Abstract
Mixed neuroendocrine non-neuroendocrine neoplasm (MiNEN) of the pancreas is a rare entity, and obtaining a preoperative diagnosis is difficult. We present a 70-year-old man in whom the possibility of MiNEN was successfully discovered preoperatively by endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA). Immunostaining revealed positive results for the neuroendocrine markers chromogranin A and synaptophysin. We considered the possibility for MiNEN before surgery. He underwent distal pancreatectomy with splenectomy. Immunohistochemical examination of the tumor cells showed a wide range of positivity for trypsin as well as for chromogranin A and synaptophysin. Considering that ≥ 30% tumors ware positive for both acinar and neuroendocrine markers, the patient was diagnosed with MiNEN. MiNEN is a malignant tumor that requires early detection and treatment but is a rare disease for which no method has been established. We found that EUS-FNA and immunostaining are effective diagnostic methods for MiNEN.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most pancreatic cancers fall into four distinct categories: ductal adenocarcinoma, intraductal papillary mucinous neoplasm with an associated invasive carcinoma, acinar cell carcinoma (ACC), and neuroendocrine neoplasm [1]. Among pancreatic cancers, pancreatic ACC is rarer than invasive pancreatic ductal carcinoma and is reported to account for < 1% of all pancreatic cancers [2]. Mixed neuroendocrine non-neuroendocrine neoplasm (MiNEN) is defined a mixture of exocrine and endocrine tumors of the pancreas in which ≥ 30% of the endocrine component is found in an exocrine cell carcinoma by the World Health Organization (WHO) classification system [1] However, it is well established that roughly one-third of ACCs express neuroendocrine markers. When neuroendocrine cells account for > 30% of the total tumor size, they are classified as MiNEN [1]. Herein, we present the case of a 70-year-old man who was diagnosed with MiNEN using endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) and who then underwent distal pancreatectomy. He was diagnosed with MiNEN on histopathological examination same to the results of preoperative immunostaining. There have been about 40 reports on MiNEN in the past. Here we discuss the detailed pathological features of MiNEN and the appropriate preoperative diagnosis using EUS-FNA. Due to its rarity, a treatment protocol for MiNEN has not yet been standardized. We also review the existing English literature here.
Case report
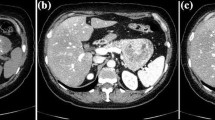
An asymptomatic 70-year-old man was referred to our hospital for examination after a pancreatic mass was identified on abdominal ultrasonography. Physical examination and laboratory test results were within normal ranges. Carcinoembryonic antigen level was 1.9 ng/mL and carbohydrate antigen 19–9 (CA19-9) was 16 U/mL, which did not increase thereafter. Serum neuron-specific enolase level was also normal. Computed tomography (CT) showed a contrast-enhanced mass with a diameter of 15 mm in the pancreatic tail (Fig. 1). Magnetic resonance imaging (MRI) showed a high-intensity mass lesion on diffusion-weighted images (Fig. 2). EUS showed a 17-mm-sized mass with an unclear boundary in the pancreatic tail. There was no capsule-like structure on the tumor margin, and the inside was hypoechoic. The central part of the tumor appeared necrotic with no echo (Fig. 3a). EUS-FNA was performed using a 22G EZ shot 3 plus. We performed two punctures in the mass during one session. Obtained specimens were subjected to tissue diagnosis after formalin fixation. The specimens revealed tumor tissues in which acidophilic cells with atypical polygonal shape formed vesicle nests and proliferated (Fig. 4). The hypoechoic region within the tumor represented cystic degeneration. There were no necrotic findings on pathological examination.
a Contrast-enhanced T1-weighted axial MR image showing a heterogeneously hypointense tumor compared to the normal pancreas (arrow). b T2-weighted axial MR image showing a heterogeneously hyperintense mass compared to the normal pancreas (arrow). c A diffusion-weighted axial MR image showing a heterogeneously hyperintense mass compared to the normal pancreas (arrow)
a Endoscopic ultrasonography (EUS) by convex scope showing a 17-mm-sized hypoechoic mass with an unclear boundary in the pancreatic tail. The central part of the mass is accompanied by a non-echoic region (yellow arrow). The mass lesion has poor flow compared to normal pancreatic parenchyma (white arrow)
Immunostaining was positive for the acinar marker α1-anti-chymotrypsin and the neuroendocrine markers chromogranin A and synaptophysin. Because both acinar and neuroendocrine markers were positive, we suspected the possibility of MiNEN preoperatively (Fig. 5).
The patient underwent distal pancreatectomy with splenectomy. The resected tumor had a maximum size of 12 mm, with a well-circumscribed shape and focal cystic change of 5 mm. In the histological findings, the tumor showed two distinct cell populations: neuroendocrine and non-neuroendocrine components, which were critically different in morphological appearances as shown by hematoxylin and eosin staining and in the patterns of special staining and immunohistochemical staining. While about 60% of the cells in the tumor showed acinar, glandular, and solid patterns, representative of acinar cell carcinoma, the other cell population (about 40%) in the tumor showed trabecular, nest, and rosette structures, suggestive of neuroendocrine carcinoma. In the special staining and immunohistochemical analyses, the former cells were positive for both periodic acid-Schiff (PAS) and PAS with diastase digestion (D-PAS) and positive for alpha-chymotrypsin, while the latter cells were positive for chromogranin A, synaptophysin, and somatostatin receptor 2 (SSTR2), which are representative markers of neuroendocrine differentiation. The Ki-67 labeling index was 1%, indicating that the tumor cells were non-proliferative or low-grade. Considering all these findings, the tumor met the histopathological criteria of mixed neuroendocrine-non-neuroendocrine neoplasm (MiNEN) with good differentiation (Fig. 6).
Histopathological features of the MiNEN. a, b Hematoxylin and eosin image of the pancreatic tumor showing a trabecular, nest, and rosette-like structure and a sheet-like structure. Acinar cell carcinoma and NET (G1) components can be observed. The 2 components are closely attached. Combined/biphasic type (100 ×) (200 ×). c Transition between ACC components and NET components (× 200). The inside of the blue circle is the NET component, and the others are the ACC components. d PAS stain (200 ×). e D-PAS stain (× 200). f α1-anti-chymotrypsin (200 ×). g Immunostaining of the pancreatic tumor for chromogranin A (200 ×). Left side: The area where the cytoplasm is stained is the NET component; right side ACC area. h: SSTR2 (200 ×). i: synaptophysin (100 ×)
According to the Union Internationale Contre le Cancer classification 8th edition of pancreatic cancer, the following staging was established: pT1, CH0, S0, RP0, RV0, A0, PL0, PCM0, DPM0, R0, N0, M0, pStageI.
The postoperative course remained uneventful. The patient was not given adjuvant chemotherapy. The patient survives for 1 year after surgery without recurrence.
Discussion
The pancreas is composed of exocrine cells consisting of pancreatic duct epithelial cells, adenocytes, and endocrine island cells. Cancer of each cell type is termed pancreatic ductal carcinoma, pancreatic ACC, and pancreatic endocrine carcinoma, respectively. Some pancreatic tumors have both exocrine and endocrine tumors mixed or coexisting within the tumor [3]; these tumors are known as MiNEN of the pancreas. All cells that compose the pancreas embryologically originate from the pancreatic duct epithelial cells, which probably explains tumors in which endocrine and exocrine masses coexist [4]. The WHO Classification 2019 collectively refers to pancreatic and gastrointestinal tumors with endocrine properties as neuroendocrine neoplasms (NEN), which are roughly categorized into well-differentiated neuroendocrine tumors (neuroendocrine neoplasm: NET) and poorly differentiated neuroendocrine tumors (Neuroendocrine carcinoma: NEC), with NET classified into NET G1, NET G2, and NET G3 based on the number of nuclear fission and the Ki-67 index. The 2019 WHO classification defined MiNEN as tumors in which ≥ 30% of the endocrine component is found in an exocrine cell carcinoma. This definition has undergone several transitions until MiNEN appeared in the 2019 WHO classification as a general term that covers various pathological conditions that do not contain adenocarcinoma components and consist of ACC and NEN components. This review includes reports the investigated mixed adeno-neuroendocrine carcinoma (MANEC) based on the 2010 WHO classification. MiNEN (including MANEC) of the pancreas is rare, with only 40 cases in the published English literature (Table 1) [2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30]. We summarized the clinicopathological features in each report in this review.
In this case report, the mass had characteristics of both acinar and endocrine cells. These findings suggest that the mass was derived from acinar cells: (1) the glandular cell line had an acinar structure, (2) α1-antitrypsin was widely positive, and (3) synaptophysin was also positive. On the other hand, findings supporting that they were derived from endocrine cells were as follows: (1) chromogranin A was extensively positive (60%) and (2) synaptophysin was also positive. We accurately suspected the possibility of MiNEN preoperatively by immunostaining the sample collected with EUS-FNA. We then performed a prompt surgery.
Since MiNEN is rare and the number of cases is limited, diagnosing MiNEN before surgery is very difficult and the diagnostic method is also controversial. Many studies have reported the correct diagnosis rate with tissue diagnosis using EUS-FNA. The pathological diagnosis of pancreatic disease by EUS-FNA was first reported by Vilmann et al. [31]. According to Agarwal et al., the sensitivity, specificity, and accuracy rate of EUS-FNA for detecting pancreatic cancer is 89, 100, and 90%, respectively, and the sensitivity for detecting endocrine carcinoma is reported to be 77–93% [32]. Based on reported cases (Table 1), this suggests that the clinical diagnosis of pancreatic MiNEN remains a big challenge. Niiya et al. [29] reported several cases of successful preoperative diagnosis of MiNEN with EUS-FNA. Among these, a definitive diagnosis of tumors with size ≤ 11 mm was rare. All reports of successful MiNEN diagnosis preoperatively using EUS-FNA were stained for both acinar and neuroendocrine markers. However, cases in which MiNEN was not diagnosed were stained only for either acinar or neuroendocrine markers [29]. They suggested that thorough immunostaining can help diagnose MiNEN. We also successfully suspecting of MiNEN preoperatively by immunostaining acinar and neuroendocrine markers.
A previous study reported that patients with MiNEN had worse overall and recurrence-free survival than those with pancreatic neuroendocrine tumor (PanNETs), but no significant difference was found between the former group and those with ACCs [33]. ACCs presented more commonly with intraductal growth than MiNEN, whereas MiNENs more often have lymph node metastasis. Patients with MiNENs and ACCs have worse survival and display more aggressive behavior than those with PanNETs. Moreover, clinicopathological behavior of MiNENs resembles that of ACCs rather than that of PanNETs [33].
Hara et al. reported that the 1-year overall survival rate was 80% and that the 3-year overall survival rate was 60% in 16 patients who underwent surgery [25]. In addition, Yu et al. reported that the larger the tumor diameter and the higher the Ki-67 index, the faster the progression rate [20]. In this case, Ki-67 was low grade in 1%, so we did not administer adjuvant chemotherapy. Moreover, Kim et al. reported that the mean survival of MiNEN patients after surgical resection of the primary tumor is 10.5 months [33]. Therefore, detection of small MiNEN and complete surgical removal are essential to achieve a cure. These results support the need for improved accuracy in the preoperative diagnosis of MiNEN.
Due to the limited number of cases of MiNEN, no effective treatment for MiNEN has been established. However, surgical resection is often performed as a curative treatment. There have also been reports of patients benefiting from surgical tumor debulking and local and systemic antiproliferative therapy [21]. In addition, no treatment has been established for unresectable or recurrent cases, and there have been reports of transcatheter hepatic artery chemoembolization for liver metastasis [20]. Moreover, an effective chemotherapy regimen is yet to be established. Yokode et al. reported that some MiNENs show a good response to S-1 chemotherapy. A multidisciplinary approach with surgery is needed for patients with advanced cases of MiNEN [26].
Preoperative diagnosis of MiNEN is difficult and is often misdiagnosed as PanNET. PanNETs are often followed up, and there is a risk that MiNEN will be overlooked. Therefore, the detection of small MiNENs and their complete surgical removal is essential to achieve cure. MiNEN is a very rare tumor of the pancreas for which surgical resection remains the only established standard treatment. Continued identification and reporting of these cases should be encouraged with the goal to better understand the disease and standardize optimal therapy.
Further cases need to be accumulated to improve the preoperative diagnostic accuracy of MiNEN and to establish an effective treatment method.
Abbreviations
- ACC:
-
Acinar cell adenocarcinoma
- CEA:
-
Carcinoembryonic antigen
- CGA:
-
Chromogranin A
- MiNEN:
-
Mixed neuroendocrine non-neuroendocrine neoplasm
- N/A:
-
Not available
- NET:
-
Neuroendocrine
- NSE:
-
Neuro-specific enolase
- PAS:
-
Periodic acid-Schiff
- SYN:
-
Synaptophysin
- TAE:
-
Transcatheter arterial embolization
References
Klimstra DS, Kloppell G, La Rosa, et al. Classification of neuroendocrine neoplasm of the digestive system. In: WHO Classification of Tumours: Digestive System Tumours, 5th ed, WHO Classification of Tumours Editorial Bord(ED), International Agency for Reserch on Cancer, Lyon 2019, p. 16–21
Klimstra DS, Adsay V. Acinar neoplasms of the pancreas—a summary of 25 years of research. Semin Diagn Pathol. 2016;33:307–18.
Kyriazi MA, Arkadopoulos N, Stafyla VK, et al. Mixed acinar-endocrine carcinoma of the pancreas: a case report and review of the literature. Cases J. 2009;2:6481.
Ohike N, Kosmahl M, Klöppel G. Mixed acinar-endocrine carcinoma of the pancreas. A clinicopathological study and comparison with acinar-cell carcinoma. Virchows Arch. 2004;445:231–5.
Ulich T, Cheng L, Lewin KJ. Acinar-endocrine cell tumor of the pancreas. Report of a pancreatic tumor containing both zymogen and neuroendocrine granules. Cancer. 1982;50:2099–105.
Ichijima K, Akaishi K, Toyoda N, et al. Carcinoma of the pancreas with endocrine component in childhood. A case report. Am J Clin Pathol. 1985;83:95–100.
Cho KJ, Kim JY, Lee SS, et al. Mixed acinar-endocrine carcinoma of the pancreas—a case report. J Korean Med Sci. 1996;11:188–92.
Shimoike T, Goto M, Nakano I, et al. Acinar-islet cell carcinoma presenting as insulinoma. J Gastroenterol. 1997;32:830–5.
Frank M, Bittinger A, Rothmund M, et al. Immunohistochemical analysis and clinical course of high-malignant composite endocrine-acinar cell carcinoma: a case report. Pancreas. 1998;17:210–2.
Ogawa T, Isaji S, Yabana T. A case of mixed acinar-endocrine carcinoma of the pancreas discovered in an asymptomatic subject. Int J Pancreatol. 2000;27:249–57.
Skacel M, Ormsby AH, Petras RE, et al. Immunohistochemistry in the differential diagnosis of acinar and endocrine pancreatic neoplasms. Appl Immunohistochem Mol Morphol. 2000;8:203–9.
Mizuno N, Naruse S, Kitagawa M, et al. Insulinoma with subsequent association of Zollinger-Ellison syndrome. Intern Med. 2001;40:386–90.
Imaoka H, Amano Y, Moriyama I, et al. Endoscopic ultrasound-guided fine-needle aspiration of a mixed acinar-endocrine carcinoma: a case report. Am J Gastroenterol. 2008;103:2659–60.
Chung WJ, Byun JH, Lee SS, et al. Imaging findings in a case of mixed acinar-endocrine carcinoma of the pancreas. Korean J Radiol. 2010;11:378–81.
Kobayashi S, Asakura T, Ohike N, et al. Mixed acinar-endocrine carcinoma of the pancreas with intraductal growth into the main pancreatic duct: report of a case. Surg Today. 2010;40:380–4.
Soubra A, Faraj W, Saab J, et al. Peri-ampullary mixed acinar-endocrine carcinoma. Rare Tumors. 2011;3:15.
Lee L, Bajor-Dattilo EB, Das K. Metastatic mixed acinar-neuroendocrine carcinoma of the pancreas to the liver: a cytopathology case report with review of the literature. Diagn Cytopathol. 2013;41:164–70.
Sullivan PS, Clebanoff JL, Hirschowitz SL. Hints to the diagnosis of mixed acinar-endocrine carcinoma on pancreatic fine-needle aspiration: avoiding a potential diagnostic pitfall. Acta Cytol. 2013;57:296–302.
Ogbonna OH, Garcon MC, Syrigos KN, et al. Mixed acinar-neuroendocrine carcinoma of the pancreas with neuroendocrine predominance. Case Rep Med. 2013. https://doi.org/10.1155/2013/705092.
Yu R, Jih L, Zhai J, Colquhoun S, Wolin E, et al. Mixed acinar-endocrine carcinoma of the pancreas: new clinical and pathological features in a contemporary series. Pancreas. 2013;42:429–35.
Kanemasa Y, Kamisawa T, Tabata T, et al. Mixed acinar-endocrine carcinoma of the pancreas treated with S-1. Clin J Gastroenterol. 2013;6:459–64.
Liu Z, Dong C, Wang C, et al. Mixed acinar-endocrine carcinoma of pancreas: a case report and brief review of the literature. OncoTargets Ther. 2015;8:1633–42.
Jakobsen M, Klöppel G, Detlefsen S. Mixed acinar-neuroendocrine carcinoma of the pancreas: a case report and a review. Histol Histopathol. 2016;31:1381–8.
Sugimoto M, Hines OJ, Dawson DW, Donahue TR, et al. Preoperative treatment with FOLFIRINOX and successful resection for a patient with mixed acinar-endocrine carcinoma of the pancreas. Pancreas. 2017;46:e32–4.
Hara T, Fujiwara Y, Takahashi H, et al. Metastatic mixed acinar-endocrine carcinoma of the pancreas treated with a multidisciplinary approach: a case report. Surg Case Rep. 2017;3:51.
Yokode M, Itai R, Yamashita Y, et al. A case report of mixed acinar-endocrine carcinoma of the pancreas treated with S-1 chemotherapy: does it work or induce endocrine differentiation? Med (Baltimore). 2017;96:e8534.
De Both A, De Man M, Troisi R, et al. Treatment of a mixed acinar-endocrine carcinoma with uptake on 68Gallium-DOTATOC positron emission tomography-computed tomography: a case report. Oncol Lett. 2017;14:547–52.
Strait AM, Sharma N, Tsapakos MJ, et al. Pancreatic mixed acinar-neuroendocrine carcinoma, a unique diagnostic challenge on FNA cytology: a small series of two cases with literature review. Diagn Cytopathol. 2018;46:971–6.
Niiya F, Takano Y, Azami T, et al. A case of pancreatic mixed acinar-neuroendocrine carcinoma successfully diagnosed with endoscopic ultrasound-guided fine needle aspiration. Clin J Gastroenterol. 2020;13:951–8.
Tang XJ, Fang XF, Zhu LZ, et al. Metastatic mixed acinar-neuroendocrine carcinoma of the pancreas treated by a multidisciplinary team: a case report and brief review of the literature. J Dig Dis. 2019;20:318–22.
Vilmann P, Jacobsen GK, Henriksen FW, et al. Endoscopic ultrasonography with guided fine needle aspiration biopsy in pancreatic disease. Gastrointest Endosc. 1992;38:172–3.
Agarwal B, Krishna NB, Labundy JL, et al. EUS and/or EUS-guided FNA in patients with CT and/or magnetic resonance imaging findings of enlarged pancreatic head or dilated pancreatic duct with or without a dilated common bile duct. Gastrointest Endosc. 2008;68:237–42 (quiz 334, 335).
Kim JY, Brosnan-Cashman JA, Kim J, et al. Pancreatic acinar cell carcinomas and mixed acinar-neuroendocrine carcinomas are more clinically aggressive than grade 1 pancreatic neuroendocrine tumours. Pathology. 2020;52:336–47.
Acknowledgements
We would like to thank Editage (www.editage.com) for English editing.
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Contributions
SH drafted the manuscript. RY performed the pathological diagnosis and pathological analysis such as immunostaining. We believe that surgery, treatment, and pathological analysis have similar importance in this case report. Therefore, SH and RY are co-first authors. MH and TI supervised the preparation of the manuscript. YM performed the surgery. TN supervised the pathological diagnosis. TS and MH performed endoscopy and biopsy. MH reviewed and modified the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Sawako Hiroi, Rie Yamamoto, Michinori Hamaoka, Masakata Hoshino, Tamito Sasaki, Yasuhiro Matsugu, Takashi Nishisaka, Hideki Nakahara and Toshiyuki Itamoto declare that they have no conflict of interest.
Human Rights
All procedures followed have been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed Consent
Informed consent was obtained from all patients for being included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hiroi, S., Yamamoto, R., Hamaoka, M. et al. Mixed neuroendocrine non-neuroendocrine neoplasm: a case report and review. Clin J Gastroenterol 15, 244–255 (2022). https://doi.org/10.1007/s12328-021-01552-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12328-021-01552-x