Abstract
Microencapsulation technology can be used to improve the probiotic viability under stress condition in the human gastrointestinal tract and during storage. The purpose of this study was to evaluate the protective effect of encapsulation materials on the survival of GABA-producing probiotics using alginate containing cassava starch nanocrystals under simulated gastrointestinal conditions and shelf storage. Lactobacillus brevis ST-69, GABA-producing probiotic strain, was isolated from kimchi and encapsulated using emulsion technique. The GABA activity, encapsulation efficiency, morphology, probiotic viability were evaluated. The encapsulation efficiency using emulsion technique was 89.72%. Probiotic encapsulated in alginate-nanocrystalline starch gel capsules showed high survival rate at 94.97% of probiotic cells under simulated gastrointestinal conditions and during long-life storage at 4 °C compared to free cells. Results showed that for improving the viability of probiotics against gastrointestinal and storage conditions, complex materials with nanocrystalline starch might be a better encapsulating matrix for the preparation of gel capsules.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Probiotics are defined as ‘‘Live microorganisms which when administered in adequate amounts confer a health benefit on the host” (FAO/WHO, 2002). Several probiotic lactic acid bacteria (LAB) strains are well-known source of food supplement in addition to gamma-aminobutyric acid (GABA) production, which exerts health effects. GABA-producing probiotic LAB strains have been isolated from the traditional fermented foods and beverages (Dhakal et al., 2012; Kook and Cho, 2013). Those probiotic LAB strains, L. brevis exhibits the highest capability to produce GABA (Shin et al., 2007; Li et al., 2010; Lim et al., 2017; Wu and Shah, 2018). Despite tremendous benefits of well-being to human, those bacteria with GABA-producing capability are susceptible to survive under hazardous conditions (i.e., manufacture, storage, and gastrointestinal tract), which would result in decreased viability under these environments (Tripathi and Giri, 2014; Chen et al., 2017). The development of several microencapsulated methods can improve the survival of GABA-producing LAB through the different material compositions and delivery systems (Yao et al., 2020).
Embedding and coating technologies used in microencapsulation processes show various success stories. The potential benefits of these methods are favorable to probiotics in terms of increased viability through the formation of a physical barrier, and nutrition support (Silva et al., 2014; Yao et al., 2020). Besides the delivery system, the material composition for its fabrication is also a critical factor for success. Alginate in combination with other materials (e.g., resistant starch, chitosan, and whey protein) can enhance the capability of microencapsulation, which increases the viability of the probiotics under gastrointestinal conditions (Sathyabama et al., 2014; Krasaekoopt and Watcharapoka, 2014; Gbassi et al., 2009; Yao et al., 2020). Currently, the concurrent use between alginate and additional material can achieve the desired outcomes to ameliorate the microencapsulation efficiency and probiotic viability, but the cost-effectiveness is likely not to be worth. Nanocrystalline starch (NCS) derived from the hydrolyzed-resistant starch (cassava starch), a low-cost material and widely used as microencapsulated material of bioactive compounds from plant extracts (Sunarti et al., 2020). However, the microencapsulation of probiotic microorganisms by NCS has never been reported. Alginate supplemented with NCS is therefore promisingly novel alternative material for probiotic microencapsulation.
The objectives of this study were evaluation the GABA-producing capability of L. brevis ST-69, establishment of suitable method and conditions for cassava starch, evaluation of the encapsulation efficiency of alginate and alginate-NCS encapsulating L. brevis ST-69, measurement the survival rate of encapsulated L. brevis ST-69 using alginate and alginate-NCS under simulated gastrointestinal conditions, and establishment the appropriate conditions for storage.
Materials and methods
Chemicals and microorganisms
Sodium alginate was purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Cassava starch was purchased from Siam Quality Starch Co., Ltd. (Chaiyaphum, Thailand). All other chemicals used in this study were of analytical grade. Probiotic Lactobacillus brevis ST-69 isolated from traditional kimchi, which was purchased from the local market in Bangkok, Thailand. L. brevis ST-69 was cultured in 5 mL of DeMan Rogosa Sharpe (MRS) broth (Himedia, Mumbai, India) under the anaerobic conditions in the anaerobic jar at 37 °C for 48 h. Subsequently, the cell suspension was sub-cultured in MRS agar containing 0.3% (w/v) calcium carbonate (Merck, Darmstadt, Germany), and incubated under the anaerobic conditions in the anaerobic jar at 37 °C for 48 h. Probiotic L. brevis ST-69 colonies, which formed a clear zone on the MRS plate were preserved in MRS broth with 50% (v/v) glycerol (Merck) and stored at −80 °C for further experiments.
Identification of GABA biosynthetic activity of Lactobacillus brevis ST-69
The GABA producing ability of probiotic L. brevis ST-69 was detected by thin layer chromatography (TLC). Probiotic was cultured in MRS broth supplemented with 5% (w/v) monosodium glutamate (MSG) (Sigma-Aldrich) under the anaerobic conditions in the anaerobic jar at 37 °C for 24 h. The probiotic culture supernatant was harvested by centrifugation at 1700 × g, 4 °C for 10 min. Then, 1.5 µL of the supernatant was spotted onto the TLC plate (Silica gel 60 F254, Merck). The TLC plate was conducted using a solvent mixture of n-butanol: acetic acid: sterile distilled water (5:3:2 v/v/v), followed by immersion into 1% (w/v) ninhydrin (Merck) in 95% (v/v) ethanol, dried and heated using the hairdryer. The colorization of the separated spot of GABA was observed compared to standard GABA (Sigma-Aldrich) position.
Preparation of nanocrystalline starch from cassava starch
Cassava starch nanocrystals were prepared by a previously described method (Kasemwong et al., 2011; Lin et al., 2011) with minor modifications. Briefly, native cassava starch was suspended in diluted sulfuric acid (3.16 M) at a concentration of 15% (w/v). The suspension was then continuously stirred at 100 rpm under 37 °C. After 7 days of hydrolysis, the suspension was filtered through a 0.45 µm membrane to separate starch granules. After that, starch granules were washed in sterile distilled water until neutrality was achieved. Neutralized starch granules were dispersed in sterile distilled water and homogenized at 14,000 rpm for 5 min to break aggregates. Nanocrystalline starch granules were lyophilized by freeze dryer (SciQuip, Newtown, UK) and stored at 25 °C in the dehumidifier. The relative crystallinity of NCS was analyzed by an X-ray diffractometer (Bruker, Karlsruhe, Germany) and the percentage of crystallinity was calculated using the following equation.
Preparation of probiotic L. brevis ST-69 suspension
Probiotic L. brevis ST-69 was grown in MRS medium under the anaerobic conditions in the anaerobic jar at 37 °C for 48 h. After incubation, they were adjusted to 1010 CFU/mL and washed twice with phosphate buffer saline (PBS), pH 7.2 by centrifugation at 1700×g, 4 °C for 10 min. Then, PBS was removed and the probiotic pellet cells were used for further experiments.
Microencapsulation of L. brevis ST-69 using emulsion technique
First, the matrix polymer was prepared by heating the mixture of 1% (w/v) sodium alginate containing either 0 or 0.1% (w/v) NCS in 100 mL of sterile distilled water at 100 °C until dissolved. After cooling, 0.1% (w/v) the probiotic pellet cells as described in the former method was added into the mixed solution and stirred at 350 rpm for 15 min. Then, the oil phase was prepared by 200 mL of sunflower oil containing 0.2% (v/v) tween 80 (Merck) as an emulsifier. The prepared matrix polymer with probiotic cells was added into the oil phase. The mixture is then stirred at 400 rpm for 30 min to form a water-in-oil emulsion. Once the emulsion is formed, the water-soluble polymer was insolubilized to form gel capsules by adding 200 mL of 0.1 M calcium lactate. The gel capsules containing probiotic cells were collected and kept at 4 °C in 0.85% (w/v) normal saline until used for further experiments.
Determination of encapsulation efficiency
Alginate probiotic gel capsules (1 g) or alginate-NCS probiotic gel capsules (1 g) were added to 10 mL of 0.06 M sodium citrate containing 0.2 M sodium bicarbonate followed by shaking for 5 min. Colony amounts of encapsulated probiotics and free cells were counted using the standard plate count method, then analyzed and represented as the percentage of viability rate of total probiotics.
Morphological characterization of gel capsules by optical and scanning electron microscopy
The size and shape of gel capsules were observed by an optical microscope (Olympus, Tokyo, Japan). The gel capsules morphology was examined by SEM (Hitachi High-Technologies, Tokyo, Japan). Briefly, the gel capsules were dried in the dehumidifier for 24 h. The gel capsules were placed on the stub with the carbon tape and then coated with gold nanoparticle for 10 nm using a sputter coater (Hitachi High-Technologies) for 2 min at the electric potential acceleration of 20 kV.
Survival rate of encapsulated L. brevis ST-69 under simulated gastrointestinal conditions
The simulated gastric juice was prepared using 0.2% (w/v) sodium chloride solution containing 0.35% (w/v) pepsin (Sigma-Aldrich), followed by adjusting pH value to 2.0 with 1 M hydrochloric acid. The simulated intestinal juice was prepared using 0.2% (w/v) sodium chloride solution containing 1.1% (w/v) sodium bicarbonate, 1.0% (w/v) ox gall (Sigma-Aldrich) and 0.1% (w/v) trypsin (Sigma-Aldrich), followed by adjusting pH value to 8.0 with 1 M sodium hydroxide. Next, the simulated gastric juice and intestinal juice were filtered using a 0.22 µm membrane. Encapsulated probiotic cells (1 g) or free cells (1 g) were added into simulated gastric juice solution and incubated at 37 °C for 3 h. After that, the totally encapsulated probiotic cells or free cells were then exposed to simulated intestinal juice solution, incubated at 37 °C for 2 h. During the incubation time, probiotic gel capsules or free cells were taken every hour and 1 g of probiotic gel capsules were released by adding into 10 mL of 0.06 M sodium citrate containing 0.2 M sodium bicarbonate, followed by shaking for 5 min. The viable encapsulated probiotic cell and free cell counts were enumerated using the standard plate count method. Results were reported as the percentage of survival rate of encapsulated probiotics and free cells.
Stability of encapsulated L. brevis ST-69 during long term storage
The encapsulated probiotic cells and free cells were stored in 0.85% (w/v) normal saline at two different temperatures (4 °C and 25 °C) for 10 weeks. Every 2 weeks of storage time, 1 g of probiotic gel capsules were released by adding into 10 mL of 0.06 M sodium citrate containing 0.2 M sodium bicarbonate, followed by shaking for 5 min. Colony amounts of the encapsulated probiotics and free cells were counted using the standard plate count method, then analyzed and represented as the percentage of viability rate of total probiotics under long term storage conditions.
Statistical analysis
All statistical analyses were performed by GraphPad Prism 8.0.2 (San Diego, CA, USA). Data were analyzed using unpaired t test and analysis of variance (ANOVA), and multiple comparisons were determined by Tamhane’s T2 test. Results were presented as mean ± standard deviation (SD) and statistically significant difference was defined at p value< 0.05. Experimental data were assembled from three repeated experiments together with replicated determinations.
Results and discussion
GABA biosynthetic activity of probiotic Lactobacillus brevis ST-69
The TLC chromatogram of L. brevis ST-69 (Fig. 1: Lane 2) showed a similar tone color and size of spot to TLC chromatogram of GABA standard (Fig. 1: Lane 1). In addition, MSG spot of L. brevis ST-69 (Fig. 1: Lane 2) was decreased when compared with MRS broth plus 5% MSG without probiotic cells (Fig. 1: Lane 3) and MSG alone (Fig. 1: Lane 4). It was clearly demonstrated that the L. brevis ST-69 can produce GABA (Fig. 1: Lane 2 vs Lane 1) through the conversion of MSG to GABA via glutamate decarboxylase (Fig. 1: Lane 2 vs Lane 3). In addition, the GABA-producing property was also found in other L. brevis strains from kimchi such as L. brevis AML15 (Shin et al., 2007) and L. brevis HYE1 (Lim et al., 2017). The result in this study suggests that the L. brevis ST-69 isolated from kimchi is an alternative choice as GABA-producing probiotics and supports the L. brevis strains isolated from kimchi as a potential source of GABA-producing probiotics.
TLC chromatogram showed the comparison of GABA-producing probiotic LAB capability. Lane 1: The GABA standard (1 mg/mL), Lane 2: Cell-free supernatant of probiotic L. brevis ST-69 cultured in MRS broth supplemented with 5% MSG at 37 °C for 24 h, Lane 3: MRS broth plus 5% MSG without probiotic cells, and Lane 4: The MSG standard (5 mg/mL). Black arrow indicates GABA spot of probiotic L. brevis ST-69 cell-free supernatant
Relative crystallinity of starch nanocrystals
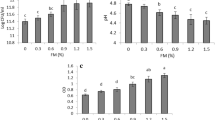
The relative percentage of crystallized region of non-hydrolyzed and hydrolyzed cassava starch with the 7-day sulfuric acid hydrolysis condition were 35.55% and 68.57%, respectively (Fig. 2). In addition, the relative percentage of crystallinity (crystalline region) was decreased with before/after 7-day hydrolysis conditions (data not shown). The suitable condition was, therefore, 7-day hydrolysis. It was explained by the susceptible of amorphous region to sulfuric acid (Jenkins and Donald, 1997; Wang et al., 2003) which could result in the low relative percentage of this region before 7-day and gradually increasing of the percentage of crystalline region. In contrast, the extended hydrolysis time over 7 days could result in the reduction of the relative percentage of crystalline region due to the destruction of this region. The determination of appropriate hydrolysis time to obtain the highest relative percentage of crystallinity is, therefore, necessary. Furthermore, the high relative percentage of crystallinity offers superior protecting capability against bile acids to a low relative percentage of crystallinity (Pankasemsuk et al., 2016). The high relative percentage of crystallinity using alginate-NCS in this study was a good option in terms of probiotic protecting agents and offered the optimization of hydrolysis time for this material.
Encapsulation efficiency
The encapsulation efficiency of encapsulated L. brevis ST-69 with alginate alone and alginate-NCS gel capsules by emulsion technique were 87.89% and 89.72%, respectively (Fig. 3). There were no significant differences between encapsulation efficiency among both treatments (p = 0.5953). Similarly, the encapsulation efficiency of L. casei using alginate-gelatinized starch and alginate-gelatinized starch coated with chitosan were not different (98.12% and 97.21% for alginate-gelatinized-chitosan and alginate-gelatinized starch, respectively) (Khosravi Zanjani et al., 2014). Therefore, alginate may be a key material enhancing the encapsulation efficiency while the additional material has less effect. However, some additional materials can improve probiotic viability such as hi-maize resistant starch (Sultana et al., 2000). In case of the encapsulation efficiency is a major concern, the encapsulated probiotic cells using alginate alone would be enough. In contrast, alginate containing the additional hi-maize starch with 2% concentration improved the encapsulation efficiency of L. casei 01 compared with alginate alone (Sultana et al., 2000). This work successfully encapsulated the probiotics with high efficiency for both alginate and alginate-NCS.
Optical and scanning electron microscopic observation
Under optical microscope, no differences in size and shape of alginate (Fig. 4A) and alginate-NCS (Fig. 4B) were found. In addition, the average diameters (size) and shape were 20 mm, and ellipse, respectively. Considering the surface of these two materials under SEM, the surface of alginate was smoother than alginate-NCS due to the distribution of NCS (starch granules) on alginate (Fig. 4C, D for alginate and alginate-NCS, respectively). The rough surface (alginate-NCS) is likely to enhance the encapsulation capability (87.89% vs 89.72%), resulting in increase of probiotics entrapment. This result was in accordance with previous reports (Lopez-Cordoba et al., 2014; Pankasemsuk et al., 2016). Furthermore, no visible probiotics were found in the surface, indicating the efficiency of encapsulation.
The morphology of probiotic gel capsules. The shape of A alginate probiotic gel capsules and B alginate-NCS probiotic gel capsules, observed by optical microscope at magnification of ×40. The microstructure of C alginate probiotic gel capsules and D alginate-NCS probiotic gel capsules, observed by SEM at magnification of ×1000
Survival rate of encapsulated probiotic cells under simulated gastric juice and simulated intestinal juice
The encapsulated probiotic L. brevis ST-69 and free cells were examined for the viability under simulated gastrointestinal conditions (Fig. 5). After 3 h of incubation under simulated gastric conditions, the viability of probiotics between encapsulated probiotic and free cells were significantly different (p < 0.05) and showed 95.84% (8.76 log CFU/mL), 96.07% (8.79 log CFU/mL), and 76.51% (7.20 log CFU/mL) for alginate, alginate-NCS, and free cells, respectively. Similarly, the viability of encapsulated probiotic L. casei 01 in alginate alone and alginate containing 1% hi-maize starch remained about 59.47% (5.45 log CFU/mL) and 62.5% (5.77 log CFU/mL), respectively, while free cells were decreased to 22.96% (2.19 log CFU/mL) under simulated gastric conditions at 2 h (Pankasemsuk et al., 2016). Previous studies showed the use of alginate as material composition may not be effective against gastric and/or intestinal juice (Cook et al., 2012; Haffner et al., 2016), resulting in decreased of probiotic viability. However, the result in this present study showed that the use of alginate or alginate supplemented with NCS can protect probiotics from the gastric environment through increased the size of microencapsulation. The effect of materials and microencapsulation size on probiotic viability were in agreement with Chandamouli and colleges (Chandramouli et al., 2004). Alginate is an applicable material for microencapsulation but its size should be properly designed.
After 5 h of incubation under simulated intestinal conditions, the viability showed that all three samples were significant differences (p < 0.05). The viability of encapsulated probiotic cells with alginate-NCS gel capsules at 5 h exhibited 94.97% (8.69 log CFU/mL), while alginate showed 87.75% (8.02 log CFU/mL) and free cells were extremely dropped to 73.11% (6.87 log CFU/mL). The result indicated the survival of probiotics between alginate and alginate-NCS were quite similar. However, the alginate-NCS showed a slightly higher percentage of survival rates. Therefore, the additional material (NCS) may support the function of alginate to protect probiotics under the simulated intestinal conditions e.g., bile salts and sodium bicarbonate. Similarly, the survival of encapsulated L. casei 01 under 2% bile salt incubation at 4 h showed 65.29% (6.03 log CFU/mL), 67.49% (6.29 log CFU/mL), and 59.71% (5.73 log CFU/mL) for alginate, alginate-1% hi-maize starch, and free cells, respectively (Pankasemsuk et al., 2016). Another reported the survival of encapsulated L. rhamnosus in alginate containing hi-maize starch was 95.47% (9.27 log CFU/mL), while free cells were reduced to 73.02% (6.44 log CFU/mL) after treated with 0.8% bile salts (Oudah et al., 2019). In contrast, the previous research mentioned that the use of alginate alone may cause an incomplete capsule wall due to the porous structure of alginate (De Araújo Etchepare et al., 2015). Hence, the additional material (NCS) may assist to fill the pores of alginate resulting in the complete capsule wall and the protection of probiotics from bile salt, which break bacterial lipid membranes (Li, 2012). Furthermore, this present study showed that NCS tended in promoting the alginate property for the protection of probiotic cells treated with simulated gastrointestinal conditions as the main problem of the probiotic cell viability.
Stability of encapsulated probiotic cells
The stability of encapsulated probiotic L. brevis ST-69 and free cells stored at 4 °C and 25 °C (room temperature) for 10 weeks are shown in Fig. 6A, B, respectively. During the first 2 weeks of storage, the viability of probiotics for both encapsulation and free cells of all temperatures were likely to be similar. For 2 to 4 weeks of storage at 25 °C, encapsulated probiotics and free cells decreased by 8.14% (0.64 log CFU/mL) and 13.13% (1.19 log CFU/mL), respectively. In addition, the viability of probiotics after 10 weeks for storage at 25 °C was poor and decreased by 55.77% (4.37 log CFU/mL) and 66.38% (6.29 log CFU/mL) for encapsulated probiotic and free cells, respectively. This result (25 °C) was in agreement with reported by Martin and colleges (decreased by 1.7 log CFU/mL at 24 h, and 100% death at 2 weeks) (Martin et al., 2013). It was noted that alginate-NCS microencapsulation in this study showed better probiotic viability than alginate-starch-microparticle microencapsulation storage (Martin et al., 2013). It can preserve the probiotic viability up to 4 weeks at room temperature with a minimal decreasing of the viable probiotics. The reduction of probiotic viability at high temperature (25 °C or room temperature) may be due to the increase of its metabolic rate (Heidebach et al., 2010), resulting in insufficient nutrition for its survival. In contrast, the viability of probiotics during storage at 4 °C sounds better. The number of viable cells for encapsulated probiotics with alginate alone and alginate-NCS were stable while the free cells decreased by 20.38% (1.91 log CFU/mL). Another reported probiotic viability for storage at 4 °C was similar to this study (the number of viable probiotics were unchanged) (Martin et al., 2013). This study confirmed that storage of probiotics at 4 °C maintained probiotics viability better than room temperature.
This current study successfully encapsulated L brevis ST-69 for both alginate and alginate-NCS materials with high encapsulated efficiency and viability. Probiotic L. brevis ST-69 can produce the GABA, suggesting that LAB strains isolated from kimchi are a potential source of GABA production. The alginate-NCS showed the best protection for encapsulated probiotics against simulated gastrointestinal conditions, supporting that alginate and NCS are appropriate materials for probiotics encapsulation. Although the alginate and alginate-NCS showed comparable survival rates during the gastric conditions, the alginate-NCS was likely favorable material against intestinal conditions. In addition, the suggested condition for keeping the L. brevis ST-69 was at 4 °C. It was noted that this condition provided good stability of probiotics over 10 weeks, whereas keeping at 25 °C also extended its stability up to 2 weeks. Therefore, alginate-NCS material would be a promising material for microencapsulation in functional foods for probiotic preservation even at room temperature. Further studies in other probiotics will be beneficial to confirm alginate-NCS application as protecting material for probiotics.
References
Chandramouli V, Kailasapathya K, Peirisb P, Jones M. An improved method of microencapsulation and its evaluation to protect Lactobacillus spp. in simulated gastric conditions. J. Microbiol. Methods 56: 27-35 (2004)
Chen HY, Li XY, Liu BJ, Meng XH. Microencapsulation of Lactobacillus bulgaricus and survival assays under simulated gastrointestinal conditions. J. Funct. Foods 29: 248-255 (2017)
Cook MT, Tzortzis G, Charalampopoulos D, Khutoryanskiy VV. Microencapsulation of probiotics for gastrointestinal delivery. J. Control. Release 162: 56-67 (2012)
Dhakal R, Bajpai VK, Baek KH. Production of GABA (gamma - aminobutyric acid) by microorganisms: a review. Braz. J. Microbiol. 43: 1230-1241 (2012)
De Araújo Etchepare M, Barin JS, Cichoski AJ, Jacob-Lopes E, Wagner R, Fries LLM, de Menezes CR. Microencapsulation of probiotics using sodium alginate. Cienc. Rural 45: 1319-1326 (2015)
FAO/WHO. Joint FAO/WHO (Food and Agriculture Organization/World Health Organization) Working Group Report on Drafting Guidelines for the Evaluation of Probiotics in Food. Ontario: London (2002)
Gbassi GK, Vandamme T, Ennahar S, Marchioni E. Microencapsulation of Lactobacillus plantarum spp in an alginate matrix coated with whey proteins. Int. J. Food Microbiol. 129: 103-105 (2009)
Haffner FB, Diab R, Pasc A. Encapsulation of probiotics: insights into academic and industrial approaches. AIMS Mater. Sci. 3: 114-136 (2016)
Heidebach T, Först P, Kulozik U. Influence of casein-based microencapsulation on freeze-drying and storage of probiotic cells. J. Food Eng. 98: 309-316 (2010)
Jenkins PJ, Donald AM. The effect of acid hydrolyis on native starch granule structure. Starch-Starke 49: 262-267 (1997)
Kasemwong K, Rungsardthong RU, Srinuanchai W, Itthisoponkul T, Sriroth K. Effect of high-pressure microfluidization on the structure of cassava starch granule. Starch-Starke 63: 160-170 (2011)
Khosravi Zanjani MA, Ghiassi Tarzi B, Sharifana A, Mohammadi N. Microencapsulation of probiotics by calcium alginate-gelatinized starch with chitosan coating and evaluation of survival in simulated human gastro-intestinal condition. Iran. J. Pharm. Res. 13: 843-852 (2014)
Kook MC, Cho SC. Production of GABA (gamma amino butyric acid) by lactic acid bacteria. Korean J. Food Sci. An. 33: 377-389 (2013)
Krasaekoopt W, Watcharapoka S. Effect of addition of inulin and galactooligosaccharide on the survival of microencapsulated probiotics in alginate beads coated with chitosan in simulated digestive system, yogurt and fruit juice. LWT Food Sci. Technol. 57: 761-766 (2014)
Li G. Intestinal probiotics: Interactions with bile salts and reduction of cholesterol. Proc. Environ. Sci. 12: 1180-1186 (2012)
Li H, Qiu T, Huang G, Cao Y. Production of gamma-aminobutyric acid by Lactobacillus brevis NCL912 using fed-batch fermentation. Microb. Cell Fact. 9: 85 (2010)
Lim HS, Cha IT, Roh SW, Shin HH, Seo MJ. Enhanced production of gamma-aminobutyric acid by optimizing culture conditions of Lactobacillus brevis HYE1 isolated from kimchi, a Korean fermented food. J. Microbiol. Biotechnol. 27: 450-459 (2017)
Lin N, Huang J, Chang PR, Anderson DP, Yu J. Preparation, modification, and application of starch nanocrystals in nanomaterials: a review. J. Nanomater. 2011: 1-13 (2011)
Lopez-Cordoba A, Deladino L, Martino M. Release of yerba mate antioxidants from corn starch-alginate capsules as affected by structure. Carbohyd. Polym. 99: 150-157 (2014)
Martin MJ, Lara-Villoslada F, Ruiz MA, Morales ME. Effect of unmodified starch on viability of alginate-encapsulated Lactobacillus fermentum CECT5716. LWT Food Sci. Technol. 53: 480-486 (2013)
Oudah MA, Rajyalakshmi K, Shabana S, Satya AK. Preservation of L. Rhamnosus with calcium alginate and it’s of survival under gastric condition and in yogurt. Acta Sci. Microbiol. 2: 70-74 (2019)
Pankasemsuk T, Apichartsrangkoon A, Worametrachanon S, Techarang J. Encapsulation of Lactobacillus casei 01 by alginate along with hi-maize starch for exposure to a simulated gut model. Food Biosci. 16: 32-36 (2016)
Sathyabama S, Ranjith Kumar M, Bruntha Devi P, Vijayabharathi R, Brindha Priyadharisini V. Co-encapsulation of probiotics with prebiotics on alginate matrix and its effect on viability in simulated gastric environment. LWT Food Sci. Technol. 57: 419-425 (2014)
Shin JW, Kim DG, Lee YW, Lee HS, Shin KS, Choi CS, Kwon GS. Isolation and characterization of Lactobacillus brevis AML15 producing γ-aminobutyric acid. J. Life Sci. 17: 970-975 (2007)
Silva P, Fries L, Menezes C, Holkem A, Schwan C, Wigmann É, Bastos J, Silva C. Microencapsulation: concepts, mechanisms, methods and some applications in food technology. Cienc. Rural 44: 1304-1311 (2014)
Sultana K, Godward G, Reynolds N, Arumugaswamy R, Peiris P, Kailasapathy K. Encapsulation of probiotic bacteria with alginate-starch and evaluation of survival in simulated gastrointestinal conditions and in yoghurt. Int. J. Food Microbiol. 62: 47-55 (2000)
Sunarti TC, Pasaribu FJ, Winarti C. Encapsulation of temulawak extract by using nanocrystalline cassava and sago starches and maltodextrin. Pharm. Sci. Res. 7: 75-80 (2020)
Tripathi MK, Giri SK. Probiotic functional foods: Survival of probiotics during processing and storage. J. Funct. Foods 9: 225-241 (2014)
Wang YJ, Truong VD, Wang L. Structures and rheological properties of corn starch as affected by acid hydrolysis. Carbohyd. Polym. 52: 327-333 (2003)
Wu Q, Shah NP. Restoration of GABA production machinery in Lactobacillus brevis by accessible carbohydrates, anaerobiosis and early acidification. Food Microbiol. 69: 151-158 (2018)
Yao M, Xie J, Du H, McClements DJ, Xiao H, Li L. Progress in microencapsulation of probiotics: A review. Compr. Rev. Food Sci. F. 19: 857-874 (2020)
Acknowledgements
This work was funded by a research Grant (Grant No.251/2562) from Faculty of Medicine, Srinakharinwirot University and National Science and Technology Development Agency Thailand, according to the supporting graduated education contract of Thailand Graduate Institute of Science and Technology (contract no. SCA-CO-2562-9697-TH).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Thangrongthong, S., Puttarat, N., Ladda, B. et al. Microencapsulation of probiotic Lactobacillus brevis ST-69 producing GABA using alginate supplemented with nanocrystalline starch. Food Sci Biotechnol 29, 1475–1482 (2020). https://doi.org/10.1007/s10068-020-00812-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-020-00812-9