Abstract
Polyploidy is an amazing evolutionary event that can be used in plant breeding to improve plant material. The present study was conducted to assess the effect of in vitro-induced polyploidy on different properties of Ajowan medicinal plant. Five different concentrations of colchicine, including 0.025, 0.05, 0.1, 0.2, and 0.5% (w/v), were applied to germinating seeds of ajowan in six different durations of exposure, including 6, 12, 24, 36, and 48 h. Chromosome counting showed successful duplication of chromosome number in tetraploid plants (2n = 4x = 36) in contrast with intact diploid plants (2n = 2x = 18). DNA content duplication in induced tetraploid plants was proved through flow cytometry analysis. The highest tetraploidy induction was achieved by applying 0.05% (w/v) colchicine for 24 h with 11.53% efficiency. The tetraploid plants achieved were larger than their diploid intact plants for plant height, leaf length, stem diameter, inflorescence length, peduncle length, and seed length characteristics. The length and width of stomata were increased in induced tetraploid plants, whereas stomata density was decreased, in contrast with initial diploid plants. Gas chromatography mass spectrometry (GC/MS) analysis showed significant increases in thymol content in essential oil of tetraploid plants (69.2%) in contrast with those of diploid plants (49.67%).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The improvement of plant material through induced polyploidy and/or used natural polyploids has been one of the major targets of plant breeding programmes in the past century. Actually, increased vigour and better performance are features that make polyploid organisms more preferred than their diploid relatives (Sattler et al. 2016). Polyploidy induction can happen through sexual polyploidization or somatic doubling. Somatic- (or mitotic-) induced polyploidy through the application of mitotic spindle poisons, such as colchicine and oryzalin, is a common plant breeding approach that has been used for many years (Dhooghe et al. 2011; Urwin 2014). Colchicine is the most widely used antimitotic agent for the induction of polyploidy in plants (Planchais et al. 2000). The main advantage of induced polyploidy is that the plants achieved usually have improved morphological and yield characteristics, such as taller height, larger tuber, rhizome or root size (Hannweg et al. 2016), increased biomass, improved photosynthetic capacity, larger flowers, fruits and seeds (Urwin 2014), than their diploid counterparts. This superiority can also occur in induced polyploid plants in contrast to their diploid relatives for resistance to abiotic stress, such as drought tolerance (Hannweg et al. 2016).
Because of the fact that many herbal medicines are free from side effects, the interest in plant products has considerably increased all over the world (Ashraf and Orooj 2006). One of these important medicinal plants is ajowan, which belongs to the Apiaceae family and mainly grows in arid and semi-arid regions of the east of India, the north-western, central and eastern parts of Iran, and also in Egypt (Ashraf and Orooj 2006; Joshi 2000; Moosavi et al. 2015; Niazian et al. 2017). Ajowan is an annual and diploid plant (2n = 18) with cross-pollination reproductive system (Malhotra and Vijay 2004). Active substances of seeds make this plant valuable for medicinal purposes (Dalkani et al. 2012). Ajowan seeds contain an essential oil with about 50% content of thymol, which has strong germicidal, anti-spasmodic and fungicidal effects (Ashraf and Orooj 2006). Many of the medicinal and aromatic plants do not have stable production in their growing areas and are usually gathered in accordance with conventional methods to meet demands (Dalkani et al. 2012). Hence, attention to stable quality and quantity of production of medicinal plants is important for responding to the growing demand for pharmaceutical needs. In addition to the aforementioned advantages of induced polyploidy in plants, this breeding method can also be used in medicinal plants to enhance their secondary metabolite production (Hannweg et al. 2016). The present study was conducted to investigate the effect of in vitro colchicine-induced polyploidy on some morphological characteristics and essential oil composition of the ajowan medicinal plant and compare the achieved tetraploid plants with their intact diploid plants.
Materials and methods
Induction of polyploidy
Germinating seeds of an Iranian ecotype of ajowan (Shahedye-Yazd), procured from the Research Institute of Forests and Rangelands of Iran, were used for in vitro polyploidy induction. Seeds with well-developed radicles (~0.8 mm–1 cm) were transferred to full strength MS medium, supplemented with different concentrations of colchicine, including 0.025, 0.05, 0.1, 0.2, and 0.5% (w/v). Before autoclaving, the medium’s pH was adjusted to 5.8 ± 0.2 with 4 mM NaOH (Niazian et al. 2017). Stock solutions of colchicine were prepared by solving the aforementioned concentrations of colchicine in sterilized distilled water and 2% (v/v) dimethyl sulfoxide (DMSO). In final, filter-sterilized colchicine was added to media after autoclaving. All cultures were maintained at 24 °C under a 16/8 h (light/dark) photoperiod with 60–65% relative humidity for different exposure times, including 6, 12, 24, 36, and 45 h. The experiment was conducted as a factorial, using a completely randomized design with two factors (a: five different concentrations of colchicine; b: five different durations of exposure) and three replications (as Petri dishes). Thirty seeds were cultivated in each replication. Following treatment, seeds were subcultured in free-colchicine full strength MS medium. All the cultures were then incubated in growth room under the aforementioned conditions. After 15 days, the seedlings that survived were first adopted and then transferred to pots contain perlite and coco pite (1:1). Ploidy analyses were conducted after 50 days in well-developed seedlings. Survival Rate was calculated by dividing the number of survived plants form each treatments on all cultivated seeds (90 seeds), and tetraploid frequency was calculated by dividing the number of achieved tetraploid plants from each treatment on all obtained tetraploid plants (26 plants).
Chromosome counting and flow cytometry assessments
After calculating the survival rate, in all treatment sets, 26 plants were analysed for ploidy induction. The direct method of chromosome counting was used to count the diploid and tetraploid plants. For this purpose, root tips (~7 mm long) were pre-treated with 8-hydroxyquinoline (0.002 M) for 5 h. To remove 8-hydroxyquinoline, samples were washed thoroughly and then placed in Carnoy’s fixative (60% ethanol, 30% acetic acid, 10% chloroform) for 24 h. The fixator solution was removed with three subsequent washings using distilled water. Samples were then incubated in chloridric acid (1 N) for 20 min to remove cell walls and subsequent washing helped remove chloridric acid. Next, the samples were stained with aceto orcein (1%) and aceto carmine (1%) solutions for light microscopy observations. The end-parts of root tips were fixed in slides with a single drop of acetic acid (45%) and then squashed. As the final step, documentation and chromosome counting of fixed samples on slides were carried out using a light microscope equipped with a camera (Olympus DP70).
Flow cytomety analysis was carried out using a piece of leaf tissue (0.5 cm2) form 10 samples in five replications. Leaf tissues were chopped with a razor blade in 500 mL of modified Galbraith’s nuclei isolation buffer (200 mM Tris, 4 mM MgCl2.6H2O, pH 7.5, 0.5% Triton X-100) for 25 s (Galbraith et al. 1983). Then, 500 mL of the staining solution—4,6-diamino-2-phenylindole—was added to samples for DNA staining. Staining solution was contain RNAse to eliminate interferences. Samples were incubated in this solution for 2 min and then nuclei were passed through a 30-µm nylon filter to eliminate cell debris. A flow cytometer (PA-I; Partec) was used for samples analysis. Standard plant of parsely was used to compare the flow cytomety results of ajowan with this standard plant.
Evaluation of morphological and stomata characteristics
To investigate the effect of colchicine-induced tetraploidy on morphological traits of ajowan, some morphological characteristics including plant height, leaf length, stem diameter, inflorescence length, peduncle length, and seed length were compared in diploid control plants and the tetraploid plants achieved in the same situations in greenhouse condition. All measurements were conducted in 10 weeks old plants using a standard ruler and in three replications. Stomata characteristics like stomata length, width and density were measured using epiderm layer of expanded leaves. To reach the epidermal layer, the expanded leaves were first dried with colourless varnish, and the epiderm was then separated from dried leaves using a piece of banderol. Separated epiderms were fixed on glass lams and then observation and documentation of fixed sections on slides were conducted with a light microscope equipped with a camera (Olympus DP70). The pictures obtained were analysed using ImageJ software (https://imagej.nih.gov/ij).
Essential oil extraction and GC/MS analysis
For the extraction of essential oils, a sample of seed from each of the diploid control plants and induced tetraploid plants (20 g) was ground using an electric grinder, and the fine powder achieved was added to 500 mL distilled water on top of a heater at 100 °C. The oil was extracted using a Clevenger-type 5 apparatus (Mirahmadi et al. 2011) for 2.5 h. Each extraction was conducted in three replications. The percentage of extracted essential oil was calculated on the basis of the crushed seeds. The major components of the essential oil were analysed using gas chromatography mass spectrometry method. The identification of the composition of the essential oil was conducted using Kovats retention index and suggested GC/MS spectra of the computer library. Finally, the relative percentage of each component was calculated in accordance with the area under the curve of GC/MS spectra.
Statistical analysis
All statistical analyses—such as ANOVA, mean comparison analysis using LSD test at 5% probability level, and paired Student’s t test—were conducted using SAS Ver. 9.1 (Cary 2004) software. Image J software was used to analyse the picture of stomata properties.
Results and discussion
Chromosome counting and flow cytometric analyses
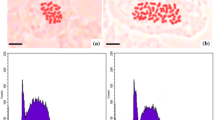
The results of direct chromosome counting of root tips showed that chromosome number of intact diploid plants (2n = 2x = 18) (Fig. 1a) was successfully duplicated after colchicine treatment at different incubation times (2n = 4x = 36) (Fig. 1b). Previous studies also reported nine pairs of chromosomes (2n = 2x = 18) for diploid plants of ajowan (Chattopadhyay and Sharma 1988; Das and Mallick 1993; Khullar et al. 1988). Colchicine is one of the mitotic spindle inhibitor that has successfully been applied for the polyploidy induction in variety of essential oil bearing plants in recent years (Hannweg et al. 2016).
Chromosomal counting and histogram of flow cytometry analysis of ajowan. a Chromosome number of diploid plants (2n = 2x = 18) (bar 10 µm). b Chromosome number of colchicine-induced tetraploid plants (2n = 4x = 36) (bar 10 µm). c Flow cytometry results for statndard diploid plant of parsely and diploid plants of ajowan. d Flow cytometry results for statndard diploid plant of parsely, diploid and tetraploid plants of ajowan
In the present study, flow cytometry analysis was also applied to determine the ploidy levels of in vitro regenerants. Flow cytometry analysis showed that diploid plants of ajowan showed a peak (Peak 2) at channel 24.87 (Fig. 1c), also standard diploid plant of parsely showed it peack (Peak 1) at channels 13.41 and 13.32 (Fig. 1c, d) whereas induced tetraploid plants showed a peak (Peak 3) at the position of channel 49.42 (Fig. 1d). Based on these results, the DNA content of putative tetraploid plants was almost two times of diploid plants of ajowan and standard plants of parsley (Peak 1 in Fig. 1 c, d). As it showen in Fig. 1c, d, coefficient of variation percentage (CV%) in flow cytometry results of ajowan plants were slightly higher than 5% which indicate to it that ajowan is a difficult species that precision measurement of its DNA content is very difficult and replicate measurements can improve its DNA content estimation reproducibility (Doležel and Bartoš 2005), similar result with CV% more than 5% in ajowan have been reported in previous study (Niazian et al. 2017). One of the fast and reliable methods widely used to determine the ploidy level, especially in polyploidy induction studies, is flow cytometry analysis. Flow cytometry has been successfully applied to verify polyploidy induction of other medicinal plants such as ginger bush (Tetradenia riparia) (Hannweg et al. 2016), Brazilian ginseng (Pfaffia glomerata [Spreng.]) (Gomes et al. 2014), purple coneflower (Echinacea purpurea L.) (Abdoli et al. 2013), cannabis (Cannabis sativa L.) (Bagheri and Mansouri 2015), and Thymus persicus (Tavan et al. 2015). This method is also applied to verify the genetic stability of regenerated plants through indirect somatic embryogenesis and indirect shoot regeneration of ajowan (Niazian et al. 2017).
Polyploidy induction efficiency
The survival rate of treated plants was evaluated after 50 days of colchicine treatment. According to the results, the survival rate of ajowan plants was decreased with the increase in colchicine dosage and also the increase in duration of exposure. The highest survival rate of treated plants was related to application of 0.025% (w/v) of colchicine for 6 h, and the lowest survival rate was related to treated plants with 0.5% (w/v) of colchicine for 36 and 48 h (Table 1). The survival rate of diploid control plants was more than all colchicine-treated plants with 99.66%. The adverse effect of higher doses of colchicine treatment on the survival rate of treated plants is also reported in other polyploidy induction studies (Abdoli et al. 2013; Bagheri and Mansouri 2015; Tavan et al. 2015). Based on karyotype and FCM analysis, and also the calculated survival rate of in vitro regenerated plants, the efficiency of polyploidy induction using different concentrations of colchicine in different exposure times was achieved (Table 1). In all treatment sets, 26 tetraploid plants were achieved. Based on evaluations, the highest polyploidy induction efficiency was achieved by applying 0.05% colchicine for 24 h (11.53%) (Table 1). Using 0.025% (w/v) colchicine for 6, 12 and 24 h was not successful for tetraploidy induction of ajowan (Table 1). Although in ploidy induction studies mixploid or plants with higher ploidy are expected but in present study only tetraploid plants were achieved in all tested plants.
Morphological characteristics of tetraploid induced plants
In the present study, the effect of tetraploidy induction was assessed on morphological traits that are usually affected by ploidy levels. The results of the analysis of variance showed the significant effect of colchicine concentration on all the investigated morphological traits of ajowan at 1% probability level (Table 2). The effect of treatment duration was significant only on inflorescence length at 5% probability level (Table 2). The interaction effects of colchicine concentration × treatment duration were only significant on peduncle length at 5% probability level (Table 2). Means comparison analysis using LSD test at 5% probability level showed that the highest mean of plant height was achieved by applying 0.1% colchicine for 6h (Table 3). The lowest plant height was related to diploid plant with 72.7 cm (Table 3). Based on the means comparison analysis, application of 0.5% colchicine led to the highest mean of leaf length in ajowan (Table 3), whereas the shortest leaf length was related to diploid plants with average of 8.33 cm (Table 3). The highest mean of stem diameter corresponded to using 0.2% of colchicine, and also the lowest mean for this trait was related to diploid plants (Table 3). According to the results of the means comparison analysis using LSD test, the application of 0.5% colchicine led to the highest means of inflorescence length, peduncle length, and seed length of ajowan (Table 3). The lowest means of inflorescence length, and seed length, were achieved in 0.025% colchicine treatment, and the lowest mean of peduncle length was related to diploid plants (Table 3). Visual evaluations were also showed the increasing effects of tetraploidy induction on plant height (Fig. 2a), leaf length (Fig. 2b), seed length (Fig. 2c), inflorescence length (Fig. 2d), and peduncle length (Fig. 2e). Abdoli et al. (2013) has also reported larger seed and flower for colchicine-induced tetraploid plants of Echinacea purpurea in contrast with diploid control plants. The application of colchicine also led to different shapes and sizes of leaves in the Tetradenia riparia medicinal plant (Hannweg et al. 2016).
Effect of tetraploidy induction on morphological charachteristics of ajowan. a Plant height of diploid (left) and tetraploid (right) plants (bar 7 cm). b Leaf length of tetraploid (left) and diploid (right) plants (bar 2 cm). c Seed length of tetraploid (left) and diploid (right) plants (bar 3 cm). d Inflorescence length of tetraploid (right) and diploid (left) plants (bar 1 cm). e Peduncle length of tetraploid (left) and diploid (right) plants (bar 1 cm)
Stomata characteristics
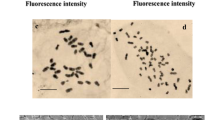
A comparison of stomata characteristics in diploid and tetraploid plants showed the increased length and width of stomata in colchicine-induced tetraploid plants of ajowan (Fig. 3a) in contrast to their diploid relatives (Fig. 3b). Microscopic observations also proved decreased stomata density in the tetraploid plants achieved (Fig. 3c) in contrast to diploid-intact plants (Fig. 3d).
Stomata charactristics of ajowan. a Stomata length and weidth of tetraploid plants of ajowan (bars 10 µm). b Stomata length and weidth of diploid plants of ajowan (bars 10 µm). c Stomata density of tetraploid plants of ajowan (bars 100 µm). d Stomata density of diploid plants of ajowan (bars 100 µm)
The results of a paired T-test analysis showed significant differences between diploid and tetraploid plants for stomata length, width, and density at 1% probability level (Table 4). The averages of the stomata length and width of tetraploid plants were 12.8 and 7.2 µm, respectively, whereas these characteristics of diploid plants were 9.2 and 5.9 µm, respectively (Table 4). Stomata density of diploid plants was 25.4 cells/mm2 whereas stomata density of induced tetraploid plants was 15.9 cells/mm2 (Table 4). One of the most appropriate features that can be used as a strong indicator of the ploidy level in plants is stomatal density (Gomes et al. 2014). Ploidy induction usually leads to lower stomatal density, most likely due to the larger stomata and epidermal cells (Gantait et al. 2011). Dramatic increases in stomata length and width and reduction in the stomata density of colchicine-induced tetraploid plants have also been reported in other ploidy induction studies (Aina et al. 2012; Majdi et al. 2010; Xu et al. 2016).
Essential oil composition
The essential oil yield of tetraploid plants was two-and-a-half times more than their diploid control plants (data not shown). On comparing the essential oil profiles achieved from GC/MS analysis, marked differences between essential oil components of diploid plants and colchicine-induced tetraploid plants were revealed (Table 5). In tetraploid plants, the percentages of all essential oils components were less than those of diploid plants, except thymol content, such that its percentage in the essential oils of tetraploid plants was more than diploid plants (Table 5). Diploids plants contained α-Terpieol in their essential oil, whereas this component was absent in the essential oil of teraploid plants. The difference between diploid plants and their corresponding tatraploid plants for their secondary metabolite profiles has been reported in several essential oil bearing plants (Dhawan and Lavania 1996). Tetraploidy induction in T. riparia medicinal plant has led to the extraction of some additional essential oil components, such as α-humulene, α-terpineol and viridiflorol, which were not present in the diploids (Hannweg et al. 2016). This alteration in secondary metabolite profiles due to ploidy induction in medicinal plants can be related to regulation of synthesis of biochemicals, functional repression of genes, and also depression of silent genes that were previously not expressed in the plant (Hannweg et al. 2016; Lavania 2005; Levy 1976). The significant outcome of the present research is that thymol content of tetraplid plants was more than diploid intact plants, so the present ploidy induction protocol can be used to increase the thymol content of ajowan, as the main secondary metabolite of this medicinal plant.
Conclusion
Chromosomal observations and flow cytometric analysis proved DNA content duplication of colchicine-induced tetraploid plants in contrast to their diploid intact plants. Colchicine treatment significantly decrease the survival rate of in vitro regenerated plants of ajowan. The highest efficiency of tetraploidy induction was achieved by applying 0.05% colchicine for 24 h. Morphological traits—such as plant height, leaf length, stem diameter, inflorescence length, peduncle length, and seed length—were increased in tetraploid plants in contrast to their diploid relatives. Stomata length and width were increased in tetraploid plants, whereas the stomata density of diploid plants was more than that in induced tetraploid plants. The secondary metabolite profile of ajowan changed with tetraploidy induction, which this result can help to study of the genes that regulate the secondary metabolism pathway in ajowan and other valuable medicinal plants. The content of thymol in the essential oil of colchicine-induced tetraploid plants was more than diploid-intact plants. In summary for the first time we established an in vitro procedure for tetraploidy induction in T. ammi that can increase its thymol content which has a significant utility at commercial and practical levels.
Abbreviations
- ANOVA:
-
Analysis of variance
- FCM:
-
Flow cytometry
- GC/MS:
-
Gas chromatography mass spectrometry
- LSD:
-
Least significant difference
- MS:
-
Murashige and Skoog medium
References
Abdoli M, Moieni A, Badi HN (2013) Morphological, physiological, cytological and phytochemical studies in diploid and colchicine-induced tetraploid plants of Echinacea purpurea (L.). Acta Physiol Plant 35(7):2075–2083
Aina O, Quesenberry K, Gallo M (2012) In vitro induction of tetraploids in Arachis paraguariensis. Plant Cell Tissue Organ Cult (PCTOC) 111(2):231–238
Ashraf M, Orooj A (2006) Salt stress effects on growth, ion accumulation and seed oil concentration in an arid zone traditional medicinal plant ajwain (Trachyspermum ammi [L.] Sprague). J Arid Environ 64(2):209–220
Bagheri M, Mansouri H (2015) Effect of induced polyploidy on some biochemical parameters in Cannabis sativa L. Appl Biochem Biotechnol 175(5):2366–2375
Cary NC (2004) SAS Institute. The SAS system for windows. Release 9.1. SAS Inst, North Carolina.
Chattopadhyay D, Sharma AK (1988) A new technique for orcein banding with acid treatment. Stain Technol 63(5):283–287
Dalkani M, Hassani A, Darvishzadeh R (2012) Determination of the genetic variation in Ajowan (Carum Copticum L.) populations using multivariate statistical techniques. Rev Ciênc Agron 43(4):698–705
Das AB, Mallick R (1993) Nuclear DNA and chromosome changes within the tribe Ammineae. Cytobios 74(298–99):197–207
Dhawan OP, Lavania UC (1996) Enhancing the productivity of secondary metabolites via induced polyploidy: a review. Euphytica 87(2):81–89
Dhooghe E, Van Laere K, Eeckhaut T, Leus L, Van Huylenbroeck J (2011) Mitotic chromosome doubling of plant tissues in vitro. Plant Cell Tissue Organ Cult (PCTOC) 104(3):359–373
Doležel J, Bartoš JAN (2005) Plant DNA flow cytometry and estimation of nuclear genome size. Ann Bot 95(1):99–110
Galbraith DW, Harkins KR, Maddox JM, Ayres NM, Sharma DP, Firoozabady E (1983) Rapid flow cytometric analysis of the cell cycle in intact plant tissues. Science 220(4601):1049–1051
Gantait S, Mandal N, Bhattacharyya S, Das PK (2011) Induction and identification of tetraploids using in vitro colchicine treatment of Gerbera jamesonii Bolus cv. Sciella. Plant Cell Tissue Organ Cult (PCTOC) 106(3):485
Gomes SSL, Saldanha CW, Neves CS, Trevizani M, Raposo NRB, Notini MM, de Oliveira Santos M, Campos JMS, Otoni WC, Viccini LF (2014) Karyotype, genome size, and in vitro chromosome doubling of Pfaffia glomerata (Spreng.) Pedersen. Plant Cell, Tissue and Organ Cult (PCTOC) 118(1):45–56
Hannweg K, Visser G, de Jager K, Bertling I (2016) In vitro-induced polyploidy and its effect on horticultural characteristics, essential oil composition and bioactivity of Tetradenia riparia. S Afr J Bot 106:186–191
Joshi SG (2000) Medicinal Plants. Oxford and IBH Publishing Co. Pvt. Ltd., New Delhi, p. 491
Khullar SP, Sharma SS, Verma SC (1988) SOCGI plant chromosome number reports. VI. J Cytol Genet 23:38–52
Lavania UC (2005) Genomic and ploidy manipulation for enhanced production of phyto-pharmaceuticals. Plant Genet Resour Charact Util 3(02):170–177
Levy M (1976) Altered glycoflavone expression in induced autotetraploids of Phlox drummondii. Biochem Syst Ecol 4(4):249–254
Majdi M, Karimzadeh G, Malboobi MA, Omidbaigi R, Mirzaghaderi G (2010) Induction of tetraploidy to feverfew (Tanacetum parthenium Schulz-Bip.): Morphological, physiological, cytological, and phytochemical changes. HortScience 45(1):16–21
Malhotra SK, Vijay OP (2004) Ajowan. In: Peter KV (ed) Handbook of Herbs and Spices, vol 2. Woodhead Publishing Limited, Cambridge, pp 107–116
Mirahmadi SF, Sefidkon F, Aalifar M, Akramian M (2011) Essential oil composition of Tanacetum polycephalum subsp. duderanum (Boiss) Podl., A Plant endemic from Iran. J Essent Oil Bear Plants 14(6):742–745
Moosavi SG, Seghatoleslami MJ, Jouyban Z, Ansarinia E, Moosavi SA (2015) Response Morphological Traits and yield of Ajowan (Carum copticum) to Water deficit stress and Nitrogen Fertilizer. In Biological Forum (Vol. 7, No. 1, p. 293). Research Trend.
Niazian M, Noori SAS, Galuszka P, Tohidfar M, Mortazavian SMM (2017) Genetic stability of regenerated plants via indirect somatic embryogenesis and indirect shoot regeneration of Carum copticum L. Ind Crops Prod 97:330–337
Planchais S, Glab N, Inze´ D, Bergonioux C (2000) Chemical inhibitors: a tool for plant cell cycle studies. FEBS Lett 476:78–83. doi:10.1016/S0014-5793(00)01675-6
Sattler MC, Carvalho CR, Clarindo WR (2016) The polyploidy and its key role in plant breeding. Planta 243(2):281–296
Tavan M, Mirjalili MH and Karimzadeh G (2015) In vitro polyploidy induction: changes in morphological, anatomical and phytochemical characteristics of Thymus persicus (Lamiaceae). Plant Cell Tissue Organ Cult (PCTOC), 122(3):573–583
Urwin NA (2014) Generation and characterisation of colchicine-induced polyploid Lavandula × intermedia. Euphytica 197(3):331–339
Xu C, Huang Z, Liao T, Li Y and Kang X (2016) In vitro tetraploid plants regeneration from leaf explants of multiple genotypes in Populus. Plant Cell Tissue Organ Cult (PCTOC), 125(1):1–9
Acknowledgements
The authors are thankful to Dr M.H Asare, the secretary of science and technological development staff of medicinal plants and traditional medicine of Islamic Republic of Iran for his kindness helps in financial support of this research. Also the authors are thankful to the Research Institute of Forests and Rangelands of Iran for procuring the seeds.
Author contributions
MNI and SASN wrote the manuscript. KS and MNO performed tetraploidy experiments. GK helped to improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest to disclose.
Ethical standards
The experiments were performed according to the current laws of Islamic Republic of Iran.
Additional information
Communicated by Maria Antonietta Germanà.
Rights and permissions
About this article
Cite this article
Sadat Noori, S.A., Norouzi, M., Karimzadeh, G. et al. Effect of colchicine-induced polyploidy on morphological characteristics and essential oil composition of ajowan (Trachyspermum ammi L.). Plant Cell Tiss Organ Cult 130, 543–551 (2017). https://doi.org/10.1007/s11240-017-1245-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-017-1245-0