Abstract
Paddy field farming remains the dominant form of growing rice in modern times as the rice is the staple food for over half the world’s population and is closely associated with food security and political stability of many countries. Record increase in rice production have been observed since the start of the Green Revolution. India is one of the largest paddy producer and exporter in the world. However, constant use of chemical herbicide like paraquat had shown adverse impact on the rice yield. Non-target organisms of the habitat including cyanobacterial paddy biofertilizer face the herbicide toxicity and are unable to perform efficiently their role as biofertilizer. Therefore, in the present study, an attempt has been made to enhance the paraquat resistance in rice biofertilizer (Microchaete sp. NCCU-342) by exogenous addition of salicylic acid. Paraquat showed toxicity in Microchaete sp. NCCU-342 in a dose-dependent manner. Concentration of paraquat >1.0 μM exhibited lethal effect since the beginning. Through successive narrow range experiment, LD50 value of paraquat was obtained as 0.6 μM. Biomass exposed to paraquat (LD50 value) and salicylic acid (0.3 mM) showed mitigation in free radical production (2.20 % MDA and 1.69 % H2O2) and enhancement in the activity of the antioxidant enzymes activity, i.e. SOD, CAT, APX (137.76 %, 87.45 %, 118 %, respectively) and osmolytes (3.8 % proline and 21.51% sucrose). Thus, for sustainable agricultural practice, especially for paddy field cyanobacterial biofertilizer, application of salicylic acid or organism with higher salicylic acid production ability may be an alternative to overcome the paraquat toxicity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the age of industrial development, the use of pesticides and metals are the most challenging pressure on the sustainable agricultural system. Herbicides, pesticides and chemical fertilizers are being frequently used to increase crop yield without paying much attention to the problem of residual toxicity. Paraquat dichloride (1, 1’-dimethyl-4, 4’-bipyridinium dichloride), or paraquat (PQ), is a quaternary ammonium salt. It is a water-soluble, broad-spectrum and non-selective contact herbicide of the methyl viologen group. It has been characterized as the class II moderately hazardous pesticide by the World Health Organization (WHO) which has been commonly used in the rice field to increase productivity. Central Insecticide Board and Registration Committee (CIBRC, India) has approved its use only for nine crops in India, viz. tea, cotton, grapes, rubber, rice, apple, maize, wheat and potato (Watts 2011). However, a high dose of PQ decreases nitrogen fixation and photosynthesis in cyanobacterial biofertilizers (non-target organism) that ultimately reduces paddy yield (Sood et al. 2011). Free radicals including superoxide anions, singlet oxygen, hydrogen peroxide, hydroxyl and peroxyl radicals were found to be induced in the presence of PQ in plants. It interferes with the photosynthetic electron transport system, stability of protein and DNA ultimately leading to cell death (Ananieva et al. 2004).
Salicylic acid (SA) is a lipophilic derivative of benzoic acid. It is considered as a phytohormone and a prominent signalling molecule whose expression is induced under stress in both prokaryotic and eukaryotic organisms. It reduces the toxic effect by modifying the physiological and biological processes of the organism (Ananieva et al. 2002). Exogenous supplementation of salicylic acid has been observed to reduce the toxic effect of herbicide, insecticide and fungicide in crops like Brassica napus (Cui et al. 2010), Triticum aestivum (Ananieva et al. 2002), Zea mays (Deef 2013), Arabidopsis thaliana (Kim et al. 2003) and Vigna radiata (Fatma et al. 2018). SA is reported to reduce the electrolyte leakage, hydrogen peroxide and malondialdehyde (MDA) level in PQ-exposed barley by increasing antioxidant (Ananieva et al. 2004). To the best of our knowledge, so far, there is no report on the effect of salicylic acid on PQ toxicity in cyanobacterial biofertilizer.
Microchaete is reported as a component of cyanobacterial natural biofertilizer of rice field that is also facing PQ toxicity (Thajamanbi et al. 2016). In the present study, an attempt has been made to work on the hypothesis that SA act as a PQ toxicity ameliorating agent in non-target cyanobacterial biofertilizer. Further, the present investigation was also planned to evaluate the effect of exogenous SA supplementation on bio-constituents of PQ exposed cyanobacterial biofertilizer that helps in coping with the herbicide toxicity.
Materials and methods
Chemicals and culture
All analytical grade chemicals used in experiments were procured from Hi-Media, Mumbai (India), and Merck & Co., New York (USA). The growth media, reagents and buffers were prepared in double-distilled water. The test organism, Microchaete sp. NCCU-342, was procured from IARI, New Delhi, India. It was maintained in BG-11 medium (without nitrogen source) at 28 ±1 °C, 12:12 light and dark cycle and shaken manually after frequent intervals to avoid clumping of cells.
Determination of growth and endogenous salicylic acid induction in Microchaete sp. NCCU-342 exposed to paraquat
Paraquat (PQ) stock solution (1mM) was prepared by dissolving 2.6 mg paraquat dichloride in 10 ml of distilled water. The stock solution was further diluted with distilled water to obtain working concentrations of PQ [(a) wide range 1.0, 1.2, 1.4, 1.6, 1.8 and 2.0 μM and (b) narrow range 0.1, 0.2, 0.4, 0.6, 0.8 and 1.0 μM] and added to the growth medium. The growth conditions for cultures were same as mentioned above. To analyse PQ toxicity, growth of cyanobacteria was monitored in its presence after every 24 h for 96 h. Biomass was harvested by centrifugation, and growth was evaluated by analysing dry biomass weight.
LD50 of PQ was determined through linear regression (probit analysis) (Singh and Zahra 2017). Specific growth rate was calculated by using the formula as given by De Oliveira et al. 1999:
where X0 is the initial dry biomass weight (in mg), X is the final dry biomass weight (in mg) at time t (in hours) and μ is the specific growth rate.
Endogenous salicylic acid production was checked in the cultures exposed at 0.6 μM PQ (LD50). Salicylic acid extraction was done according to the protocol of Raskin et al. (1989), and quantification was done by following spectrophotometry protocol of Warrier et al. (2013). For extraction of endogenous salicylic acid, 50 mg biomass was homogenized with 10 ml methanol (90%) and centrifuged at 5000 rpm. Pellet was dissolved in 10 ml methanol (100%) and centrifuged at 8000 rpm for 15 min. The supernatant was evaporated at 60 °C, and the residue was re-suspended in 5ml trichloroacetic acid (5%) and centrifuged at 5000 rpm. The supernatant was partitioned twice with double the volume of ethyl acetate (Raskin et al. 1989). The production of salicylic acid was confirmed by simple colorimetric test. To the ethyl extract (1 ml), alcoholic ferric chloride (1 ml) was added. Formation of purple-violet colour indicated the presence of salicylic acid that was measured at 540 nm. For quantifying the amount of salicylic acid, the standard curve of salicylic acid was prepared (Warrier et al. 2013). The concentration of the stock solution was 1mg/ml. It was further diluted with distilled water to obtain different working concentrations (0.01–5 μg/ml). Absorbance was taken at 540 nm. The calibration curve was described by the equation given below with regression coefficient (r2) being 0.9812. The following equation was used to calculate the amount of standard salicylic acid.
where Y is absorbance at 540 nm and X is the concentration of salicylic acid in μg/ml.
Determination of exogenous salicylic acid role in mitigating PQ toxicity
The stock solution of salicylic acid was diluted with distilled water to obtain different working concentrations (0.1, 0.3 and 0.5 mM). Culture exposed to PQ (LD50) was exogenously supplemented with salicylic acid (0.1, 0.3 and 0.5 mM) to finalize the effective dose of SA for further experiments.
The experiment was done in triplicates and consisted of four culture sets including Microchaete sp. NCCU-342 culture (control), PQ-exposed culture (PQ), SA-exposed culture (SA) and both SA and PQ exposed culture (SA+PQ). All the four culture sets were allowed to grow under the above-mentioned culture conditions. Pigments (chlorophyll, carotenoid and phycobilin), biochemical constituents (protein content, carbohydrate content), malondialdehyde (MDA) content, H2O2 content, superoxide dismutase (SOD) activity, catalase (CAT) activity, ascorbate peroxidase (APX) activity, amount of proline and sucrose were evaluated as test parameters.
The content of chlorophyll was determined by the modified protocol of Mackinney (1941). Ten milliliter of 95 % methanol was added to 5 mg dried biomass in a test tube. After vortexing, it was incubated at 65 °C for 30 min in a water bath which was then cooled to room temperature. Loss of solvent by evaporation was maintained by adding methanol and then centrifuging at 5000 rpm for 10 min. Pellets were discarded, and supernatant was collected whose absorbance was taken at 650 nm and 660 nm against 95 % methanol as blank. Total chlorophyll was calculated by using the following formula.
Carotenoid content was estimated according to the method given by Herborne (1973). Fifty milligrams of biomass was taken in the test tube to which 5 ml of 80 % acetone was added. The content was then homogenized and centrifuged at 3000 rpm for 10 min. Supernatant was collected whose absorbance was recorded at 450 nm against 80 % acetone as blank. The amount of total carotenoid was calculated by using the following formula:
where dilution factor is 4, V is the volume of extract (in ml) and W is the weight of biomass (in mg).
Phycobilins extraction was carried out by freezing and thawing in extraction buffer (0.1 M phosphate buffer, pH 6.8) (Siegelman and Kycia 1978), after which centrifugation was done at 5000 rpm for 10 min and the absorbance was taken at 562 nm, 615 nm and 652 nm. Phycocyanin, allophycocyanin and phycoerythrin contents were calculated by using the following formula:
Protein content was determined according to the method given by Lowry et al. (1951). Biomass (50 mg) was homogenized in 5 ml of extraction buffer and centrifuged at 8000 rpm for 20 min. Freshly prepared reagent A (1 ml 1% Na-K tartrate solution containing 0.5 % CuSO4 was added into 50 ml 2 % Na2CO3 solution) was added to the supernatant obtained from previous step and incubated for 10 min. Simultaneously, reagent B, i.e. Folin’s reagent (0.5 ml), was added and kept in dark for 30 min to allow the development of purple-blue colour that indicated the presence of proteins. Standard solution of bovine serum albumin (BSA) was prepared in different concentration for quantification of total protein and absorbance was taken at 650 nm. The concentration of this stock solution was 1 g/l. It was diluted with distilled water to obtain different BSA concentrations (20–250 mg/ml). The calibration curve was described by the equation given below with regression coefficient (r2) being 0.9892. This equation was used to calculate the amount of BSA.
where Y is absorbance at 650 nm and X is concentration of BSA in mg/ml.
Anthrone reagent method was used to estimate total carbohydrate (Spiro and Spiro 1966). Sample was prepared by taking 1 mg biomass, to which 1.25 ml of double distilled water and 4 ml of anthrone reagent was added and mixed properly. Then, the prepared sample was incubated in water bath at 95 °C for 8 min and then cooled under running water. Standard solution of glucose i.e. the stock solution (1 g/l) was prepared, which was further diluted with distilled water to obtain different glucose concentrations (10–150 mg/ml). Absorbance was recorded at 620 nm. The calibration curve was described by the equation given below with regression coefficient (r2) being 0.9795. This equation was used to calculate the amount of glucose.
where Y the is absorbance at 620 nm and X is the concentration of glucose in mg/ml.
For estimation of MDA, cells were homogenized in 1% TCA and centrifuged at 10,000 rpm for 15 min (Heath and Packer 1968). The supernatant was re-suspended in 0.5 % thiobarbituric acid (TBA) in 20 % TCA solution and then incubated in water bath at 95 °C for 30 min and cooled under running water. Absorbance was taken at 532 nm and 600 nm. MDA was estimated using extinction coefficient (155 mM−1 cm−1).
For estimation of hydrogen peroxide, fresh biomass (50 mg) was homogenized in 1.5 ml sodium phosphate buffer (50 mM, pH 6.5) and centrifuged at 10,000 rpm for 15 min (Jana and Choudhuri 1982). 1 ml of supernatant was mixed with 4 ml of 0.1% titanium sulphate containing 20% sulphuric acid. After the development of yellow colour, absorbance was taken at 410 nm. Hydrogen peroxide was estimated using extinction coefficient (0.28 μmol cm−1).
SOD activity was determined by monitoring the 50 % inhibition of photo-reduction of nitro blue tetrazonium chloride (NBT) (Beauchamp and Fridovich 1971). Fresh biomass (0.05 gm) was homogenized in 2 ml of phosphate buffer (0.5 M, pH 7.5) and centrifuged at 15,000 rpm at 4 °C for 15 min. The reaction mixture (3 ml) contained 2.9 ml potassium phosphate (50 mM, pH 7.8) containing 0.025% Triton-X 100, 1.17 μM riboflavin, 168 μM NBT, 10 mM methionine and 100 μl extract. Blank contained everything except the enzyme extract. Both the reaction mixture and blank were incubated for 15 min. The photo-reduction of NBT resulted in the formation of purple formazan that was measured at 560 nm. 1U of SOD activity is the amount of enzyme that causes 50% inhibition of the photo-reduction of NBT, i.e. formazan formation. SOD activity was calculated by using the given formula:
where Ac is the amount of formazan formed in the absence of enzyme, At is the amount of methionine-mediated formazan formed and dilution factor is 30.
For measuring the catalase (CAT) activity, fresh biomass (0.05 gm) was homogenized in 2 ml of phosphate buffer (0.5 M, pH 7.5) and centrifuged at 10,000 rpm at 4 °C for 15 min. Reaction mixture (2.0 ml) contained 0.1 ml of enzyme extract, 1.6 ml phosphate buffer (pH 7.3), 0.1 ml of EDTA (3 mM) and 0.2 ml of H2O2 (0.3%) which was incubated for 3 min and absorbance was taken at 240 nm (Aebi 1984). Catalase activity was calculated by taking ℇ 240 as 43.6 M−1cm−1 against a blank of same reaction mixture except hydrogen peroxide.
1U of the catalase activity is the amount of enzyme that decomposes 1 μl of hydrogen peroxide per minute at 25 °C.
For determination of ascorbate peroxidase (APX) activity, fresh biomass (0.05 gm) was homogenized in 2 ml of phosphate buffer (0.5 M, pH 7.5) and centrifuged at 10,000 rpm at 4 °C for 15 min. Reaction mixture was prepared by mixing 1 ml phosphate buffer (pH 7.3), 1 ml ascorbate (5 mM), 0.1 ml EDTA, 0.2 ml hydrogen peroxide (0.3%) and 1 ml enzyme extract. Absorbance was taken at 290 nm against blank (Asada 1997). The activity of APX was calculated by using an extinction coefficient of 2.8 mM−1cm−1:
1U of the enzyme is the amount necessary to decompose 1 μl of hydrogen peroxide per minute at 25 °C. The optical density was measured at 290 nm, and ascorbate peroxidase activity was calculated by taking ℇ 290 as 2.8 M−1cm−1 against a blank of same reaction mixture except hydrogen peroxide.
For determination of proline, 50 mg biomass was crushed in 10 ml of sulphosalicylic acid (3%) and centrifuged for 10 min at 8123 rpm. Supernatant was collected, to which 2 ml glacial acetic acid and 2 ml acid ninhydrin solution were added and heated at 100 °C for 60 min. Thereafter, 4 ml toluene was added and properly mixed by vortexing. The sample was then kept in an ice bath for 1 min for the partitioning of organic and inorganic layer. The absorbance of chromophore containing toluene solution was taken at 520 nm. Standard curve of proline was also plotted (Bates et al. 1973). The concentration of this stock solution was 1 mg/ml. It was diluted with distilled water to obtain different proline concentrations (10–150 μg/ml). The calibration curve was described by the equation given below with regression coefficient (r2) being 0.9754. This equation was used to calculate the amount of standard proline.
where Y is the absorbance at 520 nm and X is the concentration of proline in μg/ml.
For sucrose determination, 0.01 g dried biomass was dissolved in 10 ml of ethanol (80%) and placed in a water bath at boiling temperature to degrade the hexoses. The solution was then cooled, and 100 μl potassium hydroxide (30% aqueous) was added to it and kept in the boiling water bath. After cooling the samples to room temperature, anthrone reagent (3 ml) was added and then incubated at 38 °C for 20 min. By taking sucrose as standard, samples were quantified at 620 nm (Van Handel 1968). The concentration of this stock solution was 2 mg/ml. It was diluted with distilled water to obtain different sucrose concentrations (10–1000 μg/ml). The calibration curve was described by the equation given below with regression coefficient (r2) being 0.9754. This equation was used to calculate the amount of standard sucrose.
where Y is the absorbance at 620 nm and X is the concentration of sucrose in μg/ml.
Statistical analysis
All data were evaluated statistically and represented as mean ± standard deviation (SD) of three independent replicates. Means were compared by t-test and one-way ANOVA followed by least significant differences (LSD) test at levels P < 0.05. All statistical analysis was carried out by using GraphPad Prism Version 8.0.1 (244).
Results and discussion
According to the WHO, the world’s population is expected to increase from the current 7.7 billion to 9.7 billion by 2050 (Leridon 2020). To feed such a large population, there is a need to increase the crop yield, that too, using sustainable agricultural practices. Pesticide, herbicide and chemical fertilizer-based agriculture practices are not advisable due to their adverse effect on soil as well as on crop quality. It has forced the scientific community to find out alternate ways for increasing food and feed production. The present investigation is an effort towards sustainable agriculture in rice fields, using salicylic acid as a paraquat toxicity mitigator.
Relationship between Microchaete sp. NCCU-342 growth and paraquat
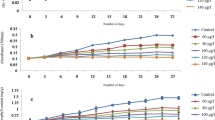
Indiscriminate application of herbicide for weed control is known to affect the physiological pathways and induces oxidative stress in non-target organisms as well. To understand the effect of PQ on Microchaete sp. NCCU-342, cultures were exposed to different PQ concentrations [(a) 1.0, 1.2, 1.4, 1.6, 1.8 and 2.0 of wide range and (b) 0.1, 0.2, 0.4, 0.6, 0.8 and 1.0 μM of narrow range] for 96 h. PQ showed the toxic effect on the growth of the test organism in a dose-dependent manner (Fig. 1 a and b). Visually, the colour of the culture changed from greenish-brown to yellowish-white. At higher PQ concentrations (≥1.0 μM), cells started to die. The cultures were grown for 96 h, and specific growth rate, as well as LD50, were determined. These values reflect the tolerance or sensitivity of the test organism against paraquat. The specific growth rate of the control culture (0.3662 h−1) was higher than the PQ exposed culture (0.1988 h−1) (Fig. 1c). The LD50 value of paraquat was obtained to be 0.6 μM that indicated the death of half of the population of culture at this concentration (Fig. 1d). Further, experiments to study the effect of PQ on biochemical constituents of Microchaete were erformed at this concentration (LD50).
Effect of paraquat (PQ) on Microchaete sp. NCCU-342. a growth in wide range, b growth in narrow range, c specific growth rate and d LD50 (as dry biomass at 96 h) of paraquat (PQ). All the values are the means of three replicates with standard deviation, and asterisks (*) indicate level of significant at P < 0.05.
Similar results have also been reported in other cyanobacterial species including Nostoc sp. N1 and Anabaena sp. A1 (Tansai et al. 2018) and Anabaena variabilis and Plectonema boryanum (Dragolova et al. 2002). A decrease in specific growth has been also observed in malathion exposed Anabaena variabilis (Ningthoujam et al. 2013). Chlorella vulgaris have been reported to show a similar LD50 of 0.67 μM (Qian et al. 2009). The higher the LD50 value, the more the tolerance against any pollutant. Scenedesmus dimorphus has been reported to be more sensitive with LD50 of 0.29 μM, whereas Selenastrum capricornutum showed more tolerance with an LD50 value of 7 μM (Ibrahim 1990). PQ has been observed to reduce the growth of Chlorella vulgaris and Chlorella pyrenoidosa by inhibiting the synthesis of chlorophyll and increasing ROS generation (Qian et al. 2009; Zhang et al. 2014). PQ accepts the electrons from PS-I, interferes with the intracellular electron transport system (ETS) and blocks NADPH formation that disrupts NADPH requiring biochemical process. Lack of NADPH could further lead to the formation of ROS, hampering the growth even more (Sood et al. 2011).
Paraquat induces endogenous salicylic acid in Microchaete sp. NCCU-342
Salicylic acid is a key endogenous secondary signalling molecule that induces defence response. PQ (0.6μM, LD50)-treated Microchaete cultures showed increased production of SA (185.78 % (significant at P ≤ 0.05)) after 96 h (Fig. 2a). This may be attributed to the cell’s efforts to overcome the PQ-imposed stress. Previous studies in higher plants have also shown that endogenous salicylic acid induced protection against abiotic and biotic stress (Mauch et al. 2001; Yang et al. 2004).
a Effect of paraquat (PQ) (0.6 μM, LD50) on production of endogenous salicylic acid in Microchaete sp. NCCU-342. Values are means of three replicates with standard deviation and asterisks (*) indicate level of significant at P < 0.05. b Effect of exogenous salicylic acid (SA) dose on the growth (as dried biomass) of Microchaete sp. NCCU-342 exposed to paraquat (PQ) (0.6 μM, LD 50). Values are means of three replicates with standard deviation, and asterisks (*) indicate level of significant at P < 0.05.
Effect of exogenously supplemented salicylic acid on PQ toxicity
To understand the role of exogenous salicylic acid in reducing PQ toxicity, cyanobacterial culture with PQ was supplemented with 0.1, 0.3 and 0.5 mM of salicylic acid and allowed to grow for 96 h. The cultures supplemented with exogenous salicylic acid showed growth patterns similar to control. The extent of protection increased from 0.1 to 0.3 mM of SA (Fig. 2b). The stimulatory effect of salicylic acid on growth has also been reported in Chlorella vulgaris (Czerpak et al. 2002). However, higher concentration of SA (0.5 mM) showed decreased growth. According to Mateo et al. (2006), the decrease in growth at higher SA concentration occurs due to increased ROS production under abiotic stress.
From growth observations, the moderate concentration of SA (0.3 mM) was selected for further studies. For understanding the interaction of SA in reducing PQ toxicity, different experimental sets were prepared, which are control (only cyanobacterial culture), culture with PQ (0.6 μM), culture with SA (0.3 mM) and culture with PQ as well as SA. These sets were allowed to grow for 96 h. Biomass obtained afterwards was used for biochemical analysis for pigment, protein, carbohydrate, free radicals, antioxidant enzymes and osmolytes.
Pigment content
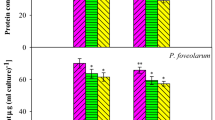
PQ-induced reduction in pigment content was observed in the present study (68 %chlorophyll, 69.34 % carotenoid, 30 % phycobilin; Fig. 3 a, b and c). Similar instances of PQ-imposed toxicity and associated reduction in chlorophyll was also reported in Chlorella vulgaris (Qian et al. 2009), Azolla microphylla (Sood et al. 2011) and Hordeum vulgare (Popova et al. 2003). Reduction in carotenoid content due to PQ toxicity has been also noticed in the pea plant in previous studies (Szafranska et al. 2016). Other pesticides like glyphosate (herbicide) have been reported to show the carotenoid breakdown in Chlorella pyrenoidosa (Hernando et al. 1989). The reduction in phycobilin pigment have been also determined in Nostoc sp. N1 and Anabaena sp. A1 under PQ toxicity (Tansai et al. 2018) and in Plectonema boryanum under endosulfan stress (Prasad et al. 2005).
Effect of 0.3 mM exogenous salicylic acid (SA) on pigments of Microchaete sp. NCCU-342 exposed to paraquat (PQ) (0.6 μM, LD50). a chlorophyll content, b carotenoid content, c phycobilin content, d protein and e carbohydrate. Values are means of three replicates with standard deviation, and asterisks (*) indicate level of significant at P < 0.05.
However, SA supplementation in PQ-exposed culture exhibited higher pigment content (108 % chlorophyll, 93.19 % carotenoid and 22 % phycobilin) as compared to PQ-treated culture, (significant at P ≤ 0.05). The results obtained in the present study were also in line with the previous studies. SA has been shown to mediate higher chlorophyll content under PQ stress in barley (Ananieva et al. 2002); under isoproturon stress in Pisum sativum (Singh et al. 2016); under mancozeb (fungicide), chlorpyrifos (insecticide) and metribuzin (herbicide) stress in Vigna radiate (Fatma et al. 2018); and under clethodim (herbicide) stress in Zea mays (Radwan and Soltan 2012).
Treatment of salicylic acid is also reported to improve carotenoid content in Pisum sativum under isoproturon stress (Singh et al. 2016); in Vicia faba under alphamethrin and endosulfan exposure (Singh et al. 2013); in peanut under Basagran® (Radwan et al. 2019); and in Vigna radiate under mancozeb (fungicide), chlorpyrifos (insecticide) and metribuzin (herbicide) (Fatma et al. 2018).
Protein and carbohydrate
The PQ-treated cultures, after 96 h, exhibited a 79.84 % decrease in protein content than control (Fig. 3d). In Chlamydomonas moewusii (Prado et al. 2009) and barley seedlings (Ananieva et al. 2002), PQ-induced decrease in the protein content were also reported. Similar observation has been recorded with other herbicides/pesticides in other organisms, e.g. in Zea mays under clethodim stress (Radwan and Soltan 2012), in Vigna radiata under metribuzin stress (Fatma et al. 2018), in Pisum sativum under isoproturon stress (Singh et al. 2016), in Anabaena sp. under Lindane stress (Babu et al. 2001) and in Lingulodinium polyedrum under polychlorinated biphenyl (PCB) stress (Leitao et al. 2003).
Supplementation of salicylic acid in PQ-exposed culture showed protective effects against PQ toxicity by enhancing the protein content by 47.82 % (significant at P ≤ 0.05) in experimental cyanobacteria compared to PQ-treated culture. SA supplementation in Zea mays under clethodim stress (Radwan and Soltan 2012) and in Salvia officinalis L. under salt stress (Sahar et al. 2011) exhibited similar results in previous studies. The increase in protein content in the presence of salicylic acid may be due to the induction of antioxidant proteins, heat shock proteins and other stress signalling proteins that aid in the defence of organism to combat stress (Ananieva et al. 2004; Radwan and Soltan 2012).
Carbohydrate content was also observed to increase [171.72 % (significant at P ≤ 0.05)] after 96 h in PQ-treated cultures as compared to control (Fig. 3e). According to Averamova and Rossler (1975), decreased protein content triggers the carbohydrate synthesis for the protection of cellular integrity. Similar results have been also observed in Nostoc kihlmani and Anabaena oscillariodes under thiobencarb toxicity (Mansour et al. 1994) and in Nostoc muscorum, Anabaena variabilis and Aulosira fertilissima under endosulfan toxicity (Kumar et al. 2008). The supplementation of SA in PQ-treated culture resulted in an almost 33.6 % decrease in carbohydrate as compared to only PQ-treated cultures (significant at P ≤ 0.05). It is believed that salicylic acid accelerates the carbohydrate and sugar metabolism and its polymerization to polysaccharides to maintain the osmolarity of cell and other cellular constituents for the protection of cells against PQ toxicity (Khodary 2004).
Oxidative damage
After 96 h, MDA and hydrogen peroxide were observed to increase significantly (4.46 % and 3.78 % respectively) in Microchaete sp. NCCU-342 under PQ stress as compared to control (significant at P ≤ 0.05) (Fig. 4 a and b), suggesting induction of oxidative stress. Previous studies have also reported an increase in MDA and hydrogen peroxide on exposure to PQ, as reported in case of Chlorella vulgaris (Qian et al. 2009), Azolla microphylla (Sood et al. 2011) and Barley (Ananieva et al. 2002). The increased level of free radical may be due to the PS-I-mediated conversion of PQ di-cation into mono-cation radical which then interacts with oxygen molecules to produce hydrogen peroxide and other reactive species that induces a cascade of toxic effects like generation of oxidative ions in ETS, protein degradation, and other alterations in cellular environment (Fuerst and Norman 1991). Moreover, pesticides other than PQ have also shown MDA and hydrogen peroxide generation and associated toxicity. For instance, it is reported that endosulfan (insecticide) toxicity in Anabaena variabilis, Aulosira fertilissima and Nostoc muscorum (Kumar et al. 2008) has shown similar results.
Cultures supplemented with both SA and PQ exhibited a decrease in MDA (2.20 %) and hydrogen peroxide (1.69 %) content. Such observations have also been noticed in barley (Ananieva et al. 2002), peanut (Radwan et al. 2019) and Brassica napus (Hasanuzzaman et al. 2014) under PQ, Basagran® and salt stress, respectively.
Antioxidant enzymes
For counteracting the toxic effects of ROS, the antioxidant system of Microchaete sp. NCCU-342 played a remarkable role. After 96 h, in PQ-exposed cultures, a significant increase in activity of SOD (78.84%), CAT (40.92 %) and APX (96 %) were recorded as compared to control (Fig. 5 a, b and c). In previous studies as well, an increase in antioxidant enzyme activity has been observed in Chlorella vulgaris (Qian et al. 2009), in Chlorella pyrenoidosa (Zhang et al. 2014), in Azolla microphylla under PQ stress (Sood et al. 2011) and in Aulosira fertilissima, Plectonema boryanum, and Anabaena variabilis (Kumar et al. 2008) and Plectonema boryanum (Prasad et al. 2005) under endosulfan toxicity.
Effect of 0.3 mM exogenous salicylic acid (SA) on antioxidant enzyme system of Microchaete sp. NCCU-342 exposed paraquat (PQ) (0.6 μM, LD50). a SOD activity, b CAT activity and c APX activity. Values are means of three replicates with standard deviation, and asterisks (*) indicate level of significant at P < 0.05.
Supplementation of SA to PQ-exposed cultures further enhanced antioxidant enzyme activities [SOD (137.76%), CAT (87.45 %), and APX (118 %) (significant at P ≤ 0.05)] as compared to PQ-treated culture. It has also been reported that exogenous SA plays protective role in the scavenging of ROS and H2O2 by augmenting the activities of SOD, CAT and APX in Oryza sativa under quinclorac and copper stress (Wang et al. 2016; Mostofa and Fujita 2013), in tomato under salt stress and in Phaseolus vulgaris L. under cadmium toxicity (Wael et al. 2015).
Osmolytes
All living cells accumulate osmolytes under stress conditions to maintain cellular osmolality and cell volume (Burg and Ferraris 2008). It is suggested that proline plays a protective role under herbicide stress by adjusting the osmotic pressure of the cell and also by scavenging the free radicals (Radwan et al. 2007). In the present study, osmolyte proline increased in PQ-treated cultures (139 %) as compared to control (significant at P ≤ 0.05). SA supplementation further increased (3.8 %) the proline content than the PQ-treated cultures after 96 h (Fig. 6a), probably to protect the cellular enzymes. According to previous reports, salicylic acid enhances the accumulation of proline under many abiotic stresses, e.g. in faba bean plant (Ahmad et al. 2018) and Gossypium hirsutum L. under salt stress (Dong et al. 2015) and in Amaranth and tomato plants under water deficit stress (Umebese et al. 2009).
Sucrose acts as a storage compound, signalling, metabolic and development molecule that plays a crucial role in defence mechanisms (Burg and Ferraris 2008). It is a major photosynthetic product in all oxygenic organisms, including microalgae (Hagemann et al. 1999). Microchaete sp. NCCU-342 total sucrose content increased in PQ-treated culture (426 %) as compared to the control (Fig. 6b). Similar trend has also been observed in Anabaena variabilis under malathion toxicity (Ningthoujam et al. 2013). In the present study, after 96 h, a 21.51 % increment in sucrose content was observed in SA+ PQ cultures than the PQ-treated culture (significant at P ≤ 0.05) (Fig. 6b). SA treatment in Brassica napus under drought stress has also shown sucrose accumulation (Lee et al. 2019). The elevated sucrose activation is believed to enhance the synthesis of metabolites needed for antioxidant defence mechanism (Hagemann et al. 1999).
Conclusion and future perspectives
PQ showed an adverse effect in the cyanobacterial biofertilizer Microchaete sp. NCCU-342 that, in turn, affects the production of rice crop (see supplementary file). Application of exogenous salicylic acid promoted the growth, pigment, protein and carbohydrate synthesis in the test organism under PQ stress by reducing ROS (MDA and H2O2), and inducing antioxidants enzymes (SOD, CAT and APX) and osmolytes. The findings of the present study suggest that in sustainable agriculture, the application of salicylic acid or the use of cyanobacteria that possess the ability to produce high salicylic acid may be used as an alternative to overcome the pesticide/herbicide-induced toxicity so that the crop yield may be enhanced.
References
Aebi H (1984) Catalase in vitro. In Methods in Enzymology Academic Press, vol 105, pp 121–126
Ahmad P, Alyemeni MN, Ahanger MA, Egamberdieva D, Wijaya L, Alam P (2018) Salicylic acid (SA) induced alterations in growth, biochemical attributes and antioxidant enzyme activity in faba bean (Vicia faba L.) seedlings under NaCl toxicity. Russ J Plant Physiol 65(1):104–114
Ananieva EA, Alexieva VS, Popova LP (2002) Treatment with salicylic acid decreases the effects of paraquat on photosynthesis. J Plant Physiol 159(7):685–693
Ananieva EA, Christov KN, Popova LP (2004) Exogenous treatment with salicylic acid leads to increased antioxidant capacity in leaves of barley plants exposed to paraquat. J Plant Physiol 161(3):319–328
Asada K (1997) The role of ascorbate peroxidase and monodehydroascorbate reductase in H2O2 scavenging in plants. Oxidatives Stress and the Molecular Biology of Antioxidant Defenses., Cold Spring Harbor Laboratory Press, 715–735
Averamova S, Rossler M (1975) Effect of various temperatures on some physiological-biochemical induces during the light phase of the life cycle of Scenedesmus sp. Appl Microbiol 5:115–120
Babu GS, Hans RK, Singh J, Viswanathan PN, Joshi PC (2001) Effect of lindane on the growth and metabolic activities of cyanobacteria. Ecotoxicol Environ Saf 48(2):219–221
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39(1):205–207
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44(1):276–287
Burg MB, Ferraris JD (2008) Intracellular organic osmolytes: function and regulation. J Biol Chem 283(12):7309–7313
Cui J, Zhang R, Wu GL, Zhu HM, Yang H (2010) Salicylic acid reduces napropamide toxicity by preventing its accumulation in rapeseed (Brassica napus L.). Arch. Environ 59(1):100–108
Czerpak R, Bajguz A, Gromek M, Kozłowska G, Nowak I (2002) Activity of salicylic acid on the growth and biochemism of Chlorella vulgaris Beijerinck. Acta Physiol Plant 24(1):45–52
Deef HE (2013) Salicylic acid and cytokinin protects maize plant against glyphosate action. Egypt J Agron 35:115–133
De Oliveira MACL, Monteiro MPC, Robbs PG, Leite SGF (1999) Growth and chemical composition of Spirulina maxima and Spirulina platensis biomass at different temperatures. Aquac Int 7(4):261–275
Dragolova D, Chaneva G, Gemishev T, Vassilev G (2002) Physiological changes in the cyanobacteria Anabaena variabilis and Plectonema boryanum caused by paraquat toxicity. Cr Acad Bulg Sci 55(5):5–85
Dong YJ, Wang ZL, Zhang JW, Liu S, He ZL, He MR (2015) Interaction effects of nitric oxide and salicylic acid in alleviating salt stress of Gossypium hirsutum L. J Soil Sci Plant Nutr 15(3):561–573
Fatma F, Kamal A, Srivastava A (2018) Exogenous application of salicylic acid mitigates the toxic effect of pesticides in Vigna radiata (L.) Wilczek. J Plant Growth Regul 37(4):1185–1194
Fuerst EP, Norman MA (1991) Interactions of herbicides with photosynthetic electron transport. Weed Sci 39:458–464
Hagemann M, Schoor A, Mikkat S, Effmert U, Zuther E, Marin K, & Probst C (1999) The biochemistry and genetics of the synthesis of osmoprotective compounds in cyanobacteria. Taylor & Francis, pp 177–186
Hasanuzzaman M, Alam MM, Nahar K, Ahamed KU, Fujita M (2014) Exogenous salicylic acid alleviates salt stress-induced oxidative damage in Brassica napus by enhancing the antioxidant defense and glyoxalase systems. Aust J Crop Sci 8(4):631–639
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125(1):189–198
Herborne JB (1973) Phytochemical methods. A guide to modern techniques of plant analysis. Chapman and Hall, London, p 119 5-11
Hernando F, Royuela M, Munoz-Rueda A, Gonzalez-Murua C (1989) Effect of glyphosate on the greening process and photosynthetic metabolism in Chlorella pyrenoidosa. J Plant Physiol 134(1):26–31
Ibrahim EA (1990) The influence of the herbicide paraquat ‘Gramaxon’on growth and metabolic activity of three chlorophytes. Water Air Soil Pollut 51(1-2):89–93
Jana S, Choudhuri MA (1982) Glycolate metabolism of three submersed aquatic angiosperms during ageing. Aquat Bot 12:345–354
Kim HS, Lim CJ, Kim JC, Jin CD, Han TJ (2003) Effects of salicylic acid on paraquat tolerance in Arabidopsis thaliana plants. J Plant Biol 46(1):31–37
Khodary SEA (2004) Effect of salicylic acid on the growth, photosynthesis and carbohydrate metabolism in salt stressed maize plants. Int J Agric Biol 6(1):5–8
Kumar S, Habib K, Fatma T (2008) Endosulfan induced biochemical changes in nitrogen-fixing cyanobacteria. Sci Total Environ 403(1-3):130–138
Lee BR, Islam MT, Park SH, Lee H, Bae DW, Kim TH (2019) Antagonistic shifting from abscisic acid-to salicylic acid-mediated sucrose accumulation contributes to drought tolerance in Brassica napus. Environ Exp Bot 162:38–47
Leitao MDS, Cardozo KH, Pinto E, Colepicolo P (2003) PCB-induced oxidative stress in the unicellular marine dinoflagellate Lingulodinium polyedrum. Arch Environ Contam Toxicol 45(1):0059–0065
Leridon H (2020) World population outlook: Explosion or implosion? Populat Soc 1:1–4
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Mackinney G (1941) Absorption of light by chlorophyll solutions. J Biol Chem 140(2):315–322
Mansour FA, Soliman AR, Shaaban-Desouki SA, Hussein MH (1994) Effect of herbicides on cyanobacteria. 1. Changes in carbohydrate content, PMase and GOT activities in Nostoc kihlmani and Anabaena oscillarioides. Phykos. New Delhi 33(1):153–162
Mostofa MG, Fujita M (2013) Salicylic acid alleviates copper toxicity in rice (Oryza sativa L.) seedlings by up-regulating antioxidative and glyoxalase systems. Ecotoxicology 22(6):959–973
Mateo A, Funck D, Mühlenbock P, Kular B, Mullineaux PM, Karpinski S (2006) Controlled levels of salicylic acid are required for optimal photosynthesis and redox homeostasis. J Exp Bot 57(8):1795–1807
Mauch F, Mauch‐Mani B, Gaille C, Kull B, Haas D, Reimmann C (2001) Manipulation of salicylate content in Arabidopsis thaliana by the expression of an engineered bacterial salicylate synthase. Plant J 25(1):67–77
Ningthoujam M, Habib K, Bano F, Zutshi S, Fatma T (2013) Exogenous osmolytes suppresses the toxic effects of malathion on Anabaena variabilis. Ecotoxicol Environ Saf 94:21–27
Popova L, Ananieva E, Hristova V, Christov K, Georgieva K, Alexieva V, Stoinova ZH (2003) Salicylic acid-and methyl jasmonate-induced protection on photosynthesis to paraquat oxidative stress. Bulg J Plant Physiol 133:152
Prado R, García R, Rioboo C, Herrero C, Abalde J, Cid A (2009) Comparison of the sensitivity of different toxicity test end points in a microalga exposed to the herbicide paraquat. Environ Int 35(2):240–247
Prasad SM, Kumar D, Zeeshan M (2005) Growth, photosynthesis, active oxygen species and antioxidants responses of paddy field cyanobacterium Plectonema boryanum to endosulfan stress. J Gen Appl Microbiol 51(2):115–123
Qian H, Chen W, Sun L, Jin Y, Liu W, Fu Z (2009) Inhibitory effects of paraquat on photosynthesis and the response to oxidative stress in Chlorella vulgaris. Ecotoxicology 18(5):537–543
Radwan DEM, Fayez KA, Mahmoud SY, Hamad A, Lu G (2007) Physiological and metabolic changes of Cucurbita pepo leave in response to zucchini yellow mosaic virus (ZYMV) infection and salicylic acid treatments. Plant Physiol Biochem 45(6-7):480–489
Radwan DEM, Soltan DM (2012) The negative effects of clethodim in photosynthesis and gas-exchange status of maize plants are ameliorated by salicylic acid pretreatment. Photosynthetica 50(2):171–179
Radwan DEM, Mohamed AK, Fayez KA, Abdelrahman AM (2019) Oxidative stress caused by Basagran® herbicide is altered by salicylic acid treatments in peanut plants. Heliyon 5(5):e01791
Raskin I, Turner IM, Melander WR (1989) Regulation of heat production in the inflorescences of an Arum lily by endogenous salicylic acid. PNAS 86(7):2214–2218
Sahar K, Amin B, Taher NM (2011) The salicylic acid effect on the Salvia officinalis L. sugar, protein and proline contents under salinity (NaCl) stress. J Stress Physiol Biochem 7(4):80–87
Singh A, Srivastava AK, Singh AK (2013) Exogenous application of salicylic acid to alleviate the toxic effects of insecticides in Vicia faba L. Environ Toxicol 28(12):666–672
Singh H, Singh NB, Singh A, Hussain I, Yadav V (2016) Physiological and biochemical effects of salicylic acid on Pisum sativum exposed to isoproturon. Arch Agron Soil Sci 62(10):1425–1436
Singh A, Zahra K (2017) LC50 assessment of cypermethrin in Heteropneustes fossilis: probit analysis. Int J Fish Aquat Stud 5(5):126–130
Siegelman H, Kycia J (1978) Algal bili-proteins: Handbook of phycological method. Cambridge University Press, Cambridge, pp 71–79
Sood A, Pabbi S, Uniyal PL (2011) Effects of paraquat on lipid peroxidation and antioxidant enzymes in aquatic fern Azolla microphylla. Russ J Plant Physiol 58(4):667–673
Spiro RG, Spiro MJ (1966) Glycoprotein biosynthesis: Studies on thyroglobulin characterization of a particulate precursor and radioisotope incorporation by thyroid slices and particle systems. J Biol Chem 241(6):1271–1282
Szafranska K, Reiter RJ, Posmyk MM (2016) Melatonin application to Pisum sativum L. seeds positively influences the function of the photosynthetic apparatus in growing seedlings during paraquat-induced oxidative stress. Front Plant Sci 7:1663
Tansai S, Issakul K, Ngearnpat N (2018) Toxicity of paraquat on growth of cyanobacteria (Nostoc sp. N1 and Anabaena sp. A1) and germination of rice seed (san-pah-twang 1). The 6th International Conference on Biochemistry and Molecular Biology, Thailand
Thajamanbi M, Rout J, Thajuddin N (2016) Blue green algae from rice fields of Karimganj district, Assam, North East India. Life 50:23
Umebese CE, Olatimilehin TO, Ogunsusi TA (2009) Salicylic acid protects nitrate reductase activity, growth and proline in amaranth and tomato plants during water deficit. Am J Agric Biol Sci 4(3):224–229. https://doi.org/10.3844/ajabssp.2009.224.229
Van Handel E (1968) Direct microdetermination of sucrose. Anal Biochem 22(2):280–283
Wang J, Lv M, Islam F, Gill RA, Yang C, Ali B, Yan G, Zhou W (2016) Salicylic acid mediates antioxidant defense system and ABA pathway related gene expression in Oryza sativa against quinclorac toxicity. Ecotoxicol Environ Saf 133:146–156
Wael MS, Mostafa MR, Taia AAEM, Saad MH, Magdi TA (2015) Alleviation of cadmium toxicity in common bean (Phaseolus vulgaris L.) plants by the exogenous application of salicylic acid. J Hortic Sci Biotechnol 90(1):83–91
Warrier RR, Paul M, Vineetha MV (2013) Estimation of salicylic acid in Eucalyptus leaves using spectrophotometric methods. Genet Plant Physiol 3(1-2):90–97
Watts M (2011) Paraquat. Pesticide Action Network Asia and the Pacific, pp 19–20. Available at ⟨http://wssroc.agron.ntu.edu.tw/note/Paraquat.pdf⟩
Yang Y, Qi M, Mei C (2004) Endogenous salicylic acid protects rice plants from oxidative damage caused by aging as well as biotic and abiotic stress. Plant J 40(6):909–919
Zhang W, Liu M, Zhang P, Yu F, Lu S, Li P, Zhou J (2014) Effects of paraquat on photosynthetic pigments, antioxidant enzymes, and gene expression in Chlorella pyrenoidosa under mixotrophic compared with autotrophic conditions. Arch Environ Contam Toxicol 67(4):593–600
Acknowledgements
Haleema Naaz is thankful to the University Grant Commission, New Delhi, India, for financial support. The authors are also thankful to the Department of Microbiology, I.A.R.I., New Delhi, for providing us Microchaete sp. NCCU-342.
Availability of data and materials
None.
Author information
Authors and Affiliations
Contributions
HN did the conceptualization, methodology, investigation, writing and original draft preparation; DY did the data curation and visualization; BA did the methodology and analysis; NS did the writing-reviewing and editing; NJK did the visualization; and TF did the supervision, validation, writing-reviewing and editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Diane Purchase
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 268 kb)
Rights and permissions
About this article
Cite this article
Naaz, H., Yasin, D., Afzal, B. et al. Exogenous salicylic acid mediated herbicide (Paraquat) resistance in cyanobacterial biofertilizer Microchaete sp. NCCU-342. Environ Sci Pollut Res 30, 25069–25079 (2023). https://doi.org/10.1007/s11356-021-15378-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-15378-0