Abstract
Introduction
To assess the cost-effectiveness of alectinib versus crizotinib as first-line treatments for advanced anaplastic lymphoma kinase (ALK)-positive non-small cell lung cancer (NSCLC) patients from the perspective of China’s healthcare system.
Methods
A Markov model was developed to assess the clinical outcomes and costs of alectinib and crizotinib, which included five health states: progression-free (PF) without central nervous system (CNS) progression, PF with CNS progression, post-progression (PP) without CNS progression, PP with CNS progression, and death. Clinical data for transition probabilities were obtained from the ALEX trial at the updated data cutoff. Healthcare resource utilization and costs were derived from clinical expert opinions and published literature. One-way sensitivity analysis and probabilistic sensitivity analysis were conducted to assess the uncertainty of the results. Scenario analyses were conducted including using clinical data from the ALESIA trial in Asian patients, using utilities from the ALEX trial, and choosing different parametric survival models.
Results
In base case analysis, alectinib yielded an additional 1.04 quality-adjusted life years (QALYs) with incremental costs of $54,827, resulting in an incremental cost-effectiveness ratio (ICER) of $52,869/QALY. In scenario analysis, the ICER was $56,787/QALY using clinical data from the ALESIA trial. In probabilistic sensitivity analysis, the probabilities of alectinib being cost-effective were 0.4% and 43.7% when the willingness-to-pay (WTP) thresholds were $28,109/QALY and $50,000/QALY, respectively.
Conclusion
Alectinib could prolong the mean time of PF and delay the time to CNS progression. However, because of its high drug cost, alectinib was unlikely to be cost-effective for untreated ALK-positive NSCLC patients in China.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer is the leading cause of cancer-related deaths in China [1], and around 85% of lung cancer cases are non-small cell lung cancer (NSCLC) [2]. Anaplastic lymphoma kinase (ALK) translocation is a potential mechanism for targeted therapy. Crizotinib, the first-generation tyrosine kinase inhibitor (TKI) for advanced-staged ALK-positive NSCLC, is the standard first-line treatment for previously untreated patients with this translocation. However, some patients have developed intolerance to crizotinib or relapse within the first year of treatment, particularly in the central nervous system (CNS) [3, 4].
Alectinib is a second-generation TKI for advanced-staged ALK-positive NSCLC, which has shown significant superiority over chemotherapy in prolonging progression-free survival (PFS) in patients with previously treated ALK-positive NSCLC [5]. The recent pivotal phase III trials (J-ALEX, ALEX, and ALESIA) further confirmed the efficacy and safety of alectinib in untreated ALK-positive NSCLC [6,7,8,9]. In the ALEX trial, at the primary data cutoff (February 9, 2017) [7], alectinib showed superior investigator-assessed PFS compared with that of crizotinib [hazard ratio (HR) 0.47, 95% confidence interval (CI) 0.34–0.65]. In addition, the time to CNS progression was significantly longer with alectinib than with crizotinib in the intention-to-treat population (cause-specific HR 0.16, 95% CI 0.10–0.28). At the updated data cutoff (December 1, 2017) [8], median PFS with alectinib was 34.8 months (95% CI 17.7–NE (not estimable)) versus 10.9 months (95% CI 9.1–12.9) with crizotinib (HR 0.43, 95% CI 0.32–0.58). In addition, the proportion of patients with grade 3–5 serious adverse events (SAE) was lower with alectinib versus crizotinib (44.7% vs. 51.0%) [8].
Despite its superior efficacy and lower toxicity in advanced-staged ALK-positive NSCLC, alectinib was also associated with high costs. To our knowledge, there were only two published economic evaluations associated with alectinib in the treatment of patients with advanced ALK-positive NSCLC [10, 11]. Both of these studies were from a US payer perspective, and found that alectinib may be considered a cost-effective treatment. However, the cost-effectiveness of alectinib for Chinese patients with untreated advanced ALK-positive NSCLC was unknown.
Given its high prevalence and ever-increasing cancer drug prices, cancer is imposing a significant pressure on China’s national health insurance system, and more attention is being given to the value of oncology drugs, which also highlights the increasingly important role of health economic evaluation for decision-making in China. The National Healthcare Security Administration (NHSA) conducted the National Reimbursement Drug List (NRDL) negotiation with pharmaceutical companies in 2018 for oncology drugs. During this negotiation, the prices of 17 cancer drugs decreased by an average of 56.7% compared with their original prices, and the price of crizotinib decreased by 70.9% [12]. Because alectinib just received approval from the China Food and Drug Administration (CFDA) in August 2018, it did not feature in this negotiation.
Therefore, our study aimed to conduct a cost-effectiveness analysis of alectinib versus crizotinib for untreated ALK-positive NSCLC from the perspective of China’s healthcare system.
Methods
Overview
We developed a Markov model to assess the cost-effectiveness of alectinib versus crizotinib as first-line treatments using Microsoft Excel. The target patient population was untreated advanced ALK-positive NSCLC patients, and a hypothetical cohort with a mean age of 55 years and an Eastern Cooperative Oncology Group (ECOG) performance status of 0–2 was assumed in this model. The model outcomes included life years (LYs), quality-adjusted life years (QALYs), and costs, with cost-effectiveness assessed through estimation of incremental cost-effectiveness ratio (ICER). The Markov cycle length was 1 week, and the time horizon was lifetime. The willingness-to-pay (WTP) threshold was three times gross domestic product (GDP) per capita in China, which was $28,109 in 2017.
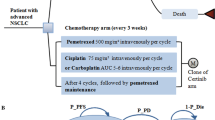
The Markov model included five health states: progression-free (PF) state without CNS progression (survival without systemic and CNS progression), PF state with CNS progression (survival without systemic progression, but with CNS oligoprogression), post-progression (PP) state without CNS progression (survival with systemic progression, but without CNS oligoprogression), PP state with CNS progression (survival with systemic and CNS progression), and death (Fig. 1). All target patients were in the PF state without CNS progression initially and could transition to progression state or death. Patients received alectinib (alectinib arm) or crizotinib (crizotinib arm) in the PF state until disease progressed. Subsequent treatments of both arms in the PP state were different on the basis of current clinical guidelines in China [13]. Patients in the alectinib arm could receive second-line platinum plus pemetrexed for up to four chemotherapy cycles, followed by pemetrexed maintenance, third-line docetaxel, and fourth-line best supportive care (BSC). Patients in the crizotinib arm could receive second-line alectinib (50%) or ceritinib (50%), third-line platinum plus pemetrexed for up to four chemotherapy cycles, followed by pemetrexed maintenance, fourth-line docetaxel, and fifth-line BSC. Patients could transition to BSC directly from any lines of therapy when disease progressed, and all patients would transition to BSC if they failed on docetaxel treatment (see Appendix 1 in the electronic supplementary material). This economic study was based on a literature review and model techniques and did not require approval by the institutional research ethics board. This article does not contain any studies with human participants or animals performed by any of the authors.
Clinical Inputs
The investigator-assessed Kaplan–Meier curves of PFS and overall survival (OS) data associated with alectinib and crizotinib were obtained from the ALEX trial at the updated data cutoff (December 1, 2017) [8]. As a result of the absence of the Kaplan–Meier data of CNS progression for both arms in the ALEX clinical literature, this data was derived from an economic evaluation by Carlson et al. [10], which reported the Kaplan–Meier curves of CNS progression for both arms from the ALEX trial. The individual patient data (IPD) then was replicated following the methodology of Guyot et al. [14]. Six commonly used parametric survival models were fitted, including exponential, Weibull, Gompertz, log-logistic, log-normal, and generalized gamma. On the basis of clinical rationality, visual fit, and statistical goodness-of-fit [Bayesian information criterion (BIC) and Akaike’s information criterion (AIC)], the exponential distribution was chosen for PFS and OS of the alectinib arm, and for OS of the crizotinib arm, while the log-normal distribution was chosen for PFS of the crizotinib arm (see Appendix 2 in the electronic supplementary material).
The mortality in the progression-free state was derived from the age-related mortality rate for the general population from Chinese life tables [15]. The elevated mortality in clinical trials was only applied in the progression state. When patients moved to the PP state, 58.3% and 52.1% of the patients would receive second- and third/fourth-line active anticancer treatment, respectively, figures which were based on the real-world treatment pattern of Chinese advanced NSCLC patients [16], and the remaining patients would receive BSC directly.
Costs and Healthcare Resource Utilization
The direct medical costs considered in this study were drug costs, follow-up costs, BSC cost, CNS progression management costs, and terminal care cost (Table 1). The drug cost of alectinib (600 mg twice a day) was based on the latest retail price and was adjusted according to the patient assistance program (PAP). The drug costs of crizotinib (250 mg twice a day) and ceritinib (750 mg once a day) were based on the latest reimbursement price set by NHSA in October 2018 [12], which included the portion paid by basic medical insurance and out-of-pocket payment by patients. The drug costs of chemotherapies were estimated on the basis on the average prices of each specification weighted by their market share in 2018 (see Appendix 3 in the electronic supplementary material). To calculate the drug costs of chemotherapy per cycle, a base case patient with a body surface area of 1.72 m2 was assumed. The cost of BSC came from a published economic evaluation in China [17].
The items for managing CNS progression included radiotherapy (stereotactic radiosurgery or whole brain radiotherapy), steroids, mannitol, and brain magnetic resonance imaging (MRI). Considering that the target patients were advanced-staged NSCLC, surgical resection was not included in the model. The proportions of stereotactic radiosurgery and whole brain radiotherapy and the associated costs per person were based on local clinical expert opinions. The steroids (i.e., dexamethasone) and mannitol were used to relieve intracranial hypertension and cerebral edema, and the dosages and schedules of both drugs were based on drug instructions. Brain MRI was performed every 3 months according to Chinese guidelines on the diagnosis and treatment of brain metastases of lung cancer [18], and the cost associated with brain MRI was derived from the local price [19].
The follow-up costs included outpatient physician visit costs, hospitalization costs, and laboratory tests, which were obtained from a published study comparing the direct medical costs of targeted treatment and chemotherapy treatment for advanced NSCLC patients in China [20]. As with previous economic evaluations, the terminal care cost during the final month of life was adopted from a cost analysis for advanced cancer patients in China [21, 22]. All costs were adjusted by Consumer Price Index for medical care services and discounted at 3% annually [23], and were presented in 2018 US dollars using an exchange rate of 6.367 (1 dollar = 6.367 Chinese yuan).
Health Utilities
Health utility values of the PF and PD states without CNS progression were obtained from a cross-sectional survey conducted at the Shanghai Chest Hospital in China [24]. The health utility for the PF state without CNS progression was 0.86 in the first-line setting, and the utility for the PP state without CNS progression was 0.74, which was the mean utility value for patients receiving second- and third- or later line treatments. In addition, the health utility for the PF state with CNS progression was 0.52 [25], which was obtained from one quality of life study that reported the utility value for advanced NSCLC patients in France and Germany with one brain metastatic site. As a result of the absence of a utility value for the PP state with CNS progression, this value was assumed to 0.40, which meant that the difference between two utilities of the PP state without and with CNS progression was consistent with that of two utilities of the PF state.
Considering that the same gene test was needed in both arms, the cost of the gene test was not included in this study. The rate of grade 3–5 SAE was low in both arms in the ALEX trial [7, 8], and the frequency of SAE requiring treatment did not exceed 5%; thus the disutility and costs of SAE associated with alectinib and crizotinib were not considered in this study.
Sensitivity Analyses
In consideration of the uncertainty of parameters and assumptions, deterministic sensitivity analysis (DSA) and probabilistic sensitivity analysis (PSA) were conducted to evaluate the robustness of the base case results. In the one-way sensitivity analyses, parameters were independently varied within a plausible range determined by either published data or by 95% CI. If not applicable, the values were varied by ± 20% of the corresponding base case value. The discount rate was varied from 0% to 8% according to Chinese guidelines for pharmacoeconomic evaluation [23]. The PSA was conducted using second-order Monte Carlo simulation by running 5000 iterations to account for uncertainty in model parameters. Gamma distributions were used for costs, whereas beta distributions were used for utilities and proportions. Uncertainty for parameters of the parametric survival model were assessed through the Cholesky decomposition if applicable. Cost-effectiveness acceptability curves were considered to show the probabilities of each arm being cost-effective at a wide range of WTP thresholds. In addition, the probabilities of alectinib being cost-effective at different drug costs of alectinib within different WTP thresholds were also shown.
The following scenario analyses were performed. For clinical efficacy of alectinib and crizotinib, the analysis was conducted on the basis of the PFS and OS data from ALESIA trial in Asian patients [9], although the PFS and OS data of both arms in this trial were still immature at the time of this study. For the parametric survival models, the exponential distribution was chosen for PFS in the crizotinib arm, which meant that the PFS and OS in both arms were all fitted by an exponential distribution. The Weibull distribution was chosen for PFS in the alectinib arm because of a slightly better statistical fit than an exponential distribution even though it produced extended tailing that probably overestimated PFS beyond the 60th month. For health utilities of the PF and PP states without CNS progression, the analysis was conducted on the basis of the health utilities from the ALEX trial, in which the utility values for the PF and PP states were 0.81 and 0.72, respectively [10]. For health utilities of the PF and PP states with CNS progression, they were assumed to have a 10% decrement over the utilities of the PF and PP states without CNS progression, which were 0.77 and 0.66, respectively. For the drug cost of alectinib, the analysis was conducted on the basis of the original price and the price discounted by 56.7%, which was the average price cut of NRDL negotiation in 2018. This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Results
Base Case Analysis
The estimated mean PFS and OS times for alectinib were 3.10 years and 5.69 years, respectively, an increase of 1.63 years and 1.13 years when compared with those for crizotinib (Table 2). The mean time without CNS progression for alectinib was 1.83 years, an increase of 1.16 year compared with that for crizotinib. When considering for the quality of life, the alectinib arm resulted in 3.26 QALYs, an increase of 1.04 QALYs compared with those of the crizotinib arm.
The estimated mean lifetime costs for patients receiving alectinib were $150,774 compared with $95,947 for crizotinib, resulting in an additional cost of $54,827. The drug costs for the alectinib arm were much higher than those for the crizotinib arm in the PF state ($115,345 vs. $43,706); however, the drug costs for the alectinib arm were lower than those for the crizotinib arm in the PP state ($5844 vs. $23,817). The brain metastasis management costs were lower with alectinib than with crizotinib ($4224 vs. $5235). The ICER for alectinib versus crizotinib was $52,869/QALY.
Sensitivity Analyses
The results of the one-way sensitivity analyses are shown in Fig. 2. The model outcome was sensitive to the drug cost of alectinib. When the cost of alectinib decreased by 20% under the PAP, the ICER would be $30,624/QALY, which was close to the WTP threshold of China. Other parameters, such as utilities in the PF state and the PP state, discount rate, and costs associated with managing CNS progression, had mild influences on the result of economic evaluation.
The cost-effectiveness acceptability curve is shown in Fig. 3. The probability of alectinib being cost-effective was 0.4% at the Chinese WTP threshold. When the WTP thresholds were $50,000/QALY and $100,000/QALY, the probabilities of alectinib being cost-effective were 43.7% and 93.0%, respectively. In addition, Fig. 4 demonstrates that the probability of alectinib being cost-effective would increase as the drug cost of alectinib decreases. When the drug cost of alectinib per week was under $555, the probability of alectinib being cost-effective would be higher than 50% at a WTP threshold of $28,019.
In the scenario analyses, when clinical data were derived from the ALESIA trial [9], the ICER was $56,787/QALY. When the exponential distribution was chosen for PFS and OS in both arms, the ICER was $54,465/QALY. When the Weibull distribution was chosen for PFS in the alectinib arm, the ICER was $67,785/QALY. When the utilities of the PF and PP states without CNS progression were derived from the ALEX trial [10], the ICER was $56,573/QALY. When the utilities of the PF and PP states with CNS progression were assumed to have a 10% decrement over the utilities of the PF and PP states without CNS progression, the ICER was $53,706/QALY. When the original price and the price discounted by 56.7% of alectinib were applied, the ICERs were $246,287/QALY and $73,554/QALY, respectively (see Appendix 4 in the electronic supplementary material). Alectinib was not cost-effective compared with crizotinib at the Chinese WTP threshold in any of the above scenarios.
Discussion
We conducted an economic analysis of alectinib versus crizotinib as first-line treatments for advanced ALK-positive NSCLC patients in China based on the updated clinical outcomes from the ALEX trial. We adopted a perspective of China’s healthcare system, used the latest drug prices of alectinib, crizotinib, and ceritinib, and obtained healthcare resource utilizations associated with CNS progression based on clinical expert opinions. The clinical pathway and treatment pattern were tailored to comply with Chinese clinical guidelines and practice. The results showed that alectinib could improve QALYs with higher costs compared with crizotinib, and the alectinib arm was not likely to be cost-effective on the basis of the commonly used WTP threshold in China. The drug cost of alectinib was the most important influencing factor.
Two key clinical values of alectinib were shown in our economic evaluation. One was that the mean time in the PF state was considerably longer with alectinib than with crizotinib (3.10 LYs vs. 1.47 LYs). Because of this, the alectinib arm had higher drug costs in the PF state. The other clinical value was that the mean time without CNS progression was significantly longer with alectinib than with crizotinib. This value on CNS progression of alectinib not only resulted in an additional 0.95 QALYs but also saved brain metastasis management costs of $1011 per patient. In addition, as patients in the crizotinib arm could receive TKIs when disease progressed, the drug costs in PP state were much higher in the crizotinib arm than those in the alectinib arm. In total, the additional costs in the PF state with alectinib were offset to some extent by the increased costs of crizotinib in the PP state and CNS progression management costs.
There was only one published economic evaluation of alectinib versus crizotinib as first-line treatments from a US payer perspective [10]. The major finding was that alectinib yielded an additional 0.87 QALYs with an additional cost of $34,151, resulting in an ICER of $39,312/QALY. The estimation of an additional 1.04 QALYs in our study was higher than that in Carlson et al.’s study [10], which was mainly due to the different data cutoff. Specifically, the median PFS by an independent review committee used in Carlson et al.’s study was 25.7 months, while the median investigator-assessed PFS was 34.8 months in our study. In addition, our finding to prefer crizotinib over alectinib was different from that of the study by Carlson et al. [10], which mostly resulted from the different WTP thresholds adopted. If a WTP threshold of $100,000/QALY had been used in our study, the probability of alectinib being cost-effective would be 93.0%, which was higher than that in Carlson et al.’s study [10].
There were some limitations in this study. First, there were no head-to-head randomized controlled trial (RCT) studies comparing alectinib with crizotinib in Chinese patients. Fortunately, the ALESIA trial included Chinese patients [9], and the primary results of this trial were consistent with the global ALEX trial, which further confirmed the clinical benefit of alectinib in Asian patients. Second, at the updated data cutoff, the OS data of alectinib and crizotinib were still immature. On the basis of the visual fit and statistical goodness-of-fit, we made a conservative estimation of long-term OS. Nevertheless, it would be essential to further confirm the current findings when mature OS data are available. Third, there was no real-world evidence of economic burden for Chinese NSCLC patients with brain metastases, and the costs associated with CNS progression management were based on clinical expert opinions. However, this uncertainty on the cost-effectiveness of alectinib was small on the basis of the result of one-way sensitivity analysis. Finally, the adoption of alectinib for untreated advanced ALK-positive NSCLC might change the clinical pathway, but the effects of alectinib on follow-up treatments were unknown. Although the treatment patterns were conducted on the basis of Chinese clinical guidelines, real-world studies would be useful to further confirm these findings.
Conclusion
Alectinib yielded an additional 1.04 QALYs with additional costs of $54,827, resulting in an ICER of $52,869/QALY using latest clinical data from the global ALEX trial in the base case analysis. In scenario analysis, the ICER was $56,787/QALY using clinical data from the ALESIA trial in Asian patients. DSA showed that the drug cost of alectinib was the most important influencing factor. PSA showed that the probability of alectinib being cost-effective was 0.4% based on the commonly used WTP threshold in China. This study might be useful for decision-making about reimbursement and for promotion of rational use of oncology drugs.
References
Chen WQ, Sun KX, Zheng RS, et al. Report of cancer incidence and mortality in different areas of China, 2014. China Cancer. 2018;27:1–14.
Kilgoz HO, Bender G, Scandura JM, Viale A, Taneri B. KRAS and the reality of personalized medicine in non-small cell lung cancer. Mol Med. 2016;22:380–7.
Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371:2167–77.
Dagogo-Jack I, Shaw AT. Crizotinib resistance: implications for therapeutic strategies. Ann Oncol. 2016;27(Suppl 3):iii42–iii50.
Novello S, Mazières J, Oh IJ, et al. Alectinib versus chemotherapy in crizotinib-pretreated anaplastic lymphoma kinase (ALK)-positive non-small-cell lung cancer: results from the phase III ALUR study. Ann Oncol. 2018;29:1409–16.
Hida T, Nokihara H, Kondo M, et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet. 2017;390:29–39.
Peters S, Camidge DR, Shaw AT, et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med. 2017;377:829–38.
Camidge DR, Peters S, Mok T, et al. Updated efficacy and safety data from the global phase III ALEX study of alectinib (ALC) vs crizotinib (CZ) in untreated advanced ALK + NSCLC. J Clin Oncol. 2018;36(15_suppl):9043.
Zhou C, Lu Y, Kim S-W, et al. Primary results of ALESIA: a randomised, phase III, open-label study of alectinib vs crizotinib in Asian patients with treatment-naïve ALK + advanced NSCLC. Ann Oncol. 2018; 29(suppl_8): mdy424.062.
Carlson JJ, Suh K, Orfanos P, Wong W. Cost-effectiveness of alectinib vs. crizotinib in first-line anaplastic lymphoma kinase-positive advanced non-small-cell lung cancer. Pharmacoeconomics. 2018;36:495–504.
Carlson JJ, Canestaro W, Ravelo A, Wong W. The cost-effectiveness of alectinib in anaplastic lymphoma kinase-positive (ALK+) advanced NSCLC previously treated with crizotinib. J Med Econ. 2017;20:671–7.
National Healthcare Security Administration. Notice on reimbursement decisions on including 17 cancer drugs in national reimbursement drug list by national healthcare security administration. http://www.gov.cn/xinwen/2018-10/10/content_5328891.htm. Accessed 10 Oct 2018.
Chinese Society of Clinical Oncology (CSCO). Primary lung cancer treatment guidelines (2018 version). http://guide.medlive.cn/guideline/16783. Accessed 23 July 2018.
Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012;12:9.
National Bureau of Statistics of China. China population and employment statistics yearbook 2017. Beijing: China Statistics Press; 2017.
Ying GZ, Chang JS, Cui LS, et al. Third-line therapy in advanced non-small cell lung cancer. J BUON. 2013;18:899–907.
Lu S, Ye M, Ding L, Tan F, Fu J, Wu B. Cost-effectiveness of gefitinib, icotinib, and pemetrexed-based chemotherapy as first-line treatments for advanced non-small cell lung cancer in China. Oncotarget. 2017;8:9996–10006.
Shi Y, Sun Y, Yu J, et al. China experts consensus on the diagnosis and treatment of brain metastases of lung cancer (2017 version). Zhongguo Fei Ai Za Zhi. 2017;20:1–13.
Beijing Municipal Commission of Development and Reform. Medical service items and price list. http://fgw.beijing.gov.cn/bjpc/mediprice/medical_price_table.jsp#top. Accessed 18 Oct 2018.
Liu B. Comparison of the cost of 4 kinds of second-line drug treatment regimes for non-small cell lung cancer. China Pharm. 2010;21:2403–6.
Cao HT, Wang JQ. Survey of the advanced cancer patients’ medical costs in registered hospice care agencies in five provinces and municipalities. Chin Gen Pract. 2010;13:3544–6. https://doi.org/10.3969/j.issn.1007-9572.2010.31.023.
Lu S, Zhang J, Ye M, Wang B, Wu B. Economic analysis of ALK testing and crizotinib therapy for advanced non-small-cell lung cancer. Pharmacogenomics. 2016;17:985–94.
Gordon L. China guidelines for pharmacoeconomic evaluation and manual. 2015th ed. Beijing: Science Press; 2015.
Shen Y, Wu B, Wang X, Zhu J. Health state utilities in patients with advanced non-small-cell lung cancer in China. J Comp Eff Res. 2018;7:443–52.
Roughley A, Damonte E, Taylor-Stokes G, Rider A, Munk VC. Impact of brain metastases on quality of life and estimated life expectancy in patients with advanced non-small cell lung cancer. Value Health. 2014;17:A650.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article. The article processing charges were funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Haijing Guan, Yanan Sheng, Wanjie Guo, Sheng Han, and Luwen Shi declare no conflicts of interest.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors
Data Availability
All data generated or analyzed during this study are included in this published article/as supplementary information files.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.7701080.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Guan, H., Sheng, Y., Guo, W. et al. Cost-Effectiveness of Alectinib for Patients with Untreated ALK-Positive Non-Small Cell Lung Cancer in China. Adv Ther 36, 1114–1125 (2019). https://doi.org/10.1007/s12325-019-00908-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-019-00908-7