Abstract
Non-dairy matrices represent 63% of the vehicles used for probiotication. However, their benefits to human health may be hindered by food processing, storage, and movement through the gastrointestinal tract. The microencapsulation of probiotic bacteria is an alternative to increase their resistance to such challenges. This review outlines the current advances in the encapsulation of probiotics using emulsification methods. The review also addresses the influence of encapsulating agents on the yield, the final size of microcapsules, and the survival rate of probiotic microorganisms. The main drying methods for probiotic microparticles, the kind of foods used for probiotication, and the emerging methods of emulsification are discussed. Emulsion microencapsulation has proven to be a viable technique for the production of probiotic microcapsules, while freeze-drying is the most suitable drying technique due to the mild process conditions. Emulsification through membranes and microfluidic devices are potential encapsulation techniques owing to their ability to control particle size and to work under mild conditions. The emulsion microencapsulation is thus a potential technique for ensuring the safe delivery of next-generation probiotics applied to non-dairy products.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Probiotics are defined as “live microorganisms, which when administered regularly provide benefits to the host health” [1]. Until recently, they were typically applied to dairy products. However, the growing number of individuals affected by lactose intolerance, milk protein allergy, galactosemia, hypercholesterolemia, or simply a change in consumers’ food preferences has considerably increased the demand for non-dairy probiotic foods [2, 3]. The food industry and food scientists are aware of this demand and are progressively developing solutions to meet the market needs.
Probiotics can provide many benefits to human health, such as the maintenance of the intestinal microbiota, the regulation of the intestine, the inhibition of pathogenic bacteria growth, the improvement of the immune system, and the increase of calcium absorption and vitamin [4]. Yet, probiotics must reach the human intestine in an active and viable way to colonize or interact with intestinal microbiota and exert these functional properties. This means that after passing through the upper digestive tract, its survival rate and metabolic activity must be maintained [5]. Prestes et al. [6] and Hill et al. [1] indicate that a microbial count equal to or greater than 6 log UFC g−1 characterizes the food as a probiotic product capable of providing benefits to human health.
One of the most important challenges for researchers and industries working with non-dairy functional foods through the incorporation of probiotics, is the maintenance of high levels of viable probiotic bacteria after food processing, during storage, and safe arrival in the intestine, which is where probiotics perform their beneficial functions for the human organism [7]. During processing, the bacteria must be stable to temperature variations caused by heat treatments such as freeze-drying, spray-drying, freezing, etc. On the other hand, during storage, probiotics must resist the intrinsic factors of the food to which they have been added, such as low pH and water activity, presence of additives, and antimicrobial substances, in addition to extrinsic factors such as temperature, relative humidity, and oxygen [8]. The human digestive tract is another obstacle that probiotics must face. Digestive enzymes, stomach acids, bile salts secreted by the duodenum, and peristaltic movements of the intestine are some of the challenges [9]. For these reasons, adequate preservation strategies to prevent bacterial damage or death are of great importance.
The microencapsulation technique proved to be a promising alternative for the protection of probiotics. Currently, there are many probiotic encapsulation technologies, such as spray-drying, freeze-drying, extrusion, fluidized bed, layer-by-layer, electrospinning, coacervation, liposome, and emulsion [10,11,12]. The encapsulation by emulsion techniques is widely used considering its cost, simplicity, mild process conditions, and the possibility of yielding microparticles [4, 13,14,15,16].
Some probiotic encapsulation methods have been systematically revised, such as complex coacervation [17], layer-by-layer [18], and electrospinning [19]. To the best of our knowledge, no review article focusing on the emulsion techniques for encapsulating probiotics has been previously published. In this review, probiotic encapsulation by emulsification will be critically discussed for an overview of the most recent published research. Information such as probiotic bacteria, encapsulating agents, encapsulation yield, drying techniques, microparticle size, non-dairy carriers, storage conditions, and probiotic survival will be presented. Then, future innovations and trends in emulsion microencapsulation, including new emulsification methods (membrane emulsification and microfluidics) and next-generation probiotics will be discussed.
Preparation of the Emulsion for Encapsulation
In encapsulation by emulsification, simple or multiple type emulsions can be formed (Fig. 1). In simple type emulsions, if the dispersed phase is aqueous, the emulsion is characterized as a water-in-oil (W/O) emulsion, while the opposite is called an oil-in-water (O/W) emulsion. If one more phase is added, multiple emulsions are obtained, such as water-in-oil-in-water (W/O/W) or oil-in-water-in-oil (O/W/O). These emulsified systems can be used to encapsulate probiotics to improve the protection of encapsulated cells. Emulsions of the (W/O) or (W/O/W) type are preferably used, due to the hydrophilic character of the probiotics [8, 20].
The technique of encapsulating probiotics by emulsion (Fig. 2) is based on the mixture of two immiscible phases, which are called dispersed or discontinuous phases, and oily or continuous phases. The dispersed phase consists of a small volume of probiotic suspension with a hydrocolloid (e.g., alginate, containing previously solubilized calcium carbonate), while the continuous phase consists of a large volume of vegetable oil, usually canola oil, corn oil, soybean oil, sunflower oil, or mineral oil [21,22,23]. An emulsifier or surfactant is added in the continuous phase to stabilize emulsions, favoring the production of smaller-sized microparticles. In the case of alginate encapsulation by emulsification, an aliquot of organic acid, usually acetic acid, is added to the mixture after emulsification to promote gelation. As the organic acid enters the aqueous phase, it interacts with calcium carbonate, releasing calcium ions and carbonic acid. Calcium ions react with alginate through complexation with the polymer's carboxyl groups, forming the structure of the “egg carton model” [11, 24, 25]. After crosslinking the hydrocolloid, the microparticles are solidified and collected by filtration. This process is known as internal polymer gelation.
The parameters of the emulsion preparation, such as the viscosity, pH, temperature, and agitation rate strongly influence the final size of the microparticles [26]. The size distribution of probiotic microparticles obtained by emulsion is often broad, between 1 µm and 100 µm [4, 14, 15, 20, 27], but can also be greater than 100 µm [28,29,30,31,32]. It is assumed that high rates of agitation during the preparation of the emulsion result in small microparticles. Rosas-Flores et al. [26] used agitation rates of 400 rpm, 800 rpm, and 1200 rpm for the production of microcapsules (wet suspensions) containing encapsulated Lactobacillus helveticus and Lactobacillus delbrueckii spp lactis. The authors verified that the size distribution range was modified by increasing the stirring rate. At 400 rpm, a bimodal behavior was observed in the range of 20–420 μm; at 800 and 1200 rpm, the behavior became unimodal and the range was from 20 to 200 μm and 20 to 160 μm, respectively.
A modification of the emulsification technique can be made to improve the survival rate of probiotic bacteria, such as the coating of microcapsules with polymers. The layer-by-layer (LbL) technique has been used to apply such coatings to probiotic microcapsules. The coating interacts with the surface of the capsule creating an additional membrane (layer) on the microcapsule [21]. The LbL is typically based on hydrogen bonding or electrostatic interactions [33]. Examples include the use of chitosan or alginate solutions, which have created additional protection for probiotic cells when exposed to the human digestive system [27, 29, 32, 34, 35], yielding a count of probiotic bacteria above 6 log UFC g−1 after in vitro simulation gastrointestinal conditions. Figure 3 shows this process in which a microcapsule produced by an anionic material (for example, alginate) is consecutively coated with a cationic material (for example, chitosan).
Chitosan coating process of a probiotic microcapsule produced with alginate [36]
The survival rate of probiotics encapsulated by emulsification may decrease during long-term storage. However, freeze-drying [4, 13, 14], spray-drying [37], critical point drying [38], or fluidized bed drying [30] can increase the cell survival rate. For instance, Beldarrain-Iznaga et al. [14] freeze-dried microcapsules of Lacticaseibacillus casei C24 (Lc), produced by emulsification. The viable cell count (7 log UFC g−1) of microencapsulated L. casei increased from 8 (control) to 17 (freeze-dried microcapsules) weeks at 4 °C.
Methods
Literature Search and Selection of Relevant Studies
The literature search method and the selection of relevant articles were carried out according to Graça et al. [39]. Four databases (Web of Science, Scopus, Science Direct, and CAPES Portal) were systematically searched in January 2021 using the following sets of keywords: emulsi* and probiotic, emulsi* and in vivo, emulsi* and lactobacillus, emulsi* and bifidobacteri*, emulsi* and “cell encapsulat*”, “cell encapsulat*” and microfluidics, “cell encapsulat*” and “membrane emulsi*”, “next-generation probiotic”. It is worth emphasizing that the CAPES Portal is a database that gathers several databases, yielding numerous scientific documents. The search was limited to peer-reviewed studies in the English language. As inclusion criteria, we selected articles that reported on the topics: (1) encapsulation of probiotic microorganisms that used the emulsion method; (2) encapsulation of microorganisms that used the membrane emulsification and microfluidics technique; and (3) papers on next-generation probiotics. On the other hand, the exclusion criteria were: (1) review, opinion, and conference articles; (2) papers that studied the emulsion technique to encapsulate non-probiotic microorganisms, enzymes, bacteriophages, bioactive compounds, and pharmaceutical drugs; (3) papers on the incorporation of probiotics in food, animal feed, and packaging, without having been previously encapsulated by the emulsion method; (4) papers that used only the complex coacervation technique; and (5) papers on tissue engineering and the medical / and or biomedical field.
Figure 4 shows a flow diagram summarizing how the bibliographic research was conducted. The searches were managed on EndNote Web, and after removing duplicate entries, a total of 876 papers were exported to the online reference management platform Rayyan.QCRI.org to proceed with title and abstract screening. Then, 188 full-texts were assessed for eligibility based on the inclusion and exclusion criteria, of which 79 were excluded, resulting in a final set of 109 articles for data extraction.
Data Extraction
Data were extracted into a standardized summary table. The extraction considered a set of general study characteristics like title, authors, year of publication, and country. The following variables were also identified: the type of microorganism, encapsulating materials, encapsulation method, and microparticle solidification technique. The response data were obtained: encapsulation yield, water activity, and size of droplets and/or solidified microparticles. Likewise, we gathered information on the type of food matrix where the microparticles were incorporated, conditions of storage of the food matrix/or microparticles, and the probiotic survival rate.
Results and Discussion
General Characteristics of the Published Literature
The literature search yielded 109 articles meeting the inclusion criteria, published between 2003 and 2020. Figure 5 shows the number of articles published over the years. Between 2003 and 2012, a small proportion (14%) of articles were published, 25% were published from 2013 to 2016, and the majority (61%) between 2017 and 2020, demonstrating that the interest in the subject of this review is growing and relevant. In addition, the bibliographic research revealed that the Asian region is the one that most publishes (42.3%), followed by Europe (29.2%), America (25.6%), Oceania (2.2%), and Africa (0.7%).
Characteristics of the Encapsulation of Probiotics by Emulsion
Probiotic Strains
Among the encapsulated probiotics, the species of Lactobacillus (62.2%) stood out, followed by the strains of Bifidobacterium (24.2%), Saccharomyces (6.3%), Enterococcus (4.2%), Pediococcus (2.1%), and Akkermansia (1%). Among the studied species, Lactiplantibacillus plantarum and Bifidobacterium BB-12 are the most used in encapsulation by emulsification.
It is notable the great use of probiotic bacteria belonging to the species of Lactobacillus and Bifidobacterium in encapsulation. Certainly, this can be associated with the wide range of potential benefits that these species can offer to human health when consumed regularly in the diet, as depicted in Fig. 6. In contrast, the survival rate of these probiotic strains in the face of heat treatments, incorporation into different food matrices (section “Probiotic microcapsule delivery vehicles”; Table 4), exposure to different storage conditions, and submission to gastrointestinal conditions, has also been explored by several authors [14,15,16, 28, 32, 40,41,42,43,44]. Beldarrain-Iznaga et al. [14] tested the thermal resistance of free and microencapsulated probiotic L. casei at 50, 70, and 90 °C, for 10 and 20 min. The viability of L. casei significantly decreased when temperature increased, and non-encapsulated cells were the most affected. The cell count of the non-encapsulated cells decreased by 4.0, 5.3, and 6.5 log CFU g−1 (from 9.71 log CFU g−1) after applying 50, 70, and 90 °C for 10 min, respectively. On the other hand, after exposing the microencapsulated probiotic at 50, 70, and 90 °C for 20 min, reductions of 1.3, 1.2, and 1.5 log CFU g− 1 were obtained, respectively. The authors concluded that the double layer formed by caseinate and sodium alginate protected the probiotic with a rigid and heat-resistant interfacial film. Similarly, Ji et al. [32] measured the thermal tolerance of free and encapsulated Bifidobacterium longum at 55, 60, and 65 °C, for 30 min. For non-encapsulated B. longum exposed to 55, 60, and 65 °C, the bacterial count decreased by 2.83, 3.31, and 4.12 log CFU g−1, respectively. The viability of alginate microcapsules decreased by 0.24, 0.53, and 1.72 log CFU g−1, while the viability of chitosan-coated microcapsules decreased by 0.20, 0.64, and 1.14 log CFU g−1 at 55, 60, and 65 °C, respectively. The authors reported that the chitosan coating could block the pores of alginate capsules, firmly immobilizing the bacteria within the microcapsules and reducing the probability of probiotic migration, thus protecting the probiotic against heat stress.
Studies have shown that alginate and caseinate [14], gelatin and gum arabic [16], pectin [42], chitosan [32], and carrageenan [28] have formed microcapsules that effectively protect cells under environmental stress such as oxygen and moisture, and internal heat diffusion. These materials have a high water-holding capacity and minimize damage to probiotic cells caused by oxygen and moisture. In addition, electrostatic interactions between wall materials (which have opposite charges) and ionic gelling form highly protective layers against environmental stresses.
Probiotic cells are typically very sensitive to the highly acidic conditions of the human stomach. Raddatz et al. [42] reported that microcapsules produced with pectin provide excellent protective barriers against stomach acidity (pH range of 1.3 to 2.5 for healthy individuals). The authors verified that the viable cell counts did not present a significant difference (p < 0.5) when comparing the initial count with the count of microorganisms released in the ileum. According to Paula et al. [16], bacteria have proton pumps in their plasma membrane as a defense mechanism to keep the cytoplasm close to its physiological pH. However, if these regulatory systems do not function properly, intracellular acidification will occur and cause a loss of viability, as seen in free cells. In the case of encapsulated cells, excess protons may not substantially affect the pH of the cell's cytoplasm, as they interact with the acid and alkali groups of the biopolymers that surround the encapsulated cells participating in the protonation equilibrium [16]. In low pH media, the amino groups of the side chain of proteins are protonated and the percentage of carboxylic groups dissociated from the polysaccharides is decreased. Therefore, electrostatic interactions between these biopolymers are enhanced so that cells are less likely to be severely affected by ambient pH. In addition, the microcapsule wall material provides a physical barrier against gastric fluids, increasing the protection of cells against adverse conditions. Coating the microcapsules with alginate [14], chitosan [32], and nanocrystalline starch [43] reduces the hydrolysis of the phospholipid layer of probiotic cells from the action of bile salts present in the duodenal phase. The additional layer limits the diffusion of bile salts into the microcapsules, delaying the interaction with probiotic bacteria and allowing them to colonize the small and large intestine providing health benefits to the host. Ma et al. [15] noticed that pepsin digested the lactoprotein layer of microcapsules containing L. plantarum LIP-1 into smaller peptide fragments. Although pepsin would damage the microcapsules resulting in a decrease in the number of living cells, the microcapsules could still effectively protect cells since the lactoprotein contains basic amino acid residues that neutralize the H+ that permeates the microcapsule, maintaining a neutral internal environment. On the other hand, the interest of researchers in discovering new probiotics (e.g., Akkermansia muciniphila), known as next-generation probiotics, is noticed. An overview of the latest studies on next-generation probiotics is shown in the following section.
Next-Generation Probiotics (NGPs)
Even though most parts of the microorganisms approved and commercialized are from the Lactobacillus species, considerable growth in the number of studies regarding the discovery of new microorganisms with probiotic potential has been observed. The next-generation of probiotics, mainly the genera Akkermansia, Enterococcus, Bacteroides, Coprococcus, Veillonella, Ruminococcus, Corynebacterium, Faecalibacterium, Lactococcus, and Eggerthellaceae, has shown promising results in the treatment of diseases such as type 2 diabetes and obesity. Moreover, some of these microorganisms exhibited potential anti-inflammatory, anticancer, antimicrobial, and antiviral properties (Table 1).
The expansion of the scope of probiotic microorganisms is very relevant for science and industry. Nevertheless, many challenges need to be overcome, like the need for higher efficacy and higher safety for consumption of these new probiotics [60], as well as the technological aspects of the incorporation of these microorganisms in food. Furthermore, the maintenance of viable cell count in the gastrointestinal tract and methods for measuring the cell survival rate are other issues that still need to be addressed. Emulsion microencapsulation technology emerges as an interesting alternative to protect cells against adverse processing conditions, intrinsic factors of the food, and hostile digestive tract environments.
So far, only six studies have assessed microencapsulation technology as a method of protecting these new probiotics, namely Akkermansia and Enterococcus [30, 48, 61,62,63,64]. Some of the studies evaluated the protective effect of microencapsulation in simulated gastrointestinal conditions and confirmed that it was effective in protecting microorganisms against stomach acids, digestive enzymes, and bile [30, 48, 63, 64]. In contrast, only two papers investigated the effect of adding these new probiotics in food matrices, Enterococcus faecium (UAM1) in sausages [61] and Akkermansia muciniphila DSM22959 in black chocolate [64]. These authors concluded that these foods served as excellent probiotic carriers, promoting counts greater than 6 log UFC g−1 after long-term storage.
The limitation of studies in the literature on the microencapsulation of new probiotics is notable. It is believed that the upcoming studies should focus on the evaluation of the protective effect of microencapsulation under different food matrices and in simulated gastrointestinal conditions. However, more research must be carried out to prove the efficacy and safety of consumption of these next-generation probiotics.
Influence of Encapsulating Agents on the Properties of Probiotic Microcapsules
Encapsulating agents are considered to be one of the most important variables for the successful microencapsulation of probiotic bacteria. Wall materials such as chitosan, alginate, gelatin, milk proteins, pectin, carrageenan, and different types of starch occupy a prominent place in microencapsulation by emulsion. Also, prebiotic materials have gained prominence as encapsulants [4, 42, 61, 65]. Table 2 presents several encapsulating agents used in microencapsulation by the emulsion of probiotic strains and the results in terms of encapsulation yield, droplet/microcapsule size, and the viable cell count of probiotic strains after storage and/or after submission to gastrointestinal conditions. The criteria for choosing a suitable encapsulating agent are mainly based on its physicochemical properties (molecular mass, solubility, glass transition temperatures, crystallinity, film formation, and emulsifying properties) [66]. A good wall material must also be easy to handle during the encapsulation process. In addition, it cannot react or injure the probiotic strain during the encapsulation and storage process and, finally, it must meet the solubility properties of the microcapsule by releasing the probiotics at the site of action [67].
Influence of Encapsulating Agents on Encapsulation Yield
The encapsulation yield is related to the concentration of cells trapped in the microparticles, and to the survival rate of the probiotic strains after the encapsulation process. Thus, high yield values are more advantageous. Variables such as the rate of agitation of the emulsion, encapsulating materials, the concentration of organic acid used in the internal gelation step, and the species of the probiotic strain influence the encapsulation yield [75]. Sodium caseinate and sodium alginate have been reported to form excellent protective barriers for the Lacticaseibacillus casei C24 (Lc) strain against stress suffered by the encapsulation through the emulsification process, resulting in a high encapsulation yield (97.3%) [14]. Similarly, a mixture of gelatin and Arabic gum was able to protect Lactiplantibacillus plantarum cells against agitation and temperature variations during the encapsulation by emulsification process, providing a 95.9% encapsulation yield [16]. A direct correlation was observed between the encapsulation yield of Lactobacillus acidophilus (PTCC 1643) and the encapsulant polymer concentration. When increasing the concentration of alginate 5% (w/v) and whey protein isolate 10% (w/v), there was an increase from 81.42 to 97.51%. This variation in the yield of the encapsulation may be due to the high concentration of the wall materials since the repetition of groups with opposite charge increases the ionic crosslinking and forms a denser membrane in the microcapsule [13]. It has also been documented that the combination of pectin with rice bran or pectin with inulin improves the survival of the Lactobacillus acidophilus LA-5 strain, promoting high yields (90.59–91.24%) [42]. The addition of prebiotics like inulin or rice bran reduces cell death by stabilizing pectin networks. Besides, inulin has excellent plasticizing properties, contributing to a more efficient coating [76]. The use of alginate with Eleutherine americana extract increased the yield of encapsulation from 67.34% (without Eleutherine americana extract) to 87.17%. It is believed that the plant extract was responsible for reducing the porosity of the microcapsules and, consequently, reduced the leakage of microencapsulated Bifidobacterium longum, ensuring good encapsulation performance [72]. Multiple type emulsions can provide a high yield in the encapsulation of probiotic microorganisms [14, 16]. The additional physical barriers formed by the encapsulating agents are mainly responsible for this increase in yield, which provides greater protection to the encapsulated probiotics. Furthermore, the presence of cells within the internal aqueous droplets limits the migration of probiotic strains out of the microcapsule, since they first need to permeate through the W/O interface and then diffuse into the oil phase, migrate through the wall material and finally reach the exterior of the capsule.
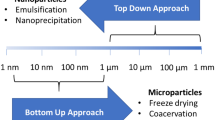
Influence of Encapsulating Agents on the Size of Droplets and Microparticles
The size of the droplets and/or microparticles is another property influenced by the encapsulating materials. In addition to the encapsulating agents, the size can be affected by the rate of agitation, since increasing the stirring speed results in decreased microparticle size, as it produces smaller emulsion droplets through stronger shear forces and increased turbulence [42]. There is no standardization regarding size limits for the classification of capsule size. However, la Cruz Pech-Canul et al. [67], Campos et al. [77], and Yao et al. [78] classified the capsules as macro (> 1000 µm), micro (1 to 1000 µm), and nanocapsules (< 0.2 μm). However, an optimal range (1—100 µm) is indicated for food application [4, 21] since microparticles above this range are sensorially perceptible, causing a gritty sensation when consumed. It is worth emphasizing that the probiotic particles must be larger than 1 μm, as microbial cells dimensions are typically in the 1—10 μm range. The reduced size (36.3 µm) of droplets of an emulsion (wet suspension) with microencapsulated Lactiplantibacillus plantarum was attributed to the use of sodium alginate since it can form a more cohesive surface structure due to the interaction between the calcium ions and alginate [14]. The combination of pectin and inulin was responsible for increasing the size (462 µm) of the microparticles (wet suspension) containing encapsulated Lactobacillus acidophilus LA-5 when compared to the size (24.4 µm) of the microcapsules produced only with pectin. The difference in size may have occurred due to the long length of the inulin chain, which when incorporated into water forms microcrystals and results in larger microparticles [42]. It has also been documented that the combination of alginate (1, 2, and 3%) with flaxseed mucilage (0.9%) produced larger probiotic microparticles (wet suspension) (60–104 µm) than those produced only with alginate (56–90 µm). This increase was associated with the increase in alginate concentration with the incorporation of flaxseed mucilage. The different polysaccharides have different hydration capacities due to the different chemical groups (COO– and SO3) that can interact with water molecules through hydrogen bonds [68]. Besides, it has been reported that multiple type emulsions tend to produce probiotic microcapsules with larger sizes, due to the formation of additional barriers by encapsulating materials [14]. Similarly, Chen et al. [34] indicated that the size of dried probiotic microparticles produced with whey protein isolate was influenced by coating with an alginate solution. After coating with the alginate solution, the size of the microcapsules increased by 40 µm. A similar finding was verified by Mokhtari et al. [79] when they produced probiotic microcapsules (wet suspension) coated with alginate. The microparticles with a single, double, and triple-layer, had a size of 54.25 µm, 77.43 µm, and 103.66 µm, respectively, directly attributed to the number/thickness of the coating layer.
Influence of Encapsulating Agents on the Survival Rate of Probiotic Strains During Storage and Passage Through the Digestive Tract
A correct choice of encapsulating agents reflects positively both on the survival rate of microencapsulated probiotic strains during storage and under gastrointestinal conditions. It is worth emphasizing that these encapsulating agents need to resist oral and stomach fluids and enzymes and dissolve in the human intestine to release the probiotics from the microcapsules. After the rupture of microparticles, probiotics must colonize or interact with intestinal microbiota to exercise their functional roles in human health. Alginate has been the wall material most widely used in emulsion microencapsulation technology [13, 30,31,32, 68]. The large use of this material is due to its low cost, biocompatibility, food grade, and the targeted delivery of probiotics (soluble in basic medium, for example in the intestine) [80]. However, microcapsules formed with this single polymer were characterized as more porous and susceptible to extreme pH values, resulting in an early release of probiotics and less efficient protection of microencapsulated strains [81]. The combination of wall materials and the formation of multiple layers through the microcapsule coating process have been reported as alternatives to overcome these limitations [14]. Chen et al. [34] reported that the high counts (> 7 log UFC g−1) of Lactobacillus bulgaricus were due to the alginate coating of the dried microparticles produced with whey protein isolate. These alginate-coated microparticles were resistant to the penetration of digestive fluids, enzymes, oxygen, and water. The coating of probiotic microparticles with other materials, such as chitosan [27, 29, 32, 35, 73, 82], xanthan gum [69], Eudragit S100 [83], whey proteins [84], and resistant starch [85], also provided counts greater than 6 log UFC g−1 after the simulation of gastrointestinal conditions, and great stability during storage.
Chitosan is frequently used in probiotics encapsulation using the emulsion technique, mainly for coating microcapsules. It is worth emphasizing that chitosan exhibited inhibitory effects on different types of lactic acid bacteria [11]. These inhibitory properties are believed to be related to a strong electrostatic interaction between chitosan, positively charged, and the cell surface of the bacterium, negatively charged. This electrostatic interaction causes changes in the functioning of the membrane cell followed by increased membrane permeability that leads to destabilization of the cell membrane and leakage of intracellular substances and, finally, cell death [86]. For this reason, chitosan is preferred to be used as a coating material, not as a single wall material. Alginate-chitosan-type microparticles are one of the most widely used microcapsules for microbial cultures due to the high compatibility between polymers, low cost, and abundance in nature. The polycationic nature of chitosan contributes to a strong interaction of the alginate carboxylic groups with the chitosan amine groups, resulting in the formation of a membrane highly resistant to digestive fluids and environmental conditions during storage [35].
Milk proteins have been reported to be good wall materials [15, 69] due to the buffering capacity of amino acids, and consequently minimizing cell death during digestion. In addition, it was reported that the combination of whey protein isolate and alginate produced dried microcapsules containing Lactobacillus acidophilus (PTCC 1643) is highly resistant to digestive fluids. The ionic interaction between alginate and β-lactoglobulin (major whey protein) can reduce the pores of the microcapsule surface and guarantee the targeted delivery of probiotics in the intestine [13].
Prebiotics such as inulin [42, 62, 65, 76], oligosaccharides [72, 87], rice bran [4, 42, 76], flaxseed mucilage [68], flour pear peel, and apple pomace flour [61] were applied with the association of other wall materials and showed promising results in the survival rate of probiotic microorganisms during storage and after the simulation of gastrointestinal conditions. These prebiotics can contain non-nitrogen compounds, mainly fibers, and monosaccharides, including xylose, galactose, arabinose, maltose, glucose, and fucose. Therefore, these substances can act in three main ways: as a carbon source promoting microbial growth; as water absorbers increasing the stability of probiotic microcapsules during storage; and as protective barriers increasing the survival rate of microorganisms during passage through the tract.
Drying Techniques for Producing Probiotic Microcapsules
Drying the probiotic microcapsules is important both from a microbiological and technological point of view, as it increases the lifetime of microorganisms. Šipailienė and Petraitytė [88] mentioned that a water activity greater than 0.25 increases the mortality rate of probiotic bacteria, supposedly due to the high molecular mobility in the matrix, which is related to the stimulation of cellular metabolism. Similarly, high humidity and high water activity favor the microparticle agglomeration during storage [89]. Another technological advantage of drying the microcapsules is the possibility of the incorporation of probiotics in a low-moisture food matrix. Therefore, choosing a suitable drying method that causes less damage to the probiotic strains is another challenge during the encapsulation by the emulsification process. Several techniques are used to dry the probiotic microcapsules obtained by emulsification. We found a total of 25 studies out of 109 eligible articles, which used some drying technique after internal or external gelation of probiotic microcapsules. Among the drying techniques used, freeze-drying (84%), spray-drying (8%), critical point drying (4%), and fluidized bed drying (4%) are highlighted.
Although the drying techniques provide technological advantages to microparticles, for example, increased probiotic stability, most studies characterized and used only wet probiotic microcapsules. As mentioned in some studies [15, 26, 29, 61, 68, 72, 81, 90,91,92,93,94], after the encapsulation process, the microparticles are filtered to remove the crosslinking solution and are washed with distilled water, peptone water, or 0.85% (w/v) saline solution to remove residual oil. After that, microparticles are stored under refrigeration (4 ± 2 °C) until analysis and incorporation into foods. On the other hand, when probiotic microcapsules are not subjected to a filtration process, they remain in emulsion [40, 41, 95, 96] or are suspended in peptone water [35] or saline solution [87] before characterization and addition to probiotic foods.
The freeze-drying technique is based on the sublimation phenomenon, which occurs in three stages: freezing, primary drying, and secondary drying. In the former stage, depending on the freezing rate and temperature, ice crystals that damage probiotic cells may be formed [80]. It is worth mentioning that, in addition to the formation of ice crystals, the chemical and osmotic damages caused by the concentration of solutes in the unfrozen fraction are highly harmful to probiotics. In the second stage, the frozen water is removed by sublimation under vacuum, while in the third stage, the non-frozen water is removed by desorption [97]. Despite the limitations, freeze-drying remains the most widely used technique to dry probiotic microcapsules. To avoid the formation of intracellular crystals, and consequently minimize cell loss, a high freezing rate is preferable (approximately 5 °C min−1, as suggested by Heylen et al. [98]), reaching a final temperature lower than -60 °C. In addition, cryoprotective agents (including lactose, sorbitol, maltodextrin, powder milk, milk proteins, glycerol, trehalose, sucrose, and mannitol) have been used to overcome the issues during freeze-drying [88]. The cryoprotective agents can be used both in the dispersed phase of the emulsion and in the coating of probiotic microcapsules. As ice crystals are formed, probiotic cells are compressed into the unfrozen fraction. The addition of these cryoprotective agents decreases the melting point of the water, and consequently increases the unfrozen fraction, giving more space to the probiotics, and thus contributing to less cellular damage due to mechanical or osmotic stress. The combination of alginate and mannitol provided slight protection to the cells of Bifidobacterium animalis ssp. lactis BB-12 after freeze-drying. The viable cell count of Bifidobacterium animalis ssp. lactis BB-12 in alginate-mannitol and alginate formulations were 6.61 and 6.34 log UFC g−1, respectively [99]. Several studies reported that the use of freeze-drying as a drying treatment after encapsulation by emulsification produced probiotic microparticles with a water activity below 0.4 [4, 14, 16, 37, 71, 76], and with good maintenance of probiotic survival rate during long-term storage.
Spray-drying is well established for large-scale industrial applications [100] and is considered economically viable. The energy consumption of the spray-drying process is considered to be 6 to 10 times lower compared to freeze-drying [11]. In this technique, the emulsion or microparticles resuspended in water are atomized in a drying chamber that contains a gas at a temperature between 47 and 200 °C [101]. The spray flow can be applied in three ways (concurrent, countercurrent, or mixed flow). However, the choice of spray flow will depend on the direction in which the air and liquid (e.g. emulsion or microparticles resuspended in water) enter the drying chamber. In the first case (co-current), the final product is in contact with the cooler air, being preferable for drying thermosensitive materials, such as probiotics. After evaporation of the solvent, the dried microcapsules are formed and separated from the drying gas using a cyclone, which deposits them in a glass collector located at the bottom of the equipment [100]. Broeckx et al. [97] detailed the main phases involved in the spray-drying process. During the process, encapsulated microorganisms can undergo several stresses, including thermal stress, dehydration, shear stress, osmotic and oxidative stress. Thermal stress and dehydration were mentioned as the main responsible for the inactivation and death of probiotic strains [102]. Gelatin, arabic gum, and cellulose acetate phthalate have been reported as protective agents capable of forming a physical barrier resistant to hot air [101]. Also, the use of disaccharides is encouraged, as they can preserve the structure of proteins and membranes of probiotic cells through a connection in places that previously interacted with water [103]. The emulsion technique was used to produce probiotic microcapsules containing sodium caseinate, okara oil, and Lactiplantibacillus plantarum CIDCA 83,114, and then the microcapsules were dried by freeze-drying or spray-drying [37]. The results revealed that spray-dried microparticles showed better physicochemical stability after storage (90 days at 4 °C) and the smallest decrease in the microbial count (1.89 log UFC g−1) when compared to freeze-drying (5 log UFC g−1).
Recently, fluidized bed drying was used to dry microcapsules produced with alginate and mineral oil, containing Saccharomyces boulardii (CGMCC No. 10381) or Enterococcus faecium (CGMCC No. 2516) microencapsulated by the emulsion technique [30]. Before the drying process, the probiotic microcapsules must be collected and separated by filtration from the oil fraction, and then dried. A heated gas (35—50 °C) with controlled speed passes through a bed of reticulated particles, suspending the microparticles in the drying air [104]. Fluidized bed drying consumes less time than freeze-drying, and operates at lower temperatures than the spray-drying process, minimizing the inactivation of probiotics by heat. Marcial-Coba et al. [80] mentioned that the osmotic stress caused by the expulsion of water during the fluidized bed drying process can affect the survival rate of probiotic strains. However, those cryoprotective agents used in freeze-drying have been reported as a solution to this problem.
The use of critical point drying was reported in only one study [38]. However, Ayama et al. [38] did not evaluate the influence of the drying technique on the survival rate of microorganisms. The effect of drying on the physicochemical and microbiological properties of probiotic microcapsules during long-term storage has also not been evaluated. Due to the mild conditions of the process, it is believed that both techniques (fluidized bed drying and critical point drying) seem to be promising for drying probiotic microcapsules obtained by emulsion, but the available literature is scarce and more research is needed to demonstrate the potential of these techniques.
Probiotic Microcapsule Delivery Vehicles
One way to deliver probiotic microcapsules to humans is through incorporation into food. Table 3 shows the different types of foods that were used as vehicles for the probiotic microparticles obtained by the emulsification technique. Only 19 articles, of 109 eligible, investigated the effect of adding probiotic microcapsules to food matrixes. This finding indicates that more research in this area must be developed because each food matrix presents new challenges due to its different compositions. In addition, 61% of the studies did not evaluate the survival rate of microorganisms in simulated gastrointestinal conditions. This evaluation can be considered one of the most relevant in the development of a functional product through the addition of probiotics, considering that probiotics must reach the colon with a count above the recommended dose (> 6 log UFC g−1) to exercise their bioactive functions.
Non-dairy products added with probiotic capsules represent 63% of probiotics delivery vehicles, 26% meat foods, 21% fruit products, and 16% bakery products. The dairy matrices represent 37% of the applications. The sausage was the delivery vehicle most used by researchers (Table 3), and good probiotic survival rate results were confirmed after long-term storage. In addition, recent researches have shown that fruit juices [94], dehydrated fruits [113, 114], and bakery products, such as cupcakes [107], guaranteed excellent results of probiotic survival rate after the study of storage, evidencing the potential of this market. Although dairy matrices are a focus of research with probiotics, there is a tendency to use these microorganisms in non-dairy matrices [115].
The main reason for the probiotication of non-dairy matrices is to serve those consumers who dislike milk and its derivatives, or who are intolerant to milk compounds. Also, probiotic products such as fruit juices are often lactose-free, soy-free, and vegan-compliant [115]. However, maintaining the survival rate of probiotic microorganisms in non-dairy matrices represents a major challenge. Intrinsic factors like the presence of fermentative bacteria, sodium chloride, nitrate and nitrite, low pH, and high water activity [41] of some meat products, for instance, represent the main obstacles for probiotic strains. Besides, the high cooking temperatures (70—72 °C) [61] negatively affect the lifetime of these microorganisms. Bakery products, such as bread and cupcakes, usually cooked at around 180 °C, represent the main difficulty in the development of functional foods. In addition, changes in pH or water activity, ethanol production, and products of the Maillard reaction are other obstacles that the probiotic strains have to face [115].
In fruit-based foods, low pH, high water activity, and the presence of phenolic acids and lactones [94] represent the main challenges for probiotic microorganisms. To overcome these challenges, the encapsulation of probiotics with different encapsulating materials has been an alternative and guaranteed good results of probiotic survival rate (Table 3). For example, flour pear peel and apple pomace flour were responsible for decreasing the humidity in sausages and providing greater thermal resistance to probiotics during cooking [61]. Alternatively, coating the microcapsules (wet suspension) with chitosan protected the probiotic cells during the baking of the bread, providing higher counts of microorganisms than the uncoated microcapsules [108]. The use of wall materials such as powder milk and lecithin proved to be effective in protecting probiotic cells in pineapple juice, but they did not protect the bacteria in strawberry-apple juice [94]. Another alternative to provide greater thermal resistance to microorganisms is the genetic manipulation of probiotic strains and their exposure to stress in sublethal temperatures [7, 116]. Table 4 shows the main challenges encountered in the probiotication of non-dairy foods, as well as the strategies that can be used to increase the survival rate of probiotics in these food matrices.
Innovations and Future Trends in the Encapsulation of Probiotics Using the Emulsion Technique
The encapsulation of probiotics using the emulsion technique is constantly advancing. Studies on new encapsulation methods that produce microparticles of small size, with high encapsulation yield, and less damage to probiotic strains during the encapsulation process should still be developed.
Membrane Emulsification
Membrane emulsification is a relatively new technology and has been reported as a method for encapsulating probiotic microorganisms in only two studies [83, 119]. Microencapsulation of probiotic strains using this technique is based on forcing the dispersed phase of an emulsion through the pores of a membrane into the continuous phase. Droplets grow at pore outlets until detaching after reaching a certain size. The detachment of these droplets from the membrane surface can also be favored by the application of shear forces [120]. As the droplets of the hydrocolloid detach, a continuous phase envelope is formed on the surface, generating the emulsion (W/O). Then, this emulsion is poured into an acetic acid solution to allow the hydrocolloid gelation, as described in "Preparation of the Emulsion for Encapsulation".
Metal membranes with a micro-sieve format are more appropriate for encapsulating probiotics since they have straight rectilinear pores in a regular array. The lack of a tortuous pore channel minimizes membrane fouling, facilitates cleaning, and yield higher fluxes [83]. Figure 7 shows the flow diagram of the process for obtaining probiotic microcapsules using membrane emulsification.
Flow diagram of the process of obtaining probiotic microcapsules using membrane emulsification [121]
Song et al. [119] used a microporous glass membrane (SPG) with a pore diameter ranging from 4—6.4 μm to encapsulate Lacticaseibacillus casei YIT 9018. The dried microcapsules obtained had a size between 31 and 52 μm and were stable during storage at 4 °C for 42 days (104–109 log UFC g−1), and resistant to gastric fluid and bile (106 log UFC g−1). Similarly, Morelli et al. [83] used a flat disk metal membrane with a pore diameter of 30 μm to encapsulate Saccharomyces cerevisiae cells. The size of the droplets (wet suspension) containing microencapsulated S. cerevisiae cells ranged between 60 and 340 μm. The size of the microcapsules favored the incorporation in food matrices (without being sensorially perceived by consumers), but the authors did not investigate this effect.
Several studies have investigated and detailed the main factors related to the membrane emulsification process (Fig. 7) and how they affect the microcapsules prepared [120, 122,123,124,125,126,127,128,129,130]. The knowledge helps researchers and industrial operators to obtain microcapsules with a uniform size distribution (monodisperse particles) and reduced size. According to Charcosset et al. [122], the factors are classified in: (i) membrane parameters (mean pore size and pore size distribution, wettability, porosity, number of active pores, permeability, and thickness); (ii) phase parameters (interfacial tension, emulsifier type and concentration, viscosity, and density of continuous and dispersed phases); (iii) process parameters (shear stress at the membrane surface, transmembrane pressure, and temperature).
Mean pore size largely affects the droplet size. It is believed that the droplet diameter, \({d}_{g}\), increases with the average membrane pore diameter, \({d}_{p}\), by a linear relationship (Eq. 1), where \(c\) depends on the operating conditions.
Monodisperse emulsions can be produced if the pore size distribution of the membrane is narrow enough. For instance, Song et al. [119] used a microporous glass membrane (SPG) with a pore diameter ranging from 4—6.4 μm to encapsulate Lacticaseibacillus casei YIT 9018. The droplets of the emulsions containing the probiotic had a diameter between 20 and 32 μm.
Membrane wettability can affect the average size and size distribution of droplets. Membranes that are not completely wetted by the continuous phase often form emulsions with a high degree of dispersion and larger average droplet size. Pore wetting with the dispersed phase should be avoided to guarantee the successful production of monodispersed emulsions [83]. Therefore, in the production of W/O emulsions, the membrane should be thoroughly wetted by the continuous oil phase, to minimize the spreading of the dispersed phase on the membrane.
The porosity of the membrane surface is essential because it determines the distance between the two adjacent pores [131]. Suárez et al. [132] recommended that the distance between the pores should be ten times the size of the pores. This rule ensures that two adjacent forming droplets do not contact each other, which could lead to coalescence.
The presence of emulsifiers or surfactants in the phases play two relevant roles in forming an emulsion: i) reduction of the interfacial tension between oil and water, facilitating the distribution of droplets and, in the case of membranes, decreasing the minimum emulsification pressure; and ii) stabilization of drops against coalescence [133]. Sorbitan monooleate, Polysorbates, PGPR, and Panodan SDK, have been the main emulsifying agents used to stabilize probiotic emulsions [4, 14, 40, 110, 113]. Furthermore, lecithin [38] and Saccharomyces cerevisiae bioemulsifier [134] have been explored as surfactants of probiotic emulsions. According to Schröder et al. [135], Tween 20 emulsifier can produce emulsions with droplet diameters about twice as large as when stabilized with Sodium Dodecyl Sulfate (SDS). So far, there are no studies in the literature that have investigated the influence of emulsifier type or emulsifier concentration on the distribution and final droplet size of probiotic emulsions obtained by membrane emulsification. Therefore, future research may focus on this field.
The viscosity of the dispersed phase also has an important effect on the performance of the membrane emulsification process. According to Darcy's law (Eq. 2) the flux of the dispersed phase (\({J}_{d}\)) is inversely proportional to the viscosity of this phase, i.e., when the viscosity is high, the flux will be low and, therefore, the droplet diameter will be large compared to the average pore diameter.
where \(K\) is the membrane permeability, \({\Delta P}_{tm}\) the transmembrane pressure, μ the dispersed phase viscosity, and \(L\) the membrane thickness.
As mentioned earlier, the dispersed phase droplets are formed at the membrane/continuous phase interface and the detachment of these droplets is favored by the application of continuous phase shear stresses [120]. According to Kobayashi et al. [136], the droplet size becomes smaller as the shear stress increases. However, in the encapsulation of probiotics, high shear rates should be avoided to minimize the death of microorganisms. High shear rates can damage cell walls, causing cell lysis. Morelli et al. [83] and Vinner et al. [121] used shear rates of 200 and 250 rpm, respectively, in an encapsulation system similar to Fig. 7 to encapsulate biological materials.
The membrane emulsification process requires hydraulic pressure to drive the dispersed phase through the membrane to the continuous phase side. The transmembrane pressure (\({\Delta P}_{tm}\)) is defined as the difference between the pressure of the phase to be dispersed (Pd) and the average pressure in the continuous phase that flows through the membrane module (Eq. 3).
where Pc,in and Pc,out are the pressure of the flowing continuous phase at the inlet and at the outlet of the membrane module, respectively.
The applied transmembrane pressure required to make the dispersed phase flow through the membrane pores can be estimated from the capillary pressure (Eq. 4), assuming that the pores are ideal cylinders:
where \({P}_{c}\) is the critical pressure, \(\gamma\) the O/W interfacial tension, \(\theta\) the contact angle of the oil droplet against the membrane surface well wetted with the continuous phase, and \({d}_{p}\) the average pore diameter.
According to Darcy’s law (Eq. 2), the dispersed phase flux, \({J}_{d}\), is related to the difference in pressure applied to the membrane, i.e., an increase in pressure will increase the flux of the dispersed phase through the membrane. The pressure applied to the membrane must be carefully chosen since high fluxes tend to form droplets with larger size distribution and diameters due to the increase in the coalescence of the droplets on the membrane surface. Also, very high fluxes will form jets of dispersed phase rather than droplets with the trapped probiotic. It is also worth emphasizing that very high pressures can damage the plasmatic membrane of probiotic cells, causing a decrease in the viable cell count. For the microporous glass membrane (SPG) with a pore diameter ranging from 4—6.4 μm, Song et al. [119] used pressures of 100 to 190 kPa to encapsulate Lacticaseibacillus casei YIT 9018.
A mathematical model (Eq. 5) has been used to predict the droplet size [127, 137,138,139] produced in a dispersion dead-end cell depicted in Fig. 7. The droplet diameter \((x)\) is calculated from a force balance of the capillary force (function of interfacial tension and pore size) and the drag force (function of shear stress and the droplet size) acting on a strongly deformed droplet at a single membrane pore.
where \({r}_{p}\) is the pore radius, \(T\) is the maximal shear stress, and \(\gamma\) is the interfacial tension. The maximal shear stress over the entire membrane area is calculated according to Eq. 6.
where \({\mu }_{c}\) is the continuous phase viscosity, \(\omega\) is the angular velocity, \({r}_{c}\) is the critical radius, which corresponds to the point where the rotation changes from a forced vortex to a free vortex, at which shear stress is greatest, calculated using Eq. 8, and \(\delta\) is the boundary layer thickness, given by Eq. 7.
where ρ is the continuous phase density.
where \(D\) is the stirrer diameter, \(T\) is the tank (cell) diameter,\(b\) is the blade height, \({n}_{b}\) is the number of impeller blades, and \(Re\) is the Reynolds number, given by Eq. 9.
Microfluidic Emulsification
Microfluidic emulsification is another emerging technology with great potential for encapsulating probiotic microorganisms. So far, to the best of our knowledge, only two studies evaluated this technology to encapsulate probiotics [95, 140]. The generation of droplets in microfluidic devices involves the injection of the dispersed phase through a single microchannel (MC) into another perpendicular MC carrying the continuous phase (T junction) or break-up of coaxial streams of immiscible liquids in a narrow orifice, which is called flow focusing. The T-junction is the simplest microfluidic structure to make droplets, and therefore the most used [141]. Figure 8 shows the flow diagram of the process for obtaining probiotic microcapsules using microfluidic emulsification. Martinez et al. [95] and Ekanem et al. [140] used microfluidic emulsification to encapsulate Saccharomyces cerevisiae cells. Ekanem et al. [140] found a high encapsulation yield (96%) and a very small dried capsule size (5–7 μm). Conversely, the droplets (wet suspension) obtained by Martinez et al. [95] remained in the size range of 60–230 μm. As with membrane emulsification, the small size of the microparticles or droplets was in the range indicated for incorporation into food. Future studies could focus on the incorporation of these microparticles in food. In addition, it is necessary to evaluate the long-term storage and in vitro digestion of these probiotic microcapsules.
To understand the mechanism of droplet breakage in the system (Fig. 8), it is usual to analyze the influence of viscous stress and pressure around the droplet. In addition, the geometry of the junction where the drop is detaching is of great importance and determines what forces are acting to cause detachment. For the T-junction structure (Fig. 8), the interfacial force tends to pull the forming droplets towards the nozzle orifice. Drop formation starts when the viscous force overcomes the clamping force due to the interfacial force and the drops are formed very close to the orifice of the capillary injection [142].
Some dimensionless numbers are important for understanding the system behavior and the drops formation. The Reynolds number (\(Re\)), Eq. 9, describes the relationship between the inertial and viscous forces and indicates the flow regime [143].
where \(\rho\) is the density of the fluid, \(V\) is the average flow velocity in the channel, \(D\) is the diameter of the channel, and \(\mu\) is the viscosity of the fluid.
Microfluidic systems operate with typically laminar flows (\(Re\) < 2000). For emulsion production systems (microfluidic emulsification) it is common to operate with numbers \(Re\) < < 1, ensuring greater droplet size stability.
The capillarity number (\(Ca\)), Eq. 10, relates the viscous forces and surface tension that act at the interface between two immiscible fluids. It is defined as a function of the continuous phase of the system [144].
where \({\mu }_{c}\) is the viscosity of the continuous phase, \({V}_{c}\) is the flow velocity of the continuous phase, which can be described in terms of the flow rate \(({Q}_{c})\) and channel geometry. \({V}_{c}= \frac{{Q}_{c}}{area}\) and \(\gamma\) is the interfacial tension between the two immiscible fluids.
As the capillarity number increases, the size of the drop is decreased. When \(Ca\) > 1, shear dominates the cutting mechanism. For \(Ca\) range between 0.01 and 1, there is a combination of shear and pressure, and for \(Ca\) < 0.1, there is a predominance of pressure on the droplet detaching mechanism.
Weber's number \((We)\), Eq. 11, is used to characterize droplet formation and is applied to the dispersed phase [145].
where \({\rho }_{d}\) is the density of the dispersed fluid, \({V}_{d}\) is the flow velocity of the dispersed fluid, and \(\gamma\) is the interfacial tension between the two immiscible fluids. Abate et al. [146] observed that with an increase in the Weber number, the drops of the dispersed phase detached faster than in lower \(We\).
Garstecki et al. [147] proposed a method (Eq. 12) to calculate the droplet diameter \({(D}_{d})\) when using a T-junction microfluidic device.
where \({D}_{d}\) is the diameter of the dispersed phase channel, \(\alpha\) is a proportionality constant dependent on the geometry of the microfluidic device, \({Q}_{d}\) is the flow rate of the dispersed phase fluid, and \({Q}_{c}\) is the flow rate of the continuous phase fluid.
Several advantages have been attributed to the use of membrane emulsification and microfluidic emulsification for the production of probiotic microcapsules: (i) production of uniformly sized and controlled-sized particles (control by appropriate membrane pore size selection), (ii) particles with a low polydispersity (CV = standard deviation / mean < 3%) [141], (iii) low shear stress, (iv) energy requirement reduction, (v) high flexible plant use and (vi) operation under mild conditions [125]. The set of these characteristics demonstrates that these methods are very promising in microencapsulation of probiotic strains, because these microorganisms are sensitive to shear and temperature, and also, the control of the final size of the microcapsule is a valuable advantage for the applications in foods.
In addition to the development of emerging emulsion methods to encapsulate probiotics, in vivo studies that demonstrate the behavior of emulsion-encapsulated probiotics during passage through the gastrointestinal tract are needed. So far, only a couple of papers in the literature have studied the survival rate of these microorganisms microencapsulated by emulsion in animal models. In the study of Oguntoye et al. [91], forty male Wistar rats were fed for fifteen days with a provitamin A cassava hydrolysate containing free and encapsulated Lacticaseibacillus rhamnosus GG (LGG) (wet suspension). The fecal microbial population was determined in rats for days 15 and 30 post-administration of provitamin A cassava hydrolysate without or with LGG. The authors found that emulsion encapsulation improved LGG survival during exposure to in vivo gastrointestinal conditions. The encapsulated LGG was able to outcompete total aerobes and other pathogenic organisms in the intestine and eventually colonize the intestine. According to Adak et al. [148], LGG is a facultative anaerobic bacterium that creates anaerobiosis in the environment, competes with pathogenic bacteria for nutrients and binding sites, and produces bacteriostatic compounds that limit the activity and growth of aerobic and other pathogenic organisms. In the study of Rodklongtan et al. [149], microcapsules (wet suspension) containing Limosilactobacillus reuteri KUB-AC were incorporated into an animal feed for chickens. A group consisting of five chickens was randomly divided into two groups consisting of two in the control and three in the probiotic treated group. The authors concluded that the encapsulation of the probiotics protected the cell from acid-induced cell death in the upper digestive tract of chickens and delivered the cells in sufficient numbers in the intestine.
Conclusions
The emulsification technique is a useful tool to improve the survival of probiotic bacteria during processing, storage, and gastrointestinal passage. When considering encapsulating agents, studies have shown that the use of alginate in combination with chitosan or prebiotics extends the life of probiotic strains during storage and ensures safe delivery to the intestine. Furthermore, the coating of the microparticles with chitosan, xanthan gum, resistant starch, or alginate, and the use of multiple emulsions improves the encapsulation performance and guarantees greater protection to probiotic microorganisms during storage and movement through the digestive tract. Nevertheless, the coating increases the final size of the microparticle. The use of by-products from the food industry, especially those with prebiotic properties, is highlighted since such a procedure can stabilize probiotic microorganisms and add value to industrial waste. Freeze-drying is the most suitable drying technique for drying probiotic microcapsules due to the mild process conditions. However, more attention should be given to fluidized bed drying and critical point drying.
Encapsulation technologies that operate under mild process conditions and that produce microcapsules with reduced size are still required. Membrane emulsification and microfluidics methods have great potential to meet such demands. The different beneficial effects of next-generation probiotics encourage future studies focusing on using technologies that guarantee the safe delivery of these microorganisms to the intestine, e.g., microencapsulation by emulsification. In addition, one of the research trends in this area is to incorporate probiotics into non-dairy foods. Non-dairy products represented 63% of applications with probiotics microencapsulated by emulsion techniques, demonstrating the researchers' concern to serve other consumer groups, e.g., vegans.
References
Hill C, Guarner F, Reid G et al (2014) The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 11:506–514
Dias CO, dos Santos Opuski de Almeida J, Pinto SS et al (2018) Development and physico-chemical characterization of microencapsulated bifidobacteria in passion fruit juice: A functional non-dairy product for probiotic delivery. Food Biosci 24:26–36
Pimentel TC, da Costa WKA, Barão CE et al (2021) Vegan probiotic products: A modern tendency or the newest challenge in functional foods. Food Res Int 140:110033
Ashwar BA, Gani A, Gani A et al (2018) Production of RS4 from rice starch and its utilization as an encapsulating agent for targeted delivery of probiotics. Food Chem 239:287–294
de Liz GR, Verruck S, Canella MHM et al (2020) Stability of bifidobacteria entrapped in goat’s whey freeze concentrate and inulin as wall materials and powder properties. Food Res Int 127:108752
Prestes AA, Verruck S, Vargas MO et al (2021) Influence of guabiroba pulp (campomanesia xanthocarpa o. berg) added to fermented milk on probiotic survival under in vitro simulated gastrointestinal conditions. Food Res Int 141:110135
Cassani L, Gomez-Zavaglia A, Simal-Gandara J (2020) Technological strategies ensuring the safe arrival of beneficial microorganisms to the gut: From food processing and storage to their passage through the gastrointestinal tract. Food Res Int 129:108852
Rodrigues FJ, Cedran MF, Bicas JL, Sato HH (2020) Encapsulated probiotic cells: Relevant techniques, natural sources as encapsulating materials and food applications – A narrative review. Food Res Int 137:109682
Ghibaudo F, Gerbino E, Campo Dall’ Orto V, Gómez-Zavaglia A (2017) Pectin-iron capsules: Novel system to stabilise and deliver lactic acid bacteria. J Funct Foods 39:299–305
Oberoi K, Tolun A, Sharma K, Sharma S (2019) Microencapsulation: An overview for the survival of probiotic bacteria. J Microbiol Biotechnol Food Sci 9:280–287
Martín MJ, Lara-Villoslada F, Ruiz MA, Morales ME (2015) Microencapsulation of bacteria: A review of different technologies and their impact on the probiotic effects. Innov Food Sci Emerg Technol 27:15–25
Ðordevic´V, Balanč B, Belščak-Cvitanovic´A et al (2015) Trends in Encapsulation Technologies for Delivery of Food Bioactive Compounds. Food Eng Rev 7:452–490
Dehkordi SS, Alemzadeh I, Vaziri AS, Vossoughi A (2020) Optimization of Alginate-Whey Protein Isolate Microcapsules for Survivability and Release Behavior of Probiotic Bacteria. Appl Biochem Biotechnol 190:182–196
Beldarrain-Iznaga T, Villalobos-Carvajal R, Leiva-Vega J, Sevillano Armesto E (2020) Influence of multilayer microencapsulation on the viability of Lactobacillus casei using a combined double emulsion and ionic gelation approach. Food Bioprod Process 124:57–71
Ma L, Shang Y, Zhu Y et al (2020) Study on microencapsulation of Lactobacillus plantarum LIP-1 by emulsification method. J Food Process Eng 43:e13437
de Paula DA, Martins EMF, de Almeida Costa N et al (2019) Use of gelatin and gum arabic for microencapsulation of probiotic cells from Lactobacillus plantarum by a dual process combining double emulsification followed by complex coacervation. Int J Biol Macromol 133:722–731
Eratte D, Dowling K, Barrow CJ, Adhikari B (2018) Recent advances in the microencapsulation of omega-3 oil and probiotic bacteria through complex coacervation: A review. Trends Food Sci Technol 71:121–131
Anselmo AC, McHugh KJ, Webster J et al (2016) Layer-by-Layer Encapsulation of Probiotics for Delivery to the Microbiome. Adv Mater 28:9486–9490
Ghorani B, Tucker N (2015) Fundamentals of electrospinning as a novel delivery vehicle for bioactive compounds in food nanotechnology. Food Hydrocoll 51:227–240
Wang L, Song M, Zhao Z et al (2020) Lactobacillus acidophilus loaded pickering double emulsion with enhanced viability and colon-adhesion efficiency. LWT - Food Sci Technol 121:108928
Heidebach T, Först P, Kulozik U (2012) Microencapsulation of Probiotic Cells for Food Applications. Crit Rev Food Sci Nutr 52:291–311
Krasaekoopt W, Bhandari B, Deeth H (2003) Evaluation of encapsulation techniques of probiotics for yoghurt. Int Dairy J 13:3–13
Lu Y, Mao L, Hou Z et al (2019) Development of Emulsion Gels for the Delivery of Functional Food Ingredients: from Structure to Functionality. Food Eng Rev 11:245–258
Cook MT, Tzortzis G, Charalampopoulos D, Khutoryanskiy VV (2012) Microencapsulation of probiotics for gastrointestinal delivery. J Control Release 162:56–67
Rokka S, Rantamäki P (2010) Protecting probiotic bacteria by microencapsulation: Challenges for industrial applications. Eur Food Res Technol 231:1–12
Rosas-Flores W, Ramos-Ramírez EG, Salazar-Montoya JA (2013) Microencapsulation of Lactobacillus helveticus and Lactobacillus delbrueckii using alginate and gellan gum. Carbohydr Polym 98:1011–1017
Eshrati M, Amadei F, Van De Wiele T et al (2018) Biopolymer-Based Minimal Formulations Boost Viability and Metabolic Functionality of Probiotics Lactobacillus rhamnosus GG through Gastrointestinal Passage. Langmuir 34:11167–11175
Dong LM, Luan NT, Thuy DTK (2020) The viability of encapsulated Lactobacillus plantarum during cupcake baking process, storage, and simulated gastric digestion. J Microbiol Biotechnol Food Sci 9:1157–1161
Khosravi Zanjani MA, Ehsani MR, Tarzi BG, Sharifan A (2018) Promoting probiotics survival by microencapsualtion with hylon starch and genipin cross-linked coatings in simulated gastro-intestinal condition and heat treatment. Iran J Pharm Res 17:753–766
Qi W, Liang X, Yun T, Guo W (2019) Growth and survival of microencapsulated probiotics prepared by emulsion and internal gelation. J Food Sci Technol 56:1398–1404
Zheng H, Gao M, Ren Y et al (2017) An improved pH-responsive carrier based on EDTA-Ca-alginate for oral delivery of Lactobacillus rhamnosus ATCC 53103. Carbohydr Polym 155:329–335
Ji R, Wu J, Zhang J et al (2019) Extending viability of Bifidobacterium longum in chitosan-coated alginate microcapsules using emulsification and internal gelation encapsulation technology. Front Microbiol 10:1–10
Ramos PE, Cerqueira MA, Teixeira JA, Vicente AA (2018) Physiological protection of probiotic microcapsules by coatings. Crit Rev Food Sci Nutr 58:1864–1877
Chen H, Li X, Liu B, Meng X (2017) Microencapsulation of Lactobacillus bulgaricus and survival assays under simulated gastrointestinal conditions. J Funct Foods 29:248–255
Kamalian N, Mirhosseini H, Mustafa S et al (2014) Effect of alginate and chitosan on viability and release behavior of Bifidobacterium pseudocatenulatum G4 in simulated gastrointestinal fluid. Carbohydr Polym 111:700–706
Calinoiu LF, Ştefanescu BE, Pop ID et al (2019) Chitosan coating applications in probiotic microencapsulation. Coatings 9:1–21
Quintana G, Gerbino E (2018) Valorization of okara oil for the encapsulation of Lactobacillus plantarum. Food Res Int 106:81–89
Ayama H, Sumpavapol P, Chanthachum S (2014) Effect of encapsulation of selected probiotic cell on survival in simulated gastrointestinal tract condition. Songklanakarin J Sci Technol 36:291–299
Graça J, Godinho CA, Truninger M (2019) Reducing meat consumption and following plant-based diets: Current evidence and future directions to inform integrated transitions. Trends Food Sci Technol 91:380–390
Cavalheiro CP, Ruiz-Capillas C, Herrero AM et al (2020) Effect of encapsulated Lactobacillus plantarum as probiotic on dry-sausages during chilled storage. Int J Food Sci Technol 55:3613–3621
Cavalheiro CP, Ruiz-Capillas C, Herrero AM et al (2019) Effect of different strategies of Lactobacillus plantarum incorporation in chorizo sausages. J Sci Food Agric 99:6706–6712
Raddatz GC, de Souza da Fonseca B, Poletto G et al (2020) Influence of the prebiotics hi-maize, inulin and rice bran on the viability of pectin microparticles containing Lactobacillus acidophilus LA-5 obtained by internal gelation/emulsification. Powder Technol 362:409–415
Thangrongthong S, Puttarat N, Ladda B et al (2020) Microencapsulation of probiotic Lactobacillus brevis ST-69 producing GABA using alginate supplemented with nanocrystalline starch. Food Sci Biotechnol 29:1475–1482
Sengsaengthong S, Oonsivilai R (2019) Effect of microencapsulation of Lactobacillus sp. 21C2-10 isolated from cassava pulp on physicochemical, sensorial and microbiological characteristics of ice cream. Int Food Res J 26:585–594
Cremon C, Barbaro MR, Ventura M, Barbara G (2018) Pre- and probiotic overview. Curr Opin Pharmacol 43:87–92
George Kerry R, Patra JK, Gouda S et al (2018) Benefaction of probiotics for human health: A review. J Food Drug Anal 26:927–939
Kirmiz N, Galindo K, Cross KL et al (2020) Comparative Genomics Guides Elucidation of Vitamin B12 Biosynthesis in Novel Human-Associated Akkermansia Strains. Appl Environ Microbiol 86:e02117-e2119
van der Ark KCH, Nugroho ADW, Berton-Carabin C et al (2017) Encapsulation of the therapeutic microbe Akkermansia muciniphila in a double emulsion enhances survival in simulated gastric conditions. Food Res Int 102:372–379
Jiang T, Wu H, Yang X et al (2020) Lactobacillus mucosae strain promoted by a high-fiber diet in genetic obese child alleviates lipid metabolism and modifies gut microbiota in apoe-/- mice on a western diet. Microorganisms 8:1–17
Namai F, Murakami A, Ueda A et al (2020) Construction of Genetically Modified Lactococcus lactis Producing Anti-human-CTLA-4 Single-Chain Fragment Variable. Mol Biotechnol 62:572–579
Vernay T, Cannie I, Gaboriau F et al (2020) Bacteroides fragilis prevents Salmonella Heidelberg translocation in co-culture model mimicking intestinal epithelium. Benef Microbes 11:391–401
Deng H, Yang S, Zhang Y et al (2018) Bacteroides fragilis Prevents Clostridium difficile Infection in a Mouse Model by Restoring Gut Barrier and Microbiome Regulation. Front Microbiol 9:1–12
Hiippala K, Kainulainen V, Suutarinen M et al (2020) Isolation of anti-inflammatory and epithelium reinforcing Bacteroides and Parabacteroides spp. From a healthy fecal donor Nutrients 12:935
Del Pulgar EMG, Benítez-Páez A, Sanz Y (2020) Safety assessment of Bacteroides uniformis CECT 7771, a symbiont of the gut microbiota in infants. Nutrients 12:551
El Hage R, Hernandez-Sanabria E, Calatayud Arroyo M et al (2019) Propionate-producing consortium restores antibiotic-induced dysbiosis in a dynamic in vitro model of the human intestinal microbial ecosystem. Front Microbiol 10:1–17
Bhat B, Bajaj BK (2018) Hypocholesterolemic and bioactive potential of exopolysaccharide from a probiotic Enterococcus faecium K1 isolated from kalarei. Bioresour Technol 254:264–267
Kanmani P, Clua P, Vizoso-Pinto MG et al (2017) Respiratory commensal bacteria Corynebacterium pseudodiphtheriticum improves resistance of infant mice to respiratory syncytial virus and Streptococcus pneumoniae superinfection. Front Microbiol 8:1–14
Martín R, Miquel S, Benevides L et al (2017) Functional characterization of novel Faecalibacterium prausnitzii strains isolated from healthy volunteers: A step forward in the use of F. prausnitzii as a next-generation probiotic. Front Microbiol 8:1–13
Selma MV, Beltrán D, Luna MC et al (2017) Isolation of human intestinal bacteria capable of producing the bioactive metabolite isourolithin a from ellagic acid. Front Microbiol 8:1–8
Saarela MH (2019) Safety aspects of next generation probiotics. Curr Opin Food Sci 30:8–13
Barragán-Martínez LP, Totosaus A, de Lourdes P-C (2020) Probiotication of cooked sausages employing agroindustrial coproducts as prebiotic co-encapsulant in ionotropic alginate–pectin gels. Int J Food Sci Technol 55:1088–1096
Krithika B, Preetha R (2019) Formulation of protein based inulin incorporated synbiotic nanoemulsion for enhanced stability of probiotic. Mater Res Express 6:114003
Marcial-Coba MS, Cieplak T, Cahú TB et al (2018) Viability of microencapsulated: Akkermansia muciniphila and Lactobacillus plantarum during freeze-drying, storage and in vitro simulated upper gastrointestinal tract passage. Food Funct 9:5868–5879
Marcial-Coba MS, Saaby L, Knøchel S, Nielsen DS (2019) Dark chocolate as a stable carrier of microencapsulated Akkermansia muciniphila and Lactobacillus casei. FEMS Microbiol Lett 366:1–6
Kumherová M, Veselá K, Jokešová K et al (2020) Influence of co-encapsulation of Bifidobacterium animalis subsp. lactis BB-12 with inulin and ascorbic acid on its viability. Czech J Food Sci 38:57–62
Gharsallaoui A, Roudaut G, Chambin O et al (2007) Applications of spray-drying in microencapsulation of food ingredients: An overview. Food Res Int 40:1107–1121
de la Cruz P-C, Ortega D, García-Triana A et al (2020) A brief review of edible coating materials for the microencapsulation of probiotics. Coatings 10:1–36
Shafizadeh A, Golestan L, Ahmadi M et al (2020) Encapsulation of Lactobacillus casei in alginate microcapsules: improvement of the bacterial viability under simulated gastrointestinal conditions using flaxseed mucilage. J Food Meas Charact 14:1901–1908
Xiao Y, Han C, Yang H et al (2020) Layer (whey protein isolate) -by-layer (xanthan gum) microencapsulation enhances survivability of L. bulgaricus and L. paracasei under simulated gastrointestinal juice and thermal conditions. Int J Biol Macromol 148:238–247
Khosravi MA, Mohammad Z, Ehsani R et al (2018) Promoting Lactobacillus casei and Bifidobacterium adolescentis survival by microencapsulation with different starches and chitosan and poly L-lysine coatings in ice cream. J Food Process Preserv 42:e13318
Holkem AT, Raddatz GC, Nunes GL et al (2016) Development and characterization of alginate microcapsules containing Bifidobacterium BB-12 produced by emulsification/internal gelation followed by freeze drying. LWT - Food Sci Technol 71:302–308
Phoem AN, Chanthachum S, Voravuthikunchai SP (2015) Preparation of Eleutherine americana-alginate complex microcapsules and application in Bifidobacterium longum. Nutrients 7:831–848
Song H, Yu W, Liu X, Ma X (2014) Improved probiotic viability in stress environments with post-culture of alginate-chitosan microencapsulated low density cells. Carbohydr Polym 108:10–16
Papagianni M, Anastasiadou S (2009) Encapsulation of Pediococcus acidilactici cells in corn and olive oil microcapsules emulsified by peptides and stabilized with xanthan in oil-in-water emulsions: Studies on cell viability under gastro-intestinal simulating conditions. Enzyme Microb Technol 45:514–522
Sánchez MT, Ruiz MA, Lasserrot A et al (2017) An improved ionic gelation method to encapsulate Lactobacillus spp. bacteria: Protection, survival and stability study. Food Hydrocoll 69:67–75
Raddatz GC, Poletto G, de Deus C et al (2020) Use of prebiotic sources to increase probiotic viability in pectin microparticles obtained by emulsification/internal gelation followed by freeze-drying. Food Res Int 130:108902
Campos E, Branquinho J, Carreira AS et al (2013) Designing polymeric microparticles for biomedical and industrial applications. Eur Polym J 49:2005–2021
Yao M, Xie J, Du H et al (2020) Progress in microencapsulation of probiotics: A review. Compr Rev Food Sci Food Saf 19:857–874
Mokhtari S, Jafari SM, Khomeiri M et al (2017) The cell wall compound of Saccharomyces cerevisiae as a novel wall material for encapsulation of probiotics. Food Res Int 96:19–26
Marcial-Coba MS, Knøchel S, Nielsen DS (2019) Low-moisture food matrices as probiotic carriers. FEMS Microbiol Lett 366:1–11
Holkem TA, Raddatz CG, Barin SJ et al (2017) Production of microcapsules containing Bifidobacterium BB-12 by emulsification / internal gelation. LWT - Food Sci Technol 76:216–221
Zhang F, Li XY, Park HJ, Zhao M (2013) Effect of microencapsulation methods on the survival of freeze-dried Bifidobacterium bifidum. J Microencapsul 30:511–518
Morelli S, Holdich RG, Dragosavac MM (2017) Microparticles for cell encapsulation and colonic delivery produced by membrane emulsification. J Memb Sci 524:377–388
Smilkov K, Petreska Ivanovska T, Petrushevska Tozi L et al (2014) Optimization of the formulation for preparing Lactobacillus casei loaded whey protein-Ca-alginate microparticles using full-factorial design. J Microencapsul 31:166–175
Bernucci BSP, Loures CMG, Sabino AP et al (2017) Effect of microencapsulation conditions on the viability and functionality of Bifidobacterium longum 51A. LWT - Food Sci Technol 80:341–347
Kwiecień I, Kwiecień M (2018) Application of Polysaccharide-Based Hydrogels as Probiotic Delivery Systems. Gels 4:47
Liu L, Li X, Zhu Y et al (2016) Effect of microencapsulation with Maillard reaction products of whey proteins and isomaltooligosaccharide on the survival of Lactobacillus rhamnosus. LWT - Food Sci Technol 73:37–43
Šipailienė A, Petraitytė S (2018) Encapsulation of Probiotics: Proper Selection of the Probiotic Strain and the Influence of Encapsulation Technology and Materials on the Viability of Encapsulated Microorganisms. Probiotics Antimicrob Proteins 10:1–10
Juárez-Trujillo N, Jiménez-Fernández M, Franco-Robles E et al (2021) Effect of three-stage encapsulation on survival of emulsified Bifidobacterium animalis subsp. Lactis during processing, storage and simulated gastrointestinal tests. LWT - Food Sci Technol 137:110468
Kataria A, Achi SC, Halami PM (2018) Effect of Encapsulation on Viability of Bifidobacterium longum CFR815j and Physiochemical Properties of Ice Cream. Indian J Microbiol 58:248–251
Oguntoye MA, Ezekiel OO, Oridupa OA (2021) Viability of Lactobacillus rhamnosus GG in provitamin A cassava hydrolysate during fermentation, storage, in vitro and in vivo gastrointestinal conditions. Food Biosci 40:100845
El Kadri H, Lalou S, Mantzouridou FT, Gkatzionis K (2018) Utilisation of water-in-oil-water (W1/O/W2) double emulsion in a set-type yogurt model for the delivery of probiotic Lactobacillus paracasei. Food Res Int 107:325–336
Gul O (2017) Microencapsulation of Lactobacillus casei Shirota by spray drying using different combinations of wall materials and application for probiotic dairy dessert. J Food Process Preserv 41:e13198
Horáčková S, Rokytová K, Bialasová K et al (2018) Fruit juices with probiotics – New type of functional foods. Czech J Food Sci 36:284–288
Martinez CJ, Kim JW, Ye C et al (2012) A Microfluidic Approach to Encapsulate Living Cells in Uniform Alginate Hydrogel Microparticles. Macromol Biosci 12:946–951
Picone CSF, Bueno AC, Michelon M, Cunha RL (2017) Development of a probiotic delivery system based on gelation of water-in-oil emulsions. LWT - Food Sci Technol 86:62–68
Broeckx G, Vandenheuvel D, Claes IJJ et al (2016) Drying techniques of probiotic bacteria as an important step towards the development of novel pharmabiotics. Int J Pharm 505:303–318
Heylen K, Hoefman S, Vekeman B et al (2012) Safeguarding bacterial resources promotes biotechnological innovation. Appl Microbiol Biotechnol 94:565–574
Dianawati D, Shah NP (2011) Survival, Acid and Bile Tolerance, and Surface Hydrophobicity of Microencapsulated B. animalis ssp. lactis BB-12 during Storage at Room Temperature. J Food Sci 76:M92–M99
Sosnik A, Seremeta KP (2015) Advantages and challenges of the spray-drying technology for the production of pure drug particles and drug-loaded polymeric carriers. Adv Colloid Interface Sci 223:40–54
Huang S, Vignolles ML, Chen XD et al (2017) Spray drying of probiotics and other food-grade bacteria: A review. Trends Food Sci Technol 63:1–17
Perdana J, Bereschenko L, Fox MB et al (2013) Dehydration and thermal inactivation of Lactobacillus plantarum WCFS1: Comparing single droplet drying to spray and freeze drying. Food Res Int 54:1351–1359
Perdana J, Fox MB, Siwei C et al (2014) Interactions between formulation and spray drying conditions related to survival of Lactobacillus plantarum WCFS1. Food Res Int 56:9–17
Stummer S, Toegel S, Rabenreither MC et al (2012) Fluidized-bed drying as a feasible method for dehydration of Enterococcus faecium M74. J Food Eng 111:156–165
Bilenler T, Karabulut I, Candogan K (2017) Effects of encapsulated starter cultures on microbial and physicochemical properties of traditionally produced and heat treated sausages (sucuks). LWT - Food Sci Technol 75:425–433
Muthukumarasamy P, Holley RA (2006) Microbiological and sensory quality of dry fermented sausages containing alginate-microencapsulated Lactobacillus reuteri. Int J Food Microbiol 111:164–169
Dong LM, Luan NT, Thuy DTK (2020) Enhancing the viability rate of probiotic by co-encapsulating with prebiotic in alginate microcapsules supplemented to cupcake production. Microbiol Biotechnol Lett 48:113–120
Seyedain-Ardabili M, Sharifan A, Tarzi BG (2016) The production of synbiotic bread by microencapsulation. Food Technol Biotechnol 54:52–59
Amine KM, Champagne CP, Raymond Y et al (2014) Survival of microencapsulated Bifidobacterium longum in Cheddar cheese during production and storage. Food Control 37:193–199
Rodríguez-Huezo ME, Estrada-Fernández AG, García-Almendárez BE et al (2014) Viability of Lactobacillus plantarum entrapped in double emulsion during Oaxaca cheese manufacture, melting and simulated intestinal conditions. LWT - Food Sci Technol 59:768–773
Ozer B, Uzun YS, Kirmaci HA (2008) Effect of microencapsulation on viability of Lactobacillus acidophilus LA-5 and Bifidobacterium bifidum BB-12 during kasar cheese ripening. Int J Dairy Technol 61:237–244
Özer B, Kirmaci HA, Şenel E et al (2009) Improving the viability of Bifidobacterium bifidum BB-12 and Lactobacillus acidophilus LA-5 in white-brined cheese by microencapsulation. Int Dairy J 19:22–29
Huerta-Vera K, Flores-Andrade E, Pérez-Sato JA et al (2017) Enrichment of Banana with Lactobacillus rhamnosus Using Double Emulsion and Osmotic Dehydration. Food Bioprocess Technol 10:1053–1062
Flores-Andrade E, Pascual-Pineda LA, Alarcón-Elvira FG et al (2017) Effect of vacuum on the impregnation of Lactobacillus rhamnosus microcapsules in apple slices using double emulsion. J Food Eng 202:18–24
De Prisco A, Mauriello G (2016) Probiotication of foods: A focus on microencapsulation tool. Trends Food Sci Technol 48:27–39
Terpou A, Papadaki A, Lappa IK et al (2019) Probiotics in Food Systems: Significance and Emerging Strategies Towards Improved Viability and Delivery of Enhanced Beneficial Value. Nutrients 11:32
Khan MI, Arshad MS, Anjum FM et al (2011) Meat as a functional food with special reference to probiotic sausages. Food Res Int 44:3125–3133
Rubio R, Jofré A, Aymerich T et al (2014) Nutritionally enhanced fermented sausages as a vehicle for potential probiotic Lactobacilli delivery. Meat Sci 96:937–942
Song SH, Cho YH, Park J (2003) Microencapsulation of Lactobacillus casei YIT 9018 using a microporous glass membrane emulsification system. J Food Sci 68:195–200
Joscelyne SM, Trägårdh G (2000) Membrane emulsification - A literature review. J Memb Sci 169:107–117
Vinner GK, Richards K, Leppanen M et al (2019) Microencapsulation of enteric bacteriophages in a pH-Responsive solid oral dosage formulation using a scalable membrane emulsification process. Pharmaceutics 11:11090475
Charcosset C (2009) Preparation of emulsions and particles by membrane emulsification for the food processing industry. J Food Eng 92:241–249
Spyropoulos F, Lloyd DM, Hancocks RD, Pawlik AK (2014) Advances in membrane emulsification. Part B: Recent developments in modelling and scale-up approaches. J Sci Food Agric 94:628–638
Vladisavljević GT (2019) Preparation of microemulsions and nanoemulsions by membrane emulsification. Colloids Surfaces A Physicochem Eng Asp 579:123709
Piacentini E, Drioli E, Giorno L (2014) Membrane emulsification technology: Twenty-five years of inventions and research through patent survey. J Memb Sci 468:410–422
Spyropoulos F, Lloyd DM, Hancocks RD, Pawlik AK (2014) Advances in membrane emulsification. Part A: Recent developments in processing aspects and microstructural design approaches. J Sci Food Agric 94:613–627
Consolli L, Hubinger MD, Dragosavac MM (2020) Encapsulation of resveratrol using Maillard conjugates and membrane emulsification. Food Res Int 137:109359
Vladisavljević GT, Williams RA (2005) Recent developments in manufacturing emulsions and particulate products using membranes. Adv Colloid Interface Sci 113:1–20
Gijsbertsen-Abrahamse AJ, Van Der Padt A, Boom RM (2004) Status of cross-flow membrane emulsification and outlook for industrial application. J Memb Sci 230:149–159
Charcosset C, Limayem I, Fessi H (2004) Review The membrane emulsification process-a review. J Chem Technol Biotechnol 79:209–218
Williams RA (1998) Controlled Production of Emulsions Using a Crossflow Membrane: Part II: Industrial Scale Manufacture. Chem Eng Res Des 76:902–910
Suárez MA, Gutiérrez G, Coca J, Pazos C (2013) Stirred tank membrane emulsification using flat metallic membranes: A dimensional analysis. Chem Eng Process Process Intensif 69:31–43
McClements DJ, Jafari SM (2018) Improving emulsion formation, stability and performance using mixed emulsifiers: A review. Adv Colloid Interface Sci 251:55–79
Dikit P, H-Kittikun A, Maneerat S (2015) Survival of encapsulated potentially probiotic Lactobacillus plantarum D6SM3 with bioemulsifier derived from spent yeast in simulated gastrointestinal conditions. Songklanakarin J Sci Technol 37:425–432
Schröder V, Behrend O, Schubert H (1998) Effect of Dynamic Interfacial Tension on the Emulsification Process Using Microporous, Ceramic Membranes. J Colloid Interface Sci 202:334–340
Kobayashi I, Yasuno M, Iwamoto S et al (2002) Microscopic observation of emulsion droplet formation from a polycarbonate membrane. Colloids Surfaces A Physicochem Eng Asp 207:185–196
Dragosavac MM, Sovilj MN, Kosvintsev SR et al (2008) Controlled production of oil-in-water emulsions containing unrefined pumpkin seed oil using stirred cell membrane emulsification. J Memb Sci 322:178–188
Piacentini E, Giorno L, Dragosavac MM et al (2013) Microencapsulation of oil droplets using cold water fish gelatine/gum arabic complex coacervation by membrane emulsification. Food Res Int 53:362–372
Kosvintsev SR, Gasparini G, Holdich RG et al (2005) Liquid-Liquid Membrane Dispersion in a Stirred Cell with and without Controlled Shear. Ind Eng Chem Res 44:9323–9330
Ekanem EE, Zhang Z, Vladisavljević GT (2017) Facile microfluidic production of composite polymer core-shell microcapsules and crescent-shaped microparticles. J Colloid Interface Sci 498:387–394
Vladisavljević GT (2016) Recent advances in the production of controllable multiple emulsions using microfabricated devices. Particuology 24:1–17
Vladisavljević GT, Al Nuumani R, Nabavi SA (2017) Microfluidic Production of Multiple Emulsions. Micromachines 8:75
Ji Y, Bellettre J, Montillet A, Massoli P (2021) Effect of cross-slot configuration in microfluidics on o/w emulsification at high throughput.Microfluid Nanofluid 25:85
Nabavi SA, Vladisavljević GT, Bandulasena MV et al (2017) Prediction and control of drop formation modes in microfluidic generation of double emulsions by single-step emulsification. J Colloid Interface Sci 505:315–324
Abate AR, Thiele J, Weitz DA (2011) One-step formation of multiple emulsions in microfluidics. Lab Chip 11:253–258
Abate AR, Thiele J, Weinhart M, Weitz DA (2010) Patterning microfluidic device wettability using flow confinement. Lab Chip 10:1774–1776
Garstecki P, Fuerstman MJ, Stone HA, Whitesides GM (2006) Formation of droplets and bubbles in a microfluidic T-junction-scaling and mechanism of break-up. Lab Chip 6:347–446
Adak A, Maity C, Ghosh K et al (2013) Dynamics of predominant microbiota in the human gastrointestinal tract and change in luminal enzymes and immunoglobulin profile during high-altitude adaptation. Folia Microbiol (Praha) 58:523–528
Rodklongtan A, La-Ongkham O, Nitisinprasert S, Chitprasert P (2014) Enhancement of Lactobacillus reuteri KUB-AC5 survival in broiler gastrointestinal tract by microencapsulation with alginate-chitosan semi-interpenetrating polymer networks. J Appl Microbiol 117:227–238
Acknowledgements
The authors are grateful to CNPq (National Council for Scientific and Technological Development, Brazil) for the financial support (420275/2018-5, 307576/2018-3), and to CAPES (Coordination of Improvement of Higher Education Personnel) for the scholarship (PROEX).
Author information
Authors and Affiliations
Contributions
Callebe Camelo-Silva: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Writing-original draft. Silvani Verruck: Conceptualization, Data curation, Formal analysis, Methodology, Writing-review & editing. Alan Ambrosi: Conceptualization, Data curation, Formal analysis, Methodology, Writing-review & editing. Marco Di Luccio: Conceptualization, Formal analysis, Methodology, Funding acquisition, Project administration, Resources, Supervision, Writing-review & editing.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Camelo-Silva, C., Verruck, S., Ambrosi, A. et al. Innovation and Trends in Probiotic Microencapsulation by Emulsification Techniques. Food Eng Rev 14, 462–490 (2022). https://doi.org/10.1007/s12393-022-09315-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12393-022-09315-1