Abstract
Introduction
Long-acting injectable (LAI) antipsychotic use may reduce healthcare resource utilization compared with oral antipsychotic use by improving adherence and reducing dosing frequency. Our goal was to examine treatment patterns, healthcare utilization, and costs among recently diagnosed schizophrenia patients receiving oral versus LAI antipsychotics.
Methods
The MarketScan Multi-state Medicaid database was used to identify schizophrenia patients aged ≥ 18 years who received an LAI or oral antipsychotic between January 1, 2011 and December 31, 2014. Primary outcomes included treatment patterns such as adherence (measured as proportion of days covered-PDC), persistence, discontinuation, switching, and healthcare resource utilization and costs. Propensity score matching (PSM) was used to control for differences in baseline characteristics between the cohorts. Outcomes were assessed over a 12-month post-index period and compared between treatment cohorts.
Results
After PSM, 2302 patients were included in each of the LAI and oral antipsychotics cohorts. There were no differences in PDC or therapy switching between the two cohorts. Compared with the oral cohort, patients receiving LAIs had lower discontinuation rates (46.1 vs. 61.6%, p < 0.001), fewer inpatient admissions (0.5 vs. 0.9, p < 0.001), hospital days (3.9 vs. 6.5, p < 0.001), and ER visits (2.4 vs. 2.9, p = 0.007), and a higher number of prescription fills (29.5 vs. 25.3, p < 0.001). Patients prescribed LAIs had lower monthly inpatient ($US4007 vs. 8769, p < 0.001) and ER visits costs ($682 vs. 891, p < 0.001) but higher monthly medication costs ($10,713 vs. $655, p < 0.001) compared with the oral cohort over the 12-month post-index period. Overall, both cohorts had similar total medical costs (LAI vs. oral: $24,988 vs. 23,887, p = 0.354) during the follow-up period.
Conclusion
Patients receiving LAIs were more likely to remain on medication compared with the oral group, which may account for reduced inpatient admissions. Hospitalization cost reductions offset the higher costs of LAI medications, resulting in no increase in total healthcare costs relative to oral antipsychotic use.
Funding
Alkermes Inc.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Schizophrenia, a chronic and debilitating mental health disorder, affects approximately 1% of the adult US population [1, 2]. The socioeconomic burden of schizophrenia is substantial, resulting from impairment in patient physical functioning and quality of life, impact on caregivers and family members, and increased healthcare resource utilization and costs [3,4,5]. The annual economic burden of schizophrenia in the US was estimated at US$155.7 billion in 2013; a diagnosis of schizophrenia was associated with an additional annual cost burden of $44,773 compared with patients without schizophrenia diagnosis [5]. While direct medical costs accounted for 25%, indirect costs accounted for 75% of the incremental costs associated with schizophrenia [5].
Antipsychotics have been the mainstay of acute and maintenance phases of schizophrenia treatment, and have been proven to reduce the number of recurrent psychotic episodes among schizophrenia patients [6, 7]. However, approximately 40–60% of schizophrenia patients are partially or totally non-adherent to oral antipsychotics, affecting successful management of the disease [8]. Physicians often try to manage non-adherence to a specific antipsychotic by adding adjunctive medications, increasing dosage of existing medications, or switching to a different antipsychotic [9]. Such measures can serve to further compound medication burden without addressing the problem of non-adherence. Non-adherence to antipsychotic medications is significantly associated with higher rehospitalization and relapse rates along with increased rates of incarceration and violent activities among schizophrenia patients [10,11,12,13]. In addition, non-adherence has also been associated with increased healthcare costs [14, 15]. A systematic review reported the national rehospitalization costs associated with antipsychotic non-adherence to be approximately $1.5 billion among Medicaid patients in 2005 [16].
Long-acting injectable (LAI) formulations of antipsychotic agents were developed as alternatives to the oral antipsychotics. Many patients with schizophrenia have difficulty with ongoing adherence to oral regimens, and may benefit by having therapeutic plasma concentrations of antipsychotics delivered as part of a scheduled visit, with injection intervals ranging from every 2 weeks to every 3 months [17, 18]. Several real-world studies have documented better adherence and reduced hospitalization with LAI treatment [19,20,21,22,23]. Despite the evidence recommending their use, LAIs remain underutilized in clinical practice [24, 25]. A potential reason is their high acquisition cost, especially for atypical LAIs, which has been cited as one of the most common reasons for restricting LAI prescriptions [26]. However, few studies have assessed whether the high prescription costs of LAIs are offset by the reduction in hospitalization and ER visits costs associated with LAI use. Furthermore, past studies have focused on comparing oral antipsychotics to conventional and atypical LAIs such as risperidone and paliperidone palmitate. Very few studies have incorporated atypical LAI agents, such as once-monthly aripiprazole. A comprehensive understanding of treatment patterns and healthcare costs associated with the use of various treatment modalities for patients with schizophrenia in real-world settings may help managed care organizations, behavioral health organizations, and state payers better address the disease burden associated with schizophrenia. The objective of the study was to provide real-world evidence regarding treatment patterns and healthcare utilization and costs among recently diagnosed schizophrenia patients receiving oral versus LAI antipsychotics (including newer atypical agents).
Methods
Data Source
This real-world observational study was conducted using Truven MarketScan® Medicaid administrative claims data from January 1, 2010 through December 31, 2015. The database contains the pooled healthcare experiences of > 44 million Medicaid enrollees from multiple states in the US, including inpatient visits, outpatient services, prescription drug claims, long-term care, and other medical care [27]. Institutional Review Board approval was not required for this study because analyses were conducted using de-identified administrative claims data. This article is based on retrospective administrative claims data and does not contain any studies with human participants or animals performed by any of the authors.
Patient Selection
Patients with a medical claim for a schizophrenia diagnosis [International Classification of Disease, 9th Revision, Clinical Modification (ICD-9-CM) code 295.xx] were identified between January 1, 2011 and December 31, 2014. The date of the first observed schizophrenia diagnosis during this period was identified as the initial diagnosis date. Patients were included if they had ≥ 2 claims for LAI or oral antipsychotics on or after the initial diagnosis date through December 31, 2014. If patients had ≥ 2 LAI claims after the initial diagnosis date, they were classified in the LAI cohort; patients with only oral antipsychotic medications claims and no LAI claim after the initial diagnosis date were classified in the oral antipsychotic cohort. Our selection criteria were based on the fact that many patients on LAIs also get orals and the usual approach is hierarchical [28]. The date of the first antipsychotic medication claim was defined as the index date.
Patients were required to be at least 18 years old as of the index date, with continuous medical and pharmacy benefits as well as mental health/substance abuse coverage for at least 12 months prior to the index date (baseline period) and 12 months after the index date (follow-up period). Patients were excluded from the study if they had at least one schizophrenia diagnosis claim at any time prior to, or at least one LAI prescription claim during, the 12-month baseline period. This selection criterion was imposed to ensure that the first occurrence of a schizophrenia diagnosis was the initial diagnosis date. Patients with dual coverage eligibility with Medicare, or at least one prescription claim for clozapine during the study period, were also excluded.
The LAI cohort included both conventional and atypical LAI antipsychotics. Conventional LAI antipsychotics included fluphenazine decanoate and haloperidol decanoate. Atypical LAI antipsychotics included risperidone, once-monthly paliperidone palmitate, olanzapine pamoate and aripiprazole; however, 3-month paliperidone palmitate and all dose forms of aripiprazole lauroxil were not included in the study because they were not approved by the US Food and Drug Administration prior to December 31, 2014, the cut-off date for the analyses. Similarly, the oral antipsychotics cohort included both conventional and atypical antipsychotics. LAIs were identified using National Drug Codes (NDCs) in the pharmacy claims database and by Healthcare Common Procedure Coding System codes in the medical claims database. Oral antipsychotics were identified using NDCs only.
Study Variables
Baseline patient demographics included age, gender, race, and health insurance plan type and were assessed for all patients. Comorbidity indices,including Charlson Comorbidity Index (CCI) score [29, 30], Elixhauser index score [31], and chronic disease score (CDS) [32], were evaluated. Catatonic and paranoid-type schizoaffective disorder and other schizophrenia subtypes during the baseline period were examined. Baseline mental health-related comorbidities of interest including bipolar disorder, depression disorder, attention deficit hyperactivity disorder (ADHD), panic disorder, post-traumatic stress disorder (PTSD), personality disorder, anxiety, suicide attempt and intentional injuries, and substance/alcohol abuse were also assessed. Other comorbid conditions included cardiovascular disease, diabetes, and obesity. All comorbid conditions were identified using ICD-9-CM codes.
Treatment patterns during the 12-month follow-up period were assessed and compared between the LAI and oral antipsychotic cohorts. Treatment patterns were measured in terms of the proportion of days covered (PDC), discontinuation, switching and persistence (in days). Inpatient days were excluded while estimating treatment patterns, as medication received during an inpatient stay is not visible in administrative claims data.
PDC was defined as the ratio of the number of days “covered” by the index drug prescription claim divided by the total number of days during the follow-up period. Patients with PDC ≥ 0.8 were considered adherent.
Discontinuation was defined as having no additional prescription claims of the index drug for a defined gap period. Given that the days supply field is often not recorded or of uncertain accuracy for LAI antipsychotics in the medical or pharmacy claims, we used the dosing recommendation guidelines indicated in the product prescribing information and the following approach to obtain LAI discontinuation gap [33, 34]: for risperidone LAI, a gap of > 29 days (14 days for the previous injection + 15 days allowable gap period) between subsequent injections [35]; for aripiprazole LAI, olanzapine pamoate LAI, and conventional LAIs, a gap of > 45 days (30 days for the previous injection + 15 days allowable gap period) between subsequent injections [36,37,38,39]; and for once-monthly paliperidone palmitate LAI, a gap of > 12 days (7 days for the first injection + 5 days allowable gap period) between the first and second injections and a gap of > 45 days (30 days for the previous injection + 15 days allowable gap period) during the maintenance phase (injection 3 onwards) [40]. The duration of oral antipsychotics was defined based on the days of supply indicated on the prescription claim. To maintain consistency and minimize bias, a 15-day allowable gap period was used when calculating the discontinuation gap among patients receiving an oral antipsychotic. Sensitivity analyses with fixed lengths of allowable gap were also conducted, where patients were considered discontinued if there was no index drug prescription claim for at least 30, 45, or 60 days after the run-out date of the most recent index drug prescription claim.
Patients were identified as switchers if they had a claim of a non-index antipsychotic treatment during the allowable gap period before discontinuation. Sensitivity analyses were conducted among switchers using 30, 45, or 60 days allowable gap period. Discontinuation and switching measures were mutually exclusive. Persistence was defined as the number of days until discontinuation.
Annual all-cause and schizophrenia-related healthcare utilization were assessed during the baseline and follow-up period, including inpatient admissions, length of inpatient stay, emergency room (ER) visits, prescription fills, and outpatient visits [physician’s office visits and other outpatient visits (e.g., hospital outpatient departments, rural health clinics, renal dialysis facilities, outpatient rehabilitation facilities, comprehensive outpatient rehabilitation facilities, and community mental health centers)]. Time to all-cause and schizophrenia-related inpatient admission and ER visits were also assessed. A medical claim was considered schizophrenia-related if it was associated with a diagnosis of schizophrenia in the primary position. Annual all-cause and schizophrenia-related healthcare costs during the follow-up period were also examined. All healthcare costs were adjusted to 2015 US dollars using the medical care component of the Consumer Price Index [41].
Statistical Methods
All study variables, including baseline and outcome measures, were analyzed descriptively and compared between the LAI and oral antipsychotics cohorts. Propensity score matching (PSM) was performed to control for observed differences between the LAI and oral antipsychotic cohorts. An unconditional logistic regression was fitted to determine the baseline characteristics associated with being treated with LAIs or oral antipsychotics. Using this model, a propensity score was developed for each patient which characterized the probability of being a member of the LAI cohort. Covariates in the propensity model included baseline demographic and clinical characteristics, such as age, sex, race, baseline schizophrenia subtype, health plan type, CCI score, CDS, Elixhauser index score, baseline comorbidities, and baseline schizophrenia-related and non-schizophrenia-related healthcare utilization and costs. Each patient in the LAI cohort was matched to a patient in the oral antipsychotics cohort with the closest propensity score (8-to-3 decimal digit—see supplementary file with the distribution of the propensity scores before and after PSM).
Mean and standard deviations were reported for continuous variables. Numbers and percentages were computed for categorical variables. Student’s t tests and Pearson’s Chi squared tests were used to test statistical significance for continuous and categorical variables, respectively, between the cohorts. Wilcoxon rank-sum tests were used to compare the follow-up healthcare utilization and costs. Standardized differences (SDs), defined as the absolute difference in sample means divided by an estimate of the pooled SD of each variable, were provided in addition to p values. The SDs help measure the effect size and are independent of sample size. To allow for easy interpretation, SDs were reported as 100 times the absolute value of the actual SDs. Any SD greater than 10 was considered significant [42, 43]. Kaplan–Meier curves were generated, and log-rank tests were used to compare the time-to-events outcomes, including time-to-discontinuation, switch, and all-cause and schizophrenia-related hospitalizations and ER visits. A Cox proportional hazards model was applied to estimate the hazard ratio (HR) and 95% confidence interval (CI) of all time-to-events outcomes.
Statistical analyses were performed using Statistical Analysis System (SAS) v.9.3 (Cary, NC, USA). The p value level of significance was set at an α-level of 0.05.
Results
Baseline Characteristics
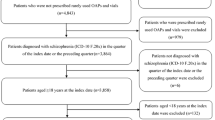
A total of 249,499 patients diagnosed with schizophrenia were identified from the Truven Medicaid database. The final analytic sample comprised a total of 22,490 eligible patients, including 2896 (12.9%) in the LAI antipsychotics cohort and 19,594 (87.1%) in the oral antipsychotics cohort (Fig. 1).
Prior to PSM, patients in the LAI cohort were younger (36.8 vs. 41.2 years, p < 0.001) and had a higher proportion of male patients (55.8 vs. 43.4%, p < 0.001) relative to the oral cohort. Additionally, the cohorts differed based on race (black patients: LAI vs. oral, 55.2 vs. 42.4%, p < 0.001) and type of health plan enrollment (comprehensive coverage: LAI vs. oral, 71.8 vs. 68.3%, p < 0.001). During the baseline period, LAI patients had fewer comorbidities demonstrated by lower mean CCI (1.7 vs. 2.5, p < 0.001) and CDS (4.4 vs. 5.1, p < 0.001) scores; and had higher rates of schizoaffective disorder (69.7 vs. 53.1%, p < 0.001) compared to those receiving oral antipsychotics. The most prevalent mental health-related comorbidities for both cohorts included major depressive disorder, substance and alcohol abuse, bipolar disorder, and anxiety. The LAI cohort had higher substance and alcohol abuse rates; however, the LAI cohort had lower rates of the aforementioned mental comorbidities than patients in the oral cohort. Other comorbid conditions including cardiovascular disease, diabetes, and obesity rates were lower among those receiving LAIs compared with those receiving oral antipsychotics. Total healthcare resource utilization and costs were slightly lower among LAI patients than those receiving oral antipsychotics (Table 1).
After conducting 1:1 PSM, most baseline characteristics were well balanced between the study cohorts, with 2302 patients in each cohort (Table 1).
Differences between the two matched cohorts in the follow-up period are presented below.
Treatment Patterns
PDC was similar for patients receiving LAIs and oral antipsychotics (0.59 vs. 0.57, p = 0.067) in the 12-month follow-up period. Similarly, 36.5% of those receiving LAIs and 34.1% of those on oral antipsychotics were adherent using PDC ≥ 0.8 criteria (p = 0.096). However, a significantly higher proportion of patients receiving oral antipsychotics discontinued treatment relative to the patients receiving LAIs (61.6 vs. 46.1%, p < 0.001; Table 2) in the 12-month follow-up period. Sensitivity analyses using 30, 45, and 60 days of allowable treatment gaps for discontinuation showed similar trends (Table 2). The proportion of schizophrenia patients who switched index therapy was also similar for the cohorts (9.0 vs. 9.0%, p = 1.000). However, sensitivity analyses using 45 and 60 days of allowable treatment gap indicated that LAI patients were significantly less likely to switch their index medication compared to those on oral antipsychotics (45-day gap: 5.3 vs. 6.9%, p = 0.023 and 60-day gap: 4.4 vs. 6.3%, p = 0.004). Persistence (mean number of days) for the LAI cohort was similar to the oral antipsychotic cohort (140.6 vs. 135.6 days, p = 0.177; Table 2). Kaplan–Meier curves (log-rank p < 0.001; HR = 1.55; 95% CI = 1.43–1.68; Fig. 2) indicated that patients on oral antipsychotics had significantly shorter time to discontinuation compared to LAI patients. No significant difference was observed in time to switch (log-rank p = 0.836; HR = 0.98; 95% CI = 0.81–1.19; Fig. 2).
Healthcare Utilization
Healthcare utilization is summarized in Table 3. Fewer LAI patients had all-cause inpatient admissions (25.6 vs. 36.9%, p < 0.001) and ER visits (56.2 vs. 61.9%, p < 0.001) compared to patients receiving oral antipsychotics. On average, LAI patients had fewer all-cause inpatient admissions (0.5 vs. 0.9, p < 0.001), hospital days (3.9 vs. 6.5, p < 0.001), and ER visits (2.4 vs. 2.9, p = 0.007) per patient than those receiving oral antipsychotics. Similar trends were observed in schizophrenia-related inpatient admissions (LAI vs. oral: 0.3 vs. 0.4, p < 0.001) and hospital days (2.3 vs. 3.1, p = 0.004). There was no difference in the number of schizophrenia-related ER visits between the two cohorts. Kaplan–Meier curves (log-rank p < 0.001; HR = 1.34; 95% CI = 1.20–1.49; Fig. 3) indicated that patients on oral antipsychotics had significantly shorter time to all-cause (mean number of days: 294.8 vs. 309.8 days) inpatient admissions relative to LAI patients. The average time to schizophrenia-related inpatient admissions was 331.8 days for patients on oral antipsychotics compared to 336.0 days for LAI patients (log-rank p = 0.015; HR = 1.21; 95% CI = 1.04–1.41; Fig. 3). Furthermore, patients in the oral antipsychotics cohort had shorter time to all-cause ER visits (mean number of days: 208.0 vs. 222.5 days) compared to LAI patients (log-rank p < 0.001; HR = 1.15; 95% CI = 1.07–1.24; Fig. 4). No significant difference was observed in time to schizophrenia-related ER visit.
There was no significant difference in all-cause outpatient office visits per patient between the two cohorts (6.1 vs. 6.2, p = 0.719; Table 3); however, LAI patients had a higher number of schizophrenia-related outpatient office visits compared to patients on oral antipsychotics (1.8 vs. 1.2, p < 0.001). Finally, patients receiving LAIs had more prescription fills (29.5 vs. 25.3, p < 0.001) compared with those receiving oral antipsychotics.
Healthcare Costs
A summary of healthcare costs for both cohorts is presented in Table 3. Annual costs of inpatient admissions were substantially lower in the LAI cohort compared with the oral antipsychotics cohort ($4007 vs. 8769; p < 0.001). Costs of schizophrenia-related inpatient admissions ($1802 vs. 2410; p = 0.038) were also lower among LAI patients, as were costs for all-cause ER visits ($682 vs. 891; p < 0.001). There was no significant difference in costs of schizophrenia-related ER visits ($83 vs. 89; p = 0.544) between the two cohorts.
Annual costs for all-cause outpatient office visits (LAI vs. oral: $410 vs. 421; p = 0.436) and other outpatient costs ($9178 vs. 9152; p = 0.965) were similar between the cohorts; however, LAI patients incurred higher schizophrenia-related outpatient office visit costs ($115 vs. 86; p < 0.001) and schizophrenia-related other outpatient costs ($2996 vs. 2071; p < 0.001) compared to patients on oral antipsychotics. Annual pharmacy costs were significantly higher among LAI patients ($10,713 vs. 4655; p < 0.001).
Overall, the average annual total healthcare costs ($24,988 vs. 23,887; p = 0.354) were similar between LAI patients and oral antipsychotics patients. The higher pharmacy costs in the LAI cohort were offset by lower inpatient admission and ER visit costs. LAI patients incurred similar schizophrenia-related healthcare costs ($4995 vs. 4656; p = 0.326) compared to oral antipsychotic patients as well. After converting the annual costs to monthly incremental cost differences, patients receiving LAIs incurred higher monthly medication costs ($505, p < 0.001) but lower monthly inpatient ($397, p < 0.001) and ER visit costs ($17, p < 0.001). Outpatient office visit, other outpatient, and total monthly costs were similar between the LAI and oral antipsychotic cohorts (Fig. 5).
Discussion
This real-world retrospective study of Medicaid claims data compared treatment patterns, healthcare resource utilization, and costs among recently diagnosed schizophrenia patients treated with LAIs versus oral antipsychotics. Overall, 13% of recently diagnosed schizophrenia patients received LAIs. After matching patients in the two cohorts using PSM, LAI patients had lower discontinuation rates compared to oral antipsychotics patients during the follow-up period. In addition, LAI patients had lower healthcare resource utilization including hospital admissions and ER visits. Lower inpatient costs offset the higher LAI medication costs, thereby resulting in similar total healthcare costs for the LAI cohort relative to the oral cohort over a 12-month follow-up period post-treatment initiation.
These findings are consistent with previous studies that analyzed treatment patterns and healthcare resource utilization and costs among patients receiving LAI and oral antipsychotics. Authors of prior research studies have reported poor adherence and high medication discontinuation rates among schizophrenia patients on oral antipsychotics compared to those on LAIs [23, 44, 45]. Moreover, relative to oral treatment, LAI antipsychotic use helps to delay non-adherence and maintain medication adherence during the length of coverage of patients who would likely otherwise discontinue their oral therapy during the therapeutic window [46]. Analyses of data from a six-state Medicaid database indicated that patients on atypical LAIs were more adherent (27.2 vs. 24.6%) and persistent (23.7 vs. 20.6%) to treatment compared to patients prescribed atypical oral antipsychotics [42]. Results from another recent study by Greene et al. suggest that schizophrenia patients who began receiving LAIs had a 5% higher adjusted mean adherence and were 20% less likely to discontinue their therapy compared with patients who switched to a different oral antipsychotic monotherapy [43].
Studies in the past have demonstrated that adherence and discontinuation of antipsychotic treatment is highly associated with healthcare resource utilization among schizophrenia patients [14,15,16, 47]. Hence, patients prescribed LAIs incurred lower healthcare utilization and costs, especially inpatient admissions and ER visits, compared to oral antipsychotics [19,20,21,22,23, 48]. Taipale et al. reported recently that, among prevalent and first-episode schizophrenic patients with up to 20 years of follow-up; LAIs were associated with lower risk of psychiatric and all-cause hospitalization than oral antipsychotics [49]. In addition, data from a single payer country showed dramatic differences in LAIs, where authors observed higher rates of prevention of relapse in schizophrenia and approximately 20–30% lower risk of rehospitalization associated with LAI treatment compared with equivalent oral antipsychotic formulations [50]. Another study based on hospital records data showed atypical LAI use was associated with a significantly lower number of rehospitalizations (1.25 vs. 1.61) and ER visits (2.33 vs. 2.67) compared with oral antipsychotics [21]. Consistent with the previous literature, discontinuation rates, inpatient admissions, and ER visits were significantly higher among patients who received oral antipsychotics compared with LAIs. However, contrary to previous studies, the current study showed similar medication adherence (measured by PDC) rates in the 1-year period following treatment initiation between LAI and oral antipsychotic patients. A possible explanation for these findings could be that this study examined recently diagnosed patients, as opposed to prevalent populations evaluated in other studies. Our study expands on existing literature by using PSM to account for observable bias which aids in reducing selection bias that may have been present in previous studies, where LAIs might have been more selectively prescribed to patients who had a history of non-adherence to oral antipsychotics. The current study methodology likely created a more homogenous sample of recently diagnosed schizophrenia patients as compared to previous studies.
Despite growing evidence and clinical recommendations advising the use of LAIs in the treatment of schizophrenia, they are still prescribed infrequently in clinical practice in the US [24]. The current analyses indicate that, among all patients prescribed antipsychotics in this population, only 13% received LAIs. This is consistent with previous real-world studies in which patients initiating LAIs ranged between 10% and 20% [19,20,21,22,23, 42]. Barriers to LAI use include psychiatrists’ belief that sufficient compliance is achieved with oral antipsychotics, as well as higher treatment costs for LAIs versus oral treatment [26]. Among patients with schizophrenia, relapses—especially inpatient admissions—are a major healthcare cost driver, and hence it is extremely important to reduce the rate of relapse in this population. The present study demonstrated that LAI patients had reduced inpatient admissions and that these reductions offset the higher cost of LAI medications.
The present study was subject to several limitations. Given the observational nature of the study, only associations without causal linkage can be inferred. Variables such as physician or patient preference, aversion to LAIs versus oral antipsychotics, clinical markers of disease severity, and patient-reported outcomes, were not captured in the database and therefore could not be measured and included in our analyses. These variables might be responsible for selection bias and consequently have substantial impact on the outcomes. In addition, schizophrenia diagnoses were identified using healthcare claims. Healthcare claims are subject to coding errors and there may be incorrectly entered diagnoses which were coded for administrative processing rather than clinical completeness; hence, some medical information may be unavailable or inaccurate. Also, schizophrenia-related healthcare resource utilization and costs may have been underestimated by capturing claims with schizophrenia diagnosis claims only in the primary position. Furthermore, the presence of a claim for a filled prescription does not indicate whether the medication was taken as prescribed or actually consumed, and therefore retrospective database studies by design might not provide an accurate representation of medication persistence and/or adherence in clinical practice. This is more applicable to oral antipsychotics than LAIs. The extent to which adherence to antipsychotics had an impact on the study outcomes were not analyzed in the current study, and future studies to assess the impact of compliance to antipsychotics on the study outcomes are warranted. Healthcare claims contained in the Truven MarketScan® Multi-State Medicaid database are not individually identifiable according to state; therefore, our study results might not be generalizable to the entire US schizophrenia population. However, health insurance claims data offer an opportunity to understand the real-world prescribing patterns of antipsychotics for schizophrenia treatment using large samples of patients in real-world settings. Conventional LAI antipsychotics and atypical LAIs are “different treatments” in terms of their efficacy, safety, and their acquisition costs. Our study results may have been influenced by treating LAI antipsychotics as one cohort and not differentiating them based on the type of LAIs, conventional or atypical. However, approximately 70% of our LAI study sample consisted of atypical LAIs which could have affected the study findings by mitigating the price difference between conventional and atypical LAIs. Finally, this study did not analyze the impact of LAI use on indirect costs due to reduced relapse rates, which were estimated at $32.4 billion in the US [51]. Further studies analyzing the impact of antipsychotic treatment on indirect costs are warranted.
Conclusion
In conclusion, antipsychotic discontinuation was common among recently diagnosed schizophrenia patients, but the results of this study suggest that patients on oral antipsychotics were more likely to discontinue than patients on LAIs. Furthermore, schizophrenia patients treated with LAIs experienced fewer hospital admissions, ER visits, and hospital days than patients treated with oral medications. Inpatient cost reductions offset the higher LAI medication costs, thereby resulting in similar total healthcare costs for patients on LAIs versus those treated with oral antipsychotics. These findings may provide insights for healthcare providers and payers, and assist in the decision-making process for effective treatment options for the management of schizophrenia.
References
Tandon R, Nasrallah HA, Keshavan MS. Schizophrenia, “just the facts” 4. Clinical features and conceptualization. Schizophr Res. 2009;110(1–3):1–23.
Schizophrenia. National Institute of Mental Health Web site. http://www.nimh.nih.gov/health/statistics/prevalence/schizophrenia.shtml. Updated November 2017. Accessed Feb 22 2018.
Jin H, Mosweu I. The societal cost of schizophrenia: a systematic review. Pharmacoeconomics. 2017;35(1):25–42.
Millier A, Schmidt U, Angermeyer MC, et al. Humanistic burden in schizophrenia: a literature review. J Psychiatr Res. 2014;54:85–93.
Cloutier M, Aigbogun MS, Guerin A, et al. The economic burden of schizophrenia in the US in 2013. J Clin Psychiatry. 2016;77(6):764–71.
Wyatt RJ. Neuroleptics and the natural course of schizophrenia. Schizophr Bull. 1991;17(2):325–51.
Leucht S, Tardy M, Komossa K, et al. Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: a systematic review and meta-analysis. Lancet. 2012;379:2063–71.
Lacro JP, Dunn LB, Dolder CR, Leckband SG, Jeste DV. Prevalence of and risk factors for medication nonadherence in patients with schizophrenia: a comprehensive review of recent literature. J Clin Psychiatry. 2002;63:892–909.
Velligan DI, Wang M, Diamond P, et al. Relationships among subjective and objective measures of adherence to oral antipsychotic medications. Psychiatr Serv. 2007;58(9):1187–92.
Pinikahana J, Happell B, Taylor M, Keks NA. Exploring the complexity of compliance in schizophrenia. Issues Ment Health Nurs. 2002;23(5):513–28.
Cramer JA, Rosenheck R. Compliance with medication regimens for mental and physical disorders. Psychiatr Serv. 1998;49(2):196–201.
Sullivan G, Wells KB, Morgenstern H, Leake B. Identifying modifiable risk factors for rehospitalization: a case-control study of seriously mentally ill persons in Mississippi. Am J Psychiatry. 1995;152(12):1749–56.
Ascher-Svanum H, Faries DE, Zhu B, Ernst FR, Swartz MS, Swanson JW. Medication adherence and long-term functional outcomes in the treatment of schizophrenia in usual care. J Clin Psychiatry. 2006;67(3):453–60.
Offord S, Lin J, Mirski D, Wong B. Impact of early nonadherence to oral antipsychotics on clinical and economic outcomes among patients with schizophrenia. Adv Ther. 2013;30(3):286–97.
Dilla T, Ciudad A, Alvarez M. Systematic review of the economic aspects of nonadherence to antipsychotic medication in patients with schizophrenia. Patient Prefer Adher. 2013;7:275–84.
Sun SX, Liu GG, Christensen DB, Fu AZ. Review and analysis of hospitalization costs associated with antipsychotic nonadherence in the treatment of schizophrenia in the US. Curr Med Res Opin. 2007;23:2305–12.
Davis JM, Matalon L, Watanabe MD, Blake L, Metalon L. Depot antipsychotic drugs. Place in therapy. Drugs. 1994;47(5):741–73.
Johnson DA. Historical perspective on antipsychotic long-acting injections. Br J Psychiatry Suppl. 2009;52:S7–12.
Marcus SC, Zummo J, Pettit AR, Stoddard J, Doshi JA. Antipsychotic adherence and rehospitalization in schizophrenia patients receiving oral versus long-acting injectable antipsychotics following hospital discharge. J Manag Care Spec Pharm. 2015;21(9):754–69.
Lin J, Wong B, Offord S, Mirski D. Healthcare cost reductions associated with the use of LAI formulations of antipsychotic medications versus oral among patients with schizophrenia. J Behav Health Serv Res. 2013;40(3):355–66.
Lafeuille MH, Laliberté-Auger F, Lefebvre P, et al. Impact of atypical long-acting injectable versus oral antipsychotics on rehospitalization rates and emergency room visits among relapsed schizophrenia patients: a retrospective database analysis. BMC Psychiatry. 2013;13:221.
Offord S, Wong B, Mirski D, et al. Healthcare resource usage of schizophrenia patients initiating long-acting injectable antipsychotics vs. oral. J Med Econ. 2013;16:231–9.
Pilon D, Tandon N, Lafeuille MH, et al. Treatment patterns, healthcare resource utilization, and spending in medicaid beneficiaries initiating second-generation long-acting injectable agents versus oral atypical antipsychotics. Clin Ther. 2017;39(10):1972–85.
West JC, Marcus SC, Wilk J, et al. Use of depot antipsychotic medications for medication nonadherence in schizophrenia. Schizophr Bull. 2008;34(5):995–1001.
Heres S. Long-acting injectable antipsychotics: an underutilized treatment option. J Clin Psychiatry. 2014;75(11):1263–5.
Heres S, Hamann J, Kissling W, Leucht S. Attitudes of psychiatrists toward antipsychotic depot medication. J Clin Psychiatry. 2006;67(12):1948–53.
Hansen L. The Truven Health MarketScan Databases for life sciences researchers. Truven Health Analytics. 2017. https://truvenhealth.com/Portals/0/Assets/2017-MarketScan-Databases-Life-Sciences-Researchers-WP.pdf. Accessed Sept 19 2017.
Lehman AF, Lieberman JA, Dixon LB, et al. Practice guideline for the treatment of patients with schizophrenia. Psychiatry Online. 2004. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/schizophrenia.pdf. Accessed Feb 19 2018.
Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–9.
Charlson ME, Charlson RE, Peterson JC, et al. The Charlson comorbidity index is adapted to predict costs of chronic disease in primary care patients. J Clin Epidemiol. 2008;61(12):1234–40.
Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27.
Von Korff M, Wagner EH, Saunders K. A chronic disease score from automated pharmacy data. J Clin Epidemiol. 1992;45(2):197–203.
Joshi K, Pan X, Wang R, Yang E, Benson C. Healthcare resource utilization of second-generation long-acting injectable antipsychotics in schizophrenia: risperidone versus paliperidone palmitate. Curr Med Res Opin. 2016;32(11):1873–81.
Campagna EJ, Muser E, Parks J, Morrato EH. Methodological considerations in estimating adherence and persistence for a long-acting injectable medication. J Manag Care Spec Pharm. 2014;20(7):756–66.
Risperdal Consta (risperidone) long-acting injection [package insert]. Janssen. 2017. http://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/RISPERDAL+CONSTA-pi.pdf. Accessed Feb 15 2018.
Abilify Maintena (aripiprazole) for extended-release injectable suspension, for intramuscular use [package insert]. Otsuka Pharmaceuticals. 2017. https://www.otsuka-us.com/media/static/Abilify-M-PI.pdf?_ga=2.155269362.833124413.1518720548-834450404.1518720548. Accessed Feb 15 2018.
Zyprexa Relprevv (olanzapine) for extended-release injectable suspension [package insert]. Lilly. 2018. http://pi.lilly.com/us/zyprexa_relprevv.pdf. Accessed Feb 15 2018.
Fluphenazine Decanoate Injection, USP [package insert]. APP Pharmaceuticals, LLC. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/071413s019lbl.pdf. Accessed Apr 12 2018.
Haloperidol Injection, USP [package insert]. Pfizer Labs. https://www.pfizer.com/files/products/uspi_haloperidol.pdf. Accessed Apr 12 2018.
Invega Sustenna (paliperidone palmitate) extended-release injectable suspension, for intramuscular use [package insert]. Janssen. 2017. http://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/INVEGA+SUSTENNA-pi.pdf. Accessed Feb 15 2018.
Crawford M, Church J, Akin B. CPI detailed report data for December 2015. Bureau of Labor Statistics website. https://www.bls.gov/cpi/cpid1412.pdf. Published 2015. Accessed Feb 15 2018.
Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput. 2009;38(6):1228–34.
Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083–107.
Pilon D, Joshi K, Tandon N, Lafeuille MH, Kamstra RL, Emond B, Lefebvre P. Treatment patterns in Medicaid patients with schizophrenia initiated on a first-or second-generation long-acting injectable versus oral antipsychotic. Patient Prefer Adherence. 2017;11:619.
Greene M, Yan T, Chang E, et al. Medication adherence and discontinuation of long-acting injectable versus oral antipsychotics in patients with schizophrenia or bipolar disorder. J Med Econ. 2018;21(2):127–34.
Brissos S, Veguilla MR, Taylor D, Balanzá-Martinez V. The role of long-acting injectable antipsychotics in schizophrenia: a critical appraisal. Ther Adv Psychopharmacol. 2014;4(5):198–219.
Ascher-Svanum H, Zhu B, Faries DE, Lacro JP, Dolder CR, Peng X. Adherence and persistence to typical and atypical antipsychotics in the naturalistic treatment of patients with schizophrenia. Patient Prefer Adherence. 2008;2:67–77.
Baser O, Xie L, Pesa J, Durkin M. Healthcare utilization and costs of Veterans Health Administration patients with schizophrenia treated with paliperidone palmitate long-acting injection or oral atypical antipsychotics. J Med Econ. 2015;18(5):357–65.
Taipale, H., Mehtälä, J., Tanskanen, A., & Tiihonen, J. (2017). Comparative effectiveness of antipsychotic drugs for rehospitalization in schizophrenia—a nationwide study with 20-year follow-up. Schizophr Bull.
Tiihonen J, Mittendorfer-Rutz E, Majak M, Mehtälä J, Hoti F, Jedenius E, Taipale H. Real-world effectiveness of antipsychotic treatments in a nationwide cohort of 29 823 patients with schizophrenia. JAMA Psychiatry. 2017;74(7):686–93.
Wu EQ, Birnbaum HG, Shi L, et al. The economic burden of schizophrenia in the United States in 2002. J Clin Pyschiatry. 2005;66(9):1122–9.
Acknowledgements
Funding
This study and the accompanying article processing charges were funded by Alkermes Inc. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analyses.
Medical Writing and/or Editorial Assistance
Editorial support was provided by Michael Moriarty of STATinMED Research.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Prior Presentations
This study was presented at the following conferences: the US Psychiatric and Mental Health Congress (USPC) New Orleans, LA, USA (Sep 16–19, 2017) and Academy Managed Care Pharmacy (AMCP) Nexus, Dallas, TX, USA (Oct 16–19, 2017).
Disclosure Statement
Ankit Shah is a full-time employee and a minor shareholder of Alkermes, Inc. Mugdha Gore was employed at Alkermes, Inc. when the study was conducted. Mugdha Gore is currently the President & CEO of Avalon Health Solutions, Inc and Founder of Samsara Healthcare Inc. Lin Xie is a full-time employee of STATinMED Research which is a paid consultant to Alkermes, Inc. Furaha Kariburyo is a full-time employee of STATinMED Research which is a paid consultant to Alkermes, Inc. Qisu Zhang is a full-time employee of STATinMED Research which is a paid consultant to Alkermes, Inc.
Compliance with Ethics Guidelines
This article is based on retrospective administrative claims data and does not contain any studies with human participants or animals performed by any of the authors
Data Availability
The datasets generated and/or analyzed during the current study are not publicly available due to a data licensing agreement with Truven Health Analytics.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced digital content
To view enhanced digital content for this article go to https://doi.org/10.6084/m9.figshare.7028381.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shah, A., Xie, L., Kariburyo, F. et al. Treatment Patterns, Healthcare Resource Utilization and Costs Among Schizophrenia Patients Treated with Long-Acting Injectable Versus Oral Antipsychotics. Adv Ther 35, 1994–2014 (2018). https://doi.org/10.1007/s12325-018-0786-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-018-0786-x