactions and can represent sources or sinks for a variety of problematic contaminants, such as nutrients and toxic trace elements (Elhatip et al. 2003). High NO3 and NaCl concentrations in springs discharging from volcanic rocks located in Kayseri were stated to arise from agricultural wastes and waste water around the springs (Baba and Ayyıldız 2006). Novais et al. (2008) reported that NO3 and Cl pollution in wells drilled in meta-sedimentary rocks in the Ervedosa region were due to agricultural and domestic wastewater.
Figure 5 presents Piper (1944) diagrams demonstrating the major anion and cation percentages of the waters discharging from the Abant Formation, Örencik Formation and alluvium. It can be seen from Fig. 5 that springs discharging from the Abant Formation and water discharging from the limestone levels of Yörük member (Örencik Formation) have Ca–HCO3 facies. Waters discharging from areas where clastic levels of the Örencik Formation and limestones of the Yörük member (Örencik Formation) are located together have mainly Ca–HCO3 facies and also high Na and Mg percentages. Water discharging from completely clastic levels of the Örencik Formation is mostly of the mixed type (Na–Ca–Mg–HCO3), whereas well EK-39 has NaHCO3 facies. The well drilled in alluvium (EK-47) is of the CaSO4 type and has high Mg and Na concentrations.
Five different primary groups are observed in the Piper diagram. Group I represents the water discharging from the Abant Formation with no NO3 pollution; Group II represents water discharging predominantly from the limestone levels of the Örencik Formation (Yörük member), containing water with less NO3 pollution; Group III represents water discharging from areas where limestone levels (Yörük member) and clastic levels of the Örencik Formation are located together containing water with medium level NO3 pollution; Group IV represents water discharging from clastic levels of the Örencik Formation and water with high levels of NO3 pollution; Group V represents water discharging from alluvium and water with NO3, Ca, and SO4 pollution. Water in which NO3 pollution is not observed and water in which NO3 pollution is observed at different ratios are separated from each other in the diagram. Furthermore, the SO4 pollution in EK-47 and Na pollution in EK-39 are clearly distinguished. When wells and springs are analyzed between each other, it can be seen from Table 4 that the total dissolved solids in the water increase throughout the general flow paths of each given group.
Figure 6 shows the NO3–Cl relationship of water in the study area and Fig. 7 shows the NO3–Mg relationship of water discharging from the Örencik Formation. It can be concluded from the figures that Cl and Mg concentrations increase along with the increase in NO3. Notwithstanding the few exceptions, because similar conditions are observed between Na, SO4 and NO3 concentrations (Table 4), it is thought that Ca, Na, Mg, Cl, and SO4 ions may originate from fertilizers used in agricultural activities in addition to water–rock interaction. In the Piper diagram, this situation is reflected in the way that the water with high NO3 concentrations is in the mixed type in terms of cations.
Conclusions and recommendations
The aim of the present study was to make a contribution to research on NO3 and trace element pollution. Investigations were performed around Eskipazar (Karabük) and NO3 pollution was recorded in water samples in areas where intensive agricultural activities are conducted. These waters are used for drinking, domestic and irrigation purposes. Pollution was particularly evident in the Örencik Formation and the Yörük member of the Örencik Formation consisting of conglomerate, sandstone, mudstone, siltstone and claystone alternations and intensive fractured-cracked limestone, and alluvium. Intensive forms of agriculture are practiced in these areas. In the wells drilled especially in the clastic levels of the formation, Na, B, Pb, Hg, Se pollutions were also detected in addition to NO3.
S, Cr, Mn, Fe, Cu, Zn, Ga, Br, Sr, Y, I, Ba, U concentrations in these waters with the highest level of NO3 are at higher levels when compared with the average values of springs discharging from Abant Formation that provides material to the clastic levels of Örencik Formation. Furthermore, the concentrations of most of these elements are higher than the average concentrations within geothermal and/or mineral waters in the study area.
There is a high amount of NO3 in EK-39, EK-40 and EK-46 wells drilled in the clastic levels of the Örencik Formation. The levels recorded are several times greater than the limit stated in the Turkish Standards for Water Intended for Human Consumption (TS 266 2005) and World Health Organization Regulations (WHO 2006). NO3 concentrations in most springs within the formation are close to the upper limit. In addition, Na, B, Pb, Hg and Se concentrations in well EK-39 exceed the regulatory limits during different periods. Pb concentration in well EK-40 does not exceed the regulatory limit during rainy periods, but is very close to this value. In addition to NO3 pollution, SO4 and Ca pollution are also observed in well EK-47, which is drilled in the alluvium in an area where agricultural activities requiring intensive irrigation are conducted.
When the factors given below are evaluated in combination, it is concluded that the main cause of NO3 and trace element pollution is agricultural activities; (1) in the study area, NO3 and trace element pollutions are observed in springs and wells discharging from areas of intensive agricultural activities, which utilize various types of pesticides and fertilizers (ammonium nitrate, ammonium sulfate, diammonium phosphate, urea (nitrogenous fertilizer), 20 × 20, 15 × 15 × 15, barnyard manure etc.). (2) There is no significant NO3 and trace element pollution in springs discharging from Abant Formation, which is the source rock of clastic levels of the Örenlice Formation. (3) In wells with the highest NO3 concentrations, concentrations of certain trace elements are mostly higher than in cold and geothermal and/or mineral waters within the study area. (4) There is mudstones in the base of the Örencik Formation, which reduces the possibility for deeper circulating water to get mixed into the wells. (5) There are no polluting sources (waste disposal area, industrial center, mineralization, etc.) within the recharge area of waters; and the sewage system of the study area is fully operational.
It is clear that these agricultural pollutants accumulating in the ground and reaching the groundwater through leaching may increase over time, depending on their usage, and may therefore constitute a significant environmental risk within the region in the future.
In the water within the study area, NO3 concentrations show a positive correlation with Mg and Cl concentrations. Notwithstanding a few exceptions, a similar correlation is observed between NO3 and Na, SO4. This is reflected in Piper diagrams in a way that these waters are of mixed types, especially in terms of cations. The waters discharging from the Örencik Formation, where NO3 and trace element pollution observed are different facies from the waters discharging from the Abant Formation, where no pollution is observed. It is suggested that the diversity stemmed from agricultural fertilizers, containing Na, Mg, Cl and SO4 in addition to water–rock interaction. To fully explore such relationships, and to determine potential local and international effects, studies of surface water, groundwater, soil, rock, fertilizers, pesticides, and chemistry involving researchers from different disciplines should be conducted on a larger scale.
Current global population expansion increases the need for meeting basic nutritional requirements. Current intensive agricultural activities are highly dependent on the use of chemical fertilizers and pesticides. Many previous studies have demonstrated the significant negative effects of such chemicals in terms of water and soil pollution. Unless the use of fertilizers and pesticides are decreased, it is likely that levels of NO3, trace elements and other pollutants in surface water, groundwater and soils will increase, and irreversible social, environmental and economic problems. Therefore, the appropriate element and compound and its amount which does not damage water quality should be determined; the proper dosages of chemicals required for every plant type should be established; conformity with standards for the use of fertilizers and pesticides should be strictly controlled; the public should be informed about the dosage use of fertilizers and pesticides, and necessary changes to agricultural methods should be immediately implemented.
Long-term monitoring studies should be carried out in regions where pollution arises from agricultural activity. Determination of the temporal distribution of NO3 and trace element increases are of crucial importance in terms of monitoring and maintaining the quality of surface and groundwater.
References
Alan İ, Aksay A (2002) 1/100.000 Ölçekli Türkiye Jeoloji Haritaları Zonguldak-F28 Paftası (1/100.000 scaled geological map of Turkey-Zonguldak F28 section). Geological Research Department, MTA (General Directorate of Mineral Research & Exploration), No. 29, Ankara, Turkey
Alloway BJ (1990) Cadmium. In: Alloway BJ (ed) Heavy metals in soils. Blackie Academic and Professional, London, pp 100–124
Alloway BJ (1995) Heavy metals in soils. Blackie Academic and Professional, London
APHA, AWWA, WPCF (1989) Standard methods for the determination of water and waste water, 15th edn. APHA
Appleton JD (2001) Mercury pollution from artisanal gold mines: a hazard to human health ?. Earthwise 17:12–13. British Geologicial Survey Publication, Nottingham
Arslan G, Akkaya C (2001) Basic problems in groundwater sources and interactions between surface and groundwater. Groundwaters and environment symposium, 21–23 March, İzmir, pp 45–54
Atabey E (2005a) Tıbbi Jeoloji (medical geology). In: Atabey E (ed) Publications of chamber of geology engineers of Turkey. TMMOB, Ankara
Atabey E (2005b) 1. Tıbbi Jeoloji Sempozyum kitabı (1th medical geology symposium book) In: Atabey E (ed).1–3 Aralık 2005, Ankara, pp 27–52
ATSDR (2003) Toxicological profile for selenium
Baba A, Ayyıldız Ö (2006) Urban groundwater pollution in Turkey, a national review of urban groundwater quality issues. In: Tellam v JH (eds) Urban groundwater management and sustainability, pp 93–110
Bilginer E, Pehlivan Ş, Aksay A (2002) 1/100.000 Ölçekli Türkiye Jeoloji Haritaları Bolu-G29 Paftası (1/100.000 scaled geological map of Turkey-Bolu G29 section). Geological Research Department, MTA (General Directorate of Mineral Research & Exploration), No. 36, Ankara, Turkey
Böhlke JK (2002) Groundwater recharge and agricultural contamination. Hydrogeol J 10:153–179
Boz A (2004) İnsan vücudundaki hazine (treasure in human body). http://www.kimyaokulu.com
Cangir C (2005) Ergene Havzası çevre düzeni planı ve bölgesel planı paneli (Panel of environment regularity and regional plan of Ergene Basin). 4th symposium on industrialization and environment. 22 p
Canter LW (1996) Nitrates in groundwater. CRC Lewis Publishers, Boca Raton
Chaney RL, Ryan JA, Kukier U, Brown SL, Siebielec G, Malik M, Angle JS (2001) Heavy metal aspects of compost use. In: Stoffella PJ, Khan BA (eds) Compost utilization in horticultural cropping systems. CRC Press LLC, Boca Raton, pp 59–324
Chen TB, Wong WC, Zhou HY, Wong MH (1997) Assessment of trace metal distribution and contamination in surface soils of Hong Kong. Environ Pollut 96:61–68
Chu LM, Wong MH (1984) Application of refuse compost: yield and metal uptake of three different food crops. Conserv Recycl 7:221–234
Danış M (2005) İthalat ve ithalatın denetimi, ağır metal kirlilikleri (Import and control of import, heavy metal pollution). Workshop of fertilizer and fertilizer raw materials, Publications of Chamber of Geology Engineers of Turkey TMMOB, 25–27 November Diyarbakır
Department of National Health and Welfare (1979) The Joint Committee on drinking water standards, Canadian drinking water standards and objectives, 1968. Catalogue No. H48-1069. Queen’s Printer for Canada, Ottawa
Dowdy RH, Volk VV (1983) Movement of heavy metals in soils. In: Nelsen DW (ed) Chemical mobility and reactivity in soil systems, SSSA Spec. Publ. 11. Madison, pp 40–227
Ekmekçi M (2005) Pesticide and nutrient contamination in the Kestel polje-Kirkgoz karst springs, Southern Turkey. Environ Geol 49:19–29
Elhatip H, Afşin M, Kuşçu İ, Dirik K, Kurmaç Y, Kavurmacı M (2003) Influences of human activities and agriculture on groundwater quality of Kayseri–Incesu–Dokuzpınar springs, Central Anatolian part of Turkey. Environ Geol 44:490–494
Eryurt A, Sekin Y (2001) Seasonal changes in groundwater around Manisa Region, hardness and nitrated compounds. Groundwater and environment symposium, March 21–23, İzmir, pp 187–193
Fergusson JE (1990) The heavy elements: chemistry, environmental impact, and health effects. Pergamon Press, New York
Fuge R (2004) Anthropogenic sources. In: Olle S (ed) Essential of medical geology, impact of the natural environment on public health, Elsevier, pp 43–60
Guo F, Jiang G, Yuan D (2007) Major ions in typical subterranean rivers and their anthropogenic impacts in southwest karst areas, China. Environ Geol 53:533–541
Güven A, Kahvecioğlu Ö, Kartal G, Timur S (2004) Metallerin çevresel etkileri-III (environmental effect of metals-III). Metalurji J (in Turkish) 138:64–71
He ZL, Yang XE, Stoffella PJ (2005) Trace elements in agroecosystems and impacts on the environment. J Trace Elem Med Biol 19:125–140
Hem JD (1985) Study and interpretation of the chemical characteristics of natural water L: US Geological Survey water-supply paper 2254. US Geological Survey, Alexandria
Herrero TC, Martin LFL (1993) Evaluation of cadmium levels in fertilized soils. Bull Environ Contam Toxicol 50:61–68
Houzim V, Vavra J, Fuksa J, Pekny V, Vrba J, Stibral J (1986) Impact of fertilizers and pesticides on groundwater quality: Impact of agricultural activities on groundwater. In: Vrba J, Rominj E (ed) International contributions to hydrogeology 5:89–132
Iyengar SS, Martens DC, Miller WP (1981) Distribution and plant availability of soil zinc fractions. Soil Sci Soc Am J 45:9–735
Kaçaroğlu F (1991) Eskişehir Ovası yeraltısuyu kirliliği incelemesi (the investigation of the groundwater pollution in the Eskişehir plain). Ph.D. theses, Graduate School of Natural and Applied Sciences, University of Hacettepe, Ankara, Turkey
Kaçaroğlu F (1997) Impacts of human activities on groundwater quality of an alluvial aquifer: a case study of the Eskişehir Plain, Turkey. Hydrogeol J 5:60–70
Kahvecioğlu Ö, Kartal G, Güven A, Timur S (2003) Metallerin çevresel etkileri-I (environmental effect of metals-I). Metalurji J (in Turkish) 136:47–53
Kara EE, Pırlak U, Özdilek G (2004) Evaluation of heavy metals (Cd, Cu, Ni, Pb, and Zn) distribution in sowing regions of patato fields in the province of Niğde, Turkey. Water Air Soil Pollut 153:173–186
Krishna AK, Govil PK (2004) Heavy metal contamination of soil around Pali Industrial Area, Rajasthan, İndia. Environ Geol 47:38–44
Kumbur H, Özsoy HD, Özer Z (2008) Mersin İli’nde tarımsal alanlarda kullanılan kimyasalların su kalitesi üzerine etkilerinin belirlenmesi (Determination of the effects of chemicals used in agricultural area on water quality in Mersin Province). Çev-Kor Ekoloji 68:54–58
Lahl U, Zeschmar B, Gabel B, Kozicki R, Podbielski A, Stachel B, Strauss S (1983) Ground-water pollution by nitrate: ground water in water resources planning, Proceeding, International Association of Hydrological Sciences (IAHS) publication 142:1159–1168
Lawrance CR (1983) Occurance and genesis of nitrate-rich groundwaters of Australia. Papers of the international conference on groundwater and man, 2: groundwater and environment, Australian Water Resources Council Conference Series 8:237–247
Manta DS, Angelone M, Bellanca A, Neri R, Sprovein M (2002) Heavy metals in urban soils: a case study from the city of Palemo (Sicily), İtaly. Sci Total Environ 300:229–243
McBride MB (2004) Molybdenum, sulfur, and other trace elements in farm soils and forages after sewage sludge application. Commun Soil Sci Plant Anal 35:517–535
McNeely RN, Neimanis VP, Dwyer L (1979) Water quality sourcebook: a guide to water quality parameters. Indland Waters Directorate, Water Quality Branch, Ottawa
Mermut AR, Jain JC, Song L, Kerrich R, Kozak L, Jana S (1996) Trace element concentrations of selected soils and fertilizers in Saskatchewan Canada. J Environ Qual 25:845–853
Mikayilov FD, Acar B (1998) Toprak ekosistemlerinde kirleticilerin taşınım mekanizmasının incelenmesi ve modellenmesi (Investigation and modelling of contaminant’s movement processes within the soil ecosystems). Çev Kor 28:20–23
Moon JW, Moon HS, Woo NC, Hahn JS, Won JS, Song Y, Lin X, Zhao Y (1999) Evaluation of heavy metal contamination and implication of multiple sources from Hunchun basin, northeastren China. Environ Geol 39:1039–1052
Moore PA Jr, Daniel TC, Gilmour JT, Shreve BR, Edwards DR, Wood BH (1998) Decreasing metal runoff from poultry litter with aluminum sulfate. J Environ Qual 27:9–92
Mortvedt JJ, Beaton JD (1995) Heavy metal and radionuclide contaminants in phosphate fertilizers. In: Tiessen H (ed) Phosphorus in the global environment: transfer, cycles and management. Wiley, New York, pp 93–106
Mühendis T, Birliği MO (2006) Mazıdağı ve fosfat gerçeği raporu (Report of the Mazı Mountain and phosphate reality). UCTEA-Union of Chambers of Turkish Engineers and Architects, Ankara, Turkey, 96 p
Nouri J, Mahvi AH, Babaei AA, Jahed GR, Ahmadpour E (2006) Investigation of heavy metals in groundwater. Pak J Biol Sci 9:377–384
Novais H, Pereira MR, Patinha C (2008) Contamination factor of the groundwater of the Ervedosa region (NE Portugal). e-Terra 5:1–18
Peker İ (1970) Endüstride İş Güvenliği (Work security in industry). İTÜ Faculty of Chemistry, İstanbul
Piper AM (1944) A graphical procedure in the geochemical interpretation of water analyses. Am Geophys Union Trans 25:914–923
P. M. (Journal) (1995) ‘Heir Liegt ein Mensch’, Marianne Oertl
Polat R, Elçi A, Şimşek C, Gündüz O (2007) İzmir-Nif Dağı çevresindeki yeraltısuyu nitrat kirliliği boyutunun mevsimsel değerlendirilmesi (Seasonal assessment of the extent of nitrate contamination in ground water around Nif Mountain in Izmir). 7th national environmental engineering congress, live environment technology, 24–27 October 2007 Izmir, Turkey, pp 482–489
Pontius FW (1993) ‘Nitrate and cancer: is there a link?’. J AWWA 85(4):12–14
Purves D (1985) Trace element contamination of the environment. Elsevier, New York
Ritter WF, Chirnside AEM (1984) Impact of land use on ground-water quality in Southern Delaware. Ground Water 22:38–47
Şahinci A (1991) Doğal Suların Jeokimyası (Geochemistry of natural waters). Reform Printing Office, Izmir
Şaroğlu F, Herece E, Sarıaslan M, Emre Ö (1995) Yeniçağa-Eskipazar-Gerede arasının jeolojisi ve Kuzey Anadolu Fayı’nın genel özellikleri (Geological of the Eskipazar-Gerede region and general features of North Anatolian Fault Zone). MTA Report no. 9873, 48 p
Schepers JS, Frank KD, Watts DG (1983) Influence of irrigation and nitrogen fertilization on groundwater quality: relation of groundwater quantity and quality. Proceeding of the Hamburg symposium, August 1983, IAHS Publ. 146:21–32
Sen E (1996) Bursa Nilüfer ve Ayvalı Havzalarındaki kaynak suyunda UV tekniği ile nitrat konsantrasyonu (Nitrate concentration with UV technic on spring water in Nilüfer and Ayvalı Basin of Bursa). Uludag University, I. Uludag environmental engineering symposium, June 24–26, Bursa, Turkey
Sevin M, Aksay A (2002) 1/100.000 Ölçekli Türkiye Jeoloji Haritaları Bolu-G28 Paftası (1/100.000 scaled geological map of Turkey-Bolu G28 section). Geological Research Department, MTA (General Directorate of Mineral Research & Exploration), No. 35, Ankara, Turkey
Sims JT, Kline JS (1991) Chemical fractionation and plant uptake of heavy metals in soils amended with cocomposed sewage sludge. J Environ Qual 20:95–387
Smith HF, Harmeson RH, Larson TE (1971) The effect of commercial fertilizer on the quality of groundwater. Groundwater pollution symposium (proceedings of the Moscow symposium, August, 1971), AISH Publ. 103:96–102
Timur E, Aksay A (2002) 1/100.000 Ölçekli Türkiye Jeoloji Haritaları Zonguldak-F29 Paftası (1/100.000 scaled geological map of Turkey-Zonguldak F29 section). Geological Research Department, MTA (General Directorate of Mineral Research and Exploration), No. 30, Ankara, Turkey
Tokay M (1973) Kuzey Anadolu Fay Zonunun Gerede ile Ilgaz arasındaki kısmında jeolojik gözlemler (Geological observations on the North Anatolian Fault Zone in the area between Gerede Ilgaz). Symposium on the north Anatolian fault and earthquake belt, Ankara, Turkey, Bulletin of MTA Institute, pp 12–29
TS 266 (2005) İnsani Tüketim Amaçlı Sular Hakkında Yönetmelik (Turkish regulation concerning water intended for human consumption), Institute of Turkish Standard, Ankara, Turkey
Uslu O, Türkman A (1987) Su kirliliği ve kontrolu (Water pollution and control). Publication series of Environment General Directorate of Prime Ministry of Turkish Republic 1:251–265
Wasik E, Bahdziewicz J, Blasszczyk M (2001) Removal of nitrates from groundwater by hybrid process of biological denitrification and microfiltration membrane. Process Biochem 37:57–64
WHO (2006) Guidelines for drinking-water quality. First addendum to 3rd edn, 1, Recommendation. Geneva, 595 p
WHO (World Health Organization) (1984) Guidelines for drinking-water quality. vol 2, Health criteria and other supporting information, WHO Publ, Geneva, 335 p
Wong MH (1990) Anaerobic digestion of pig waste mixed with sewage sludge. Biol Waste 31:223–230
Yüksel S, Nalbantçılar MT, Balkaya N (1997) Groundwater contamination in the Region of Samsun (Turkey). In: Proceeding of the international symposium on geology and environment İlyas Y (ed) İstanbul, pp 409–416
Acknowledgments
The authors would like to thank the Cumhuriyet University Scientific Research Projects Commission (CÜBAP) and Eskipazar Municipal authorities and workers for providing financial and in-field study support. The author also appreciates the assistance of reviewers and Prof. Dr. Mehmet EKMEKÇİ, who helped during the manuscript correction process, and Prof. Dr. Fikret KAÇAROĞLU for his help during the English corrections phase of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ekemen Keskin, T. Nitrate and heavy metal pollution resulting from agricultural activity: a case study from Eskipazar (Karabuk, Turkey). Environ Earth Sci 61, 703–721 (2010). https://doi.org/10.1007/s12665-009-0385-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-009-0385-x