Abstract
Background
Fluorine-18 sodium fluoride (Na[18F]F) atherosclerotic plaque uptake in positron emission tomography with computed tomography (PET-CT) identifies active microcalcification. We aim to evaluate global cardiac microcalcification activity with Na[18F]F, as a measure of unstable microcalcification burden, in high cardiovascular (CV) risk patients.
Methods and Results
Thirty-four high CV risk individuals without previous CV events were scanned with Na[18F]F PET-CT. Cardiac Na[18F]F uptake was assessed through the global molecular calcium score (GMCS), which was calculated by summing the product of the mean standardized uptake value times the area of the cardiac regions of interest times the slice thickness for all cardiac transaxial slices, divided by the total number of slices. Mean age is 63.5 ± 7.8 years and 62% male. Median GMCS is 320.9 (240.8-402.8). Individuals with more than five CV risk factors (50%) have increased GMCS [356.7 (321.0-409.6) vs. 261.1 (225.6-342.1), P = 0.01], which is positively correlated with predicted fatal CV risk by SCORE (rs = 0.32, P = 0.04). There is a positive correlation between GMCS and weight (rs = 0.61), body mass index (rs = 0.66), abdominal perimeter (rs = 0.74), thoracic fat volume (rs = 0.47), and epicardial adipose tissue (rs = 0.41), all with P ≤ 0.01. There is no correlation between GMCS and coronary calcium score nor coronary artery wall Na[18F]F uptake.
Conclusions
In a high CV risk group, the global cardiac microcalcification burden is related to CV risk factors, metabolic syndrome variables and cardiac fat. Cardiac GMCS is a promising risk stratification tool, combining a straightforward and objective methodology with a comprehensive analysis of both coronary and valvular microcalcification.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coronary artery disease (CAD) is among the leading causes of mortality and morbidity in the world. The wide range of manifestations includes asymptomatic patients with progressive and silent disease followed by a potentially fatal event. It is critical to identify those asymptomatic high-risk patients in early phases.1
Calcification plays a major role in atherogenesis and cardiac computerized tomography (CT) imaging has been used to identify coronary macrocalcification. Current guidelines give a class of recommendation IIb for the use of coronary artery calcium score (CAC) as a risk modifier for cardiovascular (CV) risk assessment of asymptomatic patients at low or moderate risk.2 However, this method is unable to either reliably detect microcalcifications nor identify active calcification.3
Positron emission tomography (PET) imaging with fluorine-18 sodium fluoride (Na[18F]F) has been studied in CV conditions as a microcalcification marker,4 particularly in atherosclerotic plaques.5 This radiotracer is incorporated into areas of calcium deposition by exchanging the hydroxyl ions of hydroxyapatite crystals with radiolabelled fluoride to form fluorapatite, identifying active calcium metabolism in osseous tissue.6 Clinical and pre-clinical data support that Na[18F]F adsorbs to the outer layer of calcified areas without deep penetration in vessels but not in other soft tissues.7 Additionally, Na[18F]F uptake in areas of active calcium metabolism was not always colocalized by macrocalcifications on CT imaging.8 Therefore, it has been suggested that Na[18F]F PET-CT recognize a different pathophysiology stage (active microcalcification), perhaps an earlier phase of atherogenesis.9 Moreover, coronary imaging with Na[18F]F PET-CT was able to localize recent plaque rupture in patients with acute myocardial infarction (acute setting) as well as recognizing coronary plaques with high-risk features on intracoronary imaging (chronic setting). Therefore, it was proposed as a potential discriminator between stable and unstable atherosclerotic plaques, thus able to identify vulnerable plaques and thereby high-risk patients.10
Studies have shown an association between Na[18F]F uptake and CV risk factors.10,11,-12 Global Molecular Calcification Score (GMCS) measures the total cardiac uptake of Na[18F]F, by identifying early molecular microcalcification at atherosclerotic sites. In patients evaluated for oncological indications, GMCS was positively correlated with the number of CV risk factors, therefore being proposed as a marker of global cardiac microcalcification burden.11
The performance of GMCS in high CV risk patients without clinically apparent CV events is unknown. This report is a retrospective analysis of our pilot study on Na[18F]F uptake in patients without manifest CV disease but at high-risk.12 In the present work, we aimed to evaluate the global cardiac microcalcification burden with GMCS, as a surrogate marker of unstable atherosclerotic disease, in high CV risk patients.
Patients and Methods
Population
Patients followed at the Arterial Hypertension outpatient clinic of a single tertiary university hospital were enrolled if they met all of the following inclusion criteria: a) ≥ 40 years; b) provided written informed consent; c) considered to be at high or very high CV risk according to the European Society of Cardiology guidelines.13 We excluded patients with previous CV events or suggestive symptoms of atherosclerotic disease (angina, heart failure symptoms, neurological complaints or claudication), malignant neoplasms in the past 5 years, chronic inflammatory disease and women of child-bearing age without contraceptive use or pregnant. Twenty five patients were part of a pilot study for an investigator’s initiative statin clinical trial with Na[18F]F PET-CT (ROPPET-NAF; ClinicalTrials.gov Identifier: NCT03233243), and the sample for the present analysis was enriched with baseline data of nine patients included in the trial. This research was conducted with the support of AstraZeneca, Produtos Farmacêuticos Lda. Patients with previous statin therapy stopped treatment for 2 weeks before enrollment as previously described.14 The protocol was approved by the Ethics Committee of Faculty of Medicine of the University of Coimbra and Portuguese National Ethics Committee for Clinical Research, with the written informed consent in accordance with the Declaration of Helsinki and with Good Clinical Practice Guidelines.
Baseline Examination
Data collection included age, gender, smoking status, diabetes mellitus, family history of premature CAD (first degree: men < 55 and women < 65 years old), blood pressure, weight, height, and waist circumference. Levels of total cholesterol, high and low-density lipoprotein cholesterol (HDL and LDL), triglycerides, creatinine, and glycated hemoglobin were determined. Individuals were grouped based on the presence or absence of ≥ 5 risk factors among the following: hypertension, dyslipidaemia, type 2 diabetes, chronic kidney disease with glomerular filtration rate under 60 ml/min/1.75 m2, obesity, abdominal obesity (> 94 cm in males or > 80 cm in females), family history of CAD, smoker. CV risk was estimated according to SCORE.13
18F-Sodium Fluoride PET
Patients underwent Na[18F]F PET-CT for identification of coronary plaques and quantification of Na[18F]F uptake. The protocol consisted in the administration of 185 megabecquerel (MBq) Na[18F]F intravenously, followed by an attenuation correction CT scan and PET imaging after 60 min (Gemini GXL Philips 16 PET/CT system). The coronary imaging protocol consisted of a cardiac-gated PET-CT with a 10 min electrocardiogram-gated acquisition with attenuation correction. An iterative reconstruction of cardiac PET scans was performed in multiple phases with the diastolic phase between 50 and 75% used for analysis.
Global cardiac uptake of Na[18F]F was assessed by GMCS.11 First, two-dimensional regions of interest (ROI) were manually drawn around the cardiac silhouette on each transverse CT slice from the base to the apex of the heart. The ROI volume was obtained by multiplying the area of the ROI by its slice thickness (4 mm). Second, quantification of Na[18F]F uptake was performed by measuring the mean standard uptake value (SUV) in the ROI of each PET-CT slice. Third, GMCS was calculated by summing the product of each ROI volume by the respective mean SUV; then, the obtained value was divided by the number of cardiac transaxial slices considered. Any activity originating from the skeleton or aorta was carefully excluded. As aortic valve calcification is correlated to CV risk,15 we included the aortic valve ROI in the measure of global cardiac microcalcification burden, termed GMCS-AV. Scans were reviewed and analyzed by experienced observers blinded to the clinical diagnosis.
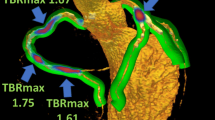
Additionally, we quantified the uptake of Na[18F]F in coronary territory as the mean of target-to-background ratio (TBR) which is reported elsewhere.16 Briefly, TBR was obtained for each individual lesion by dividing maximum SUV by mean blood-pool SUV. Quantification of coronary calcium score (CAC) was performed by one blinded reader with an off-line workstation with dedicated software for calcium scoring - GE Healthcare Advantage Workstation 4.2. Thoracic fat volume was assessed in CT using an automated software: a predefined threshold of − 190 to − 30 Hounsfield units was applied to identify the voxels consisting of fat. These voxels were summed to obtain the volume in milliliters, considering the anatomical limits previously described.17 The software automatically interpolated between the traced sections using the thresholds already described. The measurement of epicardial adipose tissue was performed using the dedicated software 3DSlicer, first by importing the CT images in DICOM format. The segmentation process was performed with manual selection tools in order to accurately trace the contours of the pericardium. The final volume was calculated automatically by the program.
Statistical Analysis
Continuous data were described using mean ± standard deviation or median and interquartile range, according to the normality of the distribution assessed by the Shapiro-Wilk test, and compared using Student T-test or Mann-Whitney U test, respectively. Correlations were analyzed with Pearson or Spearman correlation tests according to the normality. Categorical variables were represented by their frequency and compared using Fisher’s exact test or the Chi-square test. The analysis was performed with SPSS Statistics for Macintosh software, version 20.0 (IBM).
Results
Thirty-four patients were enrolled (mean age 63.5 ± 7.8 years, 62% male), of which 50% had ≥ 5 CV risk factors. Age, gender, blood pressure and type 2 diabetes are evenly distributed among GMCS terciles (Table 1). Previous medication and biochemical parameters are shown in Tables S1 and S2 in the Supplementary Material. Na[18F]F PET-CT imaging analysis is exemplified in Figs.1 and 2. Molecular imaging quantification results are presented in Table 2, with subgroups comparisons regarding the burden of CV risk factors.
High and low Na[18F]F uptake in fusion PET-CT images. High Na[18F]F uptake in the aortic valve (arrow) depicting active microcalcification in a patient with GMCS-AV of 399 (A). Low Na[18F]F uptake contrasts with visible macrocalcification (arrow) in the left anterior descending artery from another patient with GMCS-AV of 226 (B)
Median cardiac GMCS is 320.9 (240.8-402.8), and it is higher than median aortic valve GMCS (GMCS-AV), 306.4 (238.0-394.5). There is a positive correlation between GMCS and weight (rs = 0.61, P < 0.01), body mass index (rs = 0.66, P < 0.01), abdominal perimeter (rs = 0.74, P < 0.01), thoracic fat volume (rs = 0.47, P < 0.01), and epicardial adipose tissue (rs = 0.41, P = 0.01). These findings are consistent with GMCS terciles analysis, showing progressive increase of these variables with higher GMCS. GMCS-AV is positive correlated with the same aforementioned variables (Table S3). GMCS is correlated to fatal CV risk predicted by SCORE (rs = 0.32, P = 0.04). Besides c-reactive protein which is significantly higher in the superior GMCS tercile, there is no difference regarding the remaining biochemical parameters (Table S2).
Individuals with ≥ 5 CV risk factors have both higher median GMCS [356.7 (321.0-409.6) vs. 261.1(225.6-342.1), P = 0.01] and GMCS-AV [340.2 (305.1-398.7] vs. 253.1 [211.8-327.2], P = 0.01), than those with lower burden of CV risk factors (Figure 3 and Table 2).
Thoracic and epicardial fat volumes are strongly correlated (rs = 0.80, P < 0.01). Thoracic fat volume is also higher with increasing risk factors burden, but not epicardial adipose tissue volume, coronary Na[18F]F uptake (TBR), nor CAC.
There is no correlation between GMCS or GMCS-AV and CAC nor coronary Na[18F]F uptake assessed by TBR (Table S3).
Discussion
Growing evidence supports the use of Na[18F]F PET-CT to localize and quantify arterial microcalcification, as an earlier marker of unstable atherosclerotic artery disease. Despite several measurement methods explored, none is accepted as the standard.4 Previously we assessed Na[18F]F uptake in aortic, carotid and coronary territories, which was related to the burden of CV risk factors.16 We also found that patients with more than five risk factors had higher maximum Na[18F]F uptake, which was positively correlated to predicted fatal CV risk by SCORE.12 Regarding renal arteries, not only was there an association between Na[18F]F wall uptake and predicted fatal CV risk by SCORE, but also a significant inverse correlation with glomerular filtration rate.18
In the present study we retrospectively analyze a larger group of high CV risk patients, focusing on cardiac Na[18F]F PET-CT scan and applying cardiac GMCS for the first time in this population. This score has been proposed as a global assessment method of cardiac microcalcification burden.19 Patients with ≥ 5 CV risk factors have higher cardiac GMCS, which matches previous observations with oncological populations.11 We report a weak but positive correlation between cardiac GMCS and fatal CV risk predicted by SCORE. However, since nearly three quarters of the patients have diabetes, this risk score underestimates the real risk of cardiac events.20 In addition, cardiac GMCS seems to correlate to metabolic syndrome features (abdominal perimeter, obesity, and body mass index).
In this analysis, there is no correlation between GMCS and CAC score, which is consistent with the different pathophysiological stages of the disease. While the first one evaluates active microcalcification and putatively a vulnerable plaque, the latter represents a macrocalcified lesion.21 GMCS calculated from Na[18F]F PET-CT images offer some advantages compared to traditional CT imaging, providing key insights into coronary atherogenesis: earlier and comprehensive information about global cardiac microcalcification burden and risk of plaque rupture. Secondly, there is no association between cardiac GMCS and coronary artery fluoride uptake. Despite focusing on the same molecular processes, these variables are calculated according to different methods, which might partially explain the difference: GMCS uses mean SUV while TBR uses maximum SUV corrected by the blood-pool SUV. Furthermore, while in GMCS the whole cardiac silhouette is included, coronary artery fluoride uptake quantification is limited to coronary vessel’s region.
There still might not be enough data to decisively establish which Na[18F]F burden quantification method is superior for CV risk assessment; however, GMCS does offer some important theoretical advantages, such as reproducibility and a lower technical barrier of entry. Most other Na[18F]F uptake analysis methods require the precise identification of the coronary arteries, a possibly challenging feat, even for experienced physicians, in lower end PET/CT scanners, with fewer CT slices, or if contrast is not administered. In contrast, GMCS only entails the accurate delineation of the heart, which is feasible even for unexperienced users, in older PET/CT scanners or if contrast CT is not available. It should be noted, however, that this method is not suited for the identification of individual vulnerable plaques, and should be complemented with others, if such objective is to be pursued.
Epicardial fat deposition has been linked to fatal and nonfatal CV outcomes 22 and CAD.23 We reported a positive correlation between thoracic fat and fluoride uptake before.12 In the present study thoracic fat volume is strongly correlated with epicardial adipose tissue, and both are associated with GMCS, reinforcing the association between epicardial fat and Na[18F]F uptake. Thoracic fat volume is associated with pro-inflammatory states and plaque vulnerability.24 Therefore, we hypothesize that both cardiac fat variables and GMCS might help to identify higher risk patients.
The role of calcification in valvular heart disease has been extensively studied with CT imaging in patients with suspected low-flow-low-gradient aortic stenosis.25 However, there is growing evidence for Na[18F]F PET-CT imaging for valvular evaluation. Patients with aortic sclerosis or stenosis had higher Na[18F]F uptake compared to controls. In addition, Na[18F]F uptake was coincident with known regions of calcification, suggesting calcium remodeling, but it was also present in adjacent and remote regions, possibly representing an expansion of calcification.26 Not surprisingly, age and aortic Na[18F]F uptake are positively correlated.27 In addition, mitral annular Na[18F]F uptake is associated to calcification assessed by CT, at baseline and follow-up.28
In the original study that described GMCS, the aortic valve was excluded to avoid contamination by the aortic wall or calcified leaflets.11 Taking into account the association between valvular calcification and CV risk, as well as our recent report on the positive correlation between aortic valve Na[18F]F and CV risk in patients without aortic valve stenosis,15 we decided to include for the first time the aortic valve (two extra slices for most patients) in a variant of the original score termed GMCS-AV. Our methodology is distinct from studies focusing on the aortic valve 26,27 (in which a ROI was drawn around aortic valve). GMCS-AV showed a lower median value compared to GMCS, but within the same range; both scores showed similar findings regarding the association with metabolic syndrome variables, cardiac fat variables, and the burden of CV risk factors. This similar GMCS-AV was expected, since this score calculates a mean value and none of these patients has a highly calcified aortic valve. Therefore, GMCS-AV might be more useful than the original GMCS to assess the true global microcalcification burden in patients with a calcified aortic valve.
Primary prevention is the mainstay of CV medicine. Risk stratification strategies’ improvement is crucial to accurately identify asymptomatic patients who might benefit from more aggressive therapies (such as high-intensity statin). The “myth” of vulnerable plaque, that relies on focusing on individual lesions, has shifted to the concept of vulnerable patients, namely with global assessment of atherosclerotic disease and other CV risk factors.29 In that line, we hypothesize that cardiac GMCS, measured by Na[18F]F PET-CT, might evaluate the burden of active calcium metabolism in the heart, including potentially unstable atherosclerotic coronary plaques and valvular calcification, therefore being a promising risk stratification tool. In our experience, and for the previously stated reasons, cardiac GMCS determination with this approach is quicker and easier than other Na[18F]F quantification methods. In addition, GMCS might also have role in the evaluation of patients with previous CV events, by identifying those with active microcalcification despite a high-intensity statin regimen, who might benefit from other drugs regardless of the cholesterol levels. Large-scale prospective investigations are needed to confirm these findings and their association with CV outcomes.
This study had some limitations. First, a retrospective analysis of a low number of subjects limits the validity of our results, which are hypothesis generating. The limited spatial resolution of the PET system and the methods for radiotracer uptake quantification should still be optimized; further work should lead to improved standardization. Finally, PET-CT imaging is more expensive and less available than cardiac CT, which might hamper widespread clinical use.
To conclude, in a high CV risk group without clinically apparent events, the global cardiac microcalcification burden assessed by GMCS is related to the number of CV risk factors, metabolic syndrome variables and thoracic fat volume. Its assessment of coronary and valvular active microcalcification shows promise as a potential comprehensive risk stratification tool.
New Knowledge Gained
GMCS assessed by Na[18F]F PET-CT is a promising risk stratification tool that might offer advantages compared to traditional CT imaging, providing key insights into coronary atherogenesis: earlier and comprehensive information about global cardiac microcalcification burden and risk of plaque rupture.
Abbreviations
- CAC:
-
Coronary artery calcium score
- CAD:
-
Coronary artery disease
- CT:
-
Computerized tomography
- CV:
-
Cardiovascular
- GMCS:
-
Global molecular calcification score
- Na[18F]F:
-
Fluorine-18 sodium fluoride
- PET:
-
Positron emission tomography
- ROI:
-
Regions of interest
- SUV:
-
Standard uptake value
- TBR:
-
Target-to-background ratio
References
Saraste A, Barbato E, Capodanno D, Edvardsen T, Prescott E, Achenbach S, et al. Imaging in ESC clinical guidelines: Chronic coronary syndromes. Eur Heart J Cardiovasc Imaging 2019;20:1187-97.
Mach F, Baigent C, Catapano A, Koskinas K, Casula M, Badimon L, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Atherosclerosis. 2019;290:140-205.
Blaha MJ, Mortensen MB, Kianoush S, Tota-Maharaj R, Cainzos-Achirica M. Coronary artery calcium scoring: Is it time for a change in methodology? JACC Cardiovasc Imaging 2017;10:923-37.
Silva Mendes BI, Oliveira-Santos M, Vidigal Ferreira MJ. Sodium fluoride in cardiovascular disorders: A systematic review. J Nucl Cardiol 2019. https://doi.org/10.1007/s12350-019-01832-7.
Dweck MR, Chow MW, Joshi NV, Williams MC, Jones C, Fletcher AM, et al. Coronary arterial 18F-sodium fluoride uptake: A novel marker of plaque biology. J Am Coll Cardiol 2012;59:1539-48.
Czernin J, Satyamurthy N, Schiepers C. Molecular mechanisms of bone 18F-NaF deposition. J Nucl Med 2010;51:1826-9.
Irkle A, Vesey AT, Lewis DY, Skepper JN, Bird JL, Dweck MR, et al. Identifying active vascular microcalcification by (18)F-sodium fluoride positron emission tomography. Nat Commun 2015;6:7495.
Derlin T, Richter U, Bannas P, Begemann P, Buchert R, Mester J, et al. Feasibility of 18F-sodium fluoride PET/CT for imaging of atherosclerotic plaque. J Nucl Med 2010;51:862-5.
Moghbel M, Al-Zaghal A, Werner TJ, Constantinescu CM, Hoilund-Carlsen PF, Alavi A. The role of PET in evaluating atherosclerosis: A critical review. Semin Nucl Med 2018;48:488-97.
Joshi NV, Vesey AT, Williams MC, Shah AS, Calvert PA, Craighead FH, et al. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: A prospective clinical trial. Lancet 2014;383:705-13.
Beheshti M, Saboury B, Mehta NN, Torigian DA, Werner T, Mohler E, et al. Detection and global quantification of cardiovascular molecular calcification by fluoro18-fluoride positron emission tomography/computed tomography-a novel concept. Hell J Nucl Med 2011;14:114-20.
Oliveira-Santos M, Castelo-Branco M, Silva R, Gomes A, Chichorro N, Abrunhosa A, et al. Atherosclerotic plaque metabolism in high cardiovascular risk subjects: A subclinical atherosclerosis imaging study with (18)F-NaF PET-CT. Atherosclerosis 2017;260:41-6.
Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 2016;37:2315-81.
Rawlings R, Nohria A, Liu PY, Donnelly J, Creager MA, Ganz P, et al. Comparison of effects of rosuvastatin (10 mg) versus atorvastatin (40 mg) on rho kinase activity in caucasian men with a previous atherosclerotic event. Am J Cardiol 2009;103:437-41.
Domingues C, Joao Ferreira M, Silva R, Oliveira-Santos M, Andreia G, Chichorro N, et al. Aortic valve microcalcification and cardiovascular risk: An exploratory study using sodium fluoride in high cardiovascular risk patients. Int J Cardiovasc Imaging 2020;36:1593-8.
Ferreira MJV, Oliveira-Santos M, Silva R, Gomes A, Ferreira N, Abrunhosa A, et al. Assessment of atherosclerotic plaque calcification using F18-NaF PET-CT. J Nucl Cardiol 2018;25:1733-41.
Dey D, Nakazato R, Li D, Berman DS. Epicardial and thoracic fat: Noninvasive measurement and clinical implications. Cardiovasc Diagn Ther 2012;2:85-93.
Oliveira-Santos M, McMahon G, Castelo- Branco M, Silva R, Gomes A, Chichorro N, et al. Renal artery wall 18F-NaF activity and glomerular filtration rate: An exploratory analysis in a high cardiovascular risk population. Nucl Med Commun. 2020;41:126-32.
McKenney-Drake ML, Moghbel MC, Paydary K, Alloosh M, Houshmand S, Moe S, et al. (18)F-NaF and (18)F-FDG as molecular probes in the evaluation of atherosclerosis. Eur J Nucl Med Mol Imaging 2018;45:2190-200.
Grant PJ, Cosentino F. The 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: New features and the ‘Ten Commandments’ of the 2019 Guidelines are discussed by Professor Peter J. Grant and Professor Francesco Cosentino, the Task Force chairmen. Eur Heart J 2019;40:3215-7.
Chen W, Dilsizian V. Targeted PET/CT imaging of vulnerable atherosclerotic plaques: Microcalcification with sodium fluoride and inflammation with fluorodeoxyglucose. Curr Cardiol Rep 2013;15:364.
Spearman JV, Renker M, Schoepf UJ, Krazinski AW, Herbert TL, De Cecco CN, et al. Prognostic value of epicardial fat volume measurements by computed tomography: A systematic review of the literature. Eur Radiol. 2015;25:3372-81.
Tamarappoo B, Dey D, Shmilovich H, Nakazato R, Gransar H, Cheng VY, et al. Increased pericardial fat volume measured from noncontrast CT predicts myocardial ischemia by SPECT. JACC Cardiovasc Imaging 2010;3:1104-12.
Ito T, Nasu K, Terashima M, Ehara M, Kinoshita Y, Ito T, et al. The impact of epicardial fat volume on coronary plaque vulnerability: Insight from optical coherence tomography analysis. Eur Heart J Cardiovasc Imaging 2012;13:408-15.
Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017;38:2739-91.
Dweck MR, Jones C, Joshi NV, Fletcher AM, Richardson H, White A, et al. Assessment of valvular calcification and inflammation by positron emission tomography in patients with aortic stenosis. Circulation 2012;125:76-86.
Rojulpote C, Borja AJ, Zhang V, Aly M, Koa B, Seraj SM, et al. Role of (18)F-NaF-PET in assessing aortic valve calcification with age. Am J Nucl Med Mol Imaging 2020;10:47-56.
Massera D, Trivieri MG, Andrews JPM, Sartori S, Abgral R, Chapman AR, et al. Disease activity in mitral annular calcification. Circ Cardiovasc Imaging 2019;12:
Arbab-Zadeh A, Fuster V. The myth of the “vulnerable plaque”: Transitioning from a focus on individual lesions to atherosclerotic disease burden for coronary artery disease risk assessment. J Am Coll Cardiol 2015;65:846-55.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Institute of Nuclear Sciences Applied to Health (ICNAS) - Faculty of Medicine of the University of Coimbra. ROPPET-NAF trial (ClinicalTrials.gov Identifier: NCT03233243) is being conducted with the support of AstraZeneca, Produtos Farmacêuticos Lda
Disclosures
João Borges-Rosa, Manuel Oliveira-Santos, Rodolfo Silva, Nuno Pereira da Silva, Antero Abrunhosa, Miguel Castelo-Branco, Lino Gonçalves, and Maria João Ferreira have no conflict of interest to declare. This study was funded by Institute of Nuclear Sciences Applied to Health (ICNAS) - Faculty of Medicine of the University of Coimbra and with the support of AstraZeneca, Produtos Farmacêuticos Lda.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors of this article have provided a PowerPoint file, available for download at SpringerLink, which summarizes the contents of the paper and is free for re-use at meetings and presentations.
The authors have also provided an audio summary of the article, which is available to download as ESM, or to listen to via the JNC/ASNCPodcast.”
Supplementary Information
Below is the link to the Supplementary Information.
Rights and permissions
About this article
Cite this article
Borges-Rosa, J., Oliveira-Santos, M., Silva, R. et al. Cardiac microcalcification burden: Global assessment in high cardiovascular risk subjects with Na[18F]F PET-CT. J. Nucl. Cardiol. 29, 1846–1854 (2022). https://doi.org/10.1007/s12350-021-02600-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-021-02600-2