Abstract
C4 species, Amaranthus viridis L. exhibited a significant bioaccumulation of aluminium (Al) through the duration of 3- and 5-days exposure. As compared to control, Amaranthus appeared as excess-accumulator with maximum 5.85-fold bioaccumulation of Al in root. Cellular responses to Al tolerance initially scored tissue specific distribution of metal through cortical layers revealed by electron microscopy. The affected cells changed an oxidative status as read by histochemical stains, particularly, for hydrogen peroxide. Osmotic stress and its stability were scored by maximum proline and free amino acids accumulation with 1.53 and 1.59-fold increase over control. The accumulation of phenolics and flavonoids were over expressed in the ranges of 2.48–2.50-fold and 2.00–1.5-fold at 3- and 5-days respectively against control. Anti-oxidation to detoxify Al stress was facilitated by variants of peroxidases. For exclusion mechanism of metal, esterase activity significantly over expressed with maximum value of 1.80-fold at 5-days. The polymorphism of esterase exhibited few significant over produced bands, varied in numbers as detected by densitometric scanning. Moreover, plant extract was satisfactorily potential under in vitro anti-oxidation systems through assay of 1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,2′-azino-bis-3-ethylbenzthiazoline-6-sulphonic acid (ABTS), ferric chelation activity etc. Therefore, weeds like Amaranthus would be a bioprospecting in role likely involved in phytoremediation of metal.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A number of edaphic situations are most influencing to plant growth and development with their extremities. Commonly those are called environmental or abiotic stresses that leave detrimental effects to crops (Mittler 2006). Cellular responses through physiological and metabolic activities are the primary indices to react those stressors varying in growth stages. Plants are tuned with the imposed changes under those conditions and the responses are manifested into either tolerance or susceptibility. However, during the course of adaptation or acclimatization, the growth is impaired leading to loss of biological or economic yield (Singh et al. 2014). Accumulation of metals, both toxic or heavy elements in organic residues are grouped under xenobiotic stress (Memon and Schröder 2009). Thereby, survival and crop productivity, quality are faced with hazards. From the photosynthetic speciality of crops vis-a-vis plant species, C4 modules have been well efficient regardless of abiotic stress tolerance. The diversion of carbon flow and allocation in organic residues, C4 species are well attributed primarily to down-regulate the photorespiratory ROS (Wang et al. 2013). Minimization of light energy induced oxidation or photo-oxidation of these species are also well adopted to any kind of abiotic stress. Therefore, from the secondary effects of abiotic stress, oxidative injuries would be equally important to demark stress susceptibility or tolerance. So, exercise of C4 species through modulated C4 pathways would be directive as cellular biomarkers for oxidative stress (Roberts et al. 2007). Oxidative stress is undoubtedly an imbalance to the cellular redox in development of ROS either through normal life process or induced. ROS, a detrimental residue in plant tissues could also be a part of signalling cascades during drought, flooding, salinity, heavy and toxic metals, high/low temperature and intense irradiation (Czarnocka and Karpiński 2018) Therefore, a probable relationship with C4 pathways adjusting ROS metabolism would be another insight for stress recognition in environmental system. In this study, we have framed an experiment with C4 species in exercise the anti-oxidation responses against a metal stress. Aluminium (Al) could be the selected metal, not heavy one but toxic, abundant as 1/3rd of earth crust and an established phytotoxic agent (Liu et al. 2014). The exercised plant species is Amaranthus viridis L., a C4 weed herbs very often used as leafy vegetable. With this, an attempt has been taken to decipher the efficiency of Amaranthus species to extent of metal accumulation in excess along with probable in vitro anti-oxidation parameters. In brief, this study may set the Amaranthus species in bio-monitoring of Al toxicity through anti-oxidation signalling.
Materials and methods
Experimental plant material and growth condition
The whole work is conducted in Plant Physiology and Molecular Biology Research Unit, Department of Botany, University of Kalyani, Kalyani, India (22° 58′ 30″ N 88° 26′ 04″ E). Commonly the leafy weed species, slender amaranth (Amaranthus viridis L.), a C4 type was utilized for the present experiment. The plants were collected from the fallow of natural environments surrounding the University premises. Collected plants were in vegetative stage and in laboratory condition hydroponic culture, with 1/4th strength of MS media used for growth (Murashige and Skoog 1962). Initially, plants were acclimatized in the media for 5 days in simulated condition of 14/10 h photo period, 35/30 °C day/night temperature, 70% relative humidity and photosynthetically active radiation (PAR) around (900–1200) µE m−2 s−1 photon flux density. On completion of incubation period, the nutrient set was divided into 5 treatments: control (0), 120, 240, 360, 480 µM of Al concentration. Potassium aluminium sulphate dodecahydrate [AlK(SO4)2·12H2O] was used for Al source and dissolved in de-ionised water, adjusted the pH 4.8 for higher solubility of Al. Each treatment was done with three replications with standard cultural practices. The solutions were aerated and changed into fresh in 2 days interval continuing up to 5 days. Plants’ physiological condition were optimum within 5 days and thereafter, plant started to wilting and failed to survive. Thus, the samplings were done at 3 and 5-days from the first day of treatments following harvest, separated into shoots and roots, frozen into liquid nitrogen following transfer into − 80 °C for further biochemical assays.
Determination of Al bioaccumulation
Bioaccumulation of Al was estimated from 1.0 g of fresh sample. 1.0 g of fresh tissue was dried in muffle furnace and ground into fine powder. Then, the powdered samples were digested with mixture of acids (HNO3:H2SO4:PCA in 4:4:1) until the solution cleared. Filtrate was diluted by distilled water up to 50 mL and the accumulation of Al content was measured by Atomic Absorption Spectroscopy (Varian-AA240) (Zafar et al. 2010).
Histochemical detection of H2O2
Accumulation of H2O2 was detected with in vivo detection by 3′3′-diaminobenzidine (DAB) staining (Thordal-Christensen et al. 1997). Fresh leaves and roots were dipped into 5 mM freshly prepared DAB solution in phosphate buffer (pH 6.8). For completely dissolving DAB, the pH of the buffer was adjusted initially to 3.8 followed by increase to 6.8. Samples for infiltration were kept over-night in DAB solution in dark at room temperature. Samples were dipped into lactic acid-glycerol-ethanol solution (1:1:4-v/v/v) and boiled in water bath for 10–15 min to remove the chlorophyll. The brown patches developed on tissue were detected and captured with digital camera (Dewinter).
Scanning electron microscopy (SEM) analysis
For scanning electron microscopy (SEM) analysis, transverse sections of roots were done, passed through dehydrated alcohol gradation for complete dehydration. Then the sections of roots were prepared with gold plating for SEM (ZEISS) scanning aided voltage of 10.0 kV. For image capturing, magnification was set at 350 ×.
Determination of proline content
0.5 g of fresh sample was homogenised with 10 mL of 3% sulphosalicylic acid. The supernatant was collected by centrifugation at 12000 × g for 15 min at 4 °C and reacted with acid ninhydrin solution. The reaction mixture boiled for 30 min and stopped on ice bath following addition of 4 mL 10% toluene. The aqueous layer of toluene was aspirated and diluted. Then the absorbance was read at 520 nm in a UV–Vis Spectrophotometer (Cecil) and the proline content was calculated according to Bates et al. (1973) from standard of l-proline. Proline content was expressed as µM/g f. wt.
Preparation of methanolic plant extract
1.0 g of each fresh sample were grind in liquid nitrogen into fine powder and homogenised in a mortar pestle with 10 mL of 80% methanol. The crude extract was filtered several times through filter paper (Whatman 42). The filtrate was saved and evaporated to concentrate into residue of extract for further experiments (Sakanaka et al. 2005).
Determination of total phenolics
Total phenolic content was determined by Velioglu et al. (1998). 100 µL methanolic extract was added with 100 µL Folin–Ciocalteu reagent following addition of 800 µL double distilled water, incubated at 25 °C temperature for 5 min. Alkaline solution of 20% sodium carbonate was added to the reaction mixture until the effervescence ceased, kept for 40 min. Absorbance of the solution was read at 765 nm. Total phenolic content was calculated from standard of gallic acid equivalent and expressed as µg/g f. wt.
Determination of total flavonoids
250 µL of methanolic extract was added to 1.25 mL double distilled water, 5% sodium nitrite (NaNO2) solution and incubated for 5 min. Following incubation, stock solution of 1 M NaOH, 10% AlCl3 was added and kept for 10 min. Total flavonoid content was estimated by reading the absorbance at 510 nm where standard of quercetin was used and expressed as mg/g f. wt. (Zhishen et al. 1999).
Determination of total free amino acids
Free amino acids were quantified with acid Ninhydrin solution (Balasubramanian and Sadasivam 1987). 200 µL of methanolic extract was added to 1 mL ninhydrin solution (20 g ninhydrin in 500 mL methyl cellosolve, 0.8 g stannous chloride in 500 mL citrate buffer, adjusted to pH 5.0). The reactions were started in boiling water for 20 min and added with n-propanol as diluent. The colour was read at 570 nm and free amino acid was determined taking leucine as standard.
Phosphomolybdenum complex assay
Total antioxidant activity was assayed according to Prieto et al. (1999). 100 µl of methanolic extract was mixed with 1 ml of assay mixture containing 0.5 M sulfuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate. The total reaction mixture was incubated in a boiling water bath for 60 min. The assay mixture was cooled down to room temperature and absorbance was recorded at 695 nm.
DPPH scavenging assay
Free radical scavenging activity of plant extract was done using DPPH as free radical residue (Blois 1958). 100 µL of methanolic extract was mixed with freshly prepared 1 mL 0.1 mM DPPH solution and incubated in dark for 25 min at room temperature. The mixture was read for absorbance at 517 nm and decrease in absorbance indicates the higher free radical scavenging activity. BHT was used as a positive control. The activity was determined as percentage of scavenging that was calculated by the following formula
The inhibition concentration (IC50) was determined from the plots as antioxidant concentration required for providing 50% free radical scavenging activity.
ABTS scavenging assay
ABTS scavenging activity was determined according to Roberta et al. (1999). ABTS solution was prepared by 7 mM ABTS with 3 mM potassium persulfate (K2S2O8) and stored in dark for 14 h before use. 1.0 mL ABTS solution was reacted with 100 µL methanolic extract, mixed well, kept for 10 min and recorded the absorbance at 734 nm with BHT as a positive control. The free radical scavenging activity of the samples were determined using the following formula
The inhibition of concentration (IC50) was determined similarly as discussed in DPPH radical scavenging method.
Ferric chelation assay
For ferric chelation activity, 200 µL methanolic extract made up to 3 mL volume following addition of saturated 2 mM FeCl3 solution. Reaction was initiated by addition of 5 mM ferrozine solution and kept for 10 min. Then, colour development was measured at 562 nm against a blank with EDTA as a positive control (Morel et al. 1994).
Preparation of crude enzyme extract
For enzyme extraction, 1.0 g of plant sample was crushed with liquid nitrogen and homogenized with 50 mM sodium phosphate buffer (pH 7.5) containing 10 mM EDTA, 10 mM MgCl2, 10 mM DTT, 1 mM PMSF, 1% PVP (Sarkar et al. 2017). Then, the crude extract was centrifuged at 15000 × g for 15 min at 4 °C and collected supernatant was saved as enzyme protein. The protein was partially purified by 80% ammonium sulphate for 4 h at 4 °C for precipitation. The pellet recovered was finally dissolved in 10 mL phosphate buffer (pH 7.0) containing 1.0 mM DTT, 1.5 µM BSA, 1.0 mM MgCl2 following making free for ammonium salt and semidried under vacuum. Protein content of the extract was determined with Bradford reagent taking BSA as standard (Bradford 1976).
Assay of anti-oxidant enzyme activity
Guaiacol peroxidase (GPX: EC 1.11.1.7)
With an aliquot of partially purified protein, activity of GPX was determined according to Ammar et al. (2008). Assay mixture contain 100 mM phosphate buffer (pH 7.5), 0.5 mM o-dianisidine and 0.5% H2O2. By addition of freshly prepared enzyme extract, the reaction was started and the changes of absorbance by 0.1 in min was recorded at 430 nm. Enzyme activity was calculated and expressed as Unit/mg protein.
Catalase (CAT: EC 1.11.1.6)
100 µL of enzyme extract was added in a reaction mixture containing 100 mM phosphate buffer (pH 7.0), 100 mM H2O2. The assay mixture was thoroughly mixed and recorded the changes of absorbance at 240 nm for 3 min at 30 s intervals. Using molar extinction coefficient of H2O2 (39.4 M−1 cm−1), the enzyme activity was calculated and expressed as µM H2O2 oxidized/min/mg protein (Verma and Dubey 2003).
Esterase (EC 3.1.1.11)
Esterase enzyme activity was measured by Van-Asperen method (Van Asperen 1962) with some modification. 15 mM 1-naphthyle acetate was incubated in reaction mixture: 50 mM sodium phosphate buffer (pH 6.0) and 100 µL enzyme extract. Reaction mixture was incubated at 30 °C for 5 min and product as 1-naphthol was developed. To the mixture, Fast blue B salt-SDS solution was added and complex was read at 550 nm. The activity was determined taking 1-naphthol as standard.
For in-gel analysis, enzyme protein was separated in a 10% native PAGE at 4 °C through 10 V/lane. After run, the gel was incubated in a 50 mL solution containing sodium dihydrogen phosphate (0.7 g), disodium hydrogen phosphate (0.28 g), fast blue RR salt (0.05 g), 1-naphthyle acetate (0.02 g) for 30 min in room temperature in dark. Then, the reaction was stopped by adding a mixture of ethanol: methanol: water: acetic acid in the ratio 1:10:10:2. The image was captured by Gel-doc image analyser and the densitometric analysis was done by Gel Analyzer software (2010a).
Statistical analysis
Data were recorded and represented as mean ± SE of three independent replicates. Variations among different treatments were computed with one-way ANOVA following Tukey HSD (honestly significant difference) test (P ≤ 0.05). For data analysis, Windows Microsoft Excel 2016 was used.
Results
Bioaccumulation of Al and proline are dose dependent with metal toxicity
The plants had exhibited their over-accumulation of the metal in a dose dependent manner as a function of duration. The variations were significant (P ≤ 0.05) as revealed by one-way ANOVA following Tukey HSD test through the duration of days (5 and 7 days) as well as individual parameters studied (see the Supplementary Table). Thus, the maximum Al accumulation in shoots and roots were 6.74 and 6.59-fold and 5.77 and 5.85-fold on consecutive 3 and 5-days respectively (Table 1). On an average, the bioaccumulation of metal was more favoured for root regardless of concentration as well as function of duration. Thus, root had 1.60 and 1.70-fold increase over accumulation than shoot at 3 and 5-days respectively. Therefore, Amaranthus had adjusted Al avoidance while the plants restricted the metals more in roots. As a consequence of Al sensitivity, plants adjusted their osmotic potential as monitored through proline content accumulation. Interestingly, maximum amount of proline was recorded at 360 µM of Al concentration which was 1.53 and 1.33-fold respectively under 3 and 5-days against control (Fig. 1). The plants failed to recover proline at maximum Al concentration toxicity and that recovered 7.67 and 5.94% fall of proline. Responses of plants were significant (P ≤ 0.05) in proline accumulation when passed through maximum concentration of metal stress interacted with durations as elucidated from values (F = 228.071) through ANOVA.
Determination of proline content under various aluminium treatments (Control-0, 120, 240, 360, 480 μM) for 3 days and 5 days. Data represented as mean of three replicates (n = 3) ± SE and SE represented as vertical bar on each column. Different letters indicate significant differences through Tukey HSD test at P ≤ 0.05
Effects of Al toxicity on phenolics
Al tolerance in plant species is characteristically induced with anti-oxidation. In the present experiment, the stable anti-oxidants with fractions of polyphenolics were estimated. Plants had regulated the phenolic concentration in maximum at 3 days over the control. The highest phenolic concentration was also dose dependent to Al concentration and recorded 2.48-fold in 480 µM Al dose under 3 days incubation (Fig. 2). Regardless of metal concentrations, the interaction of days (3 and 5) was significant (P ≤ 0.05) as deducted from Tukey HSD test (F = 472.113). On the contrary, at 5 days, the concentration was reduced by 14.20% as compared to 3 days. Flavonoids, a form of phenolics had the compatible responses through Al doses but may vary from total phenolics in respect to pattern of accumulation. In case of flavonoids, the control set induced 1.20-fold higher accumulation at 5 days compare to 3 days (Fig. 3). Plant’s responses were interesting to adopt initial concentration of metal (120 µM) to be significant in flavonoid accumulation as derived from ANOVA table (F = 78.055). Thereafter, plants responded in a dose dependent manner through Al concentration, however, significantly more with 3 than 5-days. Thus, the changes of flavonoid contents range from 1.20-fold to 16.66%, 1.60 and 1.16-fold, 1.80 and 1.33-fold, 2.00 and 1.50-fold through Al concentrations under 3 and 5-days incubation respectively against control.
Determination of total phenolics under various aluminium treatments (Control-0, 120, 240, 360, 480 μM) for 3 days and 5 days. Data represented as mean of three replicates (n = 3) ± SE and SE represented as vertical bar on each column. Different letters indicate significant differences through Tukey HSD test at P ≤ 0.05
Determination of total flavonoids under various aluminium treatments (Control-0, 120, 240, 360, 480 μM) for 3 days and 5 days. Data represented as mean of three replicates (n = 3) ± SE and SE represented as vertical bar on each column. Different letters indicate significant differences through Tukey HSD test at P ≤ 0.05
Al induced protein hydrolysis supports metal toxicity
Plants suffered from metal toxicity for protein profiles predominantly in hydrolysis and up/down regulation of specific protein. In this experiment, the hydrolysis of protein or protease activity is indirectly monitored through release of free amino acids that recorded a linear increase through the Al doses. Duration of treatments had certainly been bothered as a significant up-regulation of free amino acid by 1.54 and 1.56-fold in 360 µM Al dose under 3 and 5-days against control (Fig. 4). With respect to concentration gradient, they recorded some significant suppression by 10.47 and 12.43% in 480 µM dose against the 360 µM Al dose under respective duration. The critical analysis through Tukey HSD test (F = 453.966) revealed a significant (P ≤ 0.05) variation for both the durations and Al concentration as plants proceeded towards maximum metal stress.
Determination of total free amino acids under various aluminium treatments (Control-0, 120, 240, 360, 480 μM) for 3 days and 5 days. Data represented as mean of three replicates (n = 3) ± SE and SE represented as vertical bar on each column. Different letters indicate significant differences through Tukey HSD test at P ≤ 0.05
Elicitation of anti-oxidation efficiency in plant extract with Al
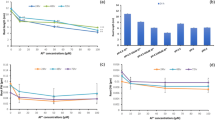
Plant extract was made as described in materials and methods and different anti-oxidation system were exercised in vitro to monitor the bio-activities. Figure 5 shows the reduction of phosphomolybdenum complex was significantly higher in 3 than 5-days, however, significant regardless of duration through the various Al doses. The peak activity of the reduction of complex was scored at 360 µM Al which were 1.54 & 1.56-fold increase over the control in 3 and 5-days respectively. Still, at highest concentration of Al, the complex formation was down-regulated by 10.47 and 12.43% than penultimate dose of Al under respective incubation days. It would be plants’ potential to response with variations to phosphor molybdenum sensitivity as deduced from significant (P ≤ 0.05) values (F = 252.070). For another mode of in vitro anti-oxidation served with free radical generating residues like DPPH and it’s percentage of inhibition was recorded in Fig. 6. Plants responded to Al and thereby, its induced anti-oxidation had taken an ascending order up to 240 µM following a gradual fall. Taking BHT as positive control, the DPPH activity had the peak values with 2.01 and 1.66-fold at 3 and 5-days. The regulation of activity was limited when the extract was made from 480 µM Al dose which curtailed the activities by 18.85 and 16.74% over its maximum values. Still, the activities of scavenging free radical were significant (P ≤ 0.05) all through the Al doses regardless of incubation days. The corresponding IC50 values were presented in the Table 2a that also recorded duration dependent with a significant variation against its concentration of Al when compared to control. In another mode of total anti-oxidant capacity, ABTS+ ions were used to determine its percentage inhibition for disappearance or decolourization. Figure 7 presents the in vitro activity of methanolic extract of ABTS solution in the same solvent with BHT as positive control. As expected, the BHT had raised the maximum inhibition of decolourization as compared to different treatments. Plants responses to ABTS+ radical was in an inductive way that for each ongoing concentrations of Al were significant (P ≤ 0.05) interacting with durations as revealed from ANOVA (F = 175.165). When control was compared, it recorded a significant rise of percentage inhibition at 360 µM Al concentration by 1.71 and 1.36-fold increased under 3 and 5-days respectively. However, the ABTS scavenging activity was regulated beyond this concentration by 16.47 and 13.51% fall to denote the maximum Al toxicity. Table 2b shows the corresponding IC50 values and that also had a significant variation at P ≤ 0.05. Therefore, the leaf extract may have some modulatory responses with the Al induction that varied through ABTS scavenging assays. In ferric chelation assay, ferrozine was used as substrate with standard EDTA was compared with tissue extracts. Under varying concentration of Al, maximum activity was scored 1.66 and 1.49-fold in 3 and 5-days respectively against control (Fig. 8). From the Table 2c, 50% reduction of chelating activity as expressed by IC50 value gradually down-regulated and thereby, showed improved chelation. The values were all significant (P ≤ 0.05) indicating the efficiency of plant extract in a linear manner according to concentration dependent Al toxicity.
Assay of phosphomolybdenum complex under various aluminium treatments (Control-0, 120, 240, 360, 480 μM) for 3 days and 5 days. Data represented as mean of three replicates (n = 3) ± SE and SE represented as vertical bar on each column. Different letters indicate significant differences through Tukey HSD test at P ≤ 0.05
DPPH scavenging activity (% inhibition) under various aluminium treatments (Control-0, 120, 240, 360, 480 μM) for 3 days and 5 days. Data represented as mean of three replicates (n = 3) ± SE and SE represented as vertical bar on each column. Different letters indicate significant differences through Tukey HSD test at P ≤ 0.05
ABTS scavenging activity (% inhibition) under various aluminium treatments (Control-0, 120, 240, 360, 480 μM) for 3 days and 5 days. Data represented as mean of three replicates (n = 3) ± SE and SE represented as vertical bar on each column. Different letters indicate significant differences through Tukey HSD test at P ≤ 0.05
Ferric chelation activity (% inhibition) under various aluminium treatments (Control-0, 120, 240, 360, 480 μM) for 3 days and 5 days. Data represented as mean of three replicates (n = 3) ± SE and SE represented as vertical bar on each column. Different letters indicate significant differences through Tukey HSD test at P ≤ 0.05
Variations in anti-oxidative enzyme activities under Al treatment
Guaiacol peroxidase (GPX) is the most common and effective enzyme within peroxidase group where a phenolic residue as electron (o-dianisidine herein the case) donor is used. A significant (P ≤ 0.05) variation between days of incubation for Al concentration was depicted when compared with control. The maximum activity of GPX was found at 360 µM Al concentration which was linearly increased by 1.78-fold against the control under 3 days (Fig. 9). Interestingly, the 5 days incubation have regulated the maximum activity within 240 and 360 µM Al dose which was 1.31-fold over the control. However, plants fail to sustain the activity beyond 360 µM dose and declined by 19.68% when tallied with its maximum activities at 480 µM Al dose under 3 days but in case of 5 days incubation, activity decreased beyond 240 µM and recorded 12.88% declined at 480 µM Al concentration. For those cases, the plants interacted metal concentration and durations in more significant (F = 281.950, P ≤ 0.05) manner towards higher concentrations of metal. Catalase (CAT) had the similar trend with GPX where the maximum activities were varied according days of incubation (3 and 5-days). For 3 days, the activity was maximised by 1.60-fold at 360 µM dose whereas extended exposure of 5 days had limited the activity by 1.36-fold within 240 µM Al concentration over control (Fig. 10). Finally, they recorded a significant (P ≤ 0.05) down-regulation of enzyme activity regardless of exposure days when it met 480 µM of Al. The fall in activity compare to its maximum value were recorded as 14.82 and 17.41% respectively for 3 and 5-days.
Assay of guaiacol peroxidase (GPX) activity under various aluminium treatments (Control-0, 120, 240, 360, 480 μM) for 3 days and 5 days. Data represented as mean of three replicates (n = 3) ± SE and SE represented as vertical bar on each column. Different letters indicate significant differences through Tukey HSD test at P ≤ 0.05
Assay of catalase (CAT) activity under various aluminium treatments (Control-0, 120, 240, 360, 480 μM) for 3 days and 5 days. Data represented as mean of three replicates (n = 3) ± SE and SE represented as vertical bar on each column. Different letters indicate significant differences through Tukey HSD test at P ≤ 0.05
Esterase activity shows maximum polymorphism under Al concentration
The activity of esterase may represent any hydrolytic activities of macro-molecules under metal stress. Plants had significantly modulated its in vitro activity and significant variations were observed. As expected, the duration of Al treatment had distinct when activities were measured at different Al concentration. Still, the peak activity was at 360 µM with a significant (P ≤ 0.05) variation by 1.47 and 1.80-fold over the control respectively at 3 and 5-days (Fig. 11). It is also interesting to note that the higher Al concentrations were more inducive for esterase activity being higher at 3 than 5-days. Moreover, plants could not sustain this hyper-activity even at maximum Al concentration (480 µM) and that reduced by 06.59 and 16.92% against its maximum activities at 3 and 5-days. This may be looked upon the threshold concentration of Al to limit the enzyme activity beyond a certain value. More so, variations in activity were inconsistent through the days of incubation as well as metal concentrations as predicted from F values (F = 435.429, P ≤ 0.05). In addition to in vitro activity of esterase, the variations in polymorphic protein banding undoubtedly sufficed the expression potential of plants through doses of Al (Fig. 12). It is clearly noted that few isomorphic bands were resolved from polyacrylamide native PAGE according to gradient of molecular weights. Regardless of duration of metal exposure, the increased concentration of metal has regulated the bands of variable weights in inversely proportionate manner. This was more clarified when densitometric analysis of the gel was scanned through intensity gradient by Gel Analyzer software (2010a) (Fig. 13). Stringently to note that plants varied not in number of isozymes but in concentrations, however, in control. This got a significant down regulation at maximum Al dose where only 3 and 2 bands also with varied concentration were resolved at 3 and 5-days respectively. Still, at intermediate Al dose with 360 µM the maximum variation was recorded through 6 and 4 bands in respectively 3 and 5-days.
Assay of esterase activity under various aluminium treatments (Control-0, 120, 240, 360, 480 μM) for 3 days and 5 days. Data represented as mean of three replicates (n = 3) ± SE and SE represented as vertical bar on each column. Different letters indicate significant differences through Tukey HSD test at P ≤ 0.05
Densitometric analysis for intensity profiles of esterase on different polymorphic bands under varying Al concentrations (Control-0, 120, 240, 360, 480 µM) in 3 and 5-days. L1, L2, L3, L4, L5 & L6 denotes the different lanes with different Al doses and incubation days on native gel run with partially purified protein
Varying H2O2 accumulation under Al toxicity detected by DAB staining
A significant gradation in H2O2 accumulation which is phenotypically visualised under DAB staining was recorded. Figure 14 represents the differentiations of H2O2-DAB complex both in leaves and roots when compared varying days (3 and 5) as well as concentrations of Al. Undoubtedly, the highest concentration of Al (480 µM) appeared as more sensitive to produced H2O2 which is captured by DAB colouration. There was recorded significant variations among the DAB intensities in leaves and roots when compared for individual Al concentration but less variations among incubation days.
Tissue lysis are evident from Al toxicity in roots
Exposure of Al salt is undoubtedly detrimental to the root tissues which is evident from SEM images (Fig. 15). Roots being sensitive zone to Al showed a significant variation at highest Al concentration over control. The metal had left the tissues more dispersed due to dissolution of cell wall following disintegration into lumens in increased volume. Image recorded the hypodermal tissues in control with more compact but disorganised and fragile under Al exposure. Therefore, either with Al specific effects or its induced oxidative exposure certainly rendered damaging effects as herein presented.
Discussion
The present study would be interesting to note that C4 species, Amaranthus viridis L. behaved as an apparent tolerant to Al stress with two predominant features. Primarily, a dose dependent relationship of Al bio-accumulation in apoplastic spaces (which had not any apparent changes in morphology; data not shown here), however, within a threshold concentration. Generally, Al is predominantly associated with root growth (Barcelo and Poschenrieder 2002). The property of metal tolerant species is the relative bio-accumulation within the limit plants could survive. Still, this is with any moderation of cellular activities. A number of Al hyper-accumulators have been well in conformity with maximum metal translocated to aerial shoots and onto non-cellular/apoplastic spaces. This may be an adoption strategy to reduce vulnerability of root tissue to Al and thereby ensures its avoidance (Horst et al. 2010). In our experiment, the plant species might have bestowed their initial defense on non-cellular space deposition of Al. A series of C4 species have moderated to high cellular translocation efficiency of heavy metals more towards leafy shoots (Gleeson 2007). Amaranthus viridis, basically a hardy weed, is thus expected to withstand the higher Al concentration by any compatible process reducing the bio-toxicity. However, the variations of Al bio-accumulation in root and shoot in Amaranthus may favour the plant for avoidance of metal toxicity. This strategy for retention of metal more in root is displayed by tolerant species (Wójcik et al. 2005). Metal stress in plants is realised at the cellular level with sort of perturbance in redox. Al toxicity is very much concurrent to induce the elevation ratio of redox more towards oxidised states. Al, not a heavy metal still, has enough potential to disburse electrons from electron transport chain (ETC) in chloroplast and mitochondria and reduce the molecular O2 into ROS. A significant amount of ROS and its distribution through roots and leaves in Amaranthus may suffice the fact in the present experiment. The histochemical detection of accumulated ROS may suit the sensitivity of plants to oxidative stress. Thus, with the ongoing concentration of Al as well as incubation days recorded fair accumulation of H2O2 shown with DAB staining in Amaranthus herein. The expression of ROS mostly in hypodermal tissues may circumvent some hyper-sensitive reactions under metal stress also (SEM). So, both metal immobilization within certain tissues and its concomitant lysis behaves as an effective resistance as revealed with Amaranthus in the present experiment. Therefore, adjustability to Al accumulation ought to be parallel to make homeostasis of oxidised and reduced redox. With this view, Amaranthus was exhibited with some in vitro anti-oxidation properties.
Plants response to anti-oxidation is not only a measure for self defense but also procures bioresource values of different anti-oxidants. In a number of plants species, the elicitation through environmental fluctuations has documented the in vitro anti-oxidation potential of that species as an elite bioresource (Zhong et al. 2010). With this notion we find Amaranthus to evoke a significant anti-oxidation activity in vitro with chemical systems. Thus, initially with phosphomolybdenum complexes irrespective of days of incubation Al had been an inducing to develop some stable anti-oxidant. This was carry forwarded to the DPPH scavenging activity where through higher concentration had the compatible activity against BHT as positive control. Phosphomolybdenum complex is the utilization of phosphate rich residues with ROS to sequester following its hydrolysis (Khurshid et al. 2018). On the other hand, DPPH scavenging is justified with direct quenching of ROS with phenolic glycosides (Moukette et al. 2015). Amaranthus has successfully competed with other artificial ROS inducing residues and qualified with a significant quenching ability. Plants possess a number of heterocyclic compounds with varying degrees of unsaturation where lone pair electrons chemically reduce the ROS (Brewer 2011). Of those residues free iron (Fe3+) are most common to chelate the xenobiotic compounds. In the present experiment, ferric chelation activity was a marked feature where a gradual increase of activities recorded all through the Al concentration. Irrespective of anti-oxidation capacities the tissue extract was more efficient obtained from minimum days of incubation. This undoubtedly showed the period dependents of Al bio-accumulation following its developed oxidative stress. Similarly, ABTS also recorded scavenging activities with gradual depletion at prolong days of incubation. ABTS covers the overall anti-oxidation for organic residues conjugated with fully or partially ROS bearing nature (Dawidowicz and Olszowy 2013). Therefore, Amaranthus may be identified as a potential bioresource for overall in vitro anti-oxidation capacity of Al toxicity. As a result of established oxidative stress through Al bio-accumulation plants suffers from peroxidation reactions. Biomolecules like lipids, proteins and nucleic acids would most targeted ones to ensure peroxidation reactions corroborating cellular disintegration. Thus, the activity of H2O2 lysing system with guaiacol mediated peroxidase must be tuned in accordance to Al toxicity. Within threshold ranges of Al, the peroxidase activity may demark a bio-indication with different isozymic forms in plants. In the present case, the activities carry for their maximum potential according to exposure duration of Al. Undoubtedly, the plateau of activities for prolonged duration would be a limit for tolerance to H2O2 which is more extended for short duration. Catalase, the enzyme has not any reported electron donors in reduction of H2O2. This is a class II peroxidase which initiates the reactions with its constituent metallic co-factor to transfer the electrons over peroxides (Mhamdi et al. 2010). In C4 plants, H2O2 derived through anaplerotic reactions in non-green plant parts is often quenched above threshold value. Amaranthus is found to have a steady up-regulation of catalase activity regardless of treatment days. This came to plateau, however, more in prolong duration. This might be either inhibitions of de-novo synthesis of catalase or/and denaturing of metal concentration to proteins crossing the sensitivity (Benavides et al. 2005).
Metal toxicity is another alternative form of water deficit stress through attainment of more negative osmotic potential in plant tissues (Poschenrieder and Barceló 1999). In the present experiment, the increased bio-accumulation of the proline with consecutive days of incubation under Al concentration is a clear indication of dual roles. Proline, a compatible solute is frequently referred to stabilize cellular hydration making an elevated solute concentration (Ashraf and Foolad 2007). Second, proline with its heterocyclic structure could also be offered in quenching of ROS rendering a minimum level of anti-oxidation. In connection with non-enzymatic anti-oxidant, a pool of phenolics appeared to be a physiological trait to encounter the ROS in diverse forms in many reactions. Amaranthus is no exception of other plant species where an over production of phenolics is expected to have a broader anti-oxidation cascade. The stabilization of phenolic content at higher Al concentration could be a feedback mechanism to diminish the excess secondary metabolite biosynthesis. In plants few rate limiting enzymes for shikimic acid biosynthesis, precursors of phenolics have been well demarked as a biomarker for metal accumulations in excess (Kumar et al. 2017). As expected, the rise up of flavonoids was quite parallel in occurrence through the course of Al concentration with free amino acids (FAA). Increase in FAA either through de-novo biosynthesis or hydrolysis of proteins is a repository for its down stream cellular reactions. Total FAA with its corresponding involvement in few reactions may include the steady supplementation in secondary metabolite biosynthetic flux. There recorded a co-linearity of FAA with shikimic acid biosynthesis in few plant species under xenobiotic toxicity (Vivancos et al. 2011). Therefore, the physiological characterization would be more biased on any of precursor or primary residues for a particular secondary metabolite biosynthetic reaction path.
Metal tolerance in few plant species is a genotypic plasticity that undergoes induction by two ways. Exclusion of metal or its sequestering is the prime one and secondarily, to minimize the metal induced specific ion effects (Vijver et al. 2004). For the later it includes the anti-oxidation cascades and its evocation to nullify the ROS under oxidative stress. However, Al has been referred earlier to be excluded or sequestered in non-cellular spaces when it is chelated with organic acids. With this notion the activity of esterase in present experiment would be supportive for Amaranthus to release organic acids from its glucosides. This would be more assumed that Amaranthus had over expressed a number of possible polymorphisms (few/any of those) would be apparent to such an activity of esterase under Al toxicity. The proportionate increase in band numbers, variations in molecular masses for esterase undoubtedly supports the wider plasticity to metal tolerance. This is an index to fall under hyper-accumulator species (Mandal et al. 2016). On this basis, Amaranthus may also be considered as a potent species for bio-accumulation linked to tolerance and esterase would be possible biomarker of Al toxicity.
Conclusion
Now the observations from data and its possible clarification may meet the conclusion for the whole research work. Amaranthus viridis L. is basically a C4 weed grown in harsh environment with limited water and intense irradiation. As C4 species it is expected to have better stress tolerance under metal toxicity, particularly, with interference of Al through varying days. At cellular level the bio-accumulator nature of Amaranthus, at least within our experimental ranges of metal was verified with suitable osmoticum development and also appeared as a good reliever of water stress. Amaranthus may also be cited as a potential performer of anti-oxidation through in vitro anti-oxidation like iron chelation, phosphomolybdenum complex assay, DPPH, ABTS scavenging assays, anti-oxidant enzyme assays etc. Thereby, it may arise the possibility of bio-resources of anti-oxidation property. Sequestering of metal by distinct polymorphism for genes recruited for chelation reaction through esterase activity also support bio-accumulation. Therefore, this weed species may aspire for phytoremediation with both bio-accumulation in excess and improved anti-oxidation properties. More attention to be paid for other inherent properties of this species allied with the metal quenching ability along with biomarkers for Al toxicity in soil.
Abbreviations
- Al:
-
Aluminium
- ROS:
-
Reactive oxygen species
- FAA:
-
Free amino acid
- ABTS:
-
2,2′-azino-bis-3-ethylbenzthiazoline-6-sulphonic acid
- DPPH:
-
1,1-diphenyl-2-picrylhydrazyl
- BHT:
-
Butylated hydroxytoluene
- H2O2 :
-
Hydrogen peroxide
- PCA:
-
Perchloric acid
- DAB:
-
3′3′-diaminobenzidine
- GPX:
-
Guaiacol peroxidase
- CAT:
-
Catalase
- DTT:
-
Dithiothreitol
- PMSF:
-
Phenylmethane sulphonyl fluoride
- EDTA:
-
Ethylenediaminetetraacetic acid
- PVP:
-
Polyvinyl pyrrolidine
- SDS:
-
Sodium dodecyl sulfate
- BSA:
-
Bovine serum albumin
References
Ammar WB, Nouairi I, Zarrouk M, Ghorbel MH, Jemal F (2008) Antioxidative response to cadmium in roots and leaves of tomato plants. Biol Plant 52:727. https://doi.org/10.1007/s10535-008-0140-2
Ashraf MF, Foolad M (2007) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot 59:206–216
Balasubramanian T, Sadasivam S (1987) Changes in starch, oil, protein and amino acids in developing seeds of okra (Abelmoschus esculentus L. Moench). Plant Food Hum Nutr 37:41–46
Barcelo J, Poschenrieder C (2002) Fast root growth responses, root exudates, and internal detoxification as clues to the mechanisms of aluminium toxicity and resistance: a review. Environ Exp Bot 48:75–92
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Benavides MP, Gallego SM, Tomaro ML (2005) Cadmium toxicity in plants. Braz J Plant Physiol 17:21–34
Blois MS (1958) Antioxidant determinations by the use of a stable free radical. Nature 181:1199. https://doi.org/10.1038/1811199a0
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brewer MS (2011) Natural antioxidants: sources, compounds, mechanisms of action, and potential applications. Compr Rev Food Sci Food Saf 10:221–247
Czarnocka W, Karpiński S (2018) Friend or foe? Reactive oxygen species production, scavenging and signaling in plant response to environmental stresses. Free Radic Biol Med 122:4–20. https://doi.org/10.1016/j.freeradbiomed.2018.01.011
Dawidowicz AL, Olszowy M (2013) The importance of solvent type in estimating antioxidant properties of phenolic compounds by ABTS assay. Eur Food Res Technol 236:1099–1105
Gleeson AM (2007) Phytoextraction of lead from contaminated soil by Panicum virgatum L. (switchgrass) and associated growth responses (Doctoral dissertation)
Horst WJ, Wang Y, Eticha D (2010) The role of the root apoplast in aluminium-induced inhibition of root elongation and in aluminium resistance of plants: a review. Ann Bot 106:185–197
Khurshid H, Sabir SM, Awan SI, Abbas SR, Irshad M (2018) Antioxidant activities of aqueous extracts from nine different rose cultivars. Int J Food Stud 7:64–75. https://doi.org/10.7455/IJFS/7.2.2018.A6
Kumar R, Bohra A, Pandey AK, Pandey MK, Kumar A (2017) Metabolomics for plant improvement: status and prospects. Front Plant Sci 8:1302
Liu J, Li Z, Wang Y, Xing D (2014) Overexpression of ALTERNATIVE OXIDASE1a alleviates mitochondria-dependent programmed cell death induced by aluminium phytotoxicity in Arabidopsis. J Exp Bot 65:4465–4478. https://doi.org/10.1093/jxb/eru222
Mandal C, Bera S, Dey N, Adak M (2016) Physiological alterations of Salvinia natans L. exposed to aluminium stress and its interaction with polyamine. Plant Sci Today 3:195–206
Memon AR, Schröder P (2009) Implications of metal accumulation mechanisms to phytoremediation. Environ Sci Pollut Res 16:162–175
Mhamdi A, Queval G, Chaouch S, Vanderauwera S, Van Breusegem F, Noctor G (2010) Catalase function in plants: a focus on Arabidopsis mutants as stress-mimic models. J Exp Bot 61:4197–4220
Mittler R (2006) Abiotic stress, the field environment and stress combination. Trends Plant Sci 11:15–19
Morel I, Lescoat G, Cillard P, Cillard J (1994) Role of flavonoids and iron chelation in antioxidant action. In: Wilchek M, Bayer EA (eds) Methods in enzymology, vol 234. Academic Press, Cambridge, pp 437–443
Moukette BM, Pieme CA, Njimou JR, Biapa CP, Marco B, Ngogang JY (2015) In vitro antioxidant properties, free radicals scavenging activities of extracts and polyphenol composition of a non-timber forest product used as spice: Monodora myristica. Biol Res 48:15. https://doi.org/10.1186/s40659-015-0003-1
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Poschenrieder CH, Barceló J (1999) Water relations in heavy metal stressed plants. In: Prasad MNV (ed) Heavy metal stress in plants. Springer, Berlin, pp 207–229
Prieto P, Pineda M, Aguilar M (1999) Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem 269:337–341
Roberta R, Nicoletta P, Anna P, Ananth P, Min Y, Catherine RE (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. J Free Radic Biol Med 26:1231–1237
Roberts K, Granum E, Leegood RC, Raven JA (2007) C3 and C4 pathways of photosynthetic carbon assimilation in marine diatoms are under genetic, not environmental, control. Plant Physiol 145:230–235
Sakanaka S, Tachibana Y, Okada Y (2005) Preparation and antioxidant properties of extracts of Japanese persimmon leaf tea (kakinoha-cha). Food Chem 89:569–575
Sarkar B, De AK, Adak MK (2017) Physiological characterization of SUB1 trait in rice under subsequent submergence and re-aeration with interaction of chemical elicitors. Plant Sci Today 4:177–190. https://doi.org/10.14719/pst.2017.4.4.351
Singh AA, Agrawal SB, Shahi JP, Agrawal M (2014) Assessment of growth and yield losses in two Zea mays L. cultivars (quality protein maize and non-quality protein maize) under projected levels of ozone. Environ Sci Pollut Res 21:2628–2641
Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB (1997) Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley–powdery mildew interaction. Plant J 11:1187–1194. https://doi.org/10.1046/j.1365-313X.1997.11061187.x
Van Asperen K (1962) A study of housefly esterases by means of a sensitive colorimetric method. J Insect Physiol 8:401–416
Velioglu YS, Mazza G, Gao L, Oomah BD (1998) Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J Agric Food Chem 46:4113–4117
Verma S, Dubey RS (2003) Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Sci 164(4):645–655
Vijver MG, Van Gestel CA, Lanno RP, Van Straalen NM, Peijnenburg WJ (2004) Internal metal sequestration and its ecotoxicological relevance: a review. Environ Sci Technol 38:4705–4712. https://doi.org/10.1021/es040354g
Vivancos PD, Driscoll SP, Bulman CA, Ying L, Emami K, Treumann A, Mauve C, Noctor G, Foyer CH (2011) Perturbations of amino acid metabolism associated with glyphosate-dependent inhibition of shikimic acid metabolism affect cellular redox homeostasis and alter the abundance of proteins involved in photosynthesis and photorespiration. Plant Physiol 157:256–268
Wang J, Nayak S, Koch K, Ming R (2013) Carbon partitioning in sugarcane (Saccharum species). Front Plant Sci 18(4):201
Wójcik M, Vangronsveld J, Tukiendorf A (2005) Cadmium tolerance in Thlaspi caerulescens: I. Growth parameters, metal accumulation and phytochelatin synthesis in response to cadmium. Environ Exp Bot 53:151–161
Zafar M, Khan MA, Ahmad M, Jan G, Sultana S, Ullah K, Marwat SK, Ahmad F, Jabeen A, Nazir A, Abbasi AM (2010) Elemental analysis of some medicinal plants used in traditional medicine by atomic absorption spectrophotometer (AAS). J Med Plants Res 4:1987–1990
Zhishen J, Mengcheng T, Jianming W (1999) The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem 64:555–559
Zhong XK, Jin X, Lai FY, Lin QS, Jiang JG (2010) Chemical analysis and antioxidant activities in vitro of polysaccharide extracted from Opuntia ficus indica Mill. cultivated in China. Carbohydr Polym 82:722–727. https://doi.org/10.1016/j.carbpol.2010.05.042
Acknowledgements
This work is acknowledged for financial support from University Grant Commission (UGC), Government of India through NET JRF Fellowship scheme to first author and this work is also supported by DST-PURSE II Program applicable to University of Kalyani.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sarkar, B., Saha, I., De, A.K. et al. Aluminium accumulation in excess and related anti-oxidation responses in C4 weed (Amaranthus viridis L.). Physiol Mol Biol Plants 26, 1583–1598 (2020). https://doi.org/10.1007/s12298-020-00840-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-020-00840-z