Abstract
Introduction
The role of vitamin D supplementation on muscle function and physical performance is still debated. Calcifediol is an available treatment for hypovitaminosis D, particularly for extra-skeletal effects. Aim of this prospective cohort study was to evaluate the effectiveness of calcifediol on serum levels of 25(OH)D3, appendicular muscle strength, physical performance, and prevention of falls in post-menopausal women.
Methods
We recruited post-menopausal women aged ≥50 years, referring to an outpatient service for the management of osteoporosis over a 18-month period. We included women with a diagnosis of osteoporosis and/or vitamin D deficiency [serum levels of 25(OH)D3 <30 ng/ml]. All the participants received calcifediol (20 μg, 4 oral drops/day) for a 6-month period. We evaluated at the baseline and after 6 months the following outcomes: serum levels of 25(OH)D3, appendicular muscle strength, using the Isometric Hand Grip Strength Test and the Knee Isometric Extension Strength Test, physical performance, using the Short Physical Performance Battery (SPPB) and the 4-m gait speed (4MGS), and the risk of falls (percentage of fallers and recurrent fallers and mean number of falls). A sub-analysis was performed in patients with vitamin D deficiency.
Results
We enrolled 113 post-menopausal women, mean aged 68.01 ± 9.13 years. After 6 months of treatment, there was a significant increase in serum levels of 25(OH)D3 (p < 0.001), appendicular muscle strength (p < 0.001), and physical performance (p = 0.002 at SPPB and p = 0.010 at 4MGS, respectively). At 6 months, the percentage of fallers was lower, although not significantly (p = 0.078), whereas there was a significant reduction both in percentage of recurrent fallers and in the mean number of falls (p < 0.001 and p = 0.020, respectively).
Conclusion
Calcifediol was significantly effective in improving serum levels of 25(OH)D3 and muscle function and in reducing the percentage of recurrent fallers and the mean number of falls in a cohort of post-menopausal women.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The role of vitamin D on skeletal muscle has been widely investigated and it was demonstrated the vitamin D receptor (VDR) expression and the direct effects of 1α,25-dihydroxy-vitamin D3 [1α,25(OH)2D3] even in human skeletal muscle precursor cells [1]. From a clinical point of view, it has been well known for a long time that vitamin D plays a significant role in the genesis of muscle strength. This concept derived from the observation that children suffering from rickets had a marked muscle weakness, particularly at proximal limb muscles, as well as the elderly suffering from osteomalacia, both clinical conditions due to severe vitamin D deficiency. It has been suggested that the therapeutic use of vitamin D could improve strength and muscle performance in these patients [2–8]. According to this concept, it has been postulated the possibility to administer the vitamin D supplements in patients with poor muscle performance (in particular older adults and/or post-menopausal women), but its effectiveness is still not yet established. A recent systematic review and meta-analysis showed that vitamin D supplementation might increase proximal muscle strength in adults with serum levels of 25-hydroxy-vitamin D3 [25(OH)D3] ≤25 nmol/l [9]. Moreover, several randomized controlled trials (RCTs) demonstrated that the supplementation with cholecalciferol plus calcium might reduce the risk of falling [10–12]. Calcifediol [25(OH)D3] is an available treatment for vitamin D deficiency, with more hydrophilic properties, shorter half-life (8–11 h) after oral administration, and faster increase of vitamin D status than the native form [13]. Indeed, in 2012, Bischoff-Ferrari et al. demonstrated the role of calcifediol in the enhancement of vitamin D status and muscle performance [14]. On the other hand, although several evidences showed an effect of vitamin D supplementation on muscle in older people, its role is still controversial [15].
Therefore, the aim of this study was to evaluate the effectiveness of 6-month calcifediol treatment on serum levels of 25(OH)D3, appendicular muscle strength, physical performance, and prevention of falls in post-menopausal women.
Methods
Participants
This cohort study respects the Declaration of Helsinki and all the criteria of a prospective study of real practice, approved by the Ethical Committee of University of Campania “Luigi Vanvitelli”. All the participants were asked to carefully read and sign an informed consent, and researchers provided to protect the participants’ privacy. We recruited consecutive post-menopausal women aged ≥50 years, referring to an outpatient service for the management of osteoporosis over a 18-month period. We included only patients with a diagnosis of osteoporosis [at least one fragility fracture and/or a low bone mineral density (BMD) with a T s < −2.5 SD] and/or hypovitaminosis D [serum levels of 25(OH)D3 <30 ng/ml]. We excluded patients with a history of fractures within the last 12 months, muscular diseases (acquired, familiar, and congenital disorders), cancer, secondary osteoporosis, cognitive impairment, chronic pain, and other conditions that might influence musculoskeletal functioning. All the participants assumed calcifediol (20 μg, 4 oral drops per day) for a 6-month period.
Outcomes
At the baseline we assessed: (1) age; (2) body mass index (BMI); (3) smoking and alcohol habits; (4) level of sun exposure; (5) level of physical activity (none, low, medium or high); (6) comorbidities, assessed by the Cumulative Illness Rating Scale (CIRS); (7) blood tests, including calcium, and parathyroid hormone (PTH); (8) urinary calcium levels; (9) pharmacological therapy; (10) previous anti-osteoporosis therapy and/or calcium and/or vitamin D intake; (11) history of osteoporotic fractures, occurred over 12 months, at any sites.
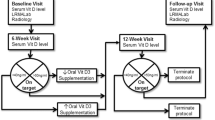
Moreover, as outcome measures, we evaluated at the baseline (T 0) and after 6 months (T 1) the following parameters: serum levels of 25(OH)D3, appendicular muscle strength, physical performance, and percentage of fallers. All participants underwent the chemiluminescent immunoassay (CLIA) technology for the quantitative determination of 25(OH)D3 in human serum. The study design is showed in Fig. 1.
The upper limb muscle strength was evaluated by the Isometric Hand Grip Strength Test (HGS), through the hand-held Jamar dynamometer (Sammons Preston Rolyan, Bolingbrook, IL, USA), considering the maximum value (in kilograms) of three consecutive measurements of the upper dominant limb (with an interval of 1 min after each measurement) [16].
Furthermore, we evaluated the lower limb muscle strength through the Knee Isometric Extension Strength Test (KES), using a hand-held dynamometer. The mean value (in kilograms) of three consecutive measurements (with a pause of 1 min after each measurement) was assumed for each patient [17].
We considered the following cut-offs to define muscle weakness: HGS <16 kg and the KES on body weight (BW) (KES/BW) ratio <0.31 [16].
Physical performance was assessed using the short physical performance battery (SPPB), that includes data on walking speed, chair stand ability, and balance, and using the 4-m gait speed (4MGS). A SPPB score ≤8 [18] or 4MGS lower than 0.8 m/s were used to define a poor muscle performance [16]. Furthermore, we reported the percentage of fallers and recurrent fallers and the mean number of falls in the previous 6 months at the baseline and at the end of the study period.
A sub-analysis was performed in patients with vitamin D deficiency.
Statistical Analysis
Data management and analyses were conducted according to a pre-specified statistical analytical plan. Statistical analysis was performed using the SPSS v.21.0 software (SPSS Inc.; Chicago, IL, USA). The continuous variables are presented as means ± standard deviations, the dichotomous variables as counts (percentages); whereas the ordinal data as medians and interquartile ranges. We performed the Shapiro–Wilk to test the normal distribution of all continuous data. As the data did not follow a normal distribution, the Wilcoxon signed rank test was used to compare the continuous variables and the McNemar’s test was used to compare the continuous variables.
Results
We enrolled 113 post-menopausal women, mean aged 68.0 ± 9.1 years, whose baseline characteristics are reported in Table 1. The 84.1% of patients have previously received vitamin D supplementation and the 88.5% were treated with anti-osteoporotic drugs. At the baseline we found a significant negative association between muscle strength and serum levels of 25(OH)D3 (r = −0.309, p < 0.001); on the other hand there was a non significant negative association between fall rate and low serum levels of 25(OH)D3 (r = −0.019, p = 0.840). At T 1 there were no statistically significant differences in serum levels of calcium (p = 0.065) and PTH (p = 0.475) and in urinary calcium levels (p = 0.256). Moreover, after 6 months of calcifediol treatment, the serum levels of 25(OH)D3 increased significantly (p < 0.001). In terms of appendicular muscle strength, we observed a statistically significant improvement both in upper (p < 0.001) and lower limbs (p < 0.001). Similar results were obtained as percentages of patients with HGS <16 (p < 0.001), and KESBW <0.31 (p = 0.005) at 6 months. Moreover, we observed a significant improvement in median SPPB score (p < 0.001) and a reduction of women with SPPB score ≤8 (p < 0.001), as well as that with 4MGS ≤0.8 m/s (p = 0.001). At 6 months, the percentage of fallers was lower, although not significantly (p = 0.078), whereas there was a significant reduction both in percentage of recurrent fallers and in the mean number of falls (p < 0.001 and p = 0.020, respectively) (see Table 2 for further details). The 92.6% of fallers at the baseline had not reported any falls at the end of the study period; the 16.3% of the non-fallers experienced an incident fall over the study period. Furthermore, in the sub-analysis performed in the 63 patients with hypovitaminosis D at the baseline (see Table 3), the calcifediol seemed to be more effective, as showed by the significant improvements of vitamin D status (p < 0.001), appendicular muscle strength (p < 0.001 for upper limbs and p = 0.001 for lower limbs), and physical performance (p = 0.002 at SPPB and p = 0.010 at 4MGS, respectively). Considering the risk of falling in these patients, the percentage of fallers and recurrent fallers and the mean number of falls were all significantly lower (p < 0.001) after 6-month calcifediol treatment. Finally, no incident falls were experienced over the study period in these patients. No adverse effects have been found during the 6-month treatment with calcifediol.
Discussion
In this prospective study performed on a cohort of post-menopausal women, after 6-month calcifediol treatment there was a significant improvement of serum levels of 25(OH)D3 (+50.9%) and muscle function. Muscle strength improved both in upper (+15.2%) and lower limbs (+34.9%) with a reduction of percentage of individuals with muscle weakness. Moreover, we found a significant improvement in muscle performance, assessed by SPPB score and 4MGS. Muscle strength and physical performance along with muscle mass concur to define sarcopenia, a syndrome commonly age-related, leading to physical disability and poor quality of life [19]. It is very interesting to have noticed a reduction of percentage of fallers after 6 months of calcifediol treatment. On the other hand, assessing patients with hypovitaminosis D at baseline, we found significant improvements of serum levels of 25(OH)D3 (+68.4%), HGS (+25.9%), and KES (+35.4%); these values were higher than those found in the whole cohort. In the last years it has been investigated the biological action of vitamin D hormone system on muscle tissue. The 1α,25(OH)2D3 exerts its action particularly on development of skeletal muscle [20], control of skeletal muscle metabolism, and contractility [21, 22]. The presence of the VDR in the skeletal muscle could explain the effects of vitamin D on muscle cells. Vitamin D metabolites might exert their action trough two mechanisms, genomic and non genomic, that both require the presence of VDR [23], whose expression seems to decline with age [24]. Regarding the development of skeletal muscle, a recent study demonstrated that VDR is significantly involved in the 1α,25(OH)2D3-induced differentiation of C2C12 cell line [25]. Moreover, Girgis et al. demonstrated in mice an increased expression of VDR mRNA after 2 days of treatment with 1α,25(OH)2D3 [26]. Capiati et al. showed that physiological doses of 1α,25(OH)2D3 could stimulate the proliferation of chicken myoblasts and also their differentiation into myotubes [27]. Moreover, Podjenic et al. have investigated the effects of metabolites of vitamin D on the expression of the VDR in human skeletal muscle cells, affirming that human primary myoblasts treated with 1α,25(OH)2D3 for 18 h significantly increased the expression of VDR after 16 weeks in older adults [28]. Therefore, it is well known that vitamin D supplementation with 4000 IU daily for 4 months in mobility-limited women aged ≥65 years increased the intra-nuclear VDR concentration in myocytes and muscle fiber size [29]. In this framework, calcifediol might play a key role in the improvement of muscle trophism and functioning inducing a rapid and sustained increase in vitamin D status also because it is more hydrophilic and with a shorter half-life than the other forms [13, 30]. Recently, it was demonstrated that in post-menopausal women the area under the concentration–time curve (AUC0–24h) was 28% higher after the first administration of 20 μg calcifediol than after the first dose of vitamin D3; after almost 4 months, this difference will be 123% higher. The authors concluded that the use of calcifediol (daily, weekly, or as a single bolus) induced a 2–3 times higher increasing in vitamin D status; moreover serum levels of 25(OH)D3 of 30 ng/ml could be quickly reached with calcifediol [31]. In clinical practice, the goal of vitamin D supplementation is to correct vitamin D deficiency as soon as possible. Several studies have investigated the role of supplementation with cholecalciferol on vitamin D status and muscle function with controversial findings [9, 32, 33], but very few studies have investigated the role of the calcifediol on the same outcomes [14, 34, 35]. Barger Lux et al. demonstrated that the oral intake of calcifediol resulted in a potent increase in serum 25(OH)D3 levels [34]. In another study, it was showed that after 10 weeks of calcifediol intake serum levels of 25(OH)D3 were about fivefold higher than after vitamin D3 intake [35]. Bischoff-Ferrari et al. [14] demonstrated that 20 µg of calcifediol are significantly more effective than 800 IU of vitamin D3 in shifting post-menopausal women from hypovitaminosis D (8–25 ng/ml) to desirable serum levels of 25(OH)D3 of at least 30 ng/ml. Moreover, patients in treatment with calcifediol showed an improvement of muscle functioning compared to vitamin D3 after 4 months of follow up. More recently, the same group showed that after 12-month administration of 24,000 IU of vitamin D3 plus calcifediol per month there was a higher increasing than groups treated with 24,000 IU of vitamin D3 per month or 60,000 IU of vitamin D3 per month; on the other hand, there was no benefit on lower limbs muscle function with also an higher risk of falls than after administration of 24,000 IU of vitamin D3 [36]. To the best of our knowledge, our prospective real practice study is original because it analyzes the role of calcifediol in improving not only the vitamin D status, but also the muscle function, including the muscle strength of upper and lower limbs, assessed by HGS and KES, respectively, physical performance, evaluated by SPPB and 4MGS, percentage of fallers and recurrent fallers, and mean number of falls. Moreover, we also considered for each outcome the specific cut-offs to define muscle weakness and poor muscle performance, as recommended by the Foundation for the National Institutes of Health Biomarkers Consortium Sarcopenia Project (FNIH) [16]. Another strength of our study was the assessment of differences after 6-month calcifediol administration in the women with hypovitaminosis D. Limitations of our study were: the absence of a control-group; the presence of only a 6-month follow up; lack of data about nutritional assessment that can contribute to hypovitaminosis D and to physical frailty [2]; concomitant therapies that can affect the muscle function.
Conclusions
In conclusion, this prospective real-practice study showed that calcifediol was significantly effective in improving vitamin D status and muscle function and in reducing the percentage of recurrent fallers and the mean number of falls in a cohort of post-menopausal women, referring to an outpatient service for the management of osteoporosis.
References
Olsson K, Saini A, Strömberg A, Alam S, Lilja M, Rullman E, Gustafsson T. Evidence for vitamin D receptor expression and direct effects of 1α,25(OH)2D3 in human skeletal muscle precursor cells. Endocrinology. 2016;157(1):98–111.
Girgis CM, Clifton-Bligh RJ, Turner N, Lau SL, Gunton JE. Effects of vitamin D in skeletal muscle: falls, strength, athletic performance and insulin sensitivity. Clin Endocrinol (Oxf). 2014;80:169–81.
Zamboni M, Zoico E, Tosoni P, Zivelonghi A, Bortolani A, Maggi S, Di Francesco V, Bosello O. Relation between vitamin D, physical performance, and disability in elderly persons. J Gerontol A Biol Sci Med Sci. 2002;57(1):M7–11.
Bischoff-Ferrari HA, Dietrich T, Orav EJ, Hu FB, Zhang Y, Karlson EW, Dawson-Hughes B. Higher 25-hydroxyvitamin D concentrations are associated with better lower-extremity function in both active and inactive persons aged > or =60 y. Am J Clin Nutr. 2004;80:752–8.
Gerdhem P, Ringsberg KAM, Obrant KJ, Akesson K. Association between 25-hydroxy vitamin D levels, physical activity, muscle strength and fractures in the prospective population-based OPRA study of elderly women. Osteoporos Int. 2005;16(11):1425–31.
Houston DK, Cesari M, Ferrucci L, Cherubini A, Maggio D, Bartali B, Johnson MA, Schwartz GG, Kritchevsky SB. Association between vitamin D status and physical performance: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2007;62(4):440–6.
Toffanello ED, Perissinotto E, Sergi G, Zambon S, Musacchio E, Maggi S, Coin A, Sartori L, Corti MC, Baggio G, Crepaldi G, Manzato E. Vitamin D and physical performance in elderly subjects: the Pro.V.A study. PLoS One. 2012;7(4):e34950.
Iolascon G, de Sire A, Calafiore D, Moretti A, Gimigliano R, Gimigliano F. Hypovitaminosis D is associated with a reduction in upper and lower limb muscle strength and physical performance in post-menopausal women: a retrospective study. Aging Clin Exp Res. 2015;27(Suppl 1):S23–30.
Stockton KA, Mengersen K, Paratz JD, Kandiah D, Bennell KL. Effect of vitamin D supplementation on muscle strength: a systematic review and meta-analysis. Osteoporos Int. 2011;22:859–71.
Pfeifer M, Begerow B, Minne HW, Suppan K, Fahrleitner-Pammer A, Dobnig H. Effects of a long-term vitamin D and calcium supplementation on falls and parameters of muscle function in community-dwelling older individuals. Osteoporos Int. 2009;20(2):315–22.
Bischoff HA, Stahelin HB, DickW Akos R, KnechtM Salis C, et al. Effects of vitamin D and calcium supplementation on falls: a randomized controlled trial. J Bone Miner Res. 2003;18:343–51.
Bischoff-Ferrari HA, Orav EJ, Dawson-Hughes B. Effect of cholecalciferol plus calcium on falling in ambulatory older men and women: a 3-year randomized controlled trial. Arch Intern Med. 2006;166:424–30.
Stamp TC. Intestinal absorption of 25-hydroxycholecalciferol. Lancet. 1974;2(7873):121–3.
Bischoff-Ferrari HA, Dawson-Hughes B, Stöcklin E, Sidelnikov E, Willett WC, Edel JO, Stähelin HB, Wolfram S, Jetter A, Schwager J, Henschkowski J, von Eckardstein A, Egli A. Oral supplementation with 25(OH)D3 versus vitamin D3: effects on 25(OH)D levels, lower extremity function, blood pressure, and markers of innate immunity. J Bone Miner Res. 2012;27(1):160–9.
Rejnmark L. Effects of vitamin D on muscle function and performance: a review of evidence from randomized controlled trials. Ther Adv Chronic Dis. 2011;2(1):25–37.
Studenski SA, Peters KW, Alley DE, Cawthon PM, McLean RR, Harris TB, Ferrucci L, Guralnik JM, Fragala MS, Kenny AM, Kiel DP, Kritchevsky SB, Shardell MD, Dam TT, Vassileva MT. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014;69(5):547–58.
Andrews AW, Thomas MW, Bohannon RW. Normative values for isometric muscle force measurements obtained with hand-held dynamometers. Phys Ther. 1996;76(3):248–59.
Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–94.
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M, European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412–23.
Endo I, Inoue D, Mitsui T, Umaki Y, Akaike M, Yoshizawa T, Kato S, Matsumoto T. Deletion of vitamin D receptor gene in mice results in abnormal skeletal muscle development with deregulated expression of myoregulatory transcription factors. Endocrinology. 2003;144(12):5138–44.
Boland R. Role of vitamin D in skeletal muscle function. Endocr Rev. 1986;7(4):434–48.
Boland R, Buitrago C, De Boland AR. Modulation of tyrosine phosphorylation signalling pathways by 1alpha,25(OH)2-vitamin D3. Trends Endocrinol Metab. 2005;16(6):280–7.
Bouillon R, Gielen E, Vanderschueren D. Vitamin D receptor and vitamin D action in muscle. Endocrinology. 2014;155(9):3210–3.
Bischoff-Ferrari HA, Borchers M, Gudat F, Dürmüller U, Stähelin HB, Dick W. Vitamin D receptor expression in human muscle tissue decreases with age. J Bone Miner Res. 2004;19(2):265–9.
Irazoqui AP, Boland RL, Buitrago CG. Actions of 1,25(OH)2-vitamin D3 on the cellular cycle depend on VDR and p38 MAPK in skeletal muscle cells. J Mol Endocrinol. 2014;53(3):331–43.
Girgis CM, Mokbel N, Cha KM, Houweling PJ, Abboud M, Fraser DR, Mason RS, Clifton-Bligh RJ, Gunton JE. The vitamin D receptor (VDR) is expressed in skeletal muscle of male mice and modulates 25-hydroxyvitamin D (25OHD) uptake in myofibers. Endocrinology. 2014;155(9):3227–37.
Capiati DA, Tellez-Inon MT. Boland RL 1999 Participation of protein kinase C a in 1,25-dihydroxy-vitamin D3 regulation of chick myoblast proliferation and differentiation. Mol Cell Endocrinol. 1999;153(1–2):39–45.
Pojednic RM, Ceglia L, Olsson K, Gustafsson T, Lichtenstein AH, Dawson-Hughes B, Fielding RA. Effects of 1,25-dihydroxyvitamin D3 and vitamin D3 on the expression of the vitamin d receptor in human skeletal muscle cells. Calcif Tissue Int. 2015;96(3):256–63.
Ceglia L, Niramitmahapanya S, da Silva Morais M, Rivas DA, Harris SS, Bischoff-Ferrari H, Fielding RA, Dawson-Hughes B. A randomized study on the effect of vitamin D3 supplementation on skeletal muscle morphology and vitamin D receptor concentration in older women. J Clin Endocrinol Metab. 2013;98(12):E1927–35.
Haddad JG Jr, Rojanasathit S. Acute administration of 25-hydroxycholecalciferol in man. J Clin Endocrinol Metab. 1976;42:284–90.
Jetter A, Egli A, Dawson-Hughes B, Staehelin HB, Stoecklin E, Goessl R, Henschkowski J, Bischoff-Ferrari HA. Pharmacokinetics of oral vitamin D(3) and calcifediol. Bone. 2014;59:14–9.
Rousseau AF, Foidart-Desalle M, Ledoux D, Remy C, Croisier JL, Damas P, Cavalier E. Effects of cholecalciferol supplementation and optimized calcium intakes on vitamin D status, muscle strength and bone health: a one-year pilot randomized controlled trial in adults with severe burns. Burns. 2015;41(2):317–25.
Cangussu LM, Nahas-Neto J, Orsatti CL, Bueloni-Dias FN, Nahas EA. Effect of vitamin D supplementation alone on muscle function in postmenopausal women: a randomized, double-blind, placebo-controlled clinical trial. Osteoporos Int. 2015;26(10):2413–21.
Barger-Lux MJ, Heaney RP, Dowell S, Chen TC, Holick MF. Vitamin D and its major metabolites: serum levels after graded oral dosing in healthy men. Osteoporos Int. 1998;8(3):222–30.
Cashman KD, Seamans KM, Lucey AJ, Stöcklin E, Weber P, Kiely M, Hill TR. Relative effectiveness of oral 25-hydroxyvitamin D3 and vitamin D3 in raising wintertime serum 25-hydroxyvitamin D in older adults. Am J Clin Nutr. 2012;95(6):1350–6.
Bischoff-Ferrari HA, Dawson-Hughes B, Orav EJ, Staehelin HB, Meyer OW, Theiler R, Dick W, Willett WC, Egli A. Monthly high-dose vitamin D treatment for the prevention of functional decline: a randomized clinical trial. JAMA Intern Med. 2016;176(2):175–83.
Acknowledgements
No funding or sponsorship was received for this study or publication of this article.
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Disclosures
Giovanni Iolascon, Antimo Moretti, Alessandro de Sire, Dario Calafiore, and Francesca Gimigliano have no disclosures for any personal, financial, commercial, or academic conflicts of interest separately.
Compliance with Ethics Guidelines
This cohort study respects the Declaration of Helsinki and all the criteria of a prospective study of real practice, approved by the Ethical Committee of University of Campania “Luigi Vanvitelli”. All the participants were asked to carefully read and sign an informed consent, and researchers provided to protect the participants’ privacy.
Data Availability
The dataset of the current study is available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/9C87F060005B90C0.
Rights and permissions
About this article
Cite this article
Iolascon, G., Moretti, A., de Sire, A. et al. Effectiveness of Calcifediol in Improving Muscle Function in Post-Menopausal Women: A Prospective Cohort Study. Adv Ther 34, 744–752 (2017). https://doi.org/10.1007/s12325-017-0492-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-017-0492-0