Abstract
The plant-specific transcription factors NACs (NAM, ATAF1, 2, CUC), one major transcription factor family, play significant roles in various physiological processes including abiotic stresses. In our study, an NAC gene from potato (Solanum tuberosum L.) was cloned and named as StNAC1 for a high-sequence similarity to SlNAC1, a well-known tomato NAC gene regulating multiple stress responses and fruit ripening. StNAC1 gene was significantly induced under salt stress. Then, we constructed StNAC1-overexpressing transgenic Nicotiana benthamiana plants and obtained three homozygous transgenic lines. The phenotypic analysis results showed that StNAC1-overexpressing transgenic plants had not only higher seed germination and green leaf rates, but also accumulated less ROS and more proline than wide-type plants under salt stress, which resulted in improving transgenic plants salt tolerance. These suggested that StNAC1 gene might function as a positive regulator in plant response to salt stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Salt stress is one of major abiotic stresses limiting plant growth and crop productivity and has been aggravated by poor irrigation practices, rising population, and industrial pollution (Ouhibi et al. 2014; Yang and Guo 2018). High salinity can cause a serious decrease in photosynthetic efficiency, destruction of ionic equilibrium, and excessive ROS accumulation, ultimately resulting in plant growth, development and crop yield, even to death (Nakashima et al. 2012). Molecular regulation mechanisms in response to salt stress include salt-stress signal perception, transduction, and gene expression, metabolic change in plant and have been intensively studied for improving plant salt tolerance (Agarwal and Jha 2010; Yoon et al. 2020). Increasing research evidence indicated that transcription factors (TFs), such as bZIP, MYB, DREB, and NAC, were involved in plant salt response (Bouaziz et al. 2013; Cheng et al. 2013, 2020; Zhao et al. 2020).
NAC TFs belong to one of the largest plant-specific TF families, represented by 138 genes in Arabidopsis (Mizzotti et al. 2018), 151 genes in rice (Nuruzzaman et al. 2010), 134 genes in wheat (Zhou et al. 2018), 148 genes in maize (Peng et al. 2015), and 180 genes in soybean (Melo et al. 2018). Typically, NAC TFs were characterized for a highly conserved N-terminal region (NAM) and a alterable C-terminus, which might functions as DNA binding and transcriptional activation, respectively (Aida et al. 1997; Ren et al. 2000; Xie et al. 2000; Duval et al. 2002; Ooka et al. 2003; Tran et al. 2004). The N-terminal region is further divided into five subdomains (A–E) (Kikuchi et al. 2000). Subdomains A, C, and D are high conserved and involved in the formation of dimeric domain and process of DNA binding, respectively (Ooka et al. 2003), while subdomains B and E are less conserved, which may indicate a functional diversification of NAC genes. Based on N-terminal region differences, The NAC TF family is divided into three subfamilies, including no apical meristem (NAM), Arabidopsis transcription activation factor (ATAF), and Cup-shaped cotyledon (CUC) (Aida et al. 1997; Duval et al. 2002; Christianson et al. 2010;).
Although NAC genes were initially discovered for their functions in plant growth and development, their roles in plant responses to abiotic and biotic stresses are drawing an increasing amount of attention (Jensen and Skriver 2014; Shao et al. 2015). The AtNAC019, AtNAC055, and AtNAC072 genes in Arabidopsis were significantly induced by salt and drought stresses. Overexpressing these genes could enhance salt and drought tolerance of transgenic plants (Tran et al. 2004; Hu et al. 2006; Hickman et al. 2013). Overexpressing NAC13 gene in poplars conferred enhanced tolerance to salt significantly, while silencing its expression impaired salt tolerance (Zhang et al. 2019). Overexpression of OsNAC5, OsNAC6, OsNAC045, SNAC1, SNAC2, and SNAC3 genes in rice enhanced drought and salt tolerance of transgenic plants via up-regulating stress-responsive genes such as POD, LEA3, and PM1, respectively (Hu et al. 2006, 2008; Nakashima et al. 2007; Zheng et al. 2009; Takasaki et al. 2010; Fang et al. 2015;). Soybean NAC TFs GmNAC11, GmNAC20, and GmNAC085 have been characterized as important transcription regulators in elevating tolerance to salt, drought, and cold stress via glutathione biosynthesis and redox balance (Hao et al. 2011; Nguyen et al. 2018). MbNAC25 and MbNAC29 genes from Malus baccata (L.) increased the activities of ROS-scavenging enzyme POD, SOD, and CAT in transgenic Arabidopsis resulting in enhanced cold and salt tolerance (Han et al. 2020a, b). Wheat TaNAC2, TaNAC67 and maize ZmSNAC1, ZmNAC55 were highly induced under drought, salt, and low-temperature, and transgenic plants overexpressing these genes showed an increased drought, salt, or low-temperature tolerance (Lu et al. 2012; Mao et al. 2012, 2016). Furthermore, a lot of studies have suggested that NACs was involved in response to abiotic stresses via hormones including ABA and ethylene. Overexpression of these NAC genes (ATAF1, OsNAC2, TIP, and ZmNAC071) results in enhanced sensitivity to ABA via down-regulating stress-responsive genes under abiotic stresses in transgenic plants (Kim et al. 2009; Marques et al. 2017; Shen et al. 2017; He et al. 2019). ATAF1 and OsNAC2 could directly bind the promoters of ABA biosynthesis-relative genes, such as NCED3, OsAP37, and OsCOX11, for regulating plant response to abiotic stresses (Jensen et al. 2013; Mao et al. 2017, 2018).
Potato (Solanum tuberosum L.) is the world's fourth most important food crop but moderately sensitive to salt stress which limits its economic yield (Jaarsma et al. 2013; Jaarsma and Boer 2018). So far, 110 NAC genes have been identified in potato via bioinformatic approaches, and some of them were showed to be induced by salt stress by Illumina RNA-seq data (Singh et al. 2013). However, none of potato NAC genes has been verified for their functions in response to salt stress except for StNAC2 (Xu et al. 2014). In this work, we isolated a potato NAC gene from the KangQing cultivar and named it as StNAC1 for a high-sequence similarity to SlNAC1 gene in tomato (Solanum lycopersicum), which was well known to regulate plant responses to multiple biotic and abiotic stresses, including salt stress. Furthermore, StNAC1 has been proved to be significantly induced by pathogen Phytophthora infestans caused late blight agent in potato (Collinge and Boller 2001). For this sake, we speculated that StNAC1 might be also involved in plant response to multiple stresses, including salt stress. In this study, the expressions of StNAC1 in potato tissues and under NaCl treatments were analyzed, followed by morphological and physiological analysis of StNAC1-overexpressing transgenic Nicotiana benthamiana under salt stress. Finally, our transgenic lines displayed significantly enhanced salt tolerance, which could provide a potential candidate to improve potato salt tolerance.
Materials and methods
Plant materials and treatments
The 10-day test-tube seedlings of Solanum tuberosum potato from a KangQing cultivar provided by Sichuan Academy of Agricultural Sciences were grown in nutritional soil for 28 days at temperature of 23 ± 1℃ (light/dark,16/8 h), 250 μmol·m−2·s−1. The plants were irrigated with different concentrations of salt solutions for different times, respectively.
The wild-type tobacco (Nicotiana benthamiana) and transgenic seeds were sterilized with 75% ethanol for 5 min, followed by 5% NaClO for 5 min, and then washed 3–5 times with sterile water. The seeds were sowed on MS/2 medium with or without salt stress in greenhouse at temperature of 23 ± 1℃ (light/dark, 16/8 h), 250 μmol·m−2·s−1. After grown on MS/2 medium for about 10 days, the seedlings were transferred to grow on nutritional soil for about 25 days in greenhouse at temperature of 28 ± 1℃. The healthy leaves of all plants were picked and immersed into different concentrations (0, 50, 100, 150, and 200 mM NaCl) of salt solution for 7 days.
Cloning and characterization of StNAC1 gene in Solanum tuberosum
The total RNAs from leaves of potato KangQing cultivar were extracted using total RNA isolation Kit (FOREGENE, RE-05021) and were quantified using a Nanodrop (Life Real, FC-1100). 1.0 μg RNA was employed for reverse transcription into cDNA using TransScript All-in-one First-Strand cDNA Synthesis SuperMix qPCR (One-Step gDNA Removal) Kit (TransGen Biotech, AT341-02). StNAC1 coding sequence (CDS) was then amplified with above cDNA as template and primers StNAC1-Forward and StNAC1-Reverse (Supplemental Table 1) designed from potato genome sequence (Gene ID: PGSC0003DMG400032555 [AJ401151/NP_001305595.1]).
The nucleotide and amino acid sequence of StNAC1 protein in solanum tuberosum were aligned with SlNAC1 protein in Solanum lycopersicum L. using DNAMAN software. The SMART (http://smart.embl-heidelberg.de/) was employed for prediction of conservative domains in StNAC1 protein.
Expression analysis of StNAC1 gene
The leaves, roots, and stems of all healthy plants (Solanum tuberosum and Nicotiana benthamiana) were separately collected and frozen in liquid nitrogen for total RNA extraction and cDNA transcription as described above. Then, the PerfectStart Green qPCR SuperMix Kit (TransGen Biotech, AQ601-02) was used for real-time fluorescence quantitative PCR (RT-qPCR). The reaction system as below: ① 95℃ for 5 min, ② 95℃ for 15 s, ③ 60℃ for 30 s, and ② ~ ③ for 40 cycles. The RT-qPCR was performed using the Ultra SYBR Mixture (CWbiotech, CW0957M) and Bio-Rad CFX96 real-time system (Thermol Fisher scientific, USA) by gene-specific primers (Supplemental Table 1). The results from RT-qPCR were calculated out using 2−ΔΔCt method ((Livak and Schmittgen 2001). The Actin gene was employed as a reference gene for target gene expression. All experiments were performed with three biological replicates and technical replicates.
Construction and identification of StNAC1 transgenic plants
The StNAC1 gene CDS amplified were inserted into plant binary vector pBI121 using restriction enzymes XbaI and SpeI (Thermo Scientific) via double enzyme digestion. The recombinant vector (pBI121-CaMV35S:: StNAC1-GFP) contained StNAC1 gene under the control of CaMV35S constitutive promoter and fused in-frame with C-terminal of the Green Fluorescent Protein (GFP). And then, all recombinant plasmids were sequenced by Tsingke Biotech (ChengDu) to confirm the correct insertion of CaMV35S:: StNAC1-GFP fragments in constructed vectors. The true recons were transformed into Agrobacterium EHA105; following introduced into Nicotiana benthamiana tobacco calluses. Finally, all positive plants obtained were identified via RT-qPCR.
The insertion sites of CaMV35S:: StNAC1-GFP fragments on chromosomes in positive transgenic plants were further confirmed for homozygous plants using Thermal Asymmetric Interlaced (TAIL-PCR). The sequencing results of PCR products were aligned with Nicotiana benthamiana genome from Sol Genomics network (https://solgenomics.net/organism/Nicotiana_benthamiana/genome). All primers used for TAIL-PCR are listed in Supplemental Table 1.
Measurement of chlorophyll content
For chlorophyll measurement, all tested leaves from wide-type and transgenic plants with or without salt treatment homogenized by liquid nitrogen and extracted via 80% acetone. The extractions were centrifuged for 5 min at 15,000 × g, and then, the supernatant was immediately subjected to spectrophotometric measurement at 663 and 645 nm by a spectrophotometer (Thermo Fisher Scientific, Varioskan LUX, USA), respectively (Lichtenthaler 1987). Statistical analyses were carried out via Duncan's test. Means and standard deviations were calculated from three independent experiments.
Histochemical and quantitative determination of ROS accumulation
Histochemical assays of ROS accumulation in Nicotiana benthamiana tobacco were conducted as described previously (Bindschedler et al. 2006). The 8-day healthy plants from wide-type and transgenic plants with or without 150 mM NaCl for different times (0, 0.5, 3, and 24 h) were incubated in 1 mg·ml−1 DAB staining buffer solution (Biosharp, D5637) at pH5.5 (vacuum for 15 min) for overnight at room temperature, respectively. Leaves were decolored in boiled bleaching solution (acetic acid:ethanol:glycerol = 1:3:1) for 15 min and then immersed in 95% ethanol until the green color in leaves was completely faded. The quantitative detection of ROS in leaves from all lines was performed using hydrogen peroxide (H2O2) detection kit (Solarbio Life Sciences, BC3595) as previously described (Chen et al. 2019). Statistical analyses were carried out via Duncan’s test. Means and standard deviations were calculated from three independent experiments.
Quantitative analysis of proline in leaf
Proline content in leaf with or without salt stress (0, 0.5, 3, and 24 h) was measured via reaction with ninhydrin (Bates et al. 1973). The leaves frozen in liquid nitrogen were ground into powders, and then mixed with 3% (w/v) sulfosalicylic acid. The samples were boiled for 30 min, followed by a centrifugation for 10 min at 3000 rpm. For colorimetric determinations, the mixed reaction solutions (supernatants:ninhydrin acid:glacial acetic acid = 1:1:1) were incubated at 100℃ for 1 h and cooled in an iced bath. The chromophore was extracted using 2 ml toluene and its absorbance at 520 nm was detected by a spectrophotometer (Thermo Fisher Scientific, Varioskan Lux, USA). All experiments were performed with three biological replicates and technical replicates. Statistical analyses were carried out via Duncan's test.
Results
Characterization of StNAC1 gene in Solanum tuberosum
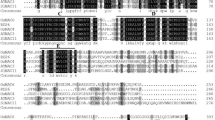
In this work, we cloned a gene (Gene ID: PGSC0003DMG400032555 [AJ401151/NP_001305595.1] in potato, which is annotated as a putative NAC domain containing protein. StNAC1 protein shared 96.37% amino acid identity with tomato SlNAC1 protein (Fig. 1a). For a high-sequence similarity to SlNAC1, we named this gene as StNAC1. An amino acid domain prediction of StNAC1 protein showed that StNAC1 protein had two NAM domains (14-137aa, 157-282aa) for protein–DNA binding and a KNOX1 domain (11-55aa) playing a role in suppressing target gene expression and homo-dimerization (Fig. 1b). Besides, 3D-spatial structure model simulation obviously showed an interaction between double-stranded DNA and StNAC1 protein (Fig. 1c). Together, these results indicated that StNAC1 gene in Solanum tuberosum L. might act as a transcription factor most and possibly play similar biological functions to tomato SlNAC1gene.
Amino acid sequence analysis of StNAC1 protein in Solanum tuberosum. a Amino acid sequence alignment of NAC1 gene in Solanum tuberosum and Solanum lycopersicum. SLNAC1 and StNAC1 correspond to accessions Solanum lycopersicum NP_001234482 and Solanum tuberosum NP_001305595. b Schematic diagram of StNAC1 protein amino acid domains predicted by SMART. The Subdomains (A–E) are shown in white box areas. c Schematic diagram of a 3D-spatial structure model simulation for StNAC1 protein and double-stranded DNA
Expression of StNAC1 in Solanum tuberosum
As it has been clarified that SlNAC1 gene was induced distinctly under salt stress (Yang et al. 2011), so we detected whether StNAC1 gene was also induced in potato under salt stress. First, we detected StNAC1 expression patterns in potato tissues. The results showed that that StNAC1 gene was mainly expressed in potato leaf comparing to roots and stems via qPCR (Fig. 2a). Furthermore, StNAC1 gene expression level in leaf was detected under salt stress. The results showed that StNAC1 gene expression was significantly induced in leaf under different salt concentrations (50 mM, 150 mM, and 250 mM), and especially the highest level at 150 mM NaCl (Fig. 2b). However, StNAC1 gene expression level induced at 150 mM NaCl for 3 h was more significantly higher than those at 150 Mm NaCl for 0.5 h and 24 h (Fig. 2c). Together, these results suggested that StNAC1 gene might play important roles in potato response to salt stress.
Fluorescence quantitative PCR (QPCR) analysis of NAC1 gene in Solanum tuberosum L. under salt. a Relative expression analysis of StNAC1 in root, stem, and leaf of Solanum tuberosum. b and c Relative expression analysis of StNAC1 in Solanum tuberosum. Leaf treated with different salt concentrations for different time, respectively. The salt treatments: roots, stems, and leaves of Solanum tuberosum. Plants grown in nutritional soil for 4 weeks were irrigated for 0 h, 0.5 h, 3 h, and 24 h with different salt concentrations (0 mM, 50 mM, 150 mM, and 250 mM NaCl), and then picked for total RNA extraction and qPCR, respectively. The experiments were repeated three times for each (nSD ≥ 6)
Construction and identification of StNAC1 gene transgenic plants
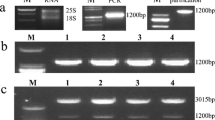
To investigate the functional roles of StNAC1 gene in response to salt stress, the CaMV35S promoter-derived StNAC1-overexpressing transgenic N. benthamiana plants were constructed by Agrobacterium-mediated transformation (Fig. 3a). The several positive transgenic plants were examined in DNA and RNA levels (Fig. 3b, c). The StNAC1 gene expression in transgenic plants were significantly higher than wide type (Fig. 3d). The total NAC1 mRNA expression levels including StNAC1 and NbNAC1 (Nicotiana benthamiana) in transgenic plants were also distinctly higher than wide type (Fig. 3e). Therefore, we obtained three independent transgenic lines OE-1, OE-2, and OE-3. The insertion sites of StNAC1 gene via Tail-PCR sequencing were further performed for homozygous transgenic plants (Fig. 4a, b). The insertion sites of StNAC1 expression box in these three transgenic lines were shown in supplementary materials (Supplemental Fig. 1).
Construction and identification of StNAC1transgenic plants in Nicotiana benthamiana. a Schematic diagram of construction of StNAC1-overexpression vector. b Identification of StNAC1transgenic plants in DNA levels. c, d and e Semi-quantitative PCR (c) and qPCR analysis of S.tNAC1 (d) and NAC1 (including StNAC1 and NbNAC1) (e) in transgenic plants. All lines grown in nutritional soil for 28 days were used for total RNA extraction and expression analysis, respectively
Identification of insertion sites on chromosomes in three StNAC1 transgenic plants. a Identification of homozygous transgenic plants by PCR electrophoresis in three transgenic plants. The leaves of StNAC1 transgenic plants in Nicotiana benthamiana grown in MS/2 medium for 10 days were picked for total RNA extraction and qPCR, respectively. b Schematic diagram of insertion sites on chromosomes in StNAC1 transgenic plants. LB: left border. RB: right border
Germination rate test of transgenic seeds under salt stress
To test whether StNAC1 overexpression could affect salt tolerance in transgenic plants, we conducted seed germination experiments under salt stress. The phenotype and statistical analysis results of seed germination showed that comparing to normal condition, the seed germinations of all lines were suppressed at 100 mM and 150 mM NaCl (Fig. 5). A prominent difference appeared between transgenic lines and wide type after 100 mM NaCl. The seed germination rates of transgenic lines are significantly higher than those of wide type at both 100 mM and 150 mM NaCl, particularly 100 mM NaCl (Fig. 5a). The seed germination rates (69.39%, 71.43%, and 73.47%) of OE-1, 2, 3 transgenic plants were more significantly than that (28.57%) of wide type under 100 mM NaCl comparing to 150 mM NaCl (Fig. 5b). These suggested that overexpressing StNAC1 gene could confer an enhancing salt tolerance in tobacco.
StNAC1 gene could increase germination abilities of OE transgenic plants under salt stress. a Phenotypes of wide-type and OE transgenic seeds under different concentrations of salt stress. Bar = 2 mm. b Germination rates (%) of wide-type and OE transgenic seeds sowed in MS/2 medium with or without 100 mM NaCl for 9 days, respectively. The experiments were repeated three times for each (nSD ≥ 49). Duncan’s test, ***P < 0.01
Leaf phenotypic analysis of transgenic plants under salt stress
The tissue expression profile above showed that StNAC1 gene mRNA expression in potato leaf exhibited a higher level than those of root and stem (Fig. 2a). Therefore, we further tested StNAC1 gene functional role in response to salt stress in leaf. As phenotypic experimental results shown, more and more green leaves in both transgenic and wide type plants were gradually turned yellow with an increasing salt concentration (Fig. 6a). However, a visible difference was beginning to appear between wide type and transgenic lines after 100 mM NaCl, and especially, it was the most obvious at 150 mM NaCl (Fig. 6a). The green leaf rates (76.67%, 71.67%, and 73.33%) from all transgenic plants were significantly more than that (23.33%) of wide type plants at 150 mM NaCl (Fig. 6a, b). All leaves were turned yellow at 200 mM NaCl (Fig. 6a). It was also confirmed from chlorophyll content (mg/g) (Fig. 6c). Together, these results suggested that StNAC1gene might act as a positive regulator in plant response to salt stress.
StNAC1 gene could regulate the salt tolerance of leaves in Nicotiana benthamiana. a Phenotype of leaves of wide-type and OE transgenic plants under different concentrations of salt stress. Bar = 5 cm. b and c Green leaves rate (%) and chlorophyll content (mg/g) of wide-type and OE transgenic plant leaves treated with or without 150 mM NaCl, respectively. The salt treatment: after wide-type and OE transgenic plants were grown for 3 weeks, the leaves were picked and immersed in different concentrations of salt solution for 7 days. The experiments were repeated three times for each (nSD ≥ 10). Duncan’s test, ***P < 0.01
ROS accumulation detection of transgenic tobacco under salt stress
To clarify the possible mechanisms of StNAC1 gene in enhancing salt tolerance in transgenic plants, we further determined ROS accumulation in both wide-type and all transgenic plants under salt stress. As a result in general, DAB staining intensities for H2O2 in all lines plants were significantly deepened under salt stress (Fig. 7a). Although there was no difference between wide type and transgenic lines plants after150 mM NaCl for 0.5 h, a change appeared after salt treatment for 3 and 24 h (Fig. 7a). The DAB staining intensities in transgenic plants were distinctly shallower than wide-type plants after 150 mM NaCl for 3 and 24 h (Fig. 7a). For NBT staining experiment, we obtained an almost similar result under salt stress (Fig. 7a). These phenotypes were identical to H2O2 and O2•− content detection results, respectively (Fig. 7b, c). Together, all above results suggested that StNAC1 gene might involve in response to enhancing salt tolerance via decreasing ROS accumulation induced under salt stress in transgenic tobacco plants.
Detection of ROS (H2O2 and O2•-) accumulations in wide-type and transgenic plants under salt stress. a DAB and NBT staining of transgenic lines and wide-type seedlings with or without 150 mM NaCl for different times (0, 0.5, 3, and 24 h). Bars = 2 mm. b and c Detection of ROS accumulations in transgenic lines and wide-type seedlings with or without 150 mM NaCl for different times (0, 0.5, 3, and 24 h). The salt treatments: 8-day seedlings grown in MS/2 medium were transferred to 150 mM NaCl solution for 0 h, 0.5 h, 3 h, and 24 h, respectively. The experiments were repeated three times for each (n ≥ 10). Duncan’s test, ***P < 0.01
Quantitative analysis of proline in transgenic plants under salt stress
Proline, as an excellent antioxidative osmolyte, plays a beneficial role in response to high salt stress (Szabados and Savouré 2010; Slama et al. 2015). In this study, we also detected proline contents in transgenic and wild-type plants under salt stress. As results shown, proline contents increased almost in all transgenic and wild-type plants at 150 mM NaCl compared to control (Fig. 8). However, it was beginning to appear an obvious difference after 150 mM NaCl for 0.5 h, 3 h, and 24 h, respectively (Fig. 8). Proline contents in all transgenic plants were significantly more than those of wide type at 150 mM NaCl (Fig. 8). These indicated a positive role of overexpressing StNAC1 gene in enhancing salt tolerance of transgenic N. benthamiana plants via proline accumulation.
Detection of proline contents (µg.g−1Fw) in wide-type and transgenic plants under salt stress. The salt treatments: 10 day seedlings grown in MS/2 medium were transferred to MS/2 medium with 150 mM NaCl for 0 h, 0.5 h, 3 h, and 24 h, respectively. The experiments were repeated three times for each (nSD ≥ 30).). Duncan’s test, ***P < 0.01
Discussion
Due to a long-term selection, the modern potato cultivars exhibit much more sensitive to salt stress compared to wild potato species with a relatively salt tolerance (Efimova et al. 2019). It is of great significance to study mechanism of potato in response to salt tolerance. Transcription factors such as WRKY, NAC, bZIP, and MYB play significant roles in salt stress responses (Lata et al. 2011). Therefore, NAC TFs were good candidates for genetically improving salt tolerance in crops because of their roles as important regulators of many stress-responsive genes to influence plant salt-stress tolerance (Wang et al. 2016). So far, several genes from 110 NACs previously identified in potato could be significantly induced under salt stress (Singh et al. 2013). However, only one potato NAC gene, StNAC2, has been described in detail involving in salt tolerance (Xu et al. 2014). In this work, we cloned and characterized a potato NAC gene from the KangQing cultivar, which showed a high-sequence similarity (96.37% amino acid identity) to tomato SlNAC1 gene encoding an NAC transcription factor. The phylogenetic tree results suggested that StNAC1 was an ATAF subfamily member rather than NAP subfamily to which StNAC2 gene belonged (Xu et al. 2014). It implied that StNAC1 gene was an SlNAC-like gene, which was independent of StNAC2 in potato. The StNAC1 protein contained an N-terminal highly conserved NAM domain for DNA binding and a C-terminus variable NAM domain for transcriptional activation, respectively. In combination with 3D-spatial structure model simulation of an interaction between StNAC1 protein and double-stranded DNA, it indicated that StNAC1 might act as an NAC transcription factor.
In this work, StNAC1 expression was detected in all three tested tissues, though with much higher expression level in leaf than in root and stem, indicating that StNAC1 might perform some basic functions in potato cells. This tissue expression pattern is very similar to that of SlNAC1 regulating salt stress responses in tomato (Yang et al. 2011; Ma et al. 2014). As StNAC1 gene was significantly induced under NaCl treatments, we presumed that StNAC1 might play a role in plant response to salt stress. A membrane-bound NAC gene NTL8 was verified to involving in regulating seed germination via salt signaling (Kim et al. 2008). Our results from seed germination experiments under salt stress showed, though there were no significant differences of seed germination rates between wild-type and transgenic plants under low salinity (50 mM NaCl), the germination rates of three transgenic plant seeds were significantly higher than wide-type plants under higher salt concentrations (100 mM and 150 mM NaCl). This suggested that StNAC1 gene was involved in improving germination rates of transgenic plant seeds. Similar results of leaf with salt treatment were also observed, and transgenic plants leaves could delay salt-induced senescence. These lines of evidences suggested that StNAC1 gene was involved in regulating salt tolerance and played a positive role in response to salt stress.
Many evidences showed overexpressing stress-inducible NAC genes could improve salt tolerance in plants via scavenging ROS and/or up-regulating osmolyte accumulation. Overexpression of chrysanthemum DgNAC1 gene could enhance salt tolerance in tobacco through regulating ROS accumulation and proline contents (Wang et al. 2017). TaNAC29 overexpression transgenic plants accumulated less malondialdehyde (MDA) and hydrogen peroxide (H2O2) through significantly improving activities of superoxide dismutase (SOD) and catalase (CAT) under salt and drought stresses (Huang et al. 2015). Overexpressing GmNAC06 could lead to proline and glycine betaine accumulations in transgenic plants to alleviate or avoid the negative effects of ROS caused by salt stress (Li et al. 2020). Studies suggested that overexpressing OsNAC45 gene in transgenic plants could more efficiently scavenge superoxide under salt stress (Yu et al. 2018). Overexpressing ThNAC7 transgenic plants in Arabidopsis showed an increased reactive oxygen species (ROS) scavenging capabilities and proline accumulation, accomplished by enhancing the activities of superoxide dismutase and peroxidase (He et al. 2019). NAC13 overexpression transgenic poplars showed lower ROS accumulation and increased proline contents in compared to wide type under salt stress (Zhang et al. 2019).
In this work, we showed that transgenic plants accumulated less H2O2 and O2•− than wide-type plants by histochemical and quantitative assays, indicating that overexpressing StNAC1 could enhanced salt tolerance in transgenic plants by decreasing ROS accumulation. These results indicated that overexpressing StNAC1 gene could be contributed to removing ROS accumulation induced by salt stress. Meanwhile, our results also showed proline contents in transgenic plants were significantly higher than that in wild-type plants under 150 mM NaCl treatment for different time points. However, quite different from that ROS accumulated continually, proline in wild-type plants did not accumulate significantly at 0.5 h compared to 0 h, and both proline contents in transgenic plants and wide-type plants did not increase significantly at 24 h compared to at 3 h. Nevertheless, it was quite clear that overexpressing StNAC1 enhanced salt tolerance of transgenic plants also by up-regulating proline contents. Besides, proline could play an excellently beneficial role as an osmolyte in maintaining cell osmotic balance and antioxidant to stabilize membrane lipid oxidation and regulate ROS levels under environmental stresses (Delauney and Verma 1993; Hayat et al. 2012). It remains to be further clarified how StNAC1 gene regulates ROS accumulation and proline contents or proline-meditated ROS scavenging under salt stress in plants.
Conclusions
In this study, we isolated and characterized a potato NAC gene, StNAC1. StNAC1 was induced by salt stress, and overexpressing StNAC1 in Nicotiana benthamiana could significantly improve plant salt tolerance, which provided a new candidate for genetically improving crop stress tolerance. In future, it is interesting to study whether StNAC1 is also responsive to other stresses, except for salt, wounding, and Phytophthora infestans infection. Most importantly, it is necessary to identify the target genes of StNAC1, which will help us understanding molecular mechanisms of StNAC1 in response to stresses.
References
Agarwal PK, Jha B (2010) Transcription factors in plants and ABA dependent and independent abiotic stress signalling. Biol Plant 54:201–212
Aida M, Ishida T, Fukaki H, Fujisawa H, Tasaka M (1997) Genes involved in organ separation in Arabidopsis an analysis of the cup-shaped cotyledon mutant. Plant Cell 9:841–857
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Bindschedler LV, Dewdney J, Blee KA, Stone JM, Asai T, Plotnikov J, Denoux C, Hayes T, Gerrish C, Davies DR, Ausubel FM, Paul Bolwell G (2006) Peroxidase-dependent apoplastic oxidative burst in Arabidopsis required for pathogen resistance. Plant J 47:851–863
Bouaziz D, Pirrello J, Charfeddine M, Hammami A, Jbir R, Dhieb A, Bouzayen M, Gargouri-Bouzid R (2013) Overexpression of StDREB1 transcription factor increases__tolerance to salt in transgenic potato plants. Mol Biotechnol 54:803–817
Chen B, Shen Z, Wu D, Xie X, Xu X, Lv L, Dai H, Chen J, Gan X (2019) Glutathione Peroxidase 1 Promotes NSCLC Resistance to Cisplatin via ROS-Induced Activation of PI3K/AKT Pathway. Biomed Res Int 2019:1–12
Cheng Y-J, Kim M-D, Deng X-P, Kwak S-S, Chen W (2013) Enhanced salt stress tolerance in transgenic potato plants expressing IbMYB1, a sweet potato transcription factor. J Microbiol Biotechnol 23:1737–1746
Cheng Z, Zhang X, Zhao K, Zhou B, Jiang T (2020) Ectopic expression of a poplar gene NAC13 confers enhanced tolerance to salinity stress in transgenic Nicotiana tabacum. J Plant Res 133:727–737
Christianson JL, Boutet E, Puri V, Chawla A, Czech MP (2010) Identification of the lipid droplet targeting domain of the Cidea protein. J Lipid Res 51:3455–3462
Collinge M, Boller T (2001) Differential induction of two potato genes, Stprx2 and StNAC, in response to infection by Phytophthora infestans and to wounding. Plant Mol Biol 46:521–529
Delauney AJ, Verma DPS (1993) Proline biosynthesis and osmoregulation in plants. Plant J 4:215–223
Duval M, Hsieh T-F, Kim SY, Thomas TL (2002) Molecular characterization of AtNAM a member of the Arabidopsis NAC domain superfamily. Plant Mol Biol 50:237–248
Efimova MV, Mukhamatdinova EA, Kovtun IS, Kabil F, Medvedeva YV, Kuznetsov VV (2019) Jasmonic acid enhances the resistance of potato plants in vitro to salt stress. General Biology 488:149–152
Fang Y, Liao K, Du H, Xu Y, Song H, Li X, Xiong L (2015) A stress-responsive NAC transcription factor SNAC3 confers heat and drought tolerance through modulation of reactive oxygen species in rice. J Exp Bot 66:6803–6817
Han D, Du M, Zhou Z, Wang S, Li T, Han J, Xu T, Yang G (2020a) An NAC transcription factor gene from Malus baccata, MbNAC29, increases cold and high salinity tolerance in Arabidopsis. Vitro Cellular & Developmental Biology - Plant 56:588–599
Han D, Du M, Zhou Z, Wang S, Li T, Han J, Xu T, Yang G (2020b) Overexpression of a Malus baccata NAC Transcription Factor Gene MbNAC25 Increases Cold and Salinity Tolerance in Arabidopsis. Int J Mol Sci 21:1198
Hao Y-J, Wei W, Song Q-X, Chen H-W, Zhang Y-Q, Wang F, Zou H-F, Lei G, Tian A-G, Zhang W-K, Ma B, Zhang J-S, Chen S-Y (2011) Soybean NAC transcription factors promote abiotic stress tolerance and lateral root formation in transgenic plants. Plant J 68:302–313
Hayat S, Hayat Q, Alyemeni MN, Wani AS, Pichtel J, Ahmad A (2012) Role of proline under changing environments. Plant Signal Behav 7:1–11
He Z, Li Z, Lu H, Huo L, Wang Z, Wang Y, Ji X (2019) The NAC protein from Tamarix hispida, ThNAC7, confers salt and osmotic stress tolerance by increasing reactive oxygen species scavenging capability. Plants 8:221
Hickman R, Hill C, Penfold CA, Breeze E, Bowden L, Moore JD, Zhang P, Jackson A, Cooke E, Bewicke-Copley F, Mead A, Beynon J, Wild DL, Denby KJ, Ott S, Buchanan-Wollaston V (2013) A local regulatory network around three NAC transcription factors in stress responses and senescence in Arabidopsis leaves. Plant J 75:26–39
Hu H, Dai M, Yao J, Xiao B, Li X, Zhang Q, Xiong L (2006) Overexpressing a NAM, ATAF, and CUC (NAC) transcription factor enhances drought resistance and salt tolerance in rice. Proc Natl Acad Sci U S A 103:12987–12992
Hu H, You J, Fang Y, Zhu X, Qi Z, Xiong L (2008) Characterization of transcription factor gene SNAC2 cofering cold and salt tolerance in rice. Plant Mol Biol 67:169–181
Huang Q, Wang Y, Li B, Chang J, Chen M, Li K, Yang G, He G (2015) TaNAC29, a NAC transcription factor from wheat, enhances salt and drought tolerance in transgenic Arabidopsis. BMC Plant Biol 15:268
Jaarsma R, De Boer AH (2018) Salinity tolerance of two potato cultivars (solanum tuberosum) correlates with differences in vacuolar transport activity. Front Plant Sci 9:737
Jaarsma R, Vries RSMd, Boer AHd (2013) Effect of Salt Stress on Growth, Na+ Accumulation and Proline Metabolism in Potato (Solanum tuberosum) Cultivars. PLoS ONE 8:e60183
Jensen MK, Skriver K (2014) NAC transcription factor gene regulatory and protein-protein interaction networks in plant stress responses and senescence. IUBMB Life 66:156–166
Jensen MK, Lindemose S, de Masi F, Reimer JJ, Nielsen M, Perera V, Workman CT, Turck F, Grant MR, Mundy J, Petersen M, Skriver K (2013) ATAF1 transcription factor directly regulates abscisic acid biosynthetic gene NCED3 in Arabidopsis thaliana. FEBS Open Bio 3:321–327
Kikuchi K, Ueguchi-Tanaka M, Yoshida KT, Nagato Y, Matsusoka M, Hirano H-Y (2000) Molecular analysis of the NAC gene family in rice. Mol Gen Genet 262:1047–1051
Kim S-G, Lee A-K, Yoon H-K, Park C-M (2008) A membrane-bound NAC transcription factor NTL8 regulates gibberellic acid-mediated salt signaling in Arabidopsis seed germination. Plant J 55:77–88
Kim MJ, Shin R, Schachtman DP (2009) A nuclear factor regulates abscisic acid responses in arabidopsis. Plant Physiol 151:1433–1445
Lata C, Yadav A, Prasad M (2011) Role of plant transcription factors in abiotic stress tolerance. Physiol Biochem Genet Persp 10:270–296
Li M, Chen R, Jiang Q, Sun X, Zhang H, Hu Z (2020) GmNAC06, a NAC domain transcription factor enhances salt stress tolerance in soybean. Plant Mol Biol 105:333–345
Lichtenthaler HK (1987) ChlorolShylls and carotenoids_ pigments of__photosynthetic biomembranes. Methods Enzymol 148:350–382
Livak KJ, Schmittgen TD (2001) Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 25:402–408
Lu M, Ying S, Zhang D-F, Shi Y-S, Song Y-C, Wang T-Y, Li Y (2012) A maize stress-responsive NAC transcription factor, ZmSNAC1, confers enhanced tolerance to dehydration in transgenic Arabidopsis. Plant Cell Rep 31:1701–1711
Ma N, Feng H, Meng X, Li D, Yang D, Wu C, Meng Q (2014) Overexpression of tomato SlNAC1 transcription factor alters fruit pigmentation and softening. BMC Plant Biol 14:351
Mao X, Zhang H, Qian X, Li A, Zhao G, Jing R (2012) TaNAC2, a NAC-type wheat transcription factor conferring enhanced multiple abiotic stress tolerances in Arabidopsis. J Exp Bot 63:2933–2946
Mao H, Yu L, Han R, Li Z, Liu H (2016) ZmNAC55, a maize stress-responsive NAC transcription factor, confers drought resistance in transgenic Arabidopsis. Plant Physiol Biochem 105:55–66
Mao C, Lu S, Lv B, Zhang B, Shen J, He J, Luo L, Xi D, Chen X, Ming F (2017) A rice NAC transcription factor promotes leaf senescence via ABA biosynthesis. Plant Physiol 174:1747–1763
Mao C, Ding J, Zhang B, Xi D, Ming F (2018) OsNAC2 positively affects salt-induced cell death and binds to the OsAP37 and OsCOX11 promoters. Plant J 94:454–468
Marques DN, Reis SPd, de Souza CRB (2017) Plant NAC transcription factors responsive to abiotic stresses. Plant Gene 11:170–179
Melo BP, Fraga OT, Silva JCF, Ferreira DO, Brustolini OJB, Carpinetti PA, Machado JPB, Reis PAB, Fontes EPB (2018) Revisiting the soybean GmNAC superfamily. Front Plant Sci 9:1864
Mizzotti C, Rotasperti L, Moretto M, Tadini L, Resentini F, Galliani BM, Galbiati M, Engelen K, Pesaresi P, Masiero S (2018) Time-course transcriptome analysis of arabidopsis siliques discloses genes essential for fruit development and maturation. Plant Physiol 178:1249–1268
Nakashima K, Tran L-SP, Van Nguyen D, Fujita M, Maruyama K, Todaka D, Ito Y, Hayashi N, Shinozaki K, Yamaguchi-Shinozaki K (2007) Functional analysis of a NAC-type transcription factor OsNAC6 involved in abiotic and biotic stress-responsive gene expression in rice. Plant J 51:617–630
Nakashima K, Takasaki H, Mizoi J, Shinozaki K, Yamaguchi-Shinozaki K (2012) NAC transcription factors in plant abiotic stress responses. Biochim Biophys Acta (BBA)-Gene Regul Mech 1819(2):97–103
Nguyen KH, Mostofa MG, Li W, Van Ha C, Watanabe Y, Le DT, Thao NP, Tran L-SP (2018) The soybean transcription factor GmNAC085 enhances drought tolerance in Arabidopsis. Environ Exp Bot 151:12–20
Nuruzzaman M, Manimekalai R, Sharoni AM, Satoh K, Kondoh H, Ooka H, Kikuchi S (2010) Genome-wide analysis of NAC transcription factor family in rice. Gene 465:30–44
Ooka H, Satoh K, Doi K, Nagata T, Otomo Y, Murakami K, Matsubara K, Osato N, Kawai J, Carninci P, Hayashizaki Y, Koji Suzuki KK, Takahara Y, Yamamoto K, Kikuchi S (2003) Comprehensive analysis of NAC family genes in Oryza sativa and Arabidopsis thaliana. DNA Res 10:239–247
Ouhibi C, Attia H, Rebah F, Msilini N, Chebbi M, Aarrouf J, Urban L, Lachaal M (2014) Salt stress mitigation by seed priming with UV-C in lettuce plants: Growth, antioxidant activity and phenolic compounds. Plant Physiol Biochem 83:126–133
Peng X, Zhao Y, Li X, Wu M, Chai W, Sheng L, Wang Y, Dong Q, Jiang H, Cheng B (2015) Genomewide identification, classification and analysis of NAC type gene family in maize. Genetics 94:377–390
Ren T, Qu F, Morris TJ (2000) HRT gene function requires interaction between a NAC protein and viral capsid protein to confer resistance to turnip crinkle virus. Plant Cell 12:1917–1926
Shao H, Wang H, Tang X (2015) NAC transcription factors in plant multiple abiotic stress responses: progress and prospects. Front Plant Sci 6:902
Shen J, Lv B, Luo L, He J, Mao C, Xi D, Ming F (2017) The NAC-type transcription factor OsNAC2 regulates ABA-dependent genes and abiotic stress tolerance in rice. Sci Rep 7(1):1–16
Singh AK, Sharma V, Pal AK, Acharya V, Ahuja PS (2013) Genome-wide organization and expression profiling of the NAC transcription factor family in potato (Solanum tuberosum L.). DNA Res 20:403–423
Slama I, Abdelly C, Bouchereau A, Flowers T, Savouré A (2015) Diversity, distribution and roles of osmoprotective compounds accumulated in halophytes under abiotic stress. Ann Bot 115:433–447
Szabados L, Savouré A (2010) Proline: a multifunctional amino acid. Trends Plant Sci 15:89–97
Takasaki H, Maruyama K, Kidokoro S, Ito Y, Fujita Y, Shinozaki K, Yamaguchi-Shinozaki K, Nakashima K (2010) The abiotic stress-responsive NAC-type transcription factor OsNAC5 regulates stress-inducible genes and stress tolerance in rice. Mol Genet Genomics 284:173–183
Tran L-SP, Nakashima K, Sakuma Y, Simpson SD, Fujita Y, Maruyama K, Fujita M, Seki M, Shinozaki K, Yamaguchi-Shinozaki K (2004) Isolation and functional analysis of arabidopsis stress-inducible NAC transcription factors that bind to a drought-responsive cis-element in the early responsive to dehydration stress 1 promoter. Plant Cell 16:2481–2498
Wang H, Wang H, Shao H, Tang X (2016) Recent advances in utilizing transcription factors to improve plant abiotic stress tolerance by transgenic technology. Front Plant Sci 7:67
Wang K, Zhong M, Wu Y-h, Bai Z-y, Liang Q-y, Liu Q-l, Pan Y-z, Zhang L, Jiang B-b, Jia Y, Liu G-l (2017) Overexpression of a chrysanthemum transcription factor gene DgNAC1 improves the salinity tolerance in chrysanthemum. Plant Cell Rep 36:571–581
Xie Q, Frugis G, Colgan D, Chua N-H (2000) Arabidopsis NAC1 transduces auxin signal downstream of TIR1 to promote lateral root development.pdf>. Genes Dev 14:3024–3036
Xu Q, He Q, Li S, Tian Z (2014) Molecular characterization of StNAC2 in potato and its overexpression confers drought and salt tolerance. Acta Physiol Plant 36:1841–1851
Yang Y, Guo Y (2018) Unraveling salt stress signaling in plants. J Integr Plant Biol 60:796–804
Yang R, Deng C, Ouyang B, Ye Z (2011) Molecular analysis of two salt-responsive NAC-family genes and their expression analysis in tomato. Mol Biol Rep 38:857–863
Yoon Y, Seo DH, Shin H, Kim HJ, Kim CM, Jang G (2020) The Role of Stress-Responsive Transcription Factors in Modulating Abiotic Stress Tolerance in Plants. Agronomy 10:788
Yu S, Huang A, Li J, Gao L, Feng Y, Pemberton E, Chen C (2018) OsNAC45 plays complex roles by mediating POD activity and the expression of development-related genes under various abiotic stresses in rice root. Plant Growth Regul 84:519–531
Zhang X, Cheng Z, Zhao K, Yao W, Sun X, Jiang T, Zhou B (2019) Functional characterization of poplar NAC13 gene in salt tolerance. Plant Sci 281:1–8
Zhao P, Ye M, Wang R, Wang D, Chen Q (2020) Systematic identification and functional analysis of potato (Solanum tuberosum L.) bZIP transcription factors and overexpression of potato bZIP transcription factor StbZIP-65 enhances salt tolerance. Int J Biol Macromol 161:155–167
Zheng X, Chen B, Lu G, Han B (2009) Overexpression of a NAC transcription factor enhances rice drought and salt tolerance. Biochem Biophys Res Commun 379:985–989
Zhou W, Qian C, Li R, Zhou S, Zhang R, Xiao J, Wang X, Zhang S, Xing L, Cao A (2018) TaNAC6s are involved in the basal and broad-spectrum resistance to powdery mildew in wheat. Plant Sci 277:218–228
Acknowledgements
WH and BZ designed research. LY conducted experiments. WH and YZ wrote the manuscript. YZ and LY performed in data analysis. All authors read and approved the manuscript. This work was supported by Sichuan Province Science and Technology Support Program (CN) (2020YFH0003).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yue, L., Zhuang, Y., Gu, Y. et al. Heterologous expression of Solanum tuberosum NAC1 gene confers enhanced tolerance to salt stress in transgenic Nicotiana benthamiana. J. Plant Biol. 64, 531–542 (2021). https://doi.org/10.1007/s12374-021-09327-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12374-021-09327-0