Abstract
Citrus limon (L.) Osbeck cultivated all over the world is a valuable source of aromatic essential oil. To develop tetraploids of C. limon, four different concentration of colchicine (0.025, 0.05, 0.1 and 0.2%; w/v) and three varied exposure time (12, 24 and 48 h) were employed. The ploidy level of diploids (2n = 2x = 18) and tetraploids (2n = 4x = 36) were determined by direct chromosome count and confirmed by flow cytometric analyses. Successful result with maximum tetraploidy frequency was observed in plantlets developed from seeds treated with 0.025% colchicine for 24 h. Morphological and stomatal characteristics indicated that tetraploids were taller with increased leaf and root length. On the other side, the leaves of tetraploids had fewer and larger stomata with a greater number of chloroplasts in guard cells in contrast with diploids. GC–GC/MS analyses showed cyclic monoterpene, limonene had increased significantly in tetraploids and was further confirmed by HPLC quantification. RT-PCR analyses revealed unaltered expression of monoterpene synthase, sesquiterpene synthase and flavone synthase and remarkable upregulation of genes such as limonene synthase, chalcone synthase and phenylalanine ammonia lyase in tetraploids. Antioxidant activity of essential oil of tetraploids was higher than diploids in all the five test systems studied. Overall, the findings of the present study prove that colchicine induced tetraploidy in C. limon could be a greater source of essential oil with improved composition and of economic significance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polyploidy is defined as the presence of more than two complete chromosome sets in a cell nucleus and is considered to be responsible for plant evolution and diversification (Soltis et al. 2009). In general, polyploid individuals have bigger cells which eventually develop larger organs such as larger flowers, fruits and seeds and often have improved traits than their diploid relatives and may have greater advantage for immediate use in cultivation (Dhooghe et al. 2011). Polyploids may be induced through either sexual polyploidization, which generates 2n gametes, or somatic doubling, which is based on the mitotic doubling of somatic cells (Ramsey and Schemske 1998). In the last century, somatic duplication using various mitotic inhibitors was the widely used method for polyploidy induction and colchicine, an alkaloid extracted from meadow saffron is the commonly used antimitotic agent for polyploidization (Planchais et al. 2000). Polyploidization under in vitro tissue culture system offers a controlled environment for induction of polyploids at higher frequency (Song et al. 2012). An efficient polyploidy induction depends on various factors such as type and concentration of antimitotic agent used, exposure time and type of explants (Sattler et al. 2016). Polyploidy manipulation for improved production of secondary metabolites like artemisinin, cichoric acid and ursolic acid have been reported earlier (Lin et al. 2011; Abdoli et al. 2013; Tavan et al. 2015).
The genus Citrus is cultivated in many countries across the world, which makes it valuable in terms of cultivation and commercial aspect. Citrus belongs to the Rutaceae family that has small evergreen aromatic trees (Goswami et al. 2013). Citrus limon (L.) Osbeck is one among the economically important species of the genus with a total production of 2.4 million tons from 248,000 ha of cultivable land in India (FAO 2017). C. limon constitute as one of the main source of essential oil used in cosmetics, medicine and food industries (Mustafa 2015). Till date, various reports on tissue culture of C. limon including nodal culture, somatic embryogenesis, adventitious bud culture, protoplast culture and nucellar callus culture are available (Orbovic et al. 2008; Perez-Tornero et al. 2010; Goswami et al. 2013). Some successful studies on autotetraploid cybrid and allotetraploid somatic hybrid of C. limon have also been reported (Romero-Aranda et al. 1997; Bosco et al. 1999). However, to the best of our knowledge polyploidy induced by colchicine in C. limon and respective enhancement of secondary metabolites production has not yet been studied. Therefore, in the present study, an effective in vitro protocol for artificial autotetraploid induction in C. limon has been developed. Furthermore, the essential oil content along with respective gene expression and antioxidant potential of the induced tetraploids and diploid parent have been reported for the first time.
Materials and methods
Induction of polyploidy
Ripened fruits of C. limon cv. Rasraj were used as the source of C. limon seeds. The seeds were germinated on full strength MS medium (Murashige and Skoog 1962) devoid of any hormones. Seeds with well developed radicles (~ 1 cm) were transferred on to MS medium supplemented with colchicine at different concentrations (0.025, 0.05, 0.1 and 0.2%; w/v) and were maintained at 25 ± 2 °C under a 16/8 h (light/dark) photoperiod of 50–60 μmol m−2 s−1 flux intensity provided by cool white fluorescent tubes for different exposure times including 12, 24 and 48 h. Each treatment consisted of three replicates and each replicate had six seeds. After treatment, to develop plantlets, the seeds were transferred to MS medium devoid of colchicine and cultured for 70 days under same culture conditions as used for colchicine treatment. The normally developed in vitro plantlets were then analyzed for ploidy level.
Ploidy analysis by chromosome count and flow cytometry
The chromosomes were counted in all survived plantlets to differentiate diploids from tetraploids. For this, the root tips (~ 1 cm) were pretreated with 0.002 M 8-hydroxyquinoline for 3 h at room temperature. The root tips were then fixed with ethanol: acetic acid (3:1) overnight at 4 °C and washed subsequently with distilled water. The root tips were hydrolyzed with 1 N HCl at 60 °C for 10–13 min and washed to remove traces of acid. Then, the root tips were squashed with 1% acetocarmine and visualized using a light microscope (Olympus CKX41) and observations were documented.
50 mg of fresh leaf samples were analyzed by flow cytometry along with a known external standard Vigna radiata. Nuclei suspension extractions were prepared according to the protocol of Lin et al. 2011. The nuclei suspension extracts were stained prior to analysis with 50 µg mL−1 propidium iodide and 10 µg mL−1 RNAse and allowed to stand for 15 min. The analyses were carried out in a Becton–Dickinson FACScalibur flow cytometer system.
Evaluation of morphological and anatomical characteristics
Morphological parameters including plant height, number of leaves, leaf length and root length were evaluated in diploid and tetraploid plants to assess the effect of colchicine treatment. Anatomical characteristics including stomata length, width, density and chloroplast density per guard cell pair were measured from the epidermal layer of completely developed leaves. For measuring stomata characteristics, epidermal strips were prepared using clear fingernail polish and for chloroplast count, the epidermal peels were prepared by staining with potassium iodide-iodine (Lugol’s iodine) solution for 1–2 min. The prepared sections were visualized and documented using a light microscope (Olympus CKX41). All measurements were carried in three replications.
Extraction of essential oil and chemical composition analysis
Essential oil was extracted from diploid and tetraploid plants (~ 30 g) using a Clevenger type apparatus for 2 h. The percentage of oil yield was calculated as
The essential oil samples were then analyzed by GC–GC/MS method. The Perkin Elmer Clarus 680 GC equipment used for the analyses had a fused silica column, packed with Elite-5 MS and the oil components were separated using helium as carrier gas at a constant flow rate of 1 mL min−1. The injector temperature was set at 260 °C and 1 μL of oil was injected. The split ratio was 10:1. The oven temperature was as follows: 60 °C for 2 min, followed by increase up to 300 °C at the rate of 10 °C min−1 and 300 °C for 6 min. The mass detector conditions were transfer line and ion source temperature at 240 °C and ionization mode electron impact at 70 eV. The components were identified by comparison with the NIST (2008) spectral library database of known compounds.
Quantification of limonene
For quantification, limonene reference standard was purchased from Sigma-Aldrich, India. The essential oil samples were analyzed according to Cataldo et al. 2004 using liquid chromatography performed on Shimadzu Prominence HPLC using a diode array detector and a C-18 column. A mixture of acetonitrile:water (80:20; v/v) was used as mobile phase at a flow rate of 1.5 mL min−1 and at a pressure of 150 bar. The detector was set at 225 nm and the peak area integration was made on the recorded chromatogram in comparison with standard.
RNA isolation and semiquantitative RT-PCR
Extraction of total RNA from 200 mg leaf samples were carried out using Trizol method. cDNA was synthesized from the extracted RNA using a reverse transcription kit purchased from HELINI (Chennai, India) following manufacturer’s instructions. cDNA template was amplified using semi-quantitative gradient thermocycler (Applied Biosystems) as follows: 94 °C for 5 min, followed by 35 cycles of amplification (92 °C for 30 s, 50–59 °C for 1 min and 72 °C for 1 min) and 72 °C for 10 min. The gene specific primers used are listed in supplementary material 1. The gene β-actin served as an internal marker.
Evaluation of antioxidant activity
The essential oil obtained from tetraploid plants was analyzed in comparison with the diploid plants using various in vitro antioxidant test systems. A stock solution of essential oils (1 mg mL−1 in 10% DMSO; v/v) was prepared and five different concentrations (25–125 µg mL−1) of oil were used for analyses. The antioxidant activity was calculated using the following formula: % inhibition = [(AB − AA)/AB] × 100; where AB is absorption of blank, AA is absorption of sample. Herein, the IC50 value indicates the effective essential oil concentration required for 50% activity in a given assay.
DPPH free radical scavenging activity
DPPH free radical scavenging activity was determined according to Tauchen et al. (2015). Different concentrations of essential oil was made up to 175 μL with DMSO and mixed with 25 μL of 1 mM DPPH. The reaction mixture was incubated at 37 °C for 30 min and then absorbance was measured at 517 nm.
Metal ion chelating activity
Essential oil of different concentrations was added to 200 μL of 0.1 mM FeSO4. Further, the reaction was initiated by addition of 400 μL of 0.25 mM ferrozine. The mixture was shaken vigorously and left to stand at room temperature for 10 min. After incubation, the absorbance was measured at 562 nm (Chew et al. 2009).
Hydroxyl radical scavenging activity
The reaction mixture included 250 μL of 1 mM FeSO4, 1.5 mL of 1 mM 1,10-phenanthroline and essential oil at different concentrations. The reaction was initiated by addition of 62.5 μL of 5 mM H2O2. After incubation at room temperature in dark for 5 min, the absorbance of the mixture was read at 510 nm (Mukhopadhyay et al. 2016).
Inhibition of linoleic acid peroxidation
Essential oil of different concentrations were mixed with 500 μL of 20 mM linoleic acid, 300 μL of 100 mM Tris-HCl (pH 7.5), 100 μL of 5 mM ascorbic acid and 100 μL of 4 mM FeSO4. The mixture was incubated at 37 °C for 60 min. After incubation, 2 mL of 10% ice cold TCA and 1 mL of 1% TBA was added to the reaction mixture, followed by heating at 95 °C for 60 min and absorbance was read at 532 nm (Choi et al. 2002).
Prevention of deoxyribose degradation
Different concentrations of essential oil were mixed with 200 μL of 15 mM deoxyribose, 200 μL of 0.1 M potassium phosphate buffer, 100 μL of 10 mM H2O2, 100 μL of 1 mM ascorbic acid and 200 μL of 0.5 mM FeCl3. The reaction mixture was incubated at 37 °C for 60 min followed by the addition of 1 mL of 1% TBA and 1 mL of 2.8% ice cold TCA. The mixture was heated in a water bath at 80 °C for 20 min and absorbance was read at 532 nm (Aruoma 1994).
Statistical analysis
All statistical analyses including mean analysis and student’s t test were conducted using SPSS Ver. 16 software. Frequency of tetraploid induction was analyzed by factorial logistic regression model since tetraploidy is a dichotomous dependent variable. ImageJ software was used to analyze the images of chromosome count and stomata parameters.
Results and discussion
Induction of polyploidy
C. limon seeds treated with colchicine at different concentrations (0.025, 0.05, 0.1 and 0.2%) for 12 – 48 h were cultured on MS medium devoid of hormones. The survival rate and efficiency of polyploidy induction were analyzed after 70 days of culture. Seeds treated with 0.025% colchicine had 100% survival rate with maximum tetraploid induction at 24 h exposure period (Table 1). It was observed that 64.4% frequency of tetraploid induction was dependent on the concentration of colchicine used. The decrease in the survival rate of the plantlets and reduced or null tetraploid induction with increase in concentration of colchicine observed in the present study is in agreement with previous reports on Echinacae purpurae and Thymus persicus (Abdoli et al. 2013; Tavan et al. 2015).
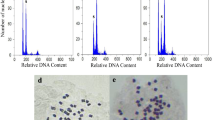
The ploidy level of the survived plantlets was screened by direct chromosome counting. The diploid plantlets had a chromosome number of 2n = 2x = 18 and the chromosome count of 2n = 4x = 36 in plantlets indicated the effective tetraploidy induction by colchicine (Fig. 1a, b). DNA content analyzed by flow cytometry confirmed the ploidy level of the plantlets (Fig. 1c, d). The mean 2C DNA content was determined in relative to V. radiata as standard and found to be 0.78 and 1.66 pg for diploid and tetraploid respectively. Our flow cytometric result is in agreement with the previous finding in which diploid C. limon had 0.81 pg 2C DNA content (Pessina et al. 2011).
Morphological and anatomical variations in induced tetraploids
The morphological traits such as plant height, number of leaves, leaf length, and root length were measured in tetraploid and diploid plants. The results showed positive changes in morphology of tetraploids in comparison with their diploid relatives (Fig. 2). The average plant height in tetraploids (13.9 cm) increased almost 80% than that of diploids. The number of leaves and its length were significantly higher in tetraploids (8.45 cm and 1.60 cm respectively) than their diploid counterparts. The tetraploids had an average root length of 5.9 cm which was less than half fold increase in relation with diploids. A similar change in the morphology of in vitro shoots of colchicine induced tetraploids of Gerbera jamesonii has been reported earlier (Gantait et al. 2011).
Morphological characterization of C. limon. a Plant and root length of diploid and tetraploid (bar 1 cm), b leaf length of diploid and tetraploid (bar 1 cm), c–f comparison between morphological traits of diploid and tetraploid plants c plant height, d number of leaves, e leaf length and f root length (values expressed as mean ± SD)
Examination of anatomical characters especially those related with stomata is an easy and reliable method to identify tetraploids in comparison with diploids (Ewald et al. 2009; Tang et al. 2010). Polyploidy is characterized by decrease in stomatal density, increase in stomata size and increase in number of guard cell chloroplast (Abdoli et al. 2013; Xu et al. 2016) which was confirmed in tetraploids induced in the present study (Fig. 3a–f). The stomatal length and width of the tetraploids showed an increase up to 48–67% than diploids. In parallel with diploids, chloroplast density per stomata of tetraploids increased 172% whereas the stomatal density dropped almost 47% in tetraploids (Fig. 3g).
Stomata characteristics of C. limon. a Stomata density of diploid, b stomata density of tetraploid, c stomata size of diploid, d stomata size of tetraploid, e guard cell chloroplast of diploid, f guard cell chloroplast of tetraploid (bars 10 µm) and g comparison of stomata characteristics of diploids and tetraploids (values expressed as mean ± SD)
Comparison of essential oil and limonene content
The comparative essential oil yield by both tetraploids and their diploid counterparts remained same but the colour of oil derived from diploids was slightly yellowish than that derived from tetraploids. This phenomenon might rise probably due to variation in the oil composition as a result of one or more of the following causes including thermal degradation, oxidation, polymerization, isomerization, and dehydrogenation (Turek and Stintzing 2013; Siddique et al. 2016; Hannweg et al. 2016). The profile of tetraploid and diploid derived essential oil obtained by GC/MS analysis had a highly significant difference in their components. The components limonene and lanceol increased drastically in tetraploids than diploids and showed presence of β-bisabolene. In converse, diploids had (Z,E)-α-farnesene in larger amount and components including trans α-bergamotene, isocarveol and geranial present in diploids were not detected in tetraploids (Table 2). This kind of change in the essential oil profile of polyploids of ginger bush and ajowan induced by colchicine with gain or reduction or even loss of components have been reported in recent time (Hannweg et al. 2016; Noori et al. 2017).
Polyploidy has shown to enhance the average secondary metabolite content of plants and are not usually predictable and are species specific (Dhawan and Lavania 1996). In our study, limonene, a cyclic monoterpene used in perfume industry, flavorings and medicines was quantified by HPLC. The results of HPLC analyses showed remarkable enhancement of limonene content in tetraploids by 7% compared with diploid counterparts (Fig. 4d). Similar results are available on enhancement of secondary metabolites by tetraploidy in Artemisia annua whose artemisinin content and in Thymus persicus whose pentacyclic triterpenoids content had increased in tetraploids (Lin et al. 2011; Tavan et al. 2015).
Gene expression profile
Polyploidy has the potential to promote variations in gene expression which often brings about beneficial changes (Lavania 2005). Expression pattern of three genes responsible for terpenes synthesis, two genes involved in flavonoid biosynthesis and one gene involved in phenylpropanoids production were studied using RT-PCR. The internal marker β-actin showed consistent expression in both diploids and tetraploids. Expression of LS, CHS and PAL increased significantly in tetraploids in comparison with diploid relatives (Fig. 5). In contrast, the genes MTS, STS and FS showed invariable stable expression in both ploidy levels. This expression pattern with elevation of particular genes and unalteration of certain genes in a specified biosynthetic pathway can be attributed to various regulatory factors such as duplication of nuclear material, suppression of certain functional genes and expression of silent genes (Levy 1976). In the present study, the increased limonene content observed in tetraploids may be related to the upregulation of LS gene. Similarly, the constant quantity of total monoterpenes and sesquiterpenes detected between both ploidy levels is in correlation with the unaltered expression of MTS and STS genes respectively.
Antioxidant activity of induced tetraploids
The essential oil from in vivo C. limon has been reported earlier to have strong antioxidant capacity (Ben Hsouna et al. 2017). It was observed that free radical scavenging activity of essential oil derived from tetraploids significantly increased in all test systems than diploids (Fig. 6). The enhanced antioxidant potential of tetraploids may be ascribed to the increase in content of limonene and lanceol, a monoterpene and a sesquiterpene respectively, as in general essential oil containing monoterpenes and sesquiterpenes are considered to have greater antioxidant properties (Lee et al. 2005; Zheljazkov et al. 2012).
Conclusion
Successful in vitro protocol for induction of tetraploidy in C. limon has been established using colchicine. The duplication of nuclear content in tetraploid plants was confirmed by chromosome count and flow cytometry. Morphological and stomata characteristics served as effective indirect indicators for identification of tetraploids of C. limon. Enhanced production of terpenes with increased antioxidant potential due to artificial tetraploidy in C. limon was validated at gene expression level. All the value added features of induced tetraploid of C. limon would definitely increase its commercial significance as tetraploids with higher essential oil yield can be grown in large number in reduced cultivable land.
Abbreviations
- MS:
-
Murashige and Skoog
- GC:
-
Gas chromatography
- GC/MS:
-
Gas chromatography/mass spectrometry
- HPLC:
-
High performance liquid chromatography
- RT-PCR:
-
Reverse transcription-polymerase chain reaction
- MTS:
-
Monoterpene synthase
- LS:
-
Limonene synthase
- STS:
-
Sesquiterpene synthase
- FS:
-
Flavone synthase
- CHS:
-
Chalcone synthase
- PAL:
-
Phenylalanine ammonia lyase
- DPPH:
-
4′,6-Diamidino-2-phenylindole
References
Abdoli M, Moieni A, Badi HN (2013) Morphological, physiological, cytological and phytochemical studies in diploid and colchicine-induced tetraploid plants of Echinacea purpurea (L.). Acta Physiol Plant 35(7):2075–2083
Aruoma OI (1994) Nutrition and health aspects of free radicals and antioxidants. Food Chem Toxicol 32:671–683
Ben Hsouna A, Ben Halima N, Smaoui S, Hamdi N (2017) Citrus limon essential oil: chemical composition, antioxidant and antimicrobial activities with its preservative effect against Listeria monocytogenes inoculated in minced beef meat. Lipids Health Dis 16:146
Bosco SFD, Tusa N, Conicella C (1999) Microsporogenesis in a Citrus interspecific tetraploid somatic hybrid and its fusion parents. Heredity 83:373–377
Cataldo F, Keheyan Y, Baccaro S (2004) Gamma-radiolysis of chiral molecules: R(+)-limonene, S(−)-limonene and R(−)-α-phellandrene. J Radioanal Nucl Chem 262(2):423–428
Chew YL, Goh JK, Lim YY (2009) Assessment of in vitro antioxidant capacity and polyphenolic composition of selected medicinal herbs from Leguminosae family in Peninsular Malaysia. Food Chem 116:13–18
Choi B, Kang S, Ha Y, Park G, Ackman RG (2002) Conjugated linoleic acid as a supplemental nutrient for common carp (Cyprinus carpio). Food Sci Biotechnol 11:457–461
Dhawan OP, Lavania UC (1996) Enhancing the productivity of secondary metabolites via induced polyploidy: a review. Euphytica 87(2):81–89
Dhooghe E, Van Laere K, Eeckhaut T, Leus L, Van Huylenbroeck J (2011) Mitotic chromosome doubling of plant tissues in vitro. Plant Cell Tissue Organ Cult 104:359–373
Ewald D, Ulrich K, Naujoks G, Schroder MB (2009) Induction of tetraploid poplar and black locust plants using colchicine: chloroplast number as an early marker for selecting polyploids in vitro. Plant Cell Tissue Organ Cult 99:353–357
FAO (2017) FAOSTAT. http://www.fao.org/faostat/en/#data. Accessed 20 May 2019
Gantait S, Mandal N, Bhattacharyya S, Das PK (2011) Induction and identification of tetraploids using in vitro colchicine treatment of Gerbera jamesonii Bolus cv. Sciella. Plant Cell Tissue Organ Cult 106:485–493
Goswami K, Sharma R, Singh PK, Singh G (2013) Micropropagation of seedless lemon (Citrus limon L. cv. Kaghzi Kalan) and assessment of genetic fidelity of micropropagated plants using RAPD markers. Physiol Mol Biol Plants 19(1):137–145
Hannweg K, Visser G, de Jager K, Bertling I (2016) In vitro-induced polyploidy and its effect on horticultural characteristics, essential oil composition and bioactivity of Tetradenia riparia. S Afr J Bot 106:186–191
Lavania UC (2005) Genomic and ploidy manipulation for enhanced production of phyto-pharmaceuticals. Plant Genet Resour 3(2):170–177
Lee S, Umano K, Shibamoto T, Lee K (2005) Identification of volatile components in basil (Ocimum basilicum L.) and thyme leaves (Thymus vulgaris L.) and their antioxidant properties. Food Chem 91:131–137
Levy M (1976) Altered glycoflavone expression in induced autotetraploids of Phlox dummondii. Biochem Syst Ecol 4:249–254
Lin X, Zhou Y, Zhang J, Lu X, Zhang F, Shen Q, Wu S, Chen Y, Wang T, Tang K (2011) Enhancement of artemisinin content in tetraploid Artemisia annua plants by modulating the expression of genes in artemisinin biosynthetic pathway. Biotechnol Appl Biochem 58(1):50–57
Mukhopadhyay D, Dasgupta P, Roy DS, Palchoudhuri S, Chatterjee I, Ali S, Dastidar SG (2016) A sensitive in vitro spectrophotometric hydrogen peroxide scavenging assay using 1,10-phenanthroline. Free Radic Antioxid 6:124–132
Murashige T, Skoog FM (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Mustafa NE (2015) Citrus essential oils: current and prospective uses in the food industry. Recent Pat Food Nutr Agric 7(2):115–127
NIST/NIH/EPA (2008) Mass spectral library with search program data version: NIST v08. Standard reference data program, National Institute of Standards and Technology, Gaithersburg
Noori SAS, Norouzi M, Karimzadeh G, Shirkool K, Niazian M (2017) Effect of colchicine-induced polyploidy on morphological characteristics and essential oil composition of ajowan (Trachyspermum ammi L.). Plant Cell Tissue Organ Cult 130(3):543–551
Orbovic V, Calovic M, Viloria Z, Nielsen B, Gmitter FG Jr, Castle WS, Grosser JW (2008) Analysis of genetic variability in various tissue culture-derived lemon plant populations using RAPD and flow cytometry. Euphytica 161:329–335
Perez-Tornero O, Tallon CI, Porras I (2010) An efficient protocol for micropropagation of lemon (Citrus limon) from mature nodal segments. Plant Cell Tissue Organ Cult 100:263–271
Pessina D, Gentili R, Barcaccia G, Nicole S, Rossi G, Barbesti S, Sgorbati S (2011) DNA content, morphometric and molecular marker analyses of Citrus limonimedica, C. limon and C. medica for the determination of their variability and genetic relationships within the genus Citrus. Sci Hortic 129:663–673
Planchais S, Glab N, Inze D, Bergonioux C (2000) Chemical inhibitors: a tool for plant cell cycle studies. FEBS Lett 476:78–83
Ramsey J, Schemske DW (1998) Pathways, mechanisms, and rates of polyploidy formation in flowering plants. Annu Rev Ecol Syst 29:467–501
Romero-Aranda R, Bondada BR, Syvertsen JP, Grosser JW (1997) Leaf characteristics and net gas exchange of diploid and autotetraploid Citrus. Ann Bot 79:153–160
Sattler MC, Carvalho CR, Clarindo WR (2016) The polyploidy and its key role in plant breeding. Planta 243(2):281–296
Siddique AB, Mizanur Rahman SM, Hossain MA (2016) Chemical composition of essential oil by different extraction methods and fatty acid analysis of the leaves of Stevia rebaudiana bertoni. Arab J Chem 9:S1185–S1189
Soltis DE, Albert VA, Leebens-Mack J, Bell CD, Paterson AH, Zheng C, Sankoff D, de Pamphilis CW, Wall PK, Soltis PS (2009) Polyploidy and angiosperm diversification. Am J Bot 96:336–348
Song C, Liu S, Xiao J, He W, Zhou Y, Qin Q, Zhang C, Liu Y (2012) Polyploid organisms. Sci China Life Sci 55:301–311
Tang ZQ, Chen DL, Song ZJ, He YC, Cai DT (2010) In vitro induction and identification of tetraploid plants of Paulownia tomentosa. Plant Cell Tissue Organ Cult 102:213–220
Tauchen J, Doskocil I, Caffi C, Lulekal E, Marsik P, Havlik J, Damme PV, Kokosk L (2015) In vitro antioxidant and anti-proliferative activity of Ethiopian medicinal plant extracts. Ind Crops Prod 74:671–679
Tavan M, Mirjalili MH, Karimzadeh G (2015) In vitro polyploidy induction: changes in morphological, anatomical and phytochemical characteristics of Thymus persicus (Lamiaceae). Plant Cell Tissue Organ Cult 122(3):573–583
Turek C, Stintzing FC (2013) Stability of essential oils: a review. Compr Rev Food Sci Food Saf 12:40–53
Xu C, Huang Z, Liao T, Li Y, Kang X (2016) In vitro tetraploid plants regeneration from leaf explants of multiple genotypes in Populus. Plant Cell Tissue Organ Cult 125(1):1–9
Zheljazkov VD, Astatkie T, Jeliazkova EA, Schlegel V (2012) Distillation time alters essential oil yield, composition, and antioxidant activity of male Juniperus scopulorum trees. J Oleo Sci 61:641–648
Acknowledgements
The authors wish to sincerely acknowledge SIF-VIT University (Vellore), CSIF-IIISM SRM Institute of Science and Technology (Katankulathur) and SAIF ICAR-SBI (Coimbatore) for providing instrumentation facilities.
Author information
Authors and Affiliations
Contributions
GB: collection of plant material, performance of experiments, data collection and manuscript preparation. RT: experimental outline, data interpretation. MG: experimental design development.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bhuvaneswari, G., Thirugnanasampandan, R. & Gogulramnath, M. Effect of colchicine induced tetraploidy on morphology, cytology, essential oil composition, gene expression and antioxidant activity of Citrus limon (L.) Osbeck. Physiol Mol Biol Plants 26, 271–279 (2020). https://doi.org/10.1007/s12298-019-00718-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-019-00718-9