Abstract
Background
The recently completed ALEX trial demonstrated that alectinib improved progression-free survival, and delayed time to central nervous system progression compared with crizotinib in patients with anaplastic lymphoma kinase-positive non-small-cell lung cancer. However, the long-term clinical and economic impact of using alectinib vs. crizotinib has not been evaluated. The objective of this study was to determine the potential cost utility of alectinib vs. crizotinib from a US payer perspective.
Methods
A cost-utility model was developed using partition survival methods and three health states: progression-free, post-progression, and death. ALEX trial data informed the progression-free and overall survival estimates. Costs included drug treatments and supportive care (central nervous system and non-central nervous system). Utility values were obtained from trial data and literature. Sensitivity analyses included one-way and probabilistic sensitivity analyses.
Results
Treatment with alectinib vs. crizotinib resulted in a gain of 0.91 life-years, 0.87 quality-adjusted life-years, and incremental costs of US$34,151, resulting in an incremental cost-effectiveness ratio of US$39,312/quality-adjusted life-year. Drug costs and utilities in the progression-free health state were the main drivers of the model in the one-way sensitivity analysis. From the probabilistic sensitivity analysis, alectinib had a 64% probability of being cost effective at a willingness-to-pay threshold of US$100,000/quality adjusted life-year.
Conclusions
Alectinib increased time in the progression-free state and quality-adjusted life-years vs. crizotinib. The marginal cost increase was reflective of longer treatment durations in the progression-free state. Central nervous system-related costs were considerably lower with alectinib. Our results suggest that compared with crizotinib, alectinib may be a cost-effective therapy for treatment-naïve patients with anaplastic lymphoma kinase-positive non-small-cell lung cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The model projected that the average time progression free and alive states were longer with alectinib vs. crizotinib. |
Alectinib, as a first-line treatment option, resulted in a gain of 0.87 quality-adjusted life-years, increased costs of US$34,151, resulting in an incremental cost-effectiveness ratio of US$39,312/quality-adjusted life-year compared with crizotinib. |
A key driver of the estimated value of alectinib was its impact on central nervous system progression. |
1 Introduction
The incidence of lung cancer in USA is the second highest among cancers in both men and women and has the highest mortality rate [1]. Non-small-cell lung cancer (NSCLC) comprises about 85% of lung cancer cases, with the majority being of the non-squamous subtype [2]. The 5-year survival rate for patients with late-stage (i.e., III/IV) NSCLC remains poor at less than 5% [2, 3]. Despite this, continuous advancement in our knowledge of the molecular nature of the disease has led to the development of targeted therapies with subsequent improvement in patient outcomes [4, 5].
The first targeted therapy in NSCLC was for mutations in the epidermal growth factor receptor [6,7,8,9,10,11,12,13]. Patients who used a tyrosine kinase inhibitor (TKI) as a targeted therapy showed clinical improvements in response rates and progression-free survival (PFS) [6,7,8,9]. As a result, clinical guidelines now recommend epidermal growth factor receptor mutation testing for patients with NSCLC, especially those with advanced non-squamous tumors [14, 15]. Anaplastic lymphoma kinase (ALK) translocation as a potential mechanism for targeted therapy soon followed, with a similar testing recommendation for all patients with NSCLC [16].
The first targeted TKI for advanced-staged ALK-positive NSCLC, crizotinib, is the standard treatment for previously untreated patients with this translocation [17]. Subsequently, other TKIs for ALK-positive NSCLC have been approved and are used in practice, as many patients develop intolerance to crizotinib or relapse within the first year of treatment, particularly in the central nervous system (CNS) [17,18,19]. Alectinib, a second-generation TKI, received approval in 2015 by the US Food and Drug Administration for the treatment of patients with ALK-positive metastatic NSCLC who progressed or were intolerant of other treatment. Two phase II trials (NP28761 and NP28673) demonstrated efficacy and safety in previously treated ALK-positive patients [20, 21].
Alectinib has more recently demonstrated positive results in a crizotinib-naïve, ALK-positive patient setting in two Japanese studies, including the phase III J-ALEX trial (JapicCTI-132316) [22, 23]. Recently, the ALEX trial (BO28984), an international randomized open-label phase III trial comparing alectinib (600 mg twice daily) with crizotinib in patients with previously untreated advanced ALK-positive NSCLC, demonstrated that alectinib is a treatment option as a first-line regimen [24]. Patients taking alectinib had significantly longer PFS [25.7 months, 95% confidence interval (CI) 19.9–not estimable] compared with crizotinib (10.4 months, 95% CI 7.7–14.6) assessed by an independent review committee, and alectinib had a significantly lower hazard ratio for disease progression or death (0.5, 95% CI 0.36–0.70) [24].
A potential advantage of alectinib is that it can cross the blood–brain barrier and is not actively exported out of the brain because it is not a p-glycoprotein substrate [25]. This was demonstrated in the NP28673 study where alectinib achieved an intracranial objective response of 75% (95% CI 48–93) with a median duration of 11.1 months (95% CI 5.8–11.1) [20]. Crizotinib, which has lower drug concentrations in the CNS, showed intracranial disease control in 56% of patients at 24 weeks [26, 27]. In ALEX, alectinib also demonstrated superior CNS activity, as the time to CNS progression was significantly delayed with alectinib in the intention-to-treat population (hazard ratio 0.16, 95% CI 0.10–0.28), and the 12-month cumulative incidence rate of CNS progression with alectinib was 9.4% (95% CI 5.4–14.7) compared with 41.4% (95% CI 33.2–49.4) with crizotinib [24].
While alectinib demonstrated superior efficacy compared with crizotinib in the BO28984 trial, the economic impact and value of alectinib have not been evaluated in the first-line setting. In a time of rising healthcare costs, increasing attention is being given to the value of prescription drugs, especially in the area of oncology [28,29,30]. Many organizations have developed value frameworks to assess treatments, including the American Society of Clinical Oncology, the National Comprehensive Cancer Network, and the Institute for Clinical and Economic Research [31]. Therefore, our objective was to estimate the cost effectiveness of alectinib vs. crizotinib in treatment-naïve patients with ALK-positive NSCLC from the US payer perspective.
2 Methods
2.1 Markov Model Overview
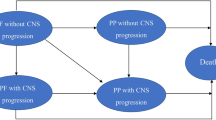
We developed an economic model in Microsoft Excel (Redmond, WA, USA) with three health states: progression-free (PF), post-progression (PP), and death (Fig. 1). Time in each health state was estimated using partition survival methods (i.e., area under the survival curves). Costs included drug therapy and supportive care, stratified by the presence or absence of CNS progression. Patients received alectinib or crizotinib until progression. Subsequent treatment was informed by clinical guidelines and a pattern of care study and included the ALK targeting TKI’s ceritinib, alectinib, and crizotinib as well as best supportive care (BSC) (see Table 1) [15, 32]. We applied a discount rate of 3%, used weekly cycles, and a lifetime time horizon. Costs from previous years were adjusted using the medical care consumer price index and reported in 2017 US dollars. For the cost-effectiveness analysis, the results were reported in terms of cost per life-year (LY) gained and cost per quality-adjusted life-year (QALY) gained.
2.2 Clinical Inputs
The most recent available data for alectinib and crizotinib PFS and overall survival (OS) were derived from the BO28984 study [24, 33]. We fit parametric survival functions to independent review committee-assessed Kaplan–Meier data. Upon review, the exponential distribution for the PFS and OS curves were most appropriate based on goodness of fit [Bayesian information criterion (BIC)] and visual fit (see Fig. 2). Clinical plausibility of the exponential distributions was also assessed with the assistance of 12 consultant oncologists specializing in lung cancer treatment in the UK, and based on their input in concert with the goodness of fit and visual fit, the exponential distributions were considered to align with progression and survival expectations. For additional details, please refer to the Electronic Supplementary Material (ESM).
2.3 Adverse Events
The grade 3–5 adverse event rate was low in both arms of the BO28984 trial with no treatable grade 3–5 adverse events occurring in greater than 5.0% of patients in either treatment arm [24]. Therefore, we did not include disutilities or costs related to adverse events in the model.
2.4 Quality-of-Life Inputs
We incorporated health-related quality of life into the model using utility values. The PF utility estimate (0.81) was derived from the BO28984 trial based on the EQ-5D-3L and applied to both initial treatments (see Table 1) [33]. Specifically, trial EQ-5D-3L data were collected every 4 weeks electronically during scheduled clinic visits. A total of 3866 questionnaires were completed out of a planned 5400 (completion rate: 72%). The data were analyzed with a linear mixed-effects model, including the following variables: sex, age, race (Asian vs. non-Asian), CNS lesions at baseline, and health state (progressed vs. non-progressed). Utility at baseline was not included, given the high percentage of missing data at baseline (> 30% in both arms). Treatment was not a significant factor in the prediction of utility (p value = 0.3912), and it was decided that a treatment-specific utility was not needed in the model, neither on the PF nor PP state.
In the progression health state, patients on treatment were assigned a health utility of 0.72 based on patients recently progressed in the BO28984 study. The model also includes a utility for patients in the progressed state on BSC based on a study by Nafees et al. that elicited utility values from patients with metastatic NSCLC receiving second-line treatment [34].
2.5 Cost Inputs
Costs included drug treatment and supportive care. Treatment costs per week are calculated using dosing schedules and unit costs for the drugs based on the average wholesale price minus 16% (see Table 1) [35, 36]. The model calculates the cost of initial drug treatment in the PF health state assuming that patients are treated until progression or death. Alectinib and crizotinib are both oral drugs and therefore no administration costs were included. The cost in the progression health state is an average weekly cost based on the assumed treatment patterns after progression [32].
Specifically, patients who progress after first-line treatment receive a second-line ALK inhibitor (70%) or BSC (30%). For the purposes of this exercise, the cost and effectiveness of second-line treatment were assumed to be similar to the use of ceritinib in second-line [37]. Patients progressing with a second-line ALK inhibitor receive an ALK inhibitor they have not previously received, such as crizotinib or alectinib (50%), depending on their first-line treatment or BSC (50%) (see Table 1). Time taking the drug was derived from clinical trials of ceritinib, crizotinib, or alectinib in the second-/third-line setting [20, 37, 38]. The mean drug cost per week in the progression health state was therefore calculated as US$770 and US$691 for patients initiating first-line alectinib and crizotinib, respectively. The slightly lower cost for patients initiating first-line treatment with crizotinib results from those patients using alectinib in the third-line setting, which is less costly than crizotinib.
Supportive care costs were also included in the PF and PP health states. The cost per week of supportive care was based on the presence or absence of CNS metastases estimated using ALEX trial data on the time to CNS progression. A log normal (best BIC across both treatments) distribution was fit to the Kaplan–Meier data and applied throughout the model time horizon (Fig. 2) [33]. The cost per week with (US$3381) and without (US$788) CNS metastases was based on a study by Guerin et al. (2015), which estimated costs using three large retrospective administrative-claims databases (Source Healthcare Analytics’ Source Lx database for June 2011–June 2013, IMS LifeLink Health Plan Claims database for January 2001–March 2013, and Truven Health Analytics MarketScan database for January 2002–September 2012) for ALK-positive patients before and after a CNS metastases diagnosis [39]. We included all non-pharmacy costs including outpatient, radiation, physician, emergency department, and inpatients costs in the supportive care estimates.
2.6 Sensitivity Analysis
To address the uncertainty in the model, we performed one-way and probabilistic sensitivity analyses. Ranges were based on 95% CIs or varying the default input by 10%. Distributional assumptions were based on recommended guidelines [40]. We ran 5000 simulations for the probabilistic sensitivity analysis in the base-case analysis. We also performed two scenario analyses. First, we used only the alectinib PP utility data in the progression health state. Second, we used the Weibull parametric function for the PFS curve fit of alectinib owing to a slightly better visual performance even though the BIC supported an exponential function. The PFS curve fits for crizotinib were not changed in this scenario as both visual and BIC assessments supported an exponential fit.
3 Results
3.1 Clinical Outcomes
In the base case, projected median PFS for alectinib was 23.08 months compared with 11.77 months for crizotinib. The projected median time to CNS progression was also greater for alectinib compared with crizotinib (16.79 vs. 7.13 months), as well as the average time spent CNS progression free (41.39 vs. 9.17 months).The average time spent progression free with first-line alectinib was 2.71 years and the average OS time was estimated at 5.21 years. This represented an incremental increase in PFS and OS time compared with crizotinib of 1.33 and 0.91 years, respectively. After accounting for health-related quality of life, first-line alectinib was estimated to result in 2.64 QALYs, which represented an increase of 0.87 QALYs compared with crizotinib.
3.2 Costs and Cost Utility
The longer PFS time with first-line alectinib resulted in higher drug costs in the PF state (US$440,631 vs. US$258,263), whereas drug costs in the progression health state were similar (US$100,249 vs. US$104,953). For non-drug costs of care, patients receiving alectinib first line incurred US$381,873 compared with US$613,707 for crizotinib, resulting in an average increase of US$231,834 over the patient’s lifetime. This difference was driven by the differential rates of CNS progression. In total, treatment with alectinib resulted in an increase of US$34,151, which translates into an incremental cost per LY gained of US$37,611 and an incremental cost per QALY gained of US$39,312 (Table 2).
3.3 Sensitivity Analysis
In the one-way sensitivity analysis, the main model drivers were drug costs and cost-of-care estimates (see Fig. 3). At the highest end of the drug costs (increase of 118% from base case) in the one-way sensitivity analysis, the incremental cost-effectiveness ratio (ICER) remained under a US$100,000/QALY threshold, indicating our results were robust. A probabilistic sensitivity analysis was performed for the base-case analysis, and the cost-effectiveness acceptability curve is shown in Fig. 4. The probabilistic sensitivity analysis demonstrated that alectinib has a 76% probability of being cost effective at a willingness-to-pay threshold of US$150,000/QALY.
In the scenario analysis, which used only alectinib PP utility data in the progression health state, an additional 0.78 QALYs were gained. This yielded an ICER of US$44,002/QALY. In the scenario analysis that used a Weibull function to fit the PFS curve of alectinib, the resultant ICER was US$144,986.
3.4 Model Validation
Appropriate steps were taken to validate our model. In line with recommendations in the literature, we assessed the validity of the conceptual model, input data, computerized model, and operational outcomes [41]. We provide a rationale for our choice in selecting our conceptual model as well as for the key input data in the ESM. We validated the computerized model by having multiple members of our research team reviewing the model calculations and by using extreme values and testing of traces to ensure the model was working as specified. Finally, we compared our results to similarly external data and other published models.
4 Discussion
Crizotinib was the first available targeted drug developed for patients with ALK-positive NSCLC. Treatments for these patients have been evolving and advancing since that time. Recently, alectinib was compared to crizotinib in treatment-naïve patients and demonstrated improved clinical outcomes in terms of PFS and delayed time to CNS progression. We assessed the potential cost effectiveness of alectinib vs. crizotinib in this setting from a US payer perspective. While the willingness-to-pay threshold of an ICER is organizationally dependent, our estimated ICER of US$39,312 per QALY gained may be considered a cost-effective option compared with crizotinib in USA compared with commonly used thresholds (i.e., < US$100,000/QALY or US$150,000/QALY) [42]. Across all the sensitivity and scenario analyses we performed, the resultant ICERs were all below a US$150,000/QALY threshold, highlighting the robustness of our model results. While this analysis did not directly assess the budget impact of using alectinib in the first line, owing to the low prevalence of cases of ALK-positive advanced NSCLC coupled with the similar treatment costs among the drugs evaluated, we do not anticipate any substantial impact to payers’ budgets.
With identifiable genetic alterations in NSCLC, targeted approaches to treatment have become the standard of care. One of these, the ALK translocation, is a marker that has been an important target for drug development—the products from which have led to better outcomes for this subset of patients. While alectinib was first used for patients who progressed on or did not tolerate crizotinib, it is now available for use in treatment-naïve patients. In this patient setting, alectinib has shown positive findings, highlighted by a significantly higher PFS (25.7 vs. 10.4 months) and a significantly lower disease progression or death (hazard ratio 0.5, 95% CI 0.36–0.70) [24].
In our analysis, alectinib had higher drug costs in the PF health state as a result of the aforementioned efficacy and tolerability compared with crizotinib, which translated to a higher proportion of patients remaining on treatment given that alectinib and crizotinib are both recommended to be used until progression or unacceptable toxicity [43, 44]. Our model estimated that time in the PF state and therefore time receiving therapy is about twice as long as that of crizotinib (32.5 vs. 16.6 months). The drug costs in the progression state were comparable as patients in each arm spent a similar amount of time receiving treatment given the recommended treatments for patients after initial progression on an ALK inhibitor [15].
Overall, the total cost of care for both cohorts was substantial but the higher drug costs for patients initiating with alectinib were offset by the decreased cost related to supportive care for patients with CNS progression compared with crizotinib. Using CNS progression data from the ALEX trial and ALK-positive NSCLC-specific cost of care for patients with and without CNS metastasis, our model estimated that over a patient’s lifetime, those treated with crizotinib incurred US$231,834 more in supportive care costs than those treated with alectinib. This was owing to differential CNS progression rates and the higher average cost of care for those with CNS progression [39]. This demonstrates that CNS metastasis is not only a substantial clinical burden, but also an important source of economic burden [38, 45, 46]. Therefore, prevention or delay of CNS metastasis can yield both clinical and economic returns.
There are a limited number of studies looking at the cost effectiveness of alectinib or crizotinib in the treatment-naïve setting. One study examined crizotinib in the first-line setting from a Canadian single-payer perspective and compared it to a platinum doublet treatment regimen [47]. Among patients with known ALK status, crizotinib yielded an additional 0.379 QALYs but with an additional cost of CAD$95,043 per patient, resulting in an ICER of CAD$250,632/QALY gained. The authors’ use of phase I and registry data to derive clinical inputs, comparatively low utility scores, and the setting (i.e., Canadian) limit its usefulness in terms of cross validation. For example, recent studies, including our own, demonstrate that utility values in the PF ALK inhibitor-treated setting are in the 0.8 range whereas these authors used values between 0.54 and 0.62. The differential utility scores for crizotinib vs. platinum doublet were also relatively low (~ 0.03) and contrast trial findings that show significant improvements from using ALK inhibitors compared with chemotherapy [17, 38].
In the original submission to the National Institute for Health and Care Excellence, crizotinib was denied as it was deemed clinically effective compared with a pemetrexed platinum agent combination but not cost effective [48]. In the company’s model, crizotinib was assessed using an ‘area under the curve’ analysis with the same three health states [49]. Upon revision to the price discount patient access scheme and re-assessment of the model parameters, crizotinib was deemed to provide sufficient value and given approval [48]. In comparing crizotinib to pemetrexed, the final report estimated an ICER of £47,291/QALY gained [49]. As the difference in LYs and QALYs between the treatment options were not reported, we could not make direct comparisons to our results. Finally, we previously assessed alectinib in patients receiving prior crizotinib treatment and found alectinib to be potentially cost effective vs. ceritinib [50].
There are several limitations to note in this study. There are limited data on non-drug resource use during the ALEX trial in both the PF and PP health states. To address this limitation, we used estimates that mirrored actual practice as they were from an ALK-positive-specific costing study based on chart review and claims data; the study also differentiated patient cost by CNS metastases status over time. Additionally, incomplete follow-up from the trial data required extrapolation, which injects additional uncertainty about long-term outcomes. This is particularly true in the case of OS, as there were a limited number of events at the time of this data cut. Compared with the recently updated PROFILE 1014 trial, the proportion alive at 4 years for crizotinib users is lower in our extrapolations (44 vs. 57%) [51]. This could be because of differences in PP treatments or patient population characteristics. We did, however, follow best practices for extrapolating survival data using the best model fits based on statistical and visual inspections as well as clinically plausibility. The later judgment was specifically informed by clinician feedback in support of our choice of parametric assumption. Finally, we did not include adverse event costs in our model as none of them occurred in greater than 5.0% of patients in either treatment arm and therefore would be unlikely to have a substantial impact on our results.
5 Conclusion
We estimated that treatment with alectinib in treatment-naïve patients with ALK-positive NSCLC increased time in the PF health state, increased LY, and increased QALYs vs. crizotinib. The marginal increase in costs was driven by longer treatment durations with alectinib and offset by the increased cost of CNS metastasis for patients receiving crizotinib. This model suggests that alectinib may be considered a cost-effective treatment vs. crizotinib according to commonly used thresholds in USA (i.e., < US$100,000–US$150,000/QALY) [42].
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30.
Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med. 2008;359:1367–80.
National Cancer Institute. SEER cancer statistics factsheets: lung and bronchus cancer. 2015. http://seer.cancer.gov/statfacts/html/lungb.html. Accessed 15 Aug 2017.
Reck M, Paz-Ares L. Immunologic checkpoint blockade in lung cancer. Semin Oncol. 2015;42:402–17.
Gridelli C, de Marinis F, Cappuzzo F, Di Maio M, Hirsch FR. Treatment of advanced non-small-cell lung cancer with epidermal growth factor receptor (EGFR) mutation or ALK gene rearrangement: results of an international expert panel meeting of the Italian Association of Thoracic Oncology. Clin Lung Cancer. 2014;15:173–81.
Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT. Gefitinib or carboplatin–paclitaxel in pulmonary adenocarinoma. N Engl J Med. 2009;361:947–57.
Zhou CC, Wu YL, Chen GY, Feng J, Liu XQ. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–42.
Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–46.
Wu YL, Zhou C, Hu CP, Feng J, Lu S. Afatinib versus cisplatin plus gemcitabine for firstline treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:213–22.
Fukuoka M, Yano S, Giaccone G, Tamura T, Nakagawa K, Douillard J. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (the IDEAL 1 trial). J Clin Oncol. 2003;21:2237–46.
Kris M, Natale R, Herbst R, Lynch T, Prager D, Belani C. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. JAMA. 2003;290:2149–58.
Herbst R, Prager D, Hermann R, Fehrenbacher L, Johnson B, Sandler A, et al. TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol. 2005;23:5892–9.
Gatzemeier U, Pluzanska A, Szczesna A, Kaukel E, Roubec J, De Rosa F. Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small-cell lung cancer: the Tarceva Lung Cancer Investigation Trial. J Clin Oncol. 2007;25:1545–52.
Leighl NB, Rekhtman N, Biermann WA, Huang J, Mino-Kenudson M. Molecular testing for selection of patients with lung cancer for epidermal growth factor receptor and anaplastic lymphoma kinase tyrosine kinase inhibitors: American Society of Clinical Oncology endorsement of the College of American Pathologists/International Association for the study of lung cancer/association for molecular pathology guideline. J Clin Oncol. 2014;32:3673–9.
Network National Comprehensive Cancer. NCCN clinical practice guidelines in oncology non-small cell lung cancer. Fort Washington: NCCN, Inc.; 2017.
Lindeman NI, Cagle PT, Beasly MB, Chitale DA, Dacic S. Molecular testing guideline for selection of lung cancer patients with EGFR and ALK tyrosine kinase inhibitors. J Thorac Oncol. 2013;8:823–59.
Solomon B, Mok T, Kim D, Wu YL, Nakagawa K, Mekhail T, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371(23):2167–77.
Doebele RC, Pilling AB, Aisner DL, Kutateladze TG. Mechanisms of resistance to crizotinib in patients with ALK gene rearranged non-small cell lung cancer. Clin Cancer Res. 2012;18:1472–82.
Katayama R, Friboulet L, Koike S, Lockerman EL, Khan TM, Gainor JF, et al. Two novel ALK mutations mediate acquired resistance to the next-generation ALK inhibitor alectinib. Clin Cancer Res. 2014;20:5686–96.
Shaw AT, Gandhi L, Gadgeel S, Riely GJ, Cetnar J, West H, et al. Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: a single-group, multicentre, phase 2 trial. Lancet Oncol. 2016;17(2):234–42.
Ou SI, Ahn JS, De Petris L, Govindan R, Yang JC. Alectinib in crizotinib-refractory ALK-rearranged non-small-cell lung cancer: a phase II global study. J Clin Oncol. 2016;34:661–8.
Seto T, Kiura K, Nishio M, Nakagawa K, Maemondo M. CH5424802 (RO5424802) for patients with ALK-rearranged advanced non-small-cell lung cancer (AF-001JP study): a single-arm, open-label, phase 1–2 study. Lancet Oncol. 2013;14:590–8.
Hida T, Nokihara H, Kondo M, Kim YH, Azuma K. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet. 2017;390:29–39.
Peters S, Camidge DR, Shaw AT, Gadgeel S, Ahn JS, Kim D-W, et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med. 2017;377(9):829–38.
Kodama T, Hasegawa M, Takanashi K, Sakurai Y, Kondoh O. Antitumor activity of the selective ALK inhibitor alectinib in models of intracranial metastases. Cancer Chemother Pharmacol. 2014;74:1023–8.
Costa DB, Kobayashi S, Pandya SS, Yeo WL, Shen Z. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol. 2011;29:e443–5.
Enriqueta Felip, Dong-Wan Kim, Ranee Mehra, Daniel S.W. Tan, Laura Q.M. Chow, D. Ross Camidge, et al. Efficacy and safety of ceritinib in patients with advanced anaplastic lymphoma kinase (ALK)-rearranged (ALK+) non-small cell lung cancer (NSCLC): an update of ASCEND-1. European Society of Medical Oncology Congress, 26 Sep 2014, Madrid.
Ganz PA. Improving the quality and value of cancer care: a work in progress. The 2016 Joseph V. Simone award and lecture. J Oncol Pract. 2016;12:876–9.
Young RC. Value-based cancer care. N Engl J Med. 2015;373:2593–5.
Albaba H, Lim C, Leighl N. Economic considerations in the use of novel targeted therapies for lung cancer: review of current literature. Pharmacoeconomics. 2017;35(12):1195–209.
Neumann PJ, Cohen JT. Measuring the value of prescription drugs. N Engl J Med. 2015;373:2595–7.
McKay C, Burke T, Cao X, Abernethy AP, Carbone DP. Treatment patterns for advanced non-small-cell lung cancer after platinum-containing therapy in US community oncology clinical practice. Clin Lung Cancer. 2016;17(5):449–60.
Genentech. Data on file. 2017.
Nafees B, Stafford M, Gavriel S, Bhalla S, Watkins J. Health state utilities for non small cell lung cancer. Health Qual Life Outcomes. 2008;6:84.
First DataBank Inc. Analy$ource Online: the online resource for drug pricing and deal information. 2017. https://www.analysource.com. Accessed 10 Aug 2017.
Curtiss F, Lettrich P, Fairman K. What is the price benchmark to replace average wholesale price (AWP)? J Manag Care Pharm. 2010;16:492–501.
Shaw A, Kim D, Mehra R, Tan D, Felip E, Chow LQ, et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med. 2014;370(13):1189–97.
Shaw A, Kim D, Nakagawa K, Seto T, Crino L, Ahn MJ, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368(25):2385–94.
Guerin A, Sasane M, Wakelee H, Zhang J, Culver K, Dea K, et al. Treatment, overall survival, and costs in patients with ALK-positive non-small-cell lung cancer after crizotinib monotherapy. Curr Med Res Opin. 2015;31(8):1587–97.
Briggs AH, Claxton K, Schulpher MJ. Decision modelling for health economic evaluation. Oxford: Oxford University Press; 2006.
Vemer P, Corro Ramos I, van Voorn G, Al M, Feenstra T. AdViSHE: a validation-assessment tool of health-economic models for decision makers and model users. Pharmacoeconomics. 2016;34(4):349–61.
Neumann P, Cohen J, Weinstein M. Updating cost-effectiveness: the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371:796–7.
Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448(7153):561–6.
Shaw AT, Friboulet L, Leshchiner I, Gainor JF, Bergqvist S, Brooun A, et al. Resensitization to crizotinib by the lorlatinib ALK resistance mutation L1198F. N Engl J Med. 2016;374(1):54–61.
Rangachari D, Yamaguchi N, VanderLaan PA, Folch E, Mahadevan A. Brain metastases in patients with EGFR-mutated or ALK-rearranged non-small-cell lung cancers. Lung Cancer. 2015;88:108–11.
Costa DB, Shaw AT, Ou SH, Solomon BJ, Riely GJ. Clinical experience with crizotinib in patients with advanced ALK-rearranged non-small-cell lung cancer and brain metastases. J Clin Oncol. 2015;33:1881–8.
Djalalov S, Beca J, Hoch JS, Krahn M, Tsao M-S. Cost effectiveness of EML4-ALK fusion testing and first-line crizotinib treatment for patients with advanced ALK-postive non-small-cell lung cancer. J Clin Oncol. 2014;32:1012–9.
Morgan P, Woolacott N, Biswas M, Mebrahtu T, Harden M. Crizotinib for untreated anaplastic lymphoma kinase-positive non-small-cell lung cancer: an evidence review group perspective of a NICE single technology appraisal. Pharmacoeconomics. 2017;35(9):909–19.
National Institute for Health and Care Excellence. Crizotinib for untreated anaplastic lymphoma kinase-positive advanced non-small-cell lung cancer. 2016. http://www.nice.org.uk/guidance/ta406. Accessed 15 Aug 2017.
Carlson J, Canestaro W, Ravelo A, Wong W. The cost-effectiveness of alectinib in anaplastic lymphoma kinase-positive (ALK+) advanced NSCLC previously treated with crizotinib. J Med Econ. 2017;20(7):671–7.
Mok T, Kim D, Wu Y, Nakagawa K, Mekhail T, Felip E, et al. Overall survival (OS) for first-line crizotinib versus chemotherapy in ALK+ lung cancer: updated results from PROFILE 1014. Ann Oncol. 2017;28(Suppl. 5):v605–49.
Author information
Authors and Affiliations
Contributions
All authors contributed in designing the study. JC and KS wrote the first draft of the manuscript. PO and WW provided input and revisions to the draft. All authors reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Funding
This work was funded by Genentech, Inc.
Conflict of interest
Josh J. Carlson has been a consultant for Genentech, Pfizer, and Seattle Genetics. Kangho Suh has been a consultant for Genentech and Pfizer. William Wong is an employee of Genentech. Panos Orfanos is an employee of Roche, the principal company of Genentech.
Data availability
The underlying data are not available as they are considered proprietary.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Carlson, J.J., Suh, K., Orfanos, P. et al. Cost Effectiveness of Alectinib vs. Crizotinib in First-Line Anaplastic Lymphoma Kinase-Positive Advanced Non-Small-Cell Lung Cancer. PharmacoEconomics 36, 495–504 (2018). https://doi.org/10.1007/s40273-018-0625-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-018-0625-6