Abstract
Sporisorium scitamineum, a biotrophic basidiomycete fungus, causes the ravaging sugarcane smut disease globally in almost all sugarcane growing countries. The fungus spans around three distinct developmental stages, viz., diploid teliospores, haploid sporidia and dikaryotic mycelia, during its life cycle. The yeast-like and non-pathogenic haploid sporidia represent two opposite mating types: MAT-1 and MAT-2. Discrimination of these mating type haploids is essential for long-term preservation and maintenance of true-to-type isolate/pathotype, genetic investigations and to understand how the fusion of non-pathogenic, compatible mating type haploids turn into virulent dikaryotic mycelia. Hitherto, only the mating-based phenotyping assay is used for discrimination of opposite mating type haploids. In the present study, a quick and reliable PCR-based assay was developed for the first time for discriminating the distinct mating types (MAT-1 and MAT-2) of the S. scitamineum by targeting the unique regions of bE/bW mating type genes. Among the different primer sets evaluated, two sets, viz., bE1F1/bE1-2R1 and bE2F2/bE1-2R1 that target bE mating type genes, precisely differentiated the MAT-1 and MAT-2 mating type haploids of two S. scitamineum isolates, Ss97009 (high virulent) and SsV89101 (less virulent). The pathogenicity of distinct developmental stages, viz., haploid sporidia, dikaryotic mycelia and teliospores, and the life cycle transitions of these two S. scitamineum isolates in planta were analyzed by phenotyping and microscopy, respectively. Quantitative real-time PCR was used to estimate the pathogen biomass of plants inoculated with individual haploid sporidia (MAT-1 and MAT-2), mixture of haploid sporidia and teliospores of Ss97009 and SsV89101. These results altogether have substantiated the virulence nature of these two isolates and established the significance of fusion of opposite mating type haploids in causing smut disease in sugarcane. Overall, this simple and efficient PCR-based discrimination assay of haploids would serve as a valuable tool for genomic and functional studies of S. scitamineum.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sporisorium scitamineum (Syd.) is the causative agent of sugarcane smut, which is one of the most prevalent and devastating diseases in sugarcane-producing countries worldwide (Lee-Lovick 1978; Sundar et al. 2012). It causes substantial yield loss and decrease in cane quality by making unproductive tillers, leading to a significant economic loss in sugarcane production (Zekarias et al. 2010; Magarey et al. 2011). The life cycle of S. scitamineum is similar to that of the corn smut fungus—Ustilago maydis, which has been well established as a model pathogenic fungus and it involves transitions between three cell types: diploid teliospores, haploid sporidia and dikaryotic mycelia (Kamper et al. 2006). The diploid teliospores formed in host tissues are the resting cell type and are mainly disseminated by wind or rain splashes infecting the buds in the sprouting stage (Sundar et al. 2012). Unlike U. maydis, S. scitamineum possess a bipolar mating system with two mating types, “ + ” and “−”, which were designated as MAT-1 and MAT-2 (Yan et al. 2016a). The pathogenic dikaryotic mycelia that exhibit filamentous growth, penetrate through the young bud surface and proliferate within the stalk of sugarcane, resulting in the formation of a whip-like sorus bearing diploid teliospores to complete the pathogenic life cycle (Trione 1980).
In U. maydis, mating and pathogenic development are mediated by a pair of unlinked mating type loci that are termed a and b (Kronstad and Staben 1997). Recognition of compatible mating types and haploid fusion events are controlled by the biallelic a locus that codes for a pheromone and a pheromone receptor system (Bolker et al. 1995). The multiallelic b locus controls the formation of dikaryotic mycelia and pathogenicity, and it encodes a pair of distinct homeodomain proteins, bE and bW, that function as transcription factors. The bE/bW transcription factor would be functional only when the bE and bW genes are acquired from different alleles of MAT-1 and MAT-2 (Feldbrugge et al. 2004; Wahl et al. 2010). Yan et al. (2016a) investigated the molecular mechanism of mating reaction of S. scitamineum by deleting the mating-specific U. maydis locus b ortholog in S. scitamineum and found that the b locus is crucial for mating, filamentous growth and pathogenicity. To further understand the role of the b mating type locus, a comparative stage-specific transcriptome analysis was performed, which indicated that the bE/bW transcription factors encoded by the b locus could control sexual mating and/or mycelial development in S. scitamineum (Yan et al. 2016b).
In S. scitamineum, compatible opposite mating type haploids of genetically diverse pathotypes often fuse together to produce new variants and apparently contribute for the emergence of new pathotypes with altered virulence (Barnabas et al. 2018; Nalayeni et al. 2021). Due to this phenomenon, the preservation of true-to-type of each isolate/pathotype gains significance. However, the maintenance and preservation of S. scitamineum in the form of teliospores or dikaryotic mycelia for long term is not feasible, and hence, isolation and identification of haploid sporidia for storage remains the only alternative option. Presently, the mating-based phenotyping assay is the only method used for the discrimination of opposite mating type haploids of S. scitamineum. On the genomic front, molecular identification of S. scitamineum is carried out with bE mating type gene-specific PCR, which was first developed by Albert and Schenck (1996) from the orthologous bE mating type gene of U. maydis. Subsequently, many PCR-based assays were developed for more accurate and rapid detection of S. scitamineum in sugarcane tissues (Chen et al. 2015). However, such molecular method for detection and discrimination of opposite mating type haploid sporidia is not available.
Owing to the global importance of the sugarcane smut, several research projects on genomics have been commenced in the recent past. The complete genome of a Brazilian S. scitamineum mating type strain SSC39B carrying MAT-1 specific loci was sequenced using next-generation sequencing technology (Taniguti et al. 2015). Subsequently, the genome sequence of a South African S. scitamineum haploid strain SscI8 carrying MAT-2 specific loci was reported (Dutheil et al. 2016). With this genomic information, here we developed a simple PCR-based assay to distinguish the distinct mating types of S. scitamineum using primers targeting bE/bW mating type genes. Further, the transitions that occur during distinct developmental stages in planta were documented microscopically and their pathogenicity was demonstrated by both phenotyping and qPCR analyses.
Materials and Methods
Fungal Isolate and Plant Material
Two S. scitamineum isolates, Ss97009, a high virulent isolate, and SsV89101, a low virulent isolate, collected from the sugarcane cultivars Co 97009 and CoV 89101, respectively, were used in the study. The virulence of these isolates was assessed earlier by relative virulence testing on a smut susceptible variety and differential host experiments (Barnabas et al. 2018). In our study, single bud setts of 7-month-old Co 97009, a smut susceptible variety, were used for all plant inoculation experiments.
Isolation of S. scitamineum Haploids
Isolation of distinct haploid sporidia of opposite mating types from the isolates, Ss97009 and SsV89101, was done according to the protocol described by Barnabas et al. (2017) with few modifications. Fresh teliospores collected from the infected plants were washed thrice with sterile distilled water and surface sterilized with streptomycin sulfate (500 µg/mL) for 2 min. The surface sterilized teliospores were then plated onto Potato Dextrose agar (PDA) medium amended with streptomycin sulfate (500 µg/mL) and incubated at 28 °C under dark condition for 24 to 48 h. The mycelial colonies obtained were again serially diluted, plated and incubated as described above to isolate haploid colonies.
Random Mating Assay
Single haploid sporidial cultures thus obtained were then transferred to Yeast Malt broth (YM broth—3 g/L yeast extract, 3 g/L malt extract, 10 g/L dextrose and 5 g/L peptone) and incubated on a shaker (130 rpm) at 28 °C for 24 h. After incubation, equal volumes of random combinations of single haploid sporidial cultures were mixed and co-cultivated on the surface of PDA containing 1% charcoal and streptomycin sulfate (500 µg/mL) (Banuett and Herskowitz 1989) and the plates were incubated at 28 °C under dark condition for 48 h. The plates were analyzed for the presence of fuzzy-type colonies indicating dikaryotic mycelial formation. Cultures of the distinct haploid sporidia of both isolates were then cryopreserved in 30% glycerol and stored at -80 °C.
Extraction of Fungal Genomic DNA
DNA from the dikaryotic mycelia (DM), distinct haploid sporidia of opposite mating types and teliospores of the isolates, Ss97009 and SsV89101 were extracted individually by CTAB method as described by Abu Almakarem et al. (2012). Distinct haploid sporidial cultures grown on PDA were transferred to YM broth with streptomycin sulfate (500 µg/mL) and incubated on a shaker (130 rpm) at 28 °C for 48 h for mass multiplication. Cells were harvested by centrifugation at 10,000 rpm for 15 min at 4 °C. Obtained pellets were ground with liquid nitrogen and subjected to DNA extraction. Integrity of the extracted DNA was verified on 0.8% agarose gels and quantified using NanoDrop (Thermo Scientific, USA).
Designing of S. scitamineum Mating Type-Specific Primers
Mating type-specific sequences (bE/bW) of MAT-1 were obtained from the genome assembly of S. scitamineum isolate SSC39 (GenBank Accession: CP010914.1), and MAT-2 from the genome assembly of S. scitamineum isolate SscI8 (GenBank Accession: LK056662.1) (Taniguti et al. 2015; Dutheil et al. 2016). Veracity of the retrieved bE and bW sequences of MAT-1 and MAT-2 were assessed by amplicon resequencing, and they were aligned individually using the CLUSTAL O (1.2.1) multiple sequence alignment (https://www.ebi.ac.uk/Tools/msa/clustalo/) to map their respective unique regions. Accordingly, six pairs of bE/bW-specific primers that could distinguish between MAT-1 and MAT-2 mating types were designed using Primer-BLAST (https://www.ncbi.nlm.nih.gov/tools/primer-blast/). The details of the primers are given in Table 1.
Mating Type-Specific PCR Assay
The distinct haploid sporidia of opposite mating types derived from the isolates, Ss97009 and SsV89101, were used to examine the mating type-specific PCR assay with the bE/bW gene targeting primers. Each PCR reaction consisted of a final volume of 20 µL reaction containing 1X buffer, 200 µM dNTPs, 0.5 µM each of forward and reverse primers and 1 U Taq polymerase. PCR was performed using Applied Biosystems Veriti Thermal cycler (Applied Biosystems, USA) under the following thermocycling parameters: an initial denaturation at 94 °C for 5 min, 35 cycles of denaturation at 94 °C for 1 min, annealing at 56 °C for 30 s and polymerization at 72 °C for 1 min, followed by a final elongation at 72 °C for 5 min. The short-listed primers were also assessed for amplification in a more convenient multiplex PCR. The final PCR products were resolved in a 1.5% agarose gel in 1X TAE buffer and visualized using Syngene G:Box gel documentation system (Syngene, UK). Sequences of the amplified PCR products were also validated by Sanger sequencing.
Inoculation, Sampling and Phenotypic Evaluation
For inoculum preparation, MAT-1 and MAT-2 haploids were individually cultured in YM broth for 24 h, pelleted at 3000 rpm for 5 min at 4 °C and re-suspended in sterile distilled water so as to get a final concentration of 108 cells/mL. Seven-day-old pre-germinated buds of the smut susceptible cultivar Co 97009 were inoculated individually with MAT-1 haploid, MAT-2 haploid, equal proportion mixture of MAT-1 and MAT-2, and teliospores (4 × 106 spores/mL) of individual isolates (Ss97009 and SsV89101) by bud pasting (drop inoculation) method, and planted in pots containing sandy soil: farmyard manure (3:1) mixture (Co et al. 2008). At least thirty plants were inoculated per treatment and equal number of uninoculated plants served as the mock control. All the plants were maintained under glasshouse conditions at 28°-30 °C with 60–80% humidity, and the meristem samples were drawn in triplicates per time-interval (2 dpi, 5 dpi, 10 dpi and 80 dpi) per treatment for microscopy and qPCR analysis. Remaining plants were regularly monitored for the emergence of smut whips.
Microscopic Analysis of Distinct Developmental Stages of S. scitamineum in planta
Co 97009 inoculated with teliospores of the S. scitamineum isolates Ss97009 and SsV89101 were used for investigating the colonization stages in planta. The pathogen challenged shoot meristem tissues sampled at different time points, viz., 2 dpi, 5 dpi, 10 dpi and 80 dpi, were analyzed by light microscopy. Hand-cut sections of the fresh tissues stained with Calcofluor White stain (Sigma Aldrich, USA) were observed under the UV filter (excitation filter: BP340-380, dichromatic mirror: 400 and suppression filter: LP425) using Leica DM LB2 epifluorescence microscope (Leica Microsystems, Germany), and the images were captured with a Leica DMC2900 digital camera.
In planta Pathogen Biomass Assessment by Quantitative Real-Time PCR
Samples of Co 97009 challenged with two different isolates (Ss97009 and SsV89101) were collected in triplicates at different time intervals, viz., 2 dpi, 5 dpi, 10 dpi and 80 dpi, and used for absolute quantification of pathogen by qPCR as described by Ashwin et al. (2017). For this experiment, primer sequences qbE-F1 (5′–CAAGAAGCCGCGGAAAACTG–3′) and qbE-R1 (5′–ACTCGTGTCTGCAGTAGAGC–3′) yielding a specific amplicon of 208 bp were used. For the construction of standard curve, fivefold serial dilutions of dikaryotic mycelial DNA ranging from 500 ng to 0.8 ng was prepared and real-time PCR amplification of bE gene was performed in each qPCR run using technical triplicates. For quantification of pathogen biomass in the infected samples at different intervals, DNA was extracted by CTAB method (Abu Almakarem et al. 2012). Real-time PCR amplification was performed in AB StepOne Plus (Applied Biosystems, USA) using DNA samples adjusted to 30 ng/μL. Each real-time PCR reaction consisted of a final volume of 20 μL reaction mixture containing 1 μL DNA template (30 ng), 50 nM of each primer and 10 μL SYBR Green (MESA GREEN qPCR Mastermix Plus). Specificity of the primer was verified by melt curve analysis. The standard curve was generated by plotting Cycle Threshold (CT) values of serially diluted dikaryotic mycelial DNA against DNA concentration on a logarithmic scale. Further, the concentration of fungal DNA in the unknown samples was calculated using the standard curve equation and was expressed as ng of fungal DNA per 30 ng of total DNA. Statistical analysis of data was performed using the software IBM SPSS Statistics 21.0 (SPSS, Chicago, USA). The data from triplicate observations were analyzed using one-way analysis of variance (ANOVA) and significant differences among treatments were determined at p ≤ 0.05 based on post-hoc Tukey’s test.
Results
Isolation of Opposite Mating Types of S. scitamineum by Random Mating Assay
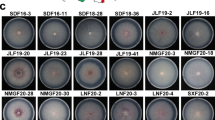
When haploid sporidia containing different a- and b loci were mixed together and co-cultivated on PDA containing 1% charcoal and streptomycin sulfate (500 µg/mL), they grew towards each other producing conjugation hyphae resulting in dikaryotic mycelial (fuzzy appearance) formation (Fig. 1). Thus, the haploid sporidia of the isolates, Ss97009 and SsV89101, were arbitrarily designated either as same or opposite mating types (+ or -) according to the smooth or fuzzy appearance, respectively, in the random mating assay.
Isolation and identification of haploid sporidia of opposite mating types. a Mycelial colonies isolated from surface sterilized teliospores of S. scitamineum. b Haploid colonies isolated from serially diluted mycelial colony. c Random plating assay for mating-type differentiation showing fused colonies of different mating types (Haploids 1,4 and 5 are of same mating type, and 2 and 3 represent opposite mating type)
Development of Mating Type-Specific PCR Assay for Discriminating Distinct Mating Types
Primers targeting the unique regions of mating type genes were designed based on the multiple sequence alignment of bE (Supplementary Fig. 1) and bW (Supplementary Fig. 2) genes of MAT-1 and MAT-2 mating types of S. scitamineum, and used for differentiating the distinct mating types of the S. scitamineum isolates Ss97009 and SsV89101. Genomic DNA extracted from the haploid sporidia were assayed by PCR using six different combinations of forward and reverse primers to identify candidates that could distinguish between the distinct mating types. Among the tested primer sets, bE1F1/bE1-2R1 and bE2F2/bE1-2R1 have shown a clear-cut distinguishing profile for both MAT-1 and MAT-2 mating types (Fig. 2). PCR amplification of bE mating type gene using the primers bE1F1 and bE1-2R1 yielded an amplicon of 668 bp with MAT-1 haploids, dikaryotic mycelia and teliospores of Ss97009 and SsV89101, but not with the MAT-2 haploids. In contrast, bE2F2 and bE1-2R1 resulted in an amplicon of 458 bp with MAT-2 haploids, dikaryotic mycelia and teliospores, but not with the MAT-1 haploids. Hence, the primers bE1F1/bE1-2R1 and bE2F2/bE1-2R1 could be used to detect the identity of MAT-1 and MAT-2 haploids, respectively. Dikaryotic mycelia and diploid teliospores which should have both mating type genes could be detected by the occurrence of both amplification products. The primer pairs, bE1F1/bE1-2R1 and bE2F2/bE1-2R1, responded with the same optimum annealing temperature (56 °C) and were utilized in a multiplex PCR, which displayed consistent results with the singleplex PCR (Supplementary Fig. 3). Among the bW-specific primers, only one primer set bW2F1/bW2R1 yielded a discriminatory profile (data not shown). Hence, it was not considered for distinguishing the distinct mating types.
Mating-type-specific PCR for differentiating distinct mating types of S. scitamineum isolates a Ss97009 and b SsV89101 using bE-specific primers. M- 100 bp ladder, lane 1 & 5- MAT-1, lane 2 & 6- MAT-2, lane 3 & 7- dikaryotic mycelia, lane 4 & 8- teliospores, and lane 9-negative control. Primer set bE1F1/bE1-2R1 yielded a specific amplicon of 668 bp with MAT-1 haploid, and bE2F2/bE1-2R1 resulted in an amplicon of 458 bp with MAT-2 haploid. Dikaryotic mycelia and teliospores which served as positive controls yielded specific amplicons with both the primers
Assessment of Pathogenicity of Distinct Developmental Stages of S. scitamineum Using Two Isolates, Ss97009 and SsV89101
The pathogenicity of distinct developmental stages of S. scitamineum, haploid sporidia (MAT-1 and MAT-2), dikaryotic mycelia and teliospores was evaluated by phenotyping the disease symptoms in a smut susceptible variety, Co 97009 using two isolates, Ss97009 (high virulent) and SsV89101 (low virulent). The plants inoculated individually with the MAT-1 and MAT-2 haploid sporidia of the isolate Ss97009 (Fig. 3a, b) did not show any disease symptom, revealing its state of non-pathogenicity. Similarly, the SsV89101 inoculated plants were symptomless when inoculated with the individual haploid sporidia (data not shown). The plants inoculated with the equal proportion mixture of compatible haploid sporidia of the isolate Ss97009 resulted in characteristic whip-shaped sorus at 80 dpi, indicating the formation of infective dikaryotic mycelia from compatible haploid sporidia and its penetration into sugarcane buds (Fig. 3c). As expected, plants inoculated with teliospores of the isolate Ss97009 also exhibited whip emergence at 80 dpi (Fig. 3d). Conversely, plants inoculated with SsV89101 did not develop any whip over a period of 80 days when inoculated with the mixture of haploid sporidia/teliospores (data not shown). The S. scitamineum isolate Ss97009 developed a total of 9 whips after 80 dpi confirming its high virulence compared to the isolate SsV89101, which exhibited the emergence of only 2 whips after 100 days.
Representative images of pathogenicity assay of distinct developmental stages of S. scitamineum isolate Ss97009. a Haploid sporidia of MAT-1 and b MAT-2 mating-type inoculated plants showing no pathogenicity in a susceptible variety Co 97,009 at 80 dpi, c plants inoculated with mixture of compatible haploid sporidia and d teliospores exhibiting characteristic whip emergence at 80 dpi. The yellow arrows indicate the whip-shaped sori
Detection of Distinct Colonization Stages of S. scitamineum in Sugarcane Through Microscopy
Distinct developmental stages in planta were monitored at different time intervals during colonization of a susceptible variety Co 97009 upon challenge inoculation with two isolates, Ss97009 and SsV89101. Teliospore germination and vigorous growth of dikaryotic mycelia were seen on the external surface of the bud surface at 2 dpi in the case of both isolates (Fig. 4a, b). At 5 dpi, hyphae with bulbous structures attempting penetration were observed on the internal surface of the bud layers and were found extending into intercellular spaces along with intracellular colonization (Fig. 4c–f). At 10 dpi, highly branched mycelia showing inter- and intracellular colonization of surrounding tissues of vascular bundles were observed in plants inoculated with Ss97009 and SsV89101 (Fig. 4g, h). Mycelia showing constrictions at the site of penetration during colonization were also noticed at this time point (Fig. 4i, j). Until this stage, the low virulent SsV89101 did not show significant difference in colonization compared to Ss97009.
Comparison of in planta colonization stages of S. scitamineum isolates Ss97009 and SsV89101 during initial stages (2, 5 and 10 dpi). Sections of the pathogen challenged shoot tissues were stained with Calcofluor White stain and observed under fluorescence microscopy. Colonization of external surface of buds showing germinating teliospores and dikaryotic mycelia at 2 dpi with a Ss97009 and b SsV89101, mycelia with appressoria-like bulbous structures at the internal surface for penetration during colonization at 5 dpi with c Ss97009 and d SsV89101, inter- and intracellular hyphal colonization at 5 dpi during colonization with e Ss97009 and f SsV89101, highly branched mycelia showing inter- and intracellular colonization of surrounding tissues of vascular bundles at 10 dpi during colonization with g Ss97009 and h SsV89101, penetrating mycelia at 10 dpi showing constrictions at the site of penetration during colonization with i Ss97009 and j SsV89101. The yellow arrows indicate the fungal structures colonizing the plant tissues
Phenotypically, S. scitamineum isolate Ss97009 exhibited characteristic whip emergence at 80 dpi, whereas no whips were observed with SsV89101 at this time point. Different parts of the whip, viz., apical and basal regions of the whip, and internode portion beneath the whip were observed under microscopy. Apical region of the whip was extensively colonized with mass of fragmented hyphae and melanized mature teliospores in the peripheral tissues of the meristem (Fig. 5a, b), whereas highly branched mycelia were observed in the central part of the meristem (Fig. 5c). The basal region of the whip with active sporogenesis displayed sporulation pockets with hyphal fragmentation and non-melanized immature teliospores in the peripheral part, and inter- and intracellular hyphal colonization in the central part of the meristem (Fig. 5d–f). The sections of the internode portion beneath sporogenesis also showed inter- and intracellular hyphal colonization of parenchyma cells and adjacent cells of vascular tissues (Fig. 5g–i).
In planta colonization stages of S. scitamineum isolate Ss97009 during whip emergence at 80 dpi. Sections of different parts, viz., (i) apical region (ii) basal region and (iii) internode portion beneath whip were stained with Calcofluor White stain and observed under fluorescence microscopy. a Apical region of the whip with mass of fragmented hyphae and melanized mature teliospores and b sporogenous hyphae in the peripheral tissues of the meristem; c longitudinal section showing highly branched hyphae in the central part of the meristem, d-e basal region of the whip with active sporogenesis in the peripheral part showing sporulation pockets with hyphal fragmentation and non-melanized immature teliospores, f inter- and intracellular hyphal colonization in the central part of the meristem, g-i internode portion beneath sporogenesis displaying inter- and intracellular colonization of parenchyma cells and surrounding tissues of vascular tissues and intracellular colonization, respectively. The yellow arrows indicate the sites of fungal colonization inside the plant tissues
Comparative in planta Quantitative Assay of Pathogen Biomass of Ss97009 and SsV89101
Fungal DNA concentration in the pathogen inoculated samples was obtained by extrapolating the CT values into the standard curve equation with a correlation coefficient (R2) of 0.994 (Supplementary Fig. 4). Results showed that a detectable quantity of fungal DNA was observed only at 2 dpi in the case of bud samples inoculated with MAT-1 or MAT-2 of Ss97009 and SsV89101 (Fig. 6). A relatively higher amount of fungal DNA was detected at 2 dpi in plants challenge inoculated with a mixture of compatible haploid sporidia and teliospores with both the isolates, owing to the presence of inoculum on the bud surface. Results indicated that the colonization of Ss97009 was significantly higher than that of SsV89101 at 5 dpi and 10 dpi. In Ss97009 teliospores inoculated plants, an average of 2.2 ng of fungal DNA was detected at 5 dpi indicating the penetration of infective dikaryotic mycelia, followed by a slight increase in the fungal inoculum density (2.9 ng) in the meristem at 10 dpi. The highest fungal DNA concentration was noticed at 80 dpi in the plant samples (~ 22 ng) infected with teliospores/ mixture of haploid sporidia of the isolate Ss97009. The pathogen biomass of Ss97009 at 80 dpi was found to be remarkably higher than the SsV89101, which showed ~ 0.1 ng fungal DNA.
Comparison of pathogen biomass in sugarcane shoot meristem during colonization with S. scitamineum isolates Ss97009 and SsV89101 by qPCR. Sugarcane cultivar Co 97009 inoculated individually with distinct developmental stages, viz., MAT-1, MAT-2, mixture of MAT-1 and MAT-2 resulting in dikaryotic mycelia (DM) and teliospores of the isolates Ss97009 and SsV89101 were analyzed at different time intervals, 2, 5, 10 and 80 dpi. Higher pathogen biomass was observed in the meristem at 80 dpi in plants inoculated with mixture of MAT-1/MAT-2 and teliospores of Ss97009 compared to that of SsV89101. The results shown represent the means and standard deviations of three replicates, and different letters represent statistically significant difference at p ≤ 0.05
Discussion
The basic pre-requisite toward understanding pathogenicity of smut fungi is to identify the distinct mating type loci. Fusion of haploid sporidia representing opposite mating types is essentially required for sexual reproduction and subsequent infection by smut fungi (Olicon-Hernandez et al. 2019). Diploid teliospores are formed only inside the host tissues (sporogenesis), particularly in the apical meristem, after successful establishment and proliferation of the dikaryotic mycelia in sugarcane. Thus, the mating nature of these haploid sporidia is an intriguing phenomenon, which plays a pivotal role in establishing pathogenicity. In S. scitamineum, the haploid sporidia of distinct mating types are morphologically identical and can only be distinguished into ‘plus’ or ‘minus’ mating types by random mating experiment on nutrient media (Singh et al. 2005; Barnabas et al. 2017). However, PCR-based methods with specific primers have been demonstrated to be quick and helpful for the differentiation of mating types in several other phytopathogenic fungi (Dyer et al. 2001; Brewer et al. 2011; Dai et al. 2018). Hence, in this study, a rapid PCR-based assay for differentiation of distinct mating types (MAT-1 and MAT-2) of S. scitamineum was developed by targeting the mating type genes (bE and bW).
Albert and Schenck (1996) designed primers to detect S. scitamineum, based on the orthologous bE gene of U. maydis, which yielded a uniform band size for both mating types. Since then, PCR amplification using bE gene has been routinely used to identify and diagnose S. scitamineum (Xu et al. 2014; Barnabas et al. 2018). In recent years, the complete genome of MAT-1 and MAT-2 mating type strains of haploid sporidia was sequenced by next-generation sequencing technology, which has advanced our understanding of the mating types of the sugarcane smut fungus (Taniguti et al. 2015; Dutheil et al. 2016). The accessibility of these publicly available genome data has facilitated the comparison of the b locus sequences (bE and bW) of the compatible mating types. The sequence and function of the b locus has been analyzed at molecular level by Yan et al. (2016a) and reported that the b locus derived from MAT-1 and MAT-2 has about 90% identity to each other. The mating type-specific PCR assay developed in this study based on the minimal dissimilar sequences of bE gene can be used reliably to differentiate between the two mating types of S. scitamineum, circumventing the need for the time-consuming random mating experiment. Besides, the assay would serve as a simple and quick tool for assessment or reassessment of the identity of haploids isolated, maintained or preserved as true-to-type.
In the present study, the pathogenicity of the distinct developmental stages, viz., haploid sporidia, dikaryotic mycelia and teliospores, was assessed by phenotyping the disease symptoms in a smut susceptible sugarcane cultivar. Plants inoculated with haploid sporidia of only one mating type (MAT-1 or MAT-2) did not result in any disease symptom indicating its non-pathogenicity, whereas the plants with the infective dikaryotic mycelia and teliospores developed characteristic whip-shaped sorus indicating its pathogenicity. Similarly, in vitro screening of tissue culture plants of three sugarcane cultivars using haploid sporidia of only one mating type (plus or minus) did not result in any symptom, but the equal proportion mixture of compatible haploid sporidia resulted in the production of whips (Singh et al. 2005). Here, the S. scitamineum isolate, Ss97009, developed higher number of whips after 80 dpi depicting its high level of virulence compared to the isolate SsV89101.
Here, the documentation of the infection process of two S. scitamineum isolates, Ss97009 and SsV89101, in a susceptible variety Co 97009 has facilitated the visualization of the natural route of the fungus during initial stages of in planta colonization of sugarcane tissues. The successful transition from teliospore to haploid sporidia and their fusion to dikaryotic mycelia were evident from the observation of mycelial colonization in the external bud surface at 2 dpi. It was reported earlier that the entry into the meristem in the bud is occurring between 6 and 36 h after the teliospores got deposited on the bud (Alexander and Ramakrishnan 1980). At 5dpi, inter- and intracellular colonization of parenchyma cells was observed, indicating the penetration of the infective dikaryotic mycelia. Hyphae showing appressoria-like bulbous structures for penetration were also observed on the internal surface of the bud layer at this time point. Inter- and intracellular colonization of parenchyma cells and vascular tissues were observed at 10 dpi, which is corroborated with the colonization of vascular tissues reported earlier (Marques et al. 2016). Interestingly, majority of the mycelial colonization was restricted to the parenchyma cells adjacent to the vascular tissues displaying the treading path toward the apical meristem. Our study has depicted a clear-cut view of developmental stages and their dimorphic transitions occurred during initial establishment of host infection. No significant difference in pathogen biomass was observed between high virulent Ss97009 and low virulent SsV89101 isolates during initial stages of in planta colonization through microscopy.
By 80 dpi, whip emergence was observed with Ss97009 indicating its high virulence compared to SsV89101 and was examined by microscopy. In the apical region of the meristem, sporogenesis in the peripheral tissues was evident by the presence of masses of fragmented sporogenous hyphae, immature non-melanized teliospores and mature teliospores. Similar observations on hyphal fragmentation and teliospore maturation have been reported previously (Trione 1980). Sporulation loci containing abundant sporogenous hyphae and non-melanized teliospores embedded in a gelatinous matrix were observed in the entire peripheral portion of the basal region of the meristem. The distribution of hyphal colonization throughout the meristem bearing the whip-shaped sorus by 80 dpi and > 100 dpi in Ss97009 and SsV89101 inoculated canes, respectively, were same except in the number of whips emerged. The results on the infection process of S. scitamineum during whip emergence have provided detailed insights into the dimorphic transitions that occur between the developmental stages during in planta colonization.
Many PCR-mediated assays including TaqMan real-time PCR assay using bE mating type gene have been widely used for diagnosis and quantitative detection of S. scitamineum in sugarcane tissues (Moosawi-Jorf and Izadi 2007; Su et al. 2013; Chen et al. 2015). Nevertheless, quantitative detection of colonization upon inoculation with the distinct developmental stage-specific inocula, viz., haploid sporidia of opposite mating types, mixture of haploids and teliospores was reported for the first time, in this study. Real-time PCR assay could detect the presence of haploid of single mating type at 2 dpi in plants discretely inoculated with each mating type of both isolates. Singh et al. (2004) reported similar findings with PCR based on bE mating type gene for smut assessment in inoculated tissue cultured sugarcane cultivars. At 5, 10 and 80 dpi, no amplification is detected in plants inoculated with single sporidia (MAT-1 or MAT-2) of both isolates, suggesting that infection is established only when the plants are inoculated with mixture of haploid sporidia or with teliospores that leads to the formation of infective dikaryotic mycelia. In plants inoculated with mixture of haploid sporidia/teliospores, the rate of colonization of Ss97009 was significantly higher compared to that of SsV89101 at 5 dpi, 10 dpi and 80 dpi. This is in line with the reports of Nalayeni et al. (2021), which demonstrated significant variation in pathogen biomass during host–pathogen interaction using the low and high virulent isolates. Real-time PCR-based absolute quantification has emphasized the application of compatible haploid mixture or teliospores for successful disease establishment and also reaffirmed the high virulence of the S. scitamineum isolate Ss97009 in comparison with SsV89101.
Overall, for the first time, we have developed a reliable and rapid PCR-based assay for differentiating the distinct mating types (MAT-1 and MAT-2) of S. scitamineum, which would serve as a valuable tool for genomic and functional studies utilizing haploid sporidia. We have presented clear-cut colonization stages in planta during initial establishment and during whip emergence by light microscopy technique. In addition, the study has provided detailed insights into the differences in virulence of the two isolates, Ss97009 and SsV89101.
References
Abu Almakarem, A.S., Katie L. Heilman, Heather L. Conger, Yury M. Shtarkman, and Scott O. Rogers. 2012. Extraction of DNA from plant and fungus tissues in situ. BMC Research Notes 5: 266. https://doi.org/10.1186/1756-0500-5-266.
Albert, Henrik H., and Susan Schenck. 1996. PCR amplification from a homolog of the bE mating-type gene as a sensitive assay for the presence of Ustilago scitaminea DNA. Plant Disease 80: 1189–1192. https://doi.org/10.1094/PD-80-1189.
Alexander, K.C., and K. Ramakrishnan. 1980. Infection of the bud, establishment in the host and production of whips in sugarcane smut (Ustilago scitaminea) of sugarcane. In Proceedings of the International Society of Sugar Cane Technologists 17: 1452–1455.
Ashwin, N.M.R., E. Leonard Barnabas, A. Ramesh Sundar, M. Muthumeena, P. Malathi, and R. Viswanathan. 2017. Disease suppressive effects of resistance-inducing agents against red rot of sugarcane. European Journal of Plant Pathology 149: 285–297. https://doi.org/10.1007/s10658-017-1181-1.
Barnabas, L., N.M.R. Ashwin, K. Nalayeni, A. Ramesh Sundar, P. Malathi, and R. Viswanathan. 2018. Genetic and pathogenic variability among the Indian isolates of Sporisorium scitamineum causing sugarcane smut. Journal of Sugarcane Research 8 (2): 138–154.
Barnabas, L., N.M.R. Ashwin, A. Ramesh Sundar, P. Malathi, and R. Viswanathan. 2017. Putative orthologs of Ustilago maydis effectors screened from the genome of sugarcane smut fungus - Sporisorium scitamineum. Australasian Plant Pathology 46: 147–156. https://doi.org/10.1007/s13313-017-0471-6.
Banuett, F., and I. Herskowitz. 1989. Different a alleles of Ustilago maydis are necessary for maintenance of filamentous growth but not for meiosis. Proceedings of the National Academy of Sciences 86: 5878–5882. https://doi.org/10.1073/pnas.86.15.5878.
Bolker, M., S. Genin, C. Lehmler, and R. Kahmann. 1995. Genetic regulation of mating and dimorphism in Ustilago maydis. Canadian Journal of Botany 73: 320–325. https://doi.org/10.1139/b95-262.
Brewer, M.T., L. Cadle-Davidson, P. Cortesi, P.D. Spanu, and M.G. Milgroom. 2011. Identification and structure of the mating-type locus and development of PCR-based markers for mating type in powdery mildew fungi. Fungal Genetics and Biology 48: 704–713. https://doi.org/10.1016/j.fgb.2011.04.004.
Chen, L.S., C.D. Liu, J.G. Tsay, and R.S. Chen. 2015. PCR-mediated detection of Sporisorium scitamineum in sugarcane based on the bE mating-type gene sequence. Tropical Plant Pathology 40: 65–69. https://doi.org/10.1007/s40858-015-0009-9.
Co, O., K. Ngugi, H. Nzioki, and S.M. Githiri. 2008. Evaluation of smut inoculation techniques in sugarcane seedlings. Sugar Tech 10: 341–345. https://doi.org/10.1007/s12355-008-0060-7.
Dai, Yu Li., Lin Gan, Hong Chun Ruan, Niu Niu Shi, Yi Xin Du, Fu Ru Chen, and Xiu Juan Yang. 2018. A PCR method to detect mating types of Cochliobolus heterostrophus. Canadian Journal of Plant Pathology 40: 358–367.https://doi.org/10.1080/07060661.2018.1481884
Dutheil, Julien Y., Gertrud Mannhaupt, Gabriel Schweizer, Christian M.K.. Sieber, Martin Munsterkotter, Ulrich Guldener, Jan Schirawski, and Regine Kahmann. 2016. A tale of genome compartmentalization: The evolution of virulence clusters in smut fungi. Genome Biology and Evolution 8: 681–704. https://doi.org/10.1093/gbe/evw026.
Dyer, P.S., P.A. Furneaux, G. Douhan, and T.D. Murray. 2001. A multiplex PCR test for determination of mating type applied to the plant pathogens Tapesia yallundae and Tapesia acuformis. Fungal Genetics and Biology 33: 173–180. https://doi.org/10.1006/fgbi.2001.1279.
Feldbrugge, M., J. Kamper, G. Steinberg, and R. Kahmann. 2004. Regulation of mating and pathogenic development in Ustilago maydis. Current Opinion in Microbiology 7: 666–672. https://doi.org/10.1016/j.mib.2004.10.006.
Kamper, J., R. Kahmann, M. Bolker, L.J. Ma, T. Brefort, B.J. Saville, F. Banuett et al. 2006. Insights from the genome of the biotrophic fungal plant pathogen Ustilago maydis. Nature 444: 97–101. https://doi.org/10.1038/nature05248.
Kronstad, J.W., and C. Staben. 1997. Mating type in filamentous fungi. Annual Review of Genetics 31: 245–276. https://doi.org/10.1146/annurev.genet.31.1.245.
Lee-Lovick, G. 1978. Smut of sugarcane - Ustilago scitaminea. Review of Plant Pathology 57: 181–188.
Magarey, R. C., T. J. Sheahan, M. Sefton, R. E. Kerkwyk, and J. I. Bull. 2011. Yield losses from sugarcane smut: Indications from Herbert mill data and from individual crop assessments. In 33rd Annual Conference of the Australian Society of Sugar Cane Technologists 33: 109–117.
Marques, Joao Paulo R., Beatriz Appezzato-da-Gloria, Meike Piepenbring, Nelson S. Massola, Claudia B. Monteiro-Vitorello, and Maria Lucia Carneiro Vieira. 2016. Sugarcane smut: shedding light on the development of the whip-shaped sorus. Annals of Botany 119: 815–827. https://doi.org/10.1093/aob/mcw169
Moosawi-Jorf, S. Ali., and Mahin B. Izadi. 2007. In vitro detection of yeast-like and mycelial colonies of Ustilago scitaminea in tissue-cultured plantlets of sugarcane using polymerase chain reaction. Journal of Applied Sciences 7: 3768–3773. https://doi.org/10.3923/jas.2007.3768.3773.
Nalayeni, Kumaravel., N. M. R. Ashwin, Leonard Barnabas, Thiyagarajan Vinodhini, V. N. Agisha, Amalraj Ramesh Sundar, Palaniyandi Malathi, and Rasappa Viswanathan. 2021. Comparative expression analysis of potential pathogenicity-associated genes of high- and low-virulent Sporisorium scitamineum isolates during interaction with sugarcane. 3 Biotech 11: 353. https://doi.org/10.1007/s13205-021-02893-7.
Olicon-Hernandez, Dario R., Minerva G. Araiza-Villanueva, Juan P. Pardo, Elisabet Aranda, and Guadalupe Guerra-Sanchez. 2019. New insights of Ustilago maydis as yeast model for genetic and biotechnological research: A review. Current Microbiology 76: 917–926. https://doi.org/10.1007/s00284-019-01629-4.
Singh, N., B.M. Somai, and D. Pillay. 2004. Smut disease assessment by PCR and microscopy in inoculated tissue cultured sugarcane cultivars. Plant Science 167: 987–994. https://doi.org/10.1016/j.plantsci.2004.05.006.
Singh, N., B.M. Somai, and D. Pillay. 2005. In vitro screening of sugarcane to evaluate smut susceptibility. Plant Cell, Tissue and Organ Culture 80: 259–266. https://doi.org/10.1007/s11240-004-1017-5.
Su, Y., S. Wang, J. Guo, B. Xue, Xu. Liping, and Y. Que. 2013. A TaqMan real-time PCR assay for detection and quantification of Sporisorium scitamineum in sugarcane. The Scientific World Journal. https://doi.org/10.1155/2013/942682.
Sundar, A. R., E. L. Barnabas, P. Malathi, and R. Viswanathan. 2012. A mini-review on smut disease of sugarcane caused by Sporisorium scitamineum. In Botany, ed. Mworia J. K, 107–128. Croatia: InTech.
Taniguti, Lucas M., Patricia D. C. Schaker, Juliana Benevenuto, Leila P. Peters, Giselle Carvalho, Alessandra Palhares, Maria C. Quecine, et al. 2015. Complete genome sequence of Sporisorium scitamineum and biotrophic interaction transcriptome with sugarcane. PLoS ONE 10: e0129318. https://doi.org/10.1371/journal.pone.0129318.
Trione, Edward J. 1980. Teliospore formation by Ustilago scitaminea in sugarcane. Phytopathology 70: 513–516. https://doi.org/10.1094/Phyto-70-513.
Wahl, R., A. Zahiri, and J. Kamper. 2010. The Ustilago maydis b mating type locus controls hyphal proliferation and expression of secreted virulence factors in planta. Molecular Microbiology 75: 208–220. https://doi.org/10.1111/j.1365-2958.2009.06984.x.
Xu, L., Lu. Yunhai, Q. You, X. Liu, M.P. Grisham, Y. Pan, and Y. Que. 2014. Biogeographical variation and population genetic structure of Sporisorium scitamineum in Mainland China: Insights from ISSR and SP-SRAP Markers. The Scientific World Journal 2014: 1–13. https://doi.org/10.1155/2014/296020.
Yan, M., G. Zhu, S. Lin, X. Xian, C. Chang, P. Xi, W. Shen et al. 2016a. The mating-type locus b of the sugarcane smut Sporisorium scitamineum is essential for mating, filamentous growth and pathogenicity. Fungal Genetics and Biology. https://doi.org/10.1016/j.fgb.2015.11.005.
Yan, M., W. Dai, E. Cai, Y.Z. Deng, C. Chang, Z. Jiang, and L.-H. Zhang. 2016b. Transcriptome analysis of Sporisorium scitamineum reveals critical environmental signals for fungal sexual mating and filamentous growth. BMC Genomics 17: 354. https://doi.org/10.1186/s12864-016-2691-5.
Zekarias, Y., M. Dejene, G. Tegegn, and F. Yirefu. 2010. Importance and status of sugarcane smut (Ustilago scitaminea) in the Ethiopian sugar estates. Ethiopian Journal of Agricultural Sciences 20: 35–46.
Acknowledgments
The authors are grateful to The Director, ICAR-Sugarcane Breeding Institute for providing facilities and continuous encouragement. The financial support received from Department of Biotechnology (DBT), Department of Science and Technology - Science and Engineering Research Board (DST-SERB) and Indian Council of Agricultural Research (ICAR), New Delhi are greatly acknowledged.
Funding
Department of Biotechnology, Ministry of Science and Technology, BT/PR12883/BPA/118/142/2015, A. Ramesh Sundar, Department of Science and Technology—Science and Engineering Research Board (DST-SERB), EMR/2016/006055, A. Ramesh Sundar.
Author information
Authors and Affiliations
Contributions
N. M. R. Ashwin and A. Ramesh Sundar conceived and designed the experiments. V. N. Agisha, Kumaravel Nalayeni, R. T. Vinodhini, K. Jeyalekshmi and Mouriya Suraj Kumar performed the experiments. V. N. Agisha and N. M. R. Ashwin wrote the manuscript. A. Ramesh Sundar, N. M. R. Ashwin, Palaniyandi Malathi and Rasappa Viswanathan shared their expertise in execution of experiments and were involved in revising and improving the intellectual content of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that they have no conflict of interest.
Human and animal rights statement
The present research did not involve human participants and/or animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Agisha, V.N., Nalayeni, K., Ashwin, N.M.R. et al. Molecular Discrimination of Opposite Mating Type Haploids of Sporisorium scitamineum and Establishing Their Dimorphic Transitions During Interaction with Sugarcane. Sugar Tech 24, 1430–1440 (2022). https://doi.org/10.1007/s12355-021-01085-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12355-021-01085-0