Abstract
A neoichnological study was done at a site west of Abha on the southern Red Sea coast of Saudi Arabia. This area covers Wadi Hali and its surroundings (180 49′35.27″N: 410 22′44.23″E). The objective of the study is to document biogenic traces that occur in the upper intertidal ripple-marked sand flats and supratidal flats that include microbial mats, mud flats, and scattered patches of mangrove stands and their muddy environments. Based on morphological descriptions, four different kinds of burrows are identified and their fossil equivalents are Psilonichnus upsilon (burrow type 1), Cylindricum (burrow types 2 and 3), and Arenicolites (burrow type 4). Tracks of land hermit crabs Coenobita clypeatus are described as ichnospecies Coenobichnus currani. Gastropod grazing trails and their fossil equivalent Planolites is identified as well. Supratidal algal mats and their microscopic contents are presented. Fossil plant roots (rhizomes) are reported from a localized fluvial deposit from a dry channel (wadi). The morphology of the traces, trace maker, and ethology and the environmental setting indicate that the ichnofauna and botanical traces belong to the Psilonichnus ichnofacies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biogenic sedimentary structures, both recent and fossil, are evidence of activities by organisms inhabiting various environments. Such structures cover the entire spectrum of substrate traces or structures that reflect a behavioral function of organisms (Pemberton et al. 2001). Bromley (1990) divided his book on trace fossils into neoichnology and paleoichnology; study of traces made by animals in modern sediments is classified under neoichnology and their fossilized equivalents in paleoichnology. He (Bromley 1990) includes the following structures as trace fossils: (a) footprints, tracks, and burrows in uncemented sediments; (b) raspings, borings, and etchings in the rigid substrates; (c) fecal pellets, pseudofeces, and coprolites; (d) plant root penetration structures; and (e) algal laminites and stromatolites. According to Shapiro (2008), stromatolites are trace fossils that recorded interaction between microbial communities and sediments for the past 3500 million years. Pemberton et al. (2001) described four categories of trace fossils; they are (1) bioturbation structures that reflect the disruption of stratification features or sediment fabrics by activity of organisms, e.g., tracks, trails, burrows; (2) biostratification structures that are stratification imparted by the activity of an organism, e.g., certain stromatolites; (3) biodepositional structures that reflect the production or concentration of sediments by the activity of an organism, e.g., fecal pellets; and (4) bioerosion structures are excavated mechanically or biochemically by an organism into a rigid substrate, e.g., borings, gnawings, scrapings, and bitings. However, for the purpose of binomial nomenclature, stromatolites are not classified as traces under the International Code of Zoological Nomenclature (Bertling et al. 2006; Rindsberg 2012). Dashtgard and Gingras (2012) further elaborated the concept of neoichnology and stated, “Neoichnology (studies of modern animal burrows, trackways and trails) details the linkage between physico-chemical stress and animal response, where animal response to various environmental factors can be assessed and the resultant trace assemblage determined.” They further elaborated, “By studying animal response, and specifically animal burrowing behavior under varying environmental conditions, neoichnologists can associate depositional parameters and organism response to the environment.”
There is a paucity of ichnological studies in the Arabian region, yet few significant papers were published in the recent past. El-Hedeny et al. (2012) reported shallow-marine trace fossils from the Callovian-Oxfordian Tuwaiq Mountain limestone and Hanifa Formations of central Saudi Arabia. Trace fossil assemblages in wet interdune deposits of the Wahiba Sand Sea record environmental changes associated with the Indian Ocean Monsoon system during the Early Holocene wet period (Rediesa et al. 2005). Significant neoichnological study in this region is by Smith et al. (2000); they examined bioturbation processes at six stations along a transect across the oxygen minimum zone (OMZ) on the Oman margin and found that bioturbation structures are good proxies for bottom-water oxygen concentrations under dysaerobic conditions. Neoichnological studies are valuable tools in paleoecological studies and offer significant insight into the depositional environments of sedimentary rocks.
The objective of the present study is to record recent biogenic traces, detect their trace makers, describe ethology (behavior of the trace maker), and the mode of their formation. This study was conducted in the area around Wadi Hali and its surroundings in the southern Red Sea coastal environments of Saudi Arabia, and numerous traces were documented from the upper intertidal ripple-marked sand flats and supratidal flats that include microbial mats, mud flats, and scattered patches of mangrove stands and their muddy environments. The present paper describes several types of burrows, tracks, trails, fecal pellets and mounds; microbial mats and traces of plant roots are reported and described.
Environment of the area
The Red Sea coast of the Arabian Peninsula is dry without much vegetation except for a few species of halophytes and mangroves (Avicennia marina) and varies from rugged marine terraces to coastal sabkhas, alluvial plains and wadis (a local term for dry river beds), and alluvial fans as well as estuaries (Khan et al. 2010). The mountains to the east and the coastal plains have a large number of fluvial channels that transport water and sediment during rains either into the lagoons or in the Red Sea. Climatically, this long coast line covers both tropical and subtropical zones. Monsoons originating in the Indian Ocean cause rainfall in southwestern Saudi Arabia. Surface water temperatures in the Red Sea increase southward in response to latitude but the salinity of surface water increases northwards indicating the intrusion of low-salinity water of Gulf of Aden into the Red Sea. The tidal amplitude along the Red Sea coast is very low which is ~ 50 cm in the northern and southern coasts that gradually decreases towards the center, close to zero near Jeddah (Kumar et al. 2010, 2011).
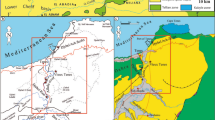
The study site is located west of the town of Abha on the southern Red Sea coast of Saudi Arabia between Jeddah in the north and Jizan in the south (Fig. 1). This area covers Wadi Hali (also spelled Haliy) and its surroundings (180 49′35.27″N: 410 22′44.23″E). Wadi Hali is a short ephemeral stream that originates in the hilly regions east of the coastal region (Fig. 2). There are several other such streams that originate in the hilly regions of the Arabian Shield and flow westward through valleys and narrow arid coastal plains and wadi deposits, at times forming braided channels and finally discharging into the Red Sea forming alluvial fans (Fig. 2). There are two alluvial fans in the area: the Wadi Hali fan in the south is larger; it covers an area of 27,167 ha and has a 38-km-long shore line, whereas the fan in the north is smaller, as it covers an area of 21,037 ha and has a 27-km-long coastline. The process of erosion and deposition of wadis and eolian sediments has been going on since Quaternary (Jado and Zölt 1984; Jado et al. 1990). Prinz (1984) describes the following surficial deposits along the Red Sea coastal plains: pediment material, a thin veneer of poorly sorted, and fine to coarse-grained sand and gravel that merges with the alluvial material. Boulders and coarse-grained sand line the channels of wadis, extensive flood plain deposits consisting of sand; silt and clay are present along the lower parts of wadis. Sabkha deposits occur extensively along the coast, and extended up to 3 km inland from the coast are brown to white salt impregnated silt. Usually, small eolian sand fields consisting of medium to fine-grained sand are characterized by low dunes and ridges. Each depositional sub-environment is characterized by its distinctive sedimentological and ichnological characters.
Geographical map of the Arabian Peninsula and the adjoining regions showing the location (red star) of the study site. (Map taken from https://www.google.ca)
Methods
A reconnaissance survey of the area around Wadi Hali surroundings and its coastal regions was conducted using Historical Landsat Enhanced Thematic Mapper (ETM) data of the year 2001 to identify various coastal sub environments along the Red Sea with special emphasis on the distribution of mangrove stands (Khan et al. 2010). A geological map of the area was used to recognize the relative ages of various Quaternary sedimentary deposits and depositional environments along the Red Sea coast (Jado et al. 1990; Prinz 1984). Extensive field observations in the area were conducted to identify the presence of biogenic structures in various Quaternary deposits and the present-day environments. Every type of biogenic sedimentary structures was recorded, measurements taken, photographed, nature of their substrates noted, and a qualitative assessment was done about their abundance. These biogenic structures were observed only on the sediment surface thus three-dimensional structures of burrows were not observed. Except for a large number of small gastropods and ghost crabs, no other animals were seen in these environments.
Results
Various types of burrows and trails were noted in different environments on different substrates. Their morphological description is provided, and animals responsible for constructing them are identified. Supratidal algal mats and their microscopic contents are presented. Fossil plant roots (rhizomes) are also described from a small fluvial deposit in a wadi. Ethology (Buatois and Mángano 2011) of each structure is discussed.
Burrows
On the basis of surface morphological features on the substrates, four types of burrows were identified.
A1. Burrow type 1 (Fig. 3c, f)
Description
Burrows with narrow circular openings of 2.0 to 2.5 cm diameter with one opening showing a slight depression and the other with sediment accumulations that look like mini volcanoes (Fig. 3f). Such burrows occur on muddy substrates, both under water and on dry but unconsolidated soft muds. When they are under shallow water as areas covered with mangrove stands, sediment accumulations at the mouth of burrows get disturbed by waves (Fig. 3c). Depth of the burrows is unknown.
a Environmental setting along the Red Sea coast of southwestern Saudi Arabia showing the intertidal region and the intertidal-subtidal boundary (location Fig. 2, ×1). b Mangrove stand on the mudflats showing burrows (location Fig. 2, ×2). c The mangrove mudflats showing burrows with and without sediment mounds Psilonichnus upsilon (location Fig. 2, ×2). d The intertidal region with localized areas covered by algal mats (location Fig. 2, ×3; pen length 12 cm). e Photomicrograph of the algal filaments of the algal mats (magnification ×400). f Burrow (Psilonichnus upsilon) opening with few volcano like (B1) sediment mounds and other simple burrows (B2) on the muddy surface (location Fig. 2, ×1; marker length 14 cm). g Gastropod (G) grazing trail (Gt) on the surface of ripple-marked sandy shore (location Fig. 2, ×1; spoon length 15 cm). h Grazing trail (Gt) made by a gastropod (G) and Nereites type trace (Nt) on the surface of ripple-marked sandy shore (location Fig. 2, ×1; spoon length 15 cm)
Trace maker
Crustaceans are predatory, scavenging, and suspension-feeding animals and commonly occur in these environments. Modern crab burrows are domiciles, unlined, with fecal matter at their openings, and their diameter and length commonly varies that produces simple U-, Y-, and J-shaped burrows described as ichnogenus Psilonichnus Fürsich, 1981(Frey et al. 1984; Dashtgard and Gingras 2012). The ghost crab Ocypode quadrata is a predatory and deposit-feeding crustacean; it is the dominant burrower in these coastal environments (Sakai and Turkay 2013). It is suggested that burrow type 1 are made by Ocypode quadrata.
Ethology
These are domicile burrows made by Ocypode quadrata ranging from straight vertical to J-, U-, and Y-shaped (Domichnia). These burrows have a high preservation potential and would form the ichnogenus Psilonichnus after fossilization (Garrison et al. 2007). The relationship between the burrows made by modern Ocypode quadrata and the ichnospecies Psilonichnus upsilon is well established (Curran 1994). Ocypode burrows in a modern unconsolidated sandy substrate on Irino Coast, Japan, and are J-, Y-, and multi-branched Y types; they have been well documented (Seike and Nara 2007). The sandy beach ichnocoenosis-4 described from the beaches of Bahama Islands and other islands of the Caribbean region, as well as Holocene calcarenites of Bahamas and Pleistocene calcarenites of Bermuda, are dominated by modern burrows of ocypodid crabs, e.g., ghost crabs and trace fossil Psilonichnus upsilon (Curran 2008). The ichnospecies Psilonichnus upsilon is typically a Y-shaped burrow but could also be U-shaped built by a juvenile ghost crab (Curran 2008; Fig. 14.6). Desjardins et al. (2012) have discussed ichnological imprints on supratidal mud flats and mangroves on the tropical and subtropical coastal regions, and similar environments occur in the present area of study where grazing gastropod trails and burrow type 1 are a common occurrence.
A2. Burrow type 2 (Fig. 4e, f)
Description
Burrows with narrow circular openings of around 1.0–1.5 cm diameter with a slight depression around the openings. These openings are surrounded with massive amounts of small fecal pellets of 3–5 mm diameter. These burrows occur on semi consolidated sandy ripple-marked substrates on the upper part of the intertidal zone.
a Grazing trail (Gt) on the surface of ripple-marked muddy shore made by gastropods (location Fig. 2, ×1; spoon length 15 cm). b Grazing trail (Gt) on the surface of ripple-marked sandy shore made by gastropods (location Fig. 2, ×1; spoon length 15 cm). c Coenobichnus currani (Cc) on the surface of ripple-marked sandy shores (location Fig. 2, ×1; spoon length 15 cm). d Traces of plant roots (Rhizomorphs R) in the section. The roots most likely belong to mangrove plants which occur commonly in the area (location Fig. 2, ×1). e Concentration of fecal pellets (Fp) around burrow openings (Br) of Psilonichnus upsilon (location Fig. 2, ×1; marker length 14 cm). f Concentration of fecal pellets (Fp) around burrow openings (Br) of Psilonichnus upsilon (location Fig. 2, ×1; marker length 14 cm). g Fecal mound (radius ~ 15 cm; height ~ 3–5 cm) (location Fig. 2, ×1). h Fecal mound (radius ~ 15 cm; height ~ 3–5 cm) (location Fig. 2, ×1)
Trace maker
Similar burrows and fecal pellets are known to be produced by earthworms, insects, beetles, and wasps.
Ethology
These are domicile burrows (Domichnia). Fecal pellets and pseudofeces commonly occur in the supratidal flats and upper zones of the tidal flats (Buatois and Mángano, 2011) and are produced by deposit and suspension feeders. These are composed of sediments that have a reasonable preservation potential as trace fossils (Bromley 1990). Fossilized forms could be such as ichnogenus Cylindricum Link 1949. These are dwelling burrows with rounded lower ends that have smooth walls. They are preserved in clusters perpendicular to a bedding plane and are formed in a variety of environments ranging from marginal marine to alluvial, floodplains, lacustrine, and eolian. The ichnogenus Cylindricum could be formed by invertebrates, insects, beetles, and sand wasps.
A3. Burrow type 3 (Fig. 5a–c)
Description
These burrows are similar to burrow type 2 but show narrow, linear, short smooth surfaces radiating from the burrow openings. Such burrows occur on the dry silty-sandy substrates without ripple marks on supratidal flats.
a Burrows and fecal pellets produced by ghost crab Ocyopoda quadrata (location Fig. 2, ×1; pen length 12 cm). b Burrows and fecal pellets produced by the ghost crab Ocyopoda quadrata (location Fig. 2, ×1; marker length 14 cm). c Burrows and fecal pellets produced by the ghost crab Ocyopoda quadrata (location Fig. 2, ×1; pen length 12 cm). d Image of a Lugworm (Arenicola sp.) from the Red Sea. (http://photos.tradeholding.com/attach/hash18/233234/fishing_lure_bait_lugworm_ragworm_sandworm03.jpg). e Burrow pattern of Arenicola marina (modified after Volkenborn, N. 2005 in http://evolutionbiology.com/articles/life-history-traits-of-the-lugworm-arenicola-marina). f Image of Ocypode quadrata, Fabricius, 1787 (Timquade at English Wikipedia in https://en.wikipedia.org/wiki/Ocypode#/media/File:FloridaGhostCrab.jpg). g The intertidal muddy areas with populations of gastropods (g) (location Fig. 2, ×1; marker length 14 cm). h The Psilonichnus ichnofacies model (modified after Buatois and Mángano, 2011)
Trace maker
Such burrows are most likely made by insects, and their fecal pellets are distributed abundantly around their openings.
Ethology
These are domicile burrows (Domichnia) made by insects on the supratidal silty-sandy substrates, and the radiating smooth surfaces from the burrow openings appear to indicate some kind of movement of the insects (probably their crawling abdomen) that smoothen the surface covered by fecal pellets. The fossilized forms of this burrow type could also be like ichnogenus Cylindricum Link 1949.
A4. Burrow type 4 (Fig. 4g, h)
Description
These burrows are identified primarily by fecal mounds formed by piles of coiled castings (excretions) of the animals that made them. Burrow openings were not observed. The dimensions of these fecal mounds range between 12 and 15 cm diameter and 3–5 cm height. These burrows occur on sandy ripple-marked surfaces under shallow water.
Trace maker
The Annelid species Arenicola marina Linnaeus 1758 (Fig. 5d).
Ethology
These are domicile burrows (Domichnia) of Arenicola marina, a large marine worm of the phylum Annelida; it is also known as lugworm or sandworm (Volkenborn 2005). The coiled castings of this animal occur on tidal flats and beaches at low tide. They produce U- and J-shaped burrows which they inhabit. Saucer-shaped depressions are formed at the head-end and a cast of coiled excretions at the tail-end (Fig. 5e). The distance between the two openings ranges between 5.1 and 7.6 cm. The lugworms are deposit feeders and ingest sand particles from their burrows and any microorganisms, and detritus stuck to the sand particles are digested as the sand passes through the worms’ gut (Tyler-Walters 2001).
Ichnogenus Arenicolites Salter, 1857 is such a burrow made by lugworms. These are vertical or oblique U- or J-shaped dwelling burrows with cylindrical and smooth walls. Burrows may also have funnel-shaped paired openings (Pemberton et al. 2001).
Tracks
Coenobichnus currani Walker et al. 2003 (Figs. 4c and 5h)
Description
These are asymmetrical tracks with the left rack larger than the right; both sides are crescent-shaped and interior drag traces that are diagnostic of modern land hermit crab walking traces (Walker et al. 2003).
Trace maker
Land hermit crabs Coenobita clypeatus (Coenobitidae) occur widely in tropical and subtropical coastal environments (Walker et al. 2003).
Ethology
This ichnospecies is a walking track (Repichnia) land hermit crab.
Trails
Gastropod Grazing Trail (Figs. 3g, h and 4a, b) and gastropod (Fig. 5g)
Description
These are simple crisscrossing meandering trails of uniform width and smooth margins and are unbranched. At times, they appear to be branched as well (Fig. 4a), but the branching may not be a true bifurcation of the trails; instead, the result of crisscrossing of trails may give the appearance of a branching trail.
Trace maker
Locomotion trails of small gastropods.
Ethology
These trails result from the grazing or foraging behavior of gastropods (Pascichnia) on the sandy as well as muddy surfaces in supratidal and upper intertidal environments.
The fossil equivalent of the grazing gastropod trail observed in this study is Ichnogenus Planolites Nicholson 1873. They are simple, unlined, straight or meandering, crisscrossing but unbranched burrows. They are usually horizontal but could also be oblique to bedding planes. They are mostly cylindrical in-filled burrows where lithology of the fill differs from that of the host rock.
Botanical structures
D1. Plant roots (rhizomorphs) (Fig. 4d)
Plant roots are fossilized in small patches of fluvial deposits found in the dry channels in the supratidal area. These fluvial deposits result from sediments deposited by ephemeral streams during the past wet periods or during seasonal rains.
Plant trace fossils are known from both marine and terrestrial facies and from aquatic and desert deposits. Sarjeant (1975) gave an initial account of the plant trace fossils; however, Bockelie (1994) discussed in detail various aspects of plant roots as trace fossils especially in the drilled cores. Fossilized mangrove roots were reported from Miocene Dam and Hofuf Formations of the eastern Arabian Peninsula that indicated a tidal-flat environment and a tropical coastal climate (Whybrow and McClure, 1980).
D2. Algal mats (Fig. 3d) and the algal filaments (Fig. 3e)
Since trace fossils record the interaction of animals and plants with their environment, stromatolites are considered as trace fossils because they record the interaction between microbial communities and sediments extending back to 3500 mybp (Shapiro 2008). Usually, microbial body fossils are not preserved in stromatolites, but they are trace fossils that record ecology on the macroscale and ethology on the microscale (Shapiro 2008). The taxonomy of trace fossils is governed by the conventions of the International Code of Zoological Nomenclature; it does not apply on stromatolites.
Since modern microbial mats are precursors of fossil stromatolites, they are being considered in this study as trace fossils. Microbial facies are represented by laminated sediments, with an upper organic-rich layer and lower mineral-rich layer; their sedimentary features are related to microbial activity. Microbial mats observed in this study are small isolated patches in the supratidal environments and appear as green areas in an otherwise dry, arid grayish brown environment. No animal activity was observed on these mats. Samples of the green microbial mats were macerated using standard palynological techniques and an abundance of simple non-septate algal filaments, and brown amorphous organic matter was observed (Fig. 3e). Unlike present microbial mats, Baucon (2008) studied microbial mats in the peritidal environments of Spiaggia al Bosco, Italy, and found a diverse and abundant ichnofauna related to this ecosystem. He discovered that insects are major trace makers and their burrows verified a number of strategies intimately centered to the microbial mat.
Discussion
The area under the present study covers mainly a mixture of marginal-marine and non-marine environments that include coastal dunes and upper intertidal and supratidal flats. Microbial mats and mud flats, scattered patches of mangrove stands and their muddy environments, sand flats, both ripple-marked and without ripples of the upper intertidal environments, form the major environments of this area. A localized fluvial deposit from a dry channel was also observed.
Usually, such environments are impacted by variations in energy levels and sediment types and thus are characterized by different suits of trace fossils. Amphibious Ocypodid crabs, both scavengers and surficial deposit feeders, dominate these environments and excavate J-, Y-, or U-shaped dwelling burrows (Chakrabarti 1981; Chan et al. 2006). These burrows when fossilized belong to the ichnogenus Psilonichnus which comprises of mainly vertical, cylindrical burrows of similar shapes with short horizontal or oblique branches. The morphology of Psilonichnus is variable and includes I-, J-, Y-, and U-shaped burrows that depend on the ontogenesis of Ocypodid crabs and environmental conditions of the substrates (Chakrabarti 1981; Chan et al. 2006; Knaust et al. 2012). Some of these U- and J-shaped burrows are Arenicolites made by lugworm Arenicola marina commonly found in the upper intertidal zones. Vertical small, unlined burrows are primarily made by terrestrial animals like insects and spiders such as Cylindricum; the ephemeral tracks, trails, and fecal pellets of insects and worms and plant roots are also common in the studied environments. This assemblage of trace fossils, their ethology and depositional environments, belongs to the Psilonichnus ichnofacies defined by Frey and Pemberton (1987).
The current concept of ichnofacies has evolved since the original idea was published by Seilacher (1953a, b), and now, it involves a deep understanding of dominant ethologies, ichnodiversity levels, and feeding strategies along with ecologic factors and depositional processes (Buatois and Mángano 2011; MacEachern et al. 2008, 2012). A schematic model of the Psilonichnus ichnofacies is presented by Buatois and Mángano (2011) which is characterized by the dominance of vertical J-, Y-, or U-shaped burrows created by ghost crabs; the presence of small unlined vertical dwelling burrows of arachnids and insects; invertebrate trails and trackways, vertebrate tracks; root traces, coproplites, and low ichnological diversity and abundance. Arachnid and insect burrows are assigned to Cylindricum and associated with Psilonichnus ichnofacies (MacEachern et al. 2008; Buatois and Mángano, 2011), and these traces (burrow type 2) are present in the upper intertidal zone of the area.
Since these ichnofacies indicate transitional conditions between marine and continental environments; variations in energy, grain size, and salinity; subaerial exposure; and periodic influx of fresh water due to rains and storm surges are significant features (Buatois and Mángano 2011). Additional environmental constraints of these ichnofacies are soft to stiff grounds, low deposition rates, and availability of food as detritus found in supratidal to upper intertidal settings, subject to low to moderate energy levels in marine or eolian settings like beach backshore and dunes, washover fans, and supratidal flats (MacEachern et al. 2012).
The above discussion about the morphology of the traces, their ethology and the animals responsible for their construction and the environmental settings, indicates that the ichnofauna and botanical traces reported in this study belong to the Psilonichnus ichnofacies.
Conclusions
-
1.
An assemblage of recent trace fossils are described from the Wadi Hali (180 49′35.27″N: 410 22′44.23″E) area of the southern Red Sea coast of Saudi Arabia
-
2.
The objectives of the study were to document and describe various biogenic traces that occur in a range of environments of this coastal region. Such studies are significant in interpreting depositional environments of the sedimentary rocks and understanding complex relationship between animals and sediments in the geological past.
-
3.
The study area is a mixture of marginal-marine and non-marine environments that include coastal dunes, upper intertidal ripple-marked sand flats and supratidal flats that include microbial mats and mud flats, and scattered patches of mangrove stands and their muddy environments.
-
4.
Such coastal environments are commonly impacted by variations in energy levels, and sediment types thus are characterized by different suits of trace fossils.
-
5.
A localized fluvial deposit from a dry channel (wadi) was observed with traces of fossil plant roots.
-
6.
Morphology of four different types of burrows (types 1, 2, 3, and 4) is described; their trace maker and ethology is discussed.
-
7.
Fossil equivalents of these burrows are Psilonichnus upsilon (type 1), Cylindricum (types 2 and 3), and Arenicolites (type 4).
-
8.
Amphibious Ocypodid crabs, both scavengers and surficial deposit feeders, dominate these environments and excavate J-, Y-, or U-shaped dwelling burrows. These burrows when fossilized belong to the ichnogenus Psilonichnus which comprises of mainly vertical, cylindrical burrows of similar shapes with short horizontal or oblique branches.
-
9.
Tracks of land hermit crabs Coenobita clypeatus are described as ichnospecies Coenobichnus currani.
-
10.
Gastropod grazing trails are common; ichnogenus Planolites represents such trails.
-
11.
Botanical traces include microbial mats and their algal filaments and plant roots.
-
12.
The morphology of the traces and their trace fossil equivalents, trace maker, and ethology and the environmental setting indicate that the ichnofauna and botanical traces belong to the Psilonichnus ichnofacies defined by Frey and Pemberton (1987).
-
13.
Since these ichnofacies indicate transitional phase between marine and continental environments, variations in energy, grain size, and salinity; subaerial exposure; and periodic influx of fresh water due to rains and storm surges are its significant features.
References
Bertling M, Brady SJ, Bromley RG, Demathieu GR, Genise J, Mikuláš R, Nielsen JK, Nielsen KKS, Rindsberg AK, Schlirf M, Uchman A (2006) Names of trace fossils: a uniform approach. Lethaia 39(3):265–286. https://doi.org/10.1080/00241160600787890
Bockelie JF (1994) Plant roots in core. In: Donovan SK (ed) The Palaeobiology of trace fossils. Johns Hopkins University Press, pp 177–199
Baucon A (2008) Neoichnology of a microbial mat in a temperate, siliciclastic environment: Spiaggia al Bosco (Grado, northern Adriatic, Italy). Studi Trent Sci Nat Acta Geol 83:183–203
Bromley RG (1990) Trace fossils: biology and taphonomy. Special topics in Palaeontology. Unwin Hyman, London
Buatois L Mángano MG (2011) Ichnology, organism-substrate interactions in space and time. Cambridge University Press, DOI: https://doi.org/10.1017/CBO9780511975622
Chakrabarti A (1981) Burrow pattern of Ocypode ceratophthalma (Pallas) and their environmental significance. J Paleontol 187:113–113
Chan BKK, Chan KKY, Leung PCM (2006) Burrow architecture of the ghost crab Ocypode ceratophthalma on a sandy shore in Hong Kong. Hydrobiol 560(1):43–49. https://doi.org/10.1007/s10750-005-1088-2
Curran HA (1994) The palaeobiology of ichnocoenoses in quaternary, Bahamian-style carbonate environments: the modern and fossil transition. In: Donovan SK (ed) Palaeobiology of trace fossils. John Wiley & Sons, pp 83–194
Curran HA (2008) Ichnofacies, Ichnocoenoses, and ichnofabrics of quaternary shallow marine to dunal tropical carbonates: a model and implications. In: Miller W III (ed) Trace fossils, concepts, problems, prospects. Elsevier, pp 232–247
Dashtgard SE, Gingras MK (2012) Marine invertebrate Neoichnology (chapter 10). In: Knaust D, Bromley RG (eds) Trace fossils as indicators of sedimentary environments. Developments in sedimentology, Elsevier, 64, pp 273–295. https://doi.org/10.1016/B978-0-444-53813-0.00010-1
Desjardins PR, Buatois LA, Mángano MG (2012) Tidal flats and supratidal sand bodies (chapter 18). In: Knaust D, Bromley RG (eds) Trace fossils as indicators of sedimentary environments. Developments in sedimentology, Elsevier, 64, pp 529–562. https://doi.org/10.1016/B978-0-444-53813-0.00018-6
El-Hedeny M, Hewaidy AGAH, Al-Kahtany K (2012) Shallow-marine trace fossils from the Callovian-Oxfordian Tuwaiq Mountain limestone and Hanifa formations, central Saudi Arabia. Australian J Basic Applied Sci 6(3):722–733
Frey RW, Pemberton SG (1987) The Psilonichnus ichnocoenose, and its relationship to adjacent marine and nonmarine ichnoecenoses along the Georgia coast. Bull Can J Petrol Geol 35:333–357
Frey RW, Curran HA, Pemberton SG (1984) Trace making activities of crabs and their environmental significance: the ichnogenus Psilonichnus. J Paleontol 58:333–350
Garrison JR, Henk B, Creel R (2007) Neoichnology of the micro-tidal Gulf Coast of Texas: implications for Paleoecological and Paleoenvironmental interpretations of ancient micro-tidal clastic shoreline systems. Gulf Coast Asso Geol Soc Ann Meeting Trans 2007, 15p
Jado AR, Zölt JG (1984) Quaternary period in Saudi Arabia, vol 2. Springer-Verlag, New York. https://doi.org/10.1007/978-3-7091-8711-1
Jado AR, Hötzl H, Roscher B (1990) Development of sedimentation along the Saudi Arabian Red Sea coast. JKAU earth Sci 3, Spe issue: 1st Saudi Symp Earth Sci Jeddah 1989:47–62
Khan AM, Kumar A, Muqtadir A (2010) Distribution of mangroves along the Red Sea coast of the Arabian Peninsula: Part 2. The Southern Coast of Western Saudi Arabia. Open Access e-J Earth Sci India 3(III):154–162
Knaust D, Curran HA, Dronov AV (2012) Shallow marine carbonates (chapter 23). In: Knaust D, Bromley RG (eds) Trace fossils as indicators of sedimentary environments. Developments in sedimentology. Elsevier, pp 705–750. https://doi.org/10.1016/B978-0-444-53813-0.00023-X
Kumar A, Khan MA, Muqtadir A (2010) Distribution of mangroves along the Red Sea coast of the Arabian peninsula: Part-1: the northern coast of western Saudi Arabia. Open Access e-J Earth Sci India 3(1):28–42
Kumar A, Khan MA, Muqtadir A (2011) Distribution of mangroves along the Red Sea coast of the Arabian Peninsula: Part-3: the coast of Yemen. Open Access e-J Earth Sci India 4(II):29–38
MacEachern JA, Pemberton SG, Gingras MK, Bann KL (2008) The ichnofacies paradigm: a fifty year retrospective (chapter 4). In: Miller W III (ed) Trace fossils, concepts, problems, prospects. Elsevier, pp 52–77
MacEachern JA, Bann KL, Gingras MK, Zonneveld JP, Dashtgard SE, Pemberton SG (2012) The ichnofacies paradigm (chapter 4). In: Knaust D, Bromley RG (eds) Trace fossils as indicators of sedimentary environments. Developments in sedimentology. Elsevier, pp 103–138. https://doi.org/10.1016/B978-0-444-53813-0.00004-6
Pemberton SG, Spila M, Pulham AJ, Saunders T, MacEachern JA, Robbins D, Sinclair IK (2001) Ichnology and sedimentology of shallow to marginal marine systems. Ben Nevis & Avalon Reservoirs, Jeanne d’Arc Basin. Geol Assoc Canada. Short Course Notes 15:1–341
Prinz WC (1984) Explanatory notes to the geologic map of the Wadi Haliy quadrangle, sheet 18E, Kingdom of Saudi Arabia (to accompany map GM-74 a.C.). Ministry petrol min res, Jeddah, Saudi Arabia, pp 1–13
Radiesa D, Hasiotisb ST, Preusserb F, Neubertc E, Mattera A (2005) Paleoclimatic significance of early Holocene faunal assemblages in wet interdune deposits of the Wahiba Sand Sea, Sultanate of Oman. J of Arid Environ 62(1):109–125. https://doi.org/10.1016/j.jaridenv.2004.09.021
Rindsberg AK (2012) Ichnotaxonomy: finding patterns in a welter of information (chapter 2). In: Knaust D, Bromley RG (eds) Trace fossils as indicators of sedimentary environments. Developments in sedimentology. Elsevier, pp 45–78. https://doi.org/10.1016/B978-0-444-53813-0.00002-2
Sakai K, Türkay M (2013) Revision of the genus Ocypode, with the description of a new genus Hoplocypode (Crustacea: Decapoda: Brachyura). Mem Queensland Mus-Nature 56(2):665–793
Sarjeant WAS (1975) Plant trace fossils. In: Frey RW (ed) The study of trace fossils. Springer-Verlag, New York, pp 163–169. https://doi.org/10.1007/978-3-642-65923-2_10
Seike K, Nara M (2007) Occurrence of bioglyphs on Ocypode crab burrows in a modern sandy beach and its palaeoenvironmental implications. Palaeogeog Palaeoclimatol Palaeoecol 252(3-4):458–463. https://doi.org/10.1016/j.palaeo.2007.05.003
Seilacher A (1953a) Studien zur Palichnologie. I Über die Methoden der Paliichnologie N Jb Geol Paläont Abh 96:421–452
Seilacher A (1953b) Studien zur Palichnologie. II. Die fossilien Ruhespuren (Cubichnia). N Jb Geol Paläont Abh 98:87–124
Shapiro RS (2008) Stromatolites: a 3.5 billion year Ichnological record (chapter 22). In: Miller IIIW (ed) Trace fossils, concepts, problems, prospects. Elsevier, pp 382–390
Smith CR, Levin LA, Hoover DJ, McMurtry G, Gage JD (2000) Variations in bioturbation across the oxygen minimum zone in the northwest Arabian Sea. Deep-Sea Research II 47(1-2):227–257. https://doi.org/10.1016/S0967-0645(99)00108-3
Tyler-Walters H (2001) Arenicola marina Blow lug. marine life information network: biology and sensitivity key information sub-programme [on-line]. Plymouth: Marine Biological Association of the United Kingdom. (November, 2002) http://www.marlin.ac.uk/species/Arenicolamarina.htm
Volkenborn N (2005) Ecosystem engineering in intertidal sand by the lugworm Arenicola marina.(http://evolutionbiology.com/articles/life-history-traits-of-the-lugworm-arenicola-marina)
Walker SE, Hollanda SM, Gardinera L (2003) Coenobichnus currani (new ichnogenus and ichnospecies): fossil trackway of a land hermit crab, early Holocene, San Salvador, Bahamas. J Paleontol 77(3):576–582. https://doi.org/10.1017/S0022336000044255
Whybrow PJ, McClure HA (1980) Fossil mangrove roots and palaeoenvironments of the Miocene of the eastern Arabian peninsula. Palaeogeogr Palaeoclimatol Palaeoecol 32:213–225. https://doi.org/10.1016/0031-0182(80)90041-3
Acknowledgements
I thank my former colleagues at King Fahad University of Petroleum and Minerals (KFUPM), Saudi Arabia. They are Drs. Osman Abdullatif, Khalid Al-Ramadan, Lamidi O. Babalola, Michael A. Kaminski, and Asif M. Khan; I thank them for their help in the field and for discussing the geology of the area. I also thank KFUPM for funding our travel to the area of the study. I thank my son Anshuman Kumar for linguistic improvements to this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, A. Recent biogenic traces from the coastal environments of the southern Red Sea coast of Saudi Arabia. Arab J Geosci 10, 500 (2017). https://doi.org/10.1007/s12517-017-3293-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-017-3293-5