Abstract
Six trace metals (chromium [Cr], nickel [Ni], copper [Cu], arsenic [As], cadmium [Cd] and lead [Pb]) were measured in sediments and soft tissues of three commonly consumed fish species (Channa punctatus, Heteropneustes fossilis, and Trichogaster fasciata) collected from three urban rivers around Dhaka City, Bangladesh. The abundance of total metals in sediments varied in the decreasing order of Cr > Ni > Pb > Cu > As > Cd. Sequential extraction tests showed that the studied metals were predominantly associated with the residual fraction followed by the organically bound phase. The range of metal concentration in fish species were as follows: Cr (0.75–4.8), Ni (0.14–3.1), Cu (1.1–7.2), As (0.091–0.53), Cd (0.008–0.13), and Pb (0.052–2.7 mg/kg wet weight [ww]). The rank of biota-sediment accumulation factor for fish species were in the descending order of Cu > As > Pb > Ni > Cr > Cd. Metal concentrations in fish exceeded the international permissible standards suggesting that these species are not safe for human consumption.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Trace-metal pollution in the environment has become a concern due to the ever-increasing contamination of water, soil, and food in many regions of the world (Tang et al. 2013; Sow et al. 2013; Rahman et al. 2014). This pollution not only poses a threat to public water supplies, it also poses an ecological and human health risk through the consumption of aquatic products (Waqar 2006; Terra et al. 2008). Therefore, studies on trace-metal pollution in fish are important for characterizing health risks (Asuquo et al. 2004). Studies have shown that urban and industrial development contributes to metal contamination in freshwater environments (Xia et al. 2011; Tao et al. 2012). Bangladesh is one of the largest delta regions in the world, formed by the Ganges, Brahmaputra, and Meghna rivers and randomly spreading across five countries, namely, Bhutan, Nepal, China, India, and Bangladesh (Sharif et al. 1993). Dhaka is the capital of Bangladesh and has many industries, built with limited planning, that discharge large amounts of untreated effluent into the adjacent rivers (Turag, Buriganga, and Shitalakha). This presents a concern in terms of the health of local aquatic ecosystems and the people inhabiting the area. Few studies have evaluated the accumulation and contamination of trace metals in these rivers (Ahmad et al. 2010; Alam et al. 2003). In recent decades, the accumulation and contamination of trace metals in these rivers have been paid less attention. These rivers are increasingly being polluted with industrial and domestic effluents (Islam et al. 2006; Ahmad et al. 2010). Trace metals have accumulated in the surface sediments of these rivers (Alam et al. 2003; Islam et al. 2014). In addition, a considerable amount of trace metals can be accumulated in the surface sediments of these rivers. In sediment, trace metals are present in a number of chemical forms, and they generally exhibit different physical and chemical behaviors in terms of chemical interaction, mobility, biological availability, and potential toxicity (Akcay et al. 2003; Singh et al. 2005). During sediment transportation, trace metals undergo various changes in their speciation due to dissolution, precipitation, sorption, and complexation phenomena (Dassenakis et al. 1998; Akcay et al. 2003; Abdel-Ghani & Elchaghaby 2007), all which affect their behavior and bioavailability (Nouri et al. 2011). The overall behavior of trace metals in the aquatic environment is strongly influenced by the association of metals with various geochemical phases (Morillo et al. 2004).
It is now widely accepted that the role of sediments as a sink for metal pollutants cannot be fully assessed by measuring the total metal concentration (Chandra Sekhar et al. 2003). Nevertheless, information on total concentration is not sufficient to assess the environmental impact of contaminated sediments (Chandra Sekhar et al. 2003). Therefore, a particular interest is focused on geochemical speciation in assessing the potential environmental impacts and ecotoxicity of trace metals (Sin et al. 2001). Chemical speciation can be defined as the process of identification and quantification of different species, forms, or phases of chemicals present in the environment (Abdallah 2007). Metal speciation in sediment is expected to influence metal bioavailability and thereby metal content in biota, in particular in the soft tissues of fish (Pempkowiase et al. 1999; Yap et al. 2002). To assess the various sediment-bound trace metals into operationally defined fractions, sequential extraction schemes have been developed by several investigators (Tessier et al. 1979; Rauret et al. 1999; Pustisek et al. 2001; Svete et al. 2001; Sutherland & Tack 2003; Oyeyiola et al. 2011). Among them, the five-stage sequential extraction schemes developed by Tessier et al. (1979) is extensively used across the world. Sequential extraction techniques would provide the history of metal input, digenetic transformation within the sediments, and reactivity of trace-metal species of both natural and anthropogenic origin (Sundaray et al. 2011).
Previous studies on the Buriganga River have focused on the river water chemistry and the physicochemical properties in the river water (Ali et al. 2008; Moniruzzaman et al. 2009), and only a few studies have assessed the seasonal and spatial distribution of trace metals in the sediment and biota of peripheral rivers in Dhaka City (Ahmad et al. 2010; Alam et al. 2003; Mohiuddin et al. 2011). No detailed studies have been completed on the trace-metal geochemical speciation in sediments and bioaccumulation in fish species in these rivers. The present study was performed to determine the seasonal variation and geochemical fractionation of metals in the sediment and to assess bioaccumulation of metals in three fish species that are commonly eaten in this area. This research work was performed to determine the seasonal variation and the geochemical fractionation of metals in sediment as well as to assess the bioaccumulation of metals in three edible fish species.
Materials and Methods
Study Area
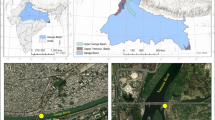
Dhaka, with a 815.8-km2 metropolitan areas, is surrounded by three major river—the Turag, the Buriganga, and the Shitalakha (Fig. 1),—which are using as a convenient means for industrial wastes disposal. Dhaka is one of the most densely populated cities in the world with 12 million people of which <25 % are served by a sewage treatment facility (Ahmad et al. 2010; Mohiuddin et al. 2011). The indiscriminate dumping of domestic and industrial wastes, combined with the failure of authorities to enforce existing regulations to protect the ecological health of these rivers, has aggravated the situation to the point where these rivers are dying biologically and hydrologically. Therefore, these rivers were selected for this study, and the basic information of the study areas is listed in Supplementary Table S1.
Sample Collection and Preparation
Sampling was performed in February through March 2012 (winter) and again in August through September 2012 (summer). During winter, there is no rainfall, and river water levels decrease; during summer, river water levels increase due to heavy rainfall. At each sampling point, composite sediment samples were collected using standard protocol (United States Environmental Protection Agency [USEPA] 2001). River bed sediment samples (approximately 200 g) were taken at a depth of 0 to 5 cm using a portable Ekman grab sampler. Each sediment sample was obtained by mixing sediments randomly collected (three times) at each sampling point, and 18 pairs of composite sediment samples were collected. Sediment samples were then freeze dried to obtain constant weight. The samples were homogenized by grinding in an agate mortar, sieved through 106-μm Aperture nylon sieve, and stored in labeled glass bottles until chemical analyses.
A total of 54 samples of 3 mostly consumable fish species—spotted snakehead (Channa punctatus), stinging catfish (Heteropneustes fossilis), and banded gourami (Trichogaster fasciata) —were collected from 3 rivers around Dhaka City, Bangladesh (Fig. 1). These three fish species can change their feeding habits and survive in polluted river conditions. C. punctatus is omnivorous—carnivorous and feeds on zooplankton, zoobenthos, and plants from the surface water body (Saikia et al. 2012). H. fossilis and T. fasciata are omnivorous and often feed on insects, larvae, crustaceans, algae, and detritus in sediment. Moreover, they live close to sediments (moreso than do C. punctatus). Thus, it is expected that these 3 fish species are differently exposed to metals and other contaminants. Each species of fish, i.e., 18 samples, were collected with the help of fishermen. Immediately after collection, fish samples were washed thoroughly with freshwater to remove mud or other fouling substances and put into plastic bag/containers; samples were then transported to the Department of Fisheries, Dhaka University, Bangladesh. After transportation to the laboratory, the fish samples were allowed to reach room temperature, and nonedible parts were removed with the help of a steam-cleaned stainless steel knife. In the present study, only fish muscles were evaluated for the elemental concentration because Bangladeshi people do not habitually consume the other parts, such as liver, kidneys, gills, and gonads. Muscle tissues of the fish samples were then washed with distilled water and cut into small pieces (2–3 cm) using the cleaned knife over a clean polyethylene sheet. Fish samples were then freeze dried to obtain constant weight and homogenized by grinding in an agate. The preprocessed samples were brought to Yokohama National University, Japan, for chemical analysis.
Analytical Methods for Physicochemical Parameters
Physicochemical parameters, such as pH, EC, % nitrogen (%N), and % carbon (%C), were measured. The pH of sediments was measured in a sediment-to-water ratio of 1:2.5. The sediment/deionized water mixture was stirred with a clean glass rod, and the slurry was allowed to equilibrate for 30 min (Ikem & Adisa 2011; Ahmed et al. 2012). The pH values were recorded using a Horiba U-23 instrument with the calibration of pH 4 and pH 7 standards. For electrical conductivity (EC) determination, 5.0 g of sediment was taken in 50-mL polypropylene tubes. Then 30 mL of distilled water was added to the tube. The lid was closed properly and was shaken for 5 min. After that, EC was measured using an EC meter (Horiba D-52) (modification after Niwa et al. 2011). Percent N and C of sediment was measured using an elemental analyzer (vario EL III; Elenemtar, Germany) at Yokohama National University, Japan. For N and C determination, sediment samples were weighed in tin or silver vessels and loaded into the integrated carousel. In a fully automatic process, transfer of the sample through the ball valve into the combustion tube was performed. Each sample was individually flushed with carrier gas to remove atmospheric N resulting in a zero blank sampling process. The catalytic combustion was performed at a permanent temperature of ≤1,200°C. The element concentration from the detector signal and the sample weight were measured on the basis of stored calibration curves. Sediment texture was determined by the hydrometer method (Ikem & Adisa 2011).
Sample Digestion and Metal Extraction
All chemicals were analytical-grade reagents, and Milli-Q (Elix UV5 and MilliQ, Millipore, USA) water was used for solution preparation. The Teflon vessel and polypropylene containers were cleaned, soaked in 5 % HNO3 for >24 h, then rinsed with Milli-Q water and dried. Samples were digested in a microwave digestion system (Berghof-MWS2; Berghof Speedwave, Germany). The microwave digestion system was designed to performed chemical digestion procedures under extreme pressure and temperature conditions. Digestion reagents used were 5 mL of 69 % HNO3 acid (Kanto Chemical Co, Japan) and 2 mL of 30 % H2O2 (Wako Chemical Co, Japan). The weighed samples (0.3 g of powdered fish) were then placed into the digestion reagent in a Teflon vessel. DAP-60 K type pressure vessels (Berghof, Germany), which are made entirely of tetrafluoromethoxylene, were used in this study. Three step-digestion procedures were followed: (1) temperature and power were maintained at 180°C and 85 %, respectively, for 15 min; (2) temperature was kept steady at 190°C for 15 min together with 90 % power; and (3) decreased temperature (100°C) and power (40 %) were used for 10 min to cool down the Teflon vessels (maximum microwave power is 1000 W when power is 100 %). After that, all vessels were kept in cold water to decrease the residual pressure inside the Teflon vessel. After digestion, the solution was then filtered using DISMIC-25HP polytetrafluoroethylene syringe filter (pore size 0.45 µm) (Toyo Roshi Kaisha, Ltd., Japan) and stored in 50-mL polypropylene centrifuge tubes (Nalgene, New York). Afterward, the vessels were cleaned with Milli-Q water and air-dried. Finally, blank digestion with 5 mL 69 % HNO3 after the described digestion procedures was performed to clean up the digestion vessels (Berghof’s Product User Manual 2008).
For chemical partitioning of metals, sediment samples were analyzed using the Tessier sequential chemical extraction procedure (Tessier et al. 1979). The sequential extraction procedure was divided into five operationally defined chemical fractions: (F1) the exchangeable fraction (readily soluble and exchangeable); (F2) the carbonate bound and specifically adsorbed fraction (carbonate-bound, specifically adsorbed and weak organic and inorganic complexes); (F3) the iron (Fe)–manganese (Mn) oxides fraction (bound to Fe and Mn oxides (Fe–Mn oxides); (F4) the organic/sulphide fraction (bound to stable organic and/or sulphide [organic] complexes); and (F5) the residual fraction (held in primary and secondary minerals within their crystal structure). The detailed geochemical fractionation procedure of sediment is presented in Fig. 2.
Instrumental Analysis and Quality Assurance
For trace metals, samples were analyzed using inductively coupled plasma mass spectrometer (Agilent 7700 series). Multielement standard XSTC-13 (Spex CertiPrep, USA) solutions were used to prepare the calibration curve. The calibration curves with R 2 > 0.999 were accepted for concentration calculation. Before starting the sequence, relative SD ([RSD] <5 %) was checked by using tuning solution purchased from Agilent. Internal calibration standard solutions containing 1.0 mg/L of indium, yttrium, beryllium, tellurium, cobalt, and thallium were purchased from Spex CertiPrep, USA. Multielement solution (Agilent), 1.0 µg/L, was used as a tuning solution covering a wide range of masses of elements. All test batches were evaluated using an internal quality approach and validated if they satisfied the defined internal quality controls. For each experiment, a run included blank, certified reference materials (CRMs), and samples, all of which were analyzed in duplicate to eliminate any batch-specific errors. The CRMs (NMIJ CRM 7303–lake sediment and DORM-2–dogfish muscle from the National Research Council, Canada) were analyzed to confirm analytical performance and good precision (RSD <20 %) of the applied method (Table S2).
Metal Bioaccumulation in Fish Species
Average metal concentrations in fish species and sediments from the studied rivers were used for calculating the (BSAF. The BSAF is an index of the ability of a fish species to accumulate a particular metal with respect to its concentration in sediment. It was calculated by the following equation (Abdallah and Abdallah 2008):
where C fish is the metal concentration in fish (mg/kg dw), and C sediment is the metal concentration in sediment (mg/kg dry weight [dw]).
Statistical Analysis
Data were statistically analyzed using the statistical package SPSS 16.0 (SPSS, USA). Means, SDs, and percentiles of metal concentrations in sediments and fish species were calculated. Multivariate post hoc Tukey tests were employed to examine the statistical significance of the differences among mean concentrations and BSAF of trace metals among the fish species.
Results and Discussion
Physicochemical Properties and Speciation of Metals in Sediment
The sites generally have pH ranging from 6.3 to 7.4 during winter and from 6.5 to 8.7 during summer, which is slightly acidic, except at the T1 site, which was alkaline (Table 1) due to the decomposition of organic matter and subsequent formation of carbonic acid (Ahmad et al. 1996). The composition of organic carbon in sediment samples was varied among the sites due to its origin in the aquatic environment. Organic carbon in sediments ranged from 0.18 % to 3.3 % (Table 1). The highest percentage of organic carbon might be attributed to the high amount of drainage water at site B3. According to the United States soil texture classification, textural analysis showed that the sediment belonged to the following classes: sandy loam, silt loam, silty clay loam, silty clay, and clay loam (Table 2). Fine-sediment textures are important for geochemical carriers, which can control metal bioavailability for the aquatic organisms exposed through dissolved metals.
The mobility and toxicity of metals are mainly dependent on metal speciation in the aquatic environment. The relative distribution of trace metals in different geochemical fractions are listed in Table 3 and Fig. 3. The metals associated with different fractions in sediments followed the descending order of Cr = residual > organic > Fe–Mn oxides > carbonate > exchangeable; Ni = residual > Fe–Mn oxides > organic > carbonate > exchangeable; Cu = organic > residual > carbonate > Fe–Mn oxides > exchangeable; As = residual > Fe–Mn oxides > organic > carbonate > exchangeable; Cd: residual > exchangeable > organic > carbonate > Fe–Mn oxides; and Pb = residual > organic > carbonate > Fe-Mn oxides > exchangeable. Considering the five defined chemical fractions, metals in sediments were greater in winter compared with summer (Table 3). Seasonal variation was due to (1 the lack of rainfall in winter, which could allow metal precipitation and resuspension and deposition (Kenniburgh et al. 1996; Islam et al. 2014) or (2) to changes in pH, redox conditions, and inorganic or organic complexation (Christl et al. 2001; Liang & Wong 2003; El Nemr et al. 2006; Bastami et al. 2012). Slightly greater level of metals in winter might be attributed to the variation in water capacity of the river when water input to the river is generally limited in winter, thus resulting in the precipitation of metals in sediment.
Chemical fractionation differentiates metals those derived from natural or anthropogenic sources. In general, results of sequential extraction indicated that the residual fraction dominated Cr (48–74 %), Ni (29–65 %), As (40–66 %), and Pb (21–44 %) contents during winter and Cr (34–63 %), Ni (38–49 %), As (43–62 %), and Pb (25–42 %) content during summer. This result suggested that Cr, Ni, As, and Pb had the strongest associations to the crystalline sedimentary components and were likely to be reflected by the geological characteristics (Hamilton et al. 1984). Cr, Ni, As, and Pb were the least mobilisable because considerable proportions of these metals were in the nonmobile fraction. This further indicates their less availability to the aquatic fauna and that they have less chance of entering into the food chain (Chandra Sekhar et al. 2003).
According to the partitioning pattern, a considerable proportion of Cd (26 % during winter and 25 % during summer) was associated with the exchangeable fraction (Fig. 3) and can be more mobile in sediments (Zhang et al. 2012). The highest proportion of Cd was bounded for the sum of exchangeable and carbonate fractions (approximately 40 %), thus placing the availability of Cd into the high-risk category, meaning that it could easily enter the food chain (Sundaray et al. 2011). Metals such as Cd and Pb represent an appreciable portion in the carbonate phase because these metals have a special affinity toward carbonate and may coprecipitate with its minerals. Substantial proportions of Cd (16 % in winter and 15 % in summer) and Pb (24 % during winter and 16 % during summer) were found in the carbonate-bound fraction (Fig. 3). This result suggests that Cd and Pb can be easily released back into the water column, which may cause secondary pollution and at the same time potential effects on the fish themselves or the organisms that consume them, including upper-trophic level organisms and humans (Burger & Gochfeld 2005; Dhanakumar et al. 2013). Numerous earlier reports have also considered the exchangeable fraction to be the most mobile and bioavailable phase present in sediments followed by the carbonate-bound fraction (Tessier et al. 1979; Ahumada et al. 1999; Howari & Banat 2001). Slight variations were observed in labile (exchangeable and carbonate-bound) and lithogenic fractions, which could be attributed to numerous factors, such as weathering, mineral transport, anthropogenic inputs, and physicochemical components of sediment (Sundaray et al. 2011). The lower association of Cr (1.2 to 19 % during winter and 1.9 to 13 % during summer) in the carbonate fraction of sediments might arise due to the inability of Cr3+ to form a precipitate or complex with carbonates (Sundaray et al. 2011).
Fe–Mn oxides and organic matter plays excellent scavenging role for the removal of trace metals from the water column in aquatic environments (Dhanakumar et al. 2013). Metals associated with Fe–Mn oxides fraction can be mobilized by changes in the redox potential of the sediment and become bioavailable in special fish that feed on sediments and/or sediment-associated benthic biota. Among the nonlithogenic fractions, Fe–Mn oxyhydroxide is the scavenger for Ni, As, and Pb (Fig. 3). This attributes to the adsorption, flocculation, and coprecipitation of trace metals with the colloids of Fe and Mn oxyhydroxide (Rath et al. 2009). Furthermore, organic-bound Cr, Cu, and Pb seem to be the second dominant fraction among the nonlithogeneous fractions. Cu was identified as the highest proportion (45 to 61 % during winter and 24 to 58 % during summer) in the organic fraction. This phenomenon can be explained by the affinity of metals to organic matter, especially humic substances, which are the component of natural organic matter and chemically active in complexing metals (Fytianos & Lourantou 2004; Jain et al. 2008). In addition, organic substances are known to exhibit a high degree of preferential selection for divalent ions (Stone & Marsalek 1996). Cu can be retained by sediment through exchange and specific adsorption, but precipitation may also be an important mechanism of retention in polluted sediments. Cu is generally adsorbed to a greater extent than other metals; the high affinity of Cu2+ ions for soluble organic ligands may greatly increase their mobility in sediments (McLean & Bledsoe 1992).
It is recognized that sequential extraction technique enables the prediction of possible metal impact on biota in aquatic ecosystems. The exchangeable and carbonate fractions are considered to be weakly bounded. These may equilibrate with the aqueous phase, thus becoming more rapidly bioavailable and causing environmental toxicity (Morrison et al. 1996; Kim et al. 1998; Karbassi & Shankar 2005). The metal in sediment of Fe–Mn oxides, as well as the organic matter bound, can be mobilized when environmental conditions become increasingly reduced or oxidized (Karbassi & Shankar 2005). Metal present in the inert fraction, being of detrital and lattice origin or primary mineral phases, can be regarded as a measure of contribution by natural sources (Salmonas & Forstner 1980) such as metal content in rocks and parent materials (Yang et al. 2009; Yi et al. 2011), anthropogenic industrial activities such as tanneries and textile factories (Mohiuddin et al. 2011), vehicle and coal combustion emissions (Li et al. 2012), treatment of agricultural land with arsenical pesticides (Fu et al. 2014), and electroplating, production of Ni–Cd batteries, waste incineration, etc. (El Nemr et al. 2006; Islam et al. 2014). During our sampling, we observed unplanned tanning activities from 270 tanneries, leachates from defused Ni–Cd batteries, Cd-plated items, and lead smelting and lead products manufacturing at the sampling sites, which can be coherent with the presence of metals in sediment.
Metal Concentrations in Fish Species
Concentrations of Cr, Ni, Cu, As, Cd, and Pb in the muscles of three fish species are listed in Table 4. The ranking order of mean concentrations of trace metals in C. punctatus Cu (3.0) > Cr (2.0) > Ni (1.0) > Pb (0.63) > As (0.19) > Cd (0.026), in H. fossilis were Cu (4.7) > Cr (2.1) > Ni (1.5) > Pb (1.1) > As (0.27) > Cd (0.033), and in T. fasciata were Cu (3.5) > Cr (2.9) > Ni (2.2) > Pb (1.3) > As (0.30) > Cd (0.045) (mg/kg ww). However, as a whole, the concentration of studied metals among the fish species were in the descending order of T. fasciata > H. fossilis > C. punctatus. Concentrations of metals varied considerably among the fish species by season, which is influenced by the age (size), growth cycle, and feeding habits of the species (Yilmaz 2005; Al Sayegh et al. 2012). In addition, the differences noted in metal concentrations in tissues of fish species between seasons could have been the result of local pollution (Dural et al. 2007). In the present study, relatively low levels of As, Cd, and Pb were found in fish, which can be due to lower levels of Cd in the environment as well as a greater proportion in the nonmobile forms of As and Pb (Campbell et al. 2005; Mazej et al. 2010).
In the present study, the greatest mean concentration of Cr was observed in T. fasciata of Buriganga River (4.5 and 2.5 mg/kg ww in winter and summer, respectively) (Table 4), which can be attributed to the wastewater coming from various industries such as dyeing and tanneries (Mohiuddin et al. 2011). Considering both seasons, the greatest mean concentrations of Ni were found in T. fasciata and H. fossilis (3.0 mg/kg ww) of Buriganga River (Table 4), which is due to the fact that Ni and its salts are used in several industrial applications, such as electroplating, storage batteries, automobiles, aircraft parts, sparks, electrodes, cooking utensils, pigments, lacquer cosmetics, water, and fabric printing (Rahman et al. 2012). Although the present study depicted that the examined fish species were not highly contaminated by Cr and/or Ni, long-term discharge of untreated industrial wastes could pollute riverine organisms. Although considerable proportions of these metals were found in the residual form, these are cumulative body poisons so their concentrations should remain as low as possible. Cu was detected in all examined fish samples, and its concentration ranged from 1.1 to 7.2 mg/kg ww with the highest mean content found in H. fossilis (6.0 mg/kg ww) during winter and the lowest mean content in C. punctatus (1.2 mg/kg ww) during summer (Table 4). Mean concentrations of As in fish species were observed 0.22–0.43 and 0.091–0.53 mg/kg ww during winter and summer, respectively. Differences in As concentration in the fish species were examined and showed that T. fasciata had higher concentrations than other two species (P < 0.05) (Table 4).
The fish species from three different rivers varied significantly for metal concentrations, wherein Buriganga River showed greater concentrations than did the other two rivers. This study has provided the evidence that effluents discharged from tanneries, dyeing, metal processing, batteries and auxiliary industries, and urban sewage system were the main sources of Cr in the river systems of the Hazaribagh area of southwestern Dhaka City, Bangladesh (Ahmad et al. 2010; Mohiuddin et al. 2011; Rahman et al. 2012; Islam et al. 2014). Concentrations of trace metals in fish samples were greater than the permissible levels in fish per Food and Agriculture Organization/World Heath Organization (FAO/WHO) guidelines (Table 4), thus indicating that consumption of these fish species might pose toxic effects on human health.
Correlation analysis of metal concentrations between fish species and sediment indicated the significant relationship between C. punctatus and sediment and H. fossilis and sediment for Cr, Ni, Cu, and Pb. Meanwhile, no relationship was observed (except for Cu) between T. fasciata and sediment (Table 5). Multivariate principal component analysis (PCA) was performed to establish the relationship between the sources and levels of metals in sediments and fish. PCA analysis incorporated the six metal concentration data of all three rivers and explored the possible similar distribution pattern of metals. In our study, two PCs extracted approximately 89 % (PC1 22 %; PC2 67 %) and 98 % (PC1 43 %; PC2 55 %) of the metal variance for fish and sediment, respectively (Fig. 4). It was estimated that all metals were strongly associated with PC2 (positive loading), mainly existing in the nonbioavailable fractions (>70 %), thus reflecting their lithogenic origin. Hence, PC2 was supposed to reflect the contribution of natural geological sources of metals into the river. Subsequently, the PCA results showed that PC1 was associated with Cu, Cd, and Pb, which might represent anthropogenic sources of metal pollution.
Metal Bioaccumulation in Fish Species
Metals contained in sediment can be bioaccumulated in fish tissues (van der Oost et al. 2003; Yi et al. 2011). Bioaccumulation of a specific metal is not only dependent on metal exposure and its environment but also different physiological and biochemical activities through which a specific organism deals with metals (Luoma & Rainbow 2008). Hence, different organisms accumulate metals from the environment depending on their filtration rate, ingestion rate, gut fluid quality, and detoxification strategies (e.g., storage in nontoxic form or elimination) (Wang & Rainbow 2008). The accumulation of metals in muscle tissues of fish could have a direct impact on health throughout the food chain. Table 6 and Fig. 5 clearly show that large variations in BSAF were observed among different fish species and metals. The ranking order of mean BSAF values of metals for C. punctatus was Cu > As > Pb > Cr > Ni > Cd; for H. fossilis was Cu > Pb > As > Cr > Ni > Cd; and for T. fasciata was Cu > Pb > As > Cr > Ni > Cd (Table 6). Among the selected six metals, Cu showed the greatest BSAF value, thus suggesting a greater rate of accumulation in fish species. At some sites, metal levels might be high but accumulation is lower than expected due to metal complexation (Hare & Tessier 1996).
BSAF of metals in fish species is regarded to decrease from readily available to unavailable form due to the strength of reagents in the extraction sequence (Tessier & Campbell 1987). Hence, the exchangeable fraction indicated the mostly bioaccumulated form in fish species. BSAF values of metals were found in the following descending order: exchangeable > carbonate > Fe–Mn oxides > organic > residual (Table 6). Metals bound in the residual phase are unlikely to be reactive during sedimentation and thus have little potential bioavailability (Jones & Turki 1997). BSAF values for Ni, As, and Pb in T. fasciata were significantly greater than those obtained for C. punctatus and H. fossilis (Fig. 5). This can be explained by their ingestion of sediment as well as omnivore-feeding behavior of T. fasciata, which may lead to the much greater BSAF value found in this study. Therefore, of the three fish species investigated in this study, T. fasciata can be used as a potential bioindicator for the contamination of trace metals in those riverine environments. The present study showed that slightly greater accumulations of metals were observed in two fish species (T. fasciata and H. fossilis). From the literature survey, it was noticed that the fish species of T. fasciata and H. fossilis are bottom dwelling, and therefore sediments could be the major sources of trace-metal accumulation in these fish species (Tao et al. 2012). Bottom dwelling fishes are found to exhibit greater concentration of trace metals than pelagic fishes (Gupta et al. 2009). Bioaccumulations of individual metals among the sampling sites were not similar in pattern due to environment-specific phenomenon. It was considered that ingested sediments found in the digestive tract of fish acted as acid ambients, which accelerated the bioaccumulation of greater metal concentrations than were expected.
Conclusion
Sequential extraction technique was applied for portioning of six trace metals in sediments of three urban rivers showing its association with various geochemical forms. The abundance of metals in chemical fractions was the following decreasing order: residual > organic > Fe–Mn oxides > carbonate > exchangeable. The concentration of trace metals in T. fasciata was slightly greater than that it C. punctatus and H. fossilis, which might be due to their mode of feeding behavior. Similarly, the calculated values of BSAF in T. fasciata were greater than in C. punctatus and H. fossilis, suggested that T. fasciata could be used as potential bioindicator for metal pollution. This study has shown that metals (Cr, Ni, Cu, Cd, and Pb) in the Turag, Buriganga, and Shitalakha rivers have accumulated in fish species (C. punctatus, H. fossilis, and T. fasciata) and exceed the permissible limits established by FAO/WHO (FAO/WHO), thus suggesting that these fish are not safe for consumption by local residents.
References
Abdallah MAM (2007) Speciation of trace metals in coastal sediments of El-Mex bay south mediterranean sea–west of alexandria (Egypt). Environ Monit Assess 132:111–123
Abdallah MAM, Abdallah AMA (2008) Biomonitoring study of heavy metals in biota and sediments in the South Eastern coast of Mediterranean sea. Egypt. Environ Monit Assess 146:139–145
Abdel-Ghani NT, Elchaghaby GA (2007) Influence of operating conditions on the removal of Cu, Zn, Cd and Pb ions from wastewater by adsorption. Int J Environ Sci Technol 4(4):451–456
Ahmad S, Siddiqui EN, Khalid S (1996) Studies on certain physico chemical properties of soil of two freshwater ponds of Darbhanga. Environ Pollut 31:31–39
Ahmad MK, Islam S, Rahman S, Haque MR, Islam MM (2010) Heavy metals in water, sediment and some fishes of Buriganga River, Bangladesh. Int J Environ Res 4(2):321–332
Ahmed F, Bibi MH, Asaeda T, Mitchell CPJ, Ishiga H, Fukushima T (2012) Elemental composition of sediments in Lake Jinzai, Japan: assessment of sources and pollution. Environ Monit Assess 184:4383–4396
Ahumada I, Mendoza J, Navarrete E, Ascar L (1999) Sequential extraction of heavy metals in soils irrigated with wastewater. Commun Soil Sci Plant Anal 30:1507–1519
Akcay H, Oguz A, Karapire C (2003) Study of heavy metal pollution and speciation in Buyak Menderes and Gediz river sediments. Water Res 37:813–822
Al Sayegh PS, Grudnik ZM, Pokorny B (2012) Heavy metals and arsenic concentrations in ten fish species from the Šalek lakes (Slovenia): assessment of potential human health risk due to fish consumption. Environ Monit Assess 184:2647–2662
Alam AMS, Islam MA, Rahman MA, Siddique MN, Matin MA (2003) Comparative study of the toxic metals and nonmetal status in the major river system of Bangladesh. Dhaka Univ J Sci 51(2):201–208
Ali MY, Amin MN, Alam K (2008) Ecological health risk of Buriganga River, Dhaka, Bangladesh. Hydro Nepal 3:25–28
Asuquo FE, Ewa-Oboho I, Asuquo EF, Udo PJ (2004) Fish species used as biomarker for heavy metal and hydrocarbon contamination for Cross River, Nigeria. Environmentalist 24:29–37
Bastami KD, Bagheri H, Haghparast S, Soltani F, Hamzehpoor A, Bastami MD (2012) Geochemical and geo-statistical assessment of selected heavy metals in the surface sediments of the Gorgan Bay Iran. Mar Pollut Bull 64:2877–2884
Burger J, Gochfeld M (2005) Heavy metals in commercial fish in New Jersey. Environ Res 99:403–412
Campbell LM, Norstroom RJ, Hobson KA, Muir DCG, Backus S, Fisk AT (2005) Mercury and other trace elements in a pelagic Arctic marine food web (Northwater Polynya, Baffin Bay). Sci Total Environ 351–352:247–263
Chandra Sekhar K, Chary NS, Kamala CT, Suman Raj DS, Sreenivasa Rao A (2003) Fractionation studies and bioaccumulation of sediment-bound heavy metals in Kolleru lake by edible fish. Environ Int 29:1001–1008
Christl I, Milne CJ, Kinniburgh DG, Kretzschmar R (2001) Relating ion binding by fulvic and humic acids to chemical composition and molecular size. 2. Metal binding. Environ Sci Technol 35:2512–2517
Dassenakis M, Scoullos M, Foufa E, Krasakopoulou E, Pavlidou A, Kloukiniotou M (1998) Effects of multiple source pollution on a small Mediterranean river. Appl Geochem 13(2):197–211
Dhanakumar S, Rutharvel Murthy K, Solaraj G, Mohanraj R (2013) Heavy metal fractionation in surface sediments of the Cauvery River estuarine region, southeastern coast of India. Arch Environ Contam Toxicol 65:14–23
Dural M, Goksu MZL, Ozak AA (2007) Investigation of heavy metal levels in economically important fish species captured from the Tuzla lagoon. Food Chem 102:415–421
El Nemr A, Khaled A, ElSikaily A (2006) Distribution and statistical analysis of leachable and total heavy metals in the sediments of the Suez Gulf. Environ Monit Assess 118:89–112
FAO/WHO (2004) Safety evaluation of certain food additives and contaminants. WHO Food Additives Series No. 52. World Health Organization, Geneva, Switzerland
Fu J, Zhao C, Luo Y, Liu C, Kyzas GZ, Luo Y et al (2014) Heavy metals in surface sediments of the Jialu River, China: Their relations to environmental factors. J Hazard Mater 270:102–109
Fytianos K, Lourantou A (2004) Speciation of elements in sediment samples collected at lakes Volvi and Koronia, N. Greece. Environ Int 30:11–17
Gupta A, Rai DK, Pandey RS, Sharma B (2009) Analysis of some heavy metals in the riverine water, sediments and fish from river Ganges at Allahabad. Environ Monit Assess 157:449–458
Hamilton RS, Revitt DM, Warren RS (1984) Levels and physicochemical associations of Cd, Cu Pb and Zn in road sediments. Sci Total Environ 33:59–74
Hare L, Tessier A (1996) Predicting animal cadmium concentrations in lakes. Nature 380:430–432
Howari FM, Banat KM (2001) Assessment of Fe, Zn, Cd, Hg, and Pb in the Jordan and Yarmouk River sediments in relation to their physicochemical properties and sequential extraction characterization. Water Air Soil Pollut 1321:43–59
Ikem A, Adisa S (2011) Runoff effect on eutrophic lake water quality and heavy metal distribution in recent littoral sediment. Chemosphere 82:259–267
Islam MM, Akhtar MK, Masud MS (2006) Prediction of environmental flow to improve the water quality in the river Buriganga. In: Proceedings of the 17th IASTED International Conference on Modelling and Simulation, Montreal, QC, Canada
Islam MS, Han S, Masunaga S (2014) Assessment of trace metal contamination in water and sediment of some rivers in Bangladesh. J Water Environ Technol 12:109–121
Jain CK, Gupta H, Chakrapani G (2008) Enrichment and fractionation of heavy metals in bed sediments of River Narmada, India. Environ Monit Assess 141:35–47
Jones B, Turki A (1997) Distribution and speciation of heavy metals in surficial sediments from the Tees Estuary, north–east England. Mar Pollut Bull 34:768–779
Karbassi AR, Shankar R (2005) Geochemistry of two sediment cores from the west coast of India. Int J Environ Sci Technol 1:307–316
Kenniburgh DG, Milne CJ, Benedetti MF, Pinheiro JP, Filius J, Koopal LK et al (1996) Metal ion binding by humic acid: Application of the NICA-`Donnan model. Environ Sci Technol 30:1687–1698
Kim KW, Myung JH, Ahn JS, Chon HT (1998) Heavy metal contamination in dusts and stream sediments in the Taejon area, Korea. J Geochem Explor 64:409–419
Li HB, Yu S, Li GL, Liu Y, Yu GB, Deng H et al (2012) Urbanization increased metal levels in lake surface sediment and catchment topsoil of waterscape parks. Sci Total Environ 432:202–209
Liang Y, Wong MH (2003) Spatial and temporal organic and heavy metal pollution at Mai Po Marshes Nature Reserve, Hong Kong. Chemosphere 52:1647–1658
Luoma SN, Rainbow PS (2008) Metal contamination in aquatic environments: Science and lateral management. Cambridge University Press, Cambridge, pp 93–123
Mazej Z, Sayegh-Petkovsek SA, Pokorny BT (2010) Heavy metal concentrations in food chain of Lake Velenjsko Jezero, Slovenia: An artificial lake from mining. Arch Environ Contam Toxicol 58:998–1007
McLean JE, Bledsoe BE (1992) Behavior of metals in soils. EPA Groundwater Issue, EPA/540/s-92/018
Mohiuddin KM, Ogawa Y, Zakir HM, Otomo K, Shikazono N (2011) Heavy metals contamination in water and sediments of an urban river in a developing country. Int J Environ Sci Technol 8(4):723–736
Moniruzzaman M, Elahi SF, Jahangir MAA (2009) Study on temporal variation of physicochemical parameters of Buriganga River Water through GIS (Geographical Information System) technology. Bangladesh J Sci Ind Res 44(3):327–334
Morillo J, Usero J, Gracia I (2004) Heavy metal distribution in marine sediments from the southwest coast of Spain. Chemosphere 55(3):431–442
Morrison HA, Gobas FAP, Lazar R, Haffner GA (1996) Development and verification of bioaccumulation model for organic contaminants in benthic invertebrates. Environ Sci Technol 30:3377–3384
Niwa Y, Sugai T, Saegusa Y, Ogami T, Sasao E (2011) Use of electrical conductivity to analyze depositional environments: Example of a Holocene delta sequence on the Nobi Plain, central Japan. Quaternary Int 230:78–86
Nouri J, Lorestani B, Yousefi N, Khorasani N, Hasani AH, Seif S et al (2011) Phytoremediation potential of native plants grown in the vicinity of Ahangaran lead–zinc mine (Hamedan, Iran). Environ Earth Sci 62(3):639–644
Oyeyiola AO, Olayinka KO, Alo BI (2011) Comparison of three sequential extraction protocols for the fractionation of potentially toxic metals in coastal sediments. Environ Monit Assess 172:319–327
Pempkowiase J, Sikora A, Biernacka E (1999) Speciation of heavy metals in marine sediments vs. their bioaccumulation by mussels. Chemosphere 39(2):313–321
Pustisek N, Milacic R, Veber M (2001) Use of the BCR three-step sequential extraction procedure for the study of the partitioning of Cd, Pb, and Zn in various soil samples. J Soil Sediments 1:25–29
Rahman MS, Molla AH, Saha N, Rahman A (2012) Study on heavy metals levels and its risk assessment in some edible fishes from Bangshi River, Savar, Dhaka, Bangladesh. Food Chem 134:1847–1854
Rahman MS, Saha N, Molla AH (2014) Potential ecological risk assessment of heavy metal contamination in sediment and water body around Dhaka export processing zone, Bangladesh. Environ Earth Sci 71:2293–2308
Rath P, Panda UC, Bhatta D, Sahu KC (2009) Use of sequential leaching, mineralogy, morphology and multivariate statistical technique for quantifying metal pollution in highly polluted aquatic sediments—A case study: Brahamani and Nandira rivers, India. J Hazard Mater 163:632–644
Rauret G, Lopez-Sanchez JF, Sahuquillo A, Rubio R, Davidson C, Ure A, Quevauviller Ph (1999) Improvement of the BCR three step sequential extraction procedure before the certification of new sediment and soil reference materials. J Environ Monit 1:57–61
Saikia AK, Abujam SKS, Biswas SP (2012) Food and feeding habit of Channa punctatus (Bloch) from the paddy field of Sivsagar District, Assam. Bull Environ Pharmacol Life Sci 1:10–15
Salmonas W, Forstner U (1980) Trace metal analysis on polluted sediments, part-II. Evaluation of environmental impact. Environ Technol Lett 1:506–517
Sharif AKM, Alamgir M, Mustafa AI, Hossain MA, Amin MN (1993) Trace element concentrations in ten species of freshwater fish of Bangladesh. Sci Total Environ 138:117–126
Sin SN, Chua H, Lo W, Ng LM (2001) Assessment of heavy metal cations in sediments of Shing Mun River, Hong Kong. Environ Int 26:297–301
Singh KP, Mohan D, Singh VK, Malik A (2005) Studies on distribution and fractionation of heavy metals in Gomati river sediments—A tributary of the Ganges, India. J Hydrol 312:14–27
Sow AY, Ismail A, Zulkifli SZ (2013) An assessment of heavy metal bioaccumulation in Asian swamp eel, Monopterus albus, during plowing stages of a paddy cycle. Bull Environ Contam Toxicol 91:6–12
Stone M, Marsalek J (1996) Trace metal composition and speciation in street sediment: Sault Ste. Marie, Canada. Water Air Soil Pollut 87:149–168
Sundaray SK, Nayak BB, Lin S, Bhatta D (2011) Geochemical speciation and risk assessment of heavy metals in the river estuarine sediments—A case study: mahanadi Basin, India. J Hazard Matter 186:1837–1846
Sutherland RA, Tack FMG (2003) Fractionation of Cu, Pb, and Zn in certified reference soils SRM 2710 and SRM 2711 using the optimized BCR sequential extraction procedure. Adv Environ Res 8:37–50
Svete P, Milacic R, Pihlar B (2001) Partitioning of Zn, Pb, and Cd in river sediments from a lead and zinc mining area using the BCR three-step sequential extraction procedure. J Environ Monit 3:586–590
Tang W, Zhao Y, Wang C, Shan B, Cui J (2013) Heavy metal contamination of overlying waters and bed sediments of Haihe Basin in China. Ecotoxicol Environ Saf 98:317–323
Tao Y, Yuan Z, Xiaona H, Wei M (2012) Distribution and bioaccumulation of heavy metals in aquatic organisms of different trophic levels and potential health risk assessment from Taihu Lake, China. Ecotoxicol Environ Saf 81:55–64
Terra BF, Araujo FG, Calza CF, Lopes RT, Teixeira TP (2008) Heavy metal in tissues of three fish species from different trophic levels in a tropical Brazilian river. Water Air Soil Pollut 187:275–284
Tessier A, Campbell FGC (1987) Partitioning of trace metals in sediments: relationships with bioavailability. Hydrobiology 149:43–52
Tessier A, Campbell PGC, Bisson M (1979) Sequential extraction procedure for the speciation of particulate trace metals. Anal Chem 51:844–851
United States Environmental Protection Agency (2001) Methods for collection, storage and manipulation of sediments for chemical and toxicological analyses. Technical manual EPA-823-B-01-002. Office of Water, USEPA, Washington, DC
van der Oost R, Beyer J, Vermeulen PEN (2003) Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ Toxicol Pharmacol 13:57–149
Wang WX, Rainbow PS (2008) Comparative approaches to understand metal bioaccumulation in aquatic animals. Comp Biochem Physiol C 148:315–323
Waqar A (2006) Levels of selected heavy metals in tuna fish. Arab J Sci Eng 31:89–92
Xia XH, Chen X, Liu RM, Liu H (2011) Heavy metals in urban soils with various types of land use in Beijing, China. J Hazard Mater 186:2043–2050
Yang ZF, Wang Y, Shen ZY, Niu JF, Tang ZW (2009) Distribution and speciation of heavy metals in sediments from the mainstream, tributaries, and lakes of the Yangtze River catchment of Wuhan, China. J Hazard Mater 166:1186–1194
Yap CK, Ismail A, Tan SG, Omar H (2002) Correlation between speciation of Cd, Cu, Pb and Zn in sediments and their concentrations in total soft tissue of green-lipped mussel Perna viridis from the west coast of Peninsular Malaysia. Environ Int 28:17–26
Yi Y, Yang Z, Zhang S (2011) Ecological risk assessment of heavy metals in sediment and human health risk assessment of heavy metals in fishes in the middle and lower reaches of the Yangtze River basin. Environ Pollut 159:2575–2585
Yilmaz A (2005) Comparison of heavy metal levels of grey mullet (Mugil cephalus L.) and sea bream (Sparus aurata L.) caught in Uskenderun Bay (Turkey). Turk J Vet Anim Sci 29:257–262
Zhang WF, Liu XP, Cheng HF, Zeng EY, Hu YN (2012) Heavy metal pollution in sediments of a typical mariculture zone in South China. Mar Pollut Bull 64:712–720
Acknowledgments
The authors are grateful for financial support from the Leadership Program in Sustainable Living with Environmental Risk (SLER) at Yokohama National University under the aid of Strategic Funds for the Promotion of Science and Technology from the Ministry of Education, Culture, Sports, Science and Technology and also for Research Collaboration Promotion Fund provided by the Graduate School of Environment and Information Sciences, Yokohama National University, Japan. Furthermore, we are thankful for the kind help from the members of Dhaka University, Bangladesh, during field sampling.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Islam, M.S., Ahmed, M.K., Raknuzzaman, M. et al. Metal Speciation in Sediment and Their Bioaccumulation in Fish Species of Three Urban Rivers in Bangladesh. Arch Environ Contam Toxicol 68, 92–106 (2015). https://doi.org/10.1007/s00244-014-0079-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-014-0079-6