Abstract
The influence of the basal medium and different plant growth regulators on micropropagation of nodal explants from mature trees of lemon cultivars was investigated. Although the basal medium did not affect any of the variables, explants on DKW medium were greener. Several combinations of 6-benzyladenine (BA) and gibberellic acid (GA) were used to optimise the proliferation phase. The number of shoots was dependent on the BA and GA concentrations and the best results were obtained with 2 mg l−1 BA and 1 or 2 mg l−1 GA. Explants length was shorter with the higher BA concentrations and, in all genotypes, shoot length was greater with 2 mg l−1 GA. The best results for productivity (number of shoots × the average shoot length) were obtained with 2 mg l−1 BA and 2 mg l−1 GA, although explants with chlorosis and narrow leaves were observed. The presence of BA and GA in the proliferation medium was essential for the explant multiplication but GA had a greater influence. The transfer of in vitro shoots to rooting media, containing different concentrations of indole butyric acid (IBA) and indole acetic acid (IAA) produced complete plantlets. Lemon shoots rooted well in all rooting combinations. The highest rooting percentages were obtained on media containing 3 mg l−1 IBA alone or IBA in combination with 1 mg l−1 IAA and on these media the highest numbers of roots were produced. The average root length was affected significantly by the IBA and IAA concentrations. Root length was greater when only 3 mg l−1 IBA was used, and in this rooting medium explants had a better appearance, with greener and larger leaves. The success during the acclimatisation was close to 100% and the plantlets exhibited normal growth in soil under greenhouse conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The genus Citrus is cultivated in more than 100 countries, making it one of the most important commercial fruit crops in terms of economic value and human nutrition (Barlass and Skene 1986). The sustainable development of the citrus industry is mainly dependent on a continuous supply of new and improved cultivars.
Micropropagation systems with high multiplication rates are not only an important asexual method that can be used for the production of clonal plants, but also form the basis for the introduction of genetic variation by genetic transformation or mutagenesis. In both cases, is necessary to be able to regenerate viable shoots, which can be propagated, by either organogenesis or somatic embryogenesis. Regeneration of plants from single cells and complex explants is therefore the key process in every genetic manipulation work; unless this can be achieved consistently and efficiently, no genetic improvement by somatic methods is possible. To accomplish this, a source of genetically homogeneous cells and tissues is necessary. Microprogation cultures are an ideal source of homogeneous cells and tissues and this system is useful for the propagation of plants emanating from experiments of genetic variation.
Several reports have been published on the micropropagation and tissue culture of explants of Citrus and citrus rootstocks (Al Khayri and Al Bahrany 2001; Chaturvedi et al. 2001; Kotsias and Roussos 2001; Carimi and De Pasquale 2003; Hassanein and Azooz 2003; Begum et al. 2004). The morphogenic responses of Citrus cultured in vitro are influenced by the genotype, explant type and culture medium (Randall 1994; Carimi and De Pasquale 2003).
In Citrus, the best results (in terms of rapid proliferation) are normally obtained using juvenile explants (Pérez-Molphe and Ochoa-Alejo 1997). Unfortunately, plants regenerated from juvenile tissues usually exhibit strong, undesirable juvenile characters and require a long period to be brought into production. Improvement of citrus species through introduction of genetic variation will have limited applications unless tissue from mature plants of elite varieties can be readily used. The use of explants from mature plants is not frequently accomplished, mainly due to the high level of contamination (Drew 1988), reduced or absence of morphogenetic ability (Bonga 1982) and poor rooting of the regenerated shoots (George 1993). In Citrus, a few protocols for the micropropagation of mature plants have been developed. Altman and Goren (1974) regenerated shoots from lateral buds collected from a mature, sweet orange plant and Barlass and Skene (1982) regenerated plantlets from nodal segments of mature plants of Cleopatra mandarin, Carrizo citrange, Rangpur lime and Sweet orange.
Like other citrus species, lemon is characterised by a long juvenile stage which is a serious obstacle to breeding programmes (Gmitter et al. 1992). Tissue culture approaches, using mature tissues, appear to be a viable option to achieve the introduction of genetic variation by genetic transformation or mutagenesis in this Citrus species.
Studies on the micropropagation of lemon explants are very scarce in comparison with other Citrus species, although successful embryo culture of immature lemon embryos has been achieved recently (Pérez-Tornero and Porras 2008). Singh et al. (1994) obtained multiple shoots from shoot tips derived from moderately-mature plants of Citrus limon cv. ‘Assam’ and Kotsias and Roussos (2001) used nodal segments of ‘Internadato’ lemon seedlings in an investigation into the effect of different plant growth-regulating compounds on in vitro culture.
The aim of this paper is to gain knowledge on the requirements of lemon shoots from adult material, cultured in vitro, for the proliferation stage and for an efficient rooting and acclimatisation of the plantlets. The micropropagated explants might be used as an ideal source of aseptic and homogeneous material in different transformation or mutagenesis experiments, and the protocol of micropropagation described in this study can be easily used for the effective propagation of genetically-modified plants.
Materials and methods
Plant material
The plant material used in this study was obtained from 15-year-old trees of the lemon cultivars ‘Fino 46’, ‘Fino 49’, ‘Fino 77’, ‘Eureka’, ‘Lisbon’ and ‘Messina’, growing in the lemon collection of the Instituto Murciano de Investigación y Desarrollo Agrario y Alimentario (IMIDA), at La Alberca, Murcia, Spain.
Explant preparation and establishment of the aseptic culture
Shoots were taken from rapidly-extending new branches during spring. The expanded leaves were removed and the stem sections were washed carefully with water and detergent (Mistol®, Henkel Ibérica, S.A.). They were shaken for 5 min in 70% ethanol and then for 20 min in a 20% solution of Domestos® (0.8% NaClO). This bleach brand also has some detergent in its composition, which acts as a surfactant. Finally, the stem sections were rinsed three times with sterile water. Node explants, 1 cm long, were cut and cultured upright with the basal end of the node inserted a few millimetres into the culture medium.
The basal medium used for the axillary shoot establishment was MS medium (Murashige and Skoog 1962), supplemented with 6-benzyladenine (BA) at 1 mg l−1, 30 g l−1 sucrose and 6 g l−1 agar (Hispanlab). After addition of plant growth regulators and adjustment of the pH to 5.7 with 1 N NaOH, the medium was dispensed in 150 mm × 25 mm culture tubes (15 ml of medium per tube) and sterilised in an autoclave at 121°C for 21 min. Cultures were grown at 25 ± 1°C with white light (5000 lux) and a 16-h photoperiod.
In this medium, the sprouting of axillary buds took place and, after removal of contaminated nodes, elongated shoots were transferred to 500-ml jars with 100 ml of establishment medium.
Effect of basal medium on the proliferation stage
The effect of two different basal media was examined in the proliferation stage: macro and micronutrients and vitamins of the MS and DKW (Driver and Kuniyuki 1984) media. Explants of ‘Fino 46’, ‘Fino 77’, ‘Eureka’ and ‘Messina’ were used in this analysis. All media were supplemented with 2 mg l−1 BA, 1 mg l−1 gibberellic acid (GA), 0.1 mg l−1 indole butyric acid (IBA), 30 g l−1 sucrose and 6 g l−1 agar. Medium sterilisation and culture conditions were as described above.
Shoot tips (1–1.5 cm long) were obtained from proliferating shoot cultures and placed in 500-ml jars with 100 ml of each medium. Each treatment consisted of four replicates with 6 shoots per jar. The explants were transferred to fresh medium every 4 weeks. The proliferation was evaluated 8 weeks after the beginning of the experiment and the number of shoots (longer than 5 mm) per explant, their length and the productivity (number of shoots × the average shoot length) were recorded. Productivity is a useful variable since it encompasses the effects of the treatment on both shoot number and length and gives a general idea of the behaviour of the in vitro shoots.
Plant growth regulators levels for optimal proliferation
DKW basal medium was used to test the optimum BA and GA levels. Three different concentrations of BA (1, 2 and 3 mg l−1) and GA (0, 1 and 2 mg l−1) were combined in 9 different treatments. The media were supplemented with 30 g l−1 sucrose and 6 g l−1 agar, and medium sterilisation and culture conditions were as described above. Proliferation (number of shoots longer than 5 mm per explant, length and productivity) was evaluated 8 weeks after the beginning of the experiment.
Effect of different auxins and their concentrations on rooting of shoots
The induction of adventitious roots in lemon was investigated. The composition of the rooting medium was DKW basal medium, 30 g l−1 sucrose and 6 g l−1 agar. Two different concentrations of IBA (1 and 3 mg l−1) and two of indole acetic acid (IAA) (0 and 1 mg l−1) were tested in 4 different treatments. Medium sterilisation and culture conditions were as described above.
Four replicates with 10 explants each were used for each treatment. Explants from proliferating plant material were at least 20 mm long and the basal callus and buds were eliminated. Explants were rooted directly in the light.
After 4 weeks in culture, the rooting percentage and number and length of roots per rooted shoot were evaluated.
Acclimatisation
Rooted explants were transferred to 250- ml pots containing a mix of peat and perlite (2:1). Acclimatisation of plants occurred within a tunnel with >85% RH inside the plant growth chamber. To harden the plants, the plastic that covered the tunnel was opened gradually, from a few minutes a day until normal greenhouse conditions could be maintained without desiccation of the plants. The acclimatisation period was 15–30 days.
Statistical analysis
ANOVA was used to analyse the influence of the basal media and the concentrations of plant growth regulators on lemon shoot proliferation and rooting. The LSD test was used to discriminate differences between different media.
The effect of treatments on rooting percentage was analysed by means of maximum likelihood ANOVA. When a significant χ2 was obtained, specific maximum likelihood contrasts were designed to examine differences between treatments.
Results and discussion
Effect of the basal medium on the proliferation stage
Although the composition of the media used for in vitro culture of Citrus is usually based on the nutrients and vitamins of the MS medium (Abdulaziz and Al Bahrany 2002; Begum et al. 2004), recently Kotsias and Roussos (2001) proposed the use of Driver-Kuniyuki medium (DKW) for the micropropagation of lemon seedlings.
In the present study, when the MS and DKW media were compared, the number of shoots and their average length and productivity were only affected significantly by the cultivar (P < 0.001 for all parameters).
When cultivars were compared using the LSD test, ‘Fino 46’ and ‘Fino 77’ were the most productive genotypes and produced the higher number of shoots. On the other hand, ‘Messina’ produced the longest shoots (Table 1).
Although the basal medium did not affect any of the variables, explants on DKW medium were greener and this medium was used in the following experiments.
Effect of BA and GA for optimal proliferation
The use of plant growth regulators in citrus tissue culture is of fundamental importance. Many studies have been conducted in order to identify the optimal composition regarding plant growth regulators, for shoot and root differentiation and plant regeneration in Citrus (Abdulaziz and Al Bahrany 2002; Begum et al. 2004). Cytokinins and auxins are the two most important plant growth regulators utilised, and gibberelins are usually used to promote shoot elongation of Citrus (Kotsias and Roussos 2001; Carimi and De Pasquale 2003).
In lemon, when different concentrations of BA and GA were used in the culture medium, the number of shoots was affected significantly by the cultivar (P < 0.001), the BA (P < 0.01) and GA (P < 0.001) concentrations and by the interactions: cultivar × BA (P < 0.01), cultivar × GA (P < 0.001) and cultivar × BA × GA (P < 0.05). The best results were obtained with 2 mg l−1 BA and 1 or 2 mg l−1 GA (Fig. 1A).
Average shoot number (A), length (B) and productivity (C) for six lemon cultivars proliferating on a culture medium with different concentrations of BA (1, 2 and 3 mg l−1) and GA (0, 1 and 2 mg l−1). Data are means ± standard error. 0 mg l−1 GA (filled circle), 1 mg l−1 GA (filled square) and 2 mg l−1 GA (filled triangle)
Given the important interactions of cultivar × BA and cultivar × GA, each genotype was studied separately. The number of shoots produced per initial explant was affected by the BA concentration only in ‘Fino 49’ (P < 0.05), ‘Fino 77’ (P < 0.001) and ‘Messina’ (P < 0.01), being highest with 1 or 2 mg l−1 BA. The GA concentrations affected all the cultivars (P < 0.001 for all); the highest shoot number was with 1 or 2 mg l−1 GA depending on the cultivar (Fig. 1A). The number of shoots or buds obtained per explant has been found to vary with the genotype (Carimi and De Pasquale 2003); thus, in calamondin, all of the explants differentiated 3.7 buds per explant in the presence of 15 mg l−1 BA (Sim et al. 1989) and in Carrizo citrange 3.1 shoots per explant were induced with 5 mg l−1 BA (Kitto and Young 1981). In lemon, the cultivar with the highest number of shoots was ‘Fino 77’, 2.78 shoots per explant were obtained with 2 mg l−1 BA and 2 mg l−1 GA.
The average shoot length was affected significantly by the cultivar (P < 0.001), the BA (P < 0.01) and GA (P < 0.001) concentrations and the interactions of cultivar × BA (P < 0.01), cultivar × GA (P < 0.001) and cultivar × BA × GA (P < 0.01)). The explant length was highest with 1 or 2 mg l−1 BA and 2 mg l−1 GA (Fig. 1B). When each genotype was studied separately, the explant length was seen to be affected by the GA concentration for all the cultivars (P < 0.001 for all); it was greatest with 2 mg l−1 GA, and the BA concentration only affected the explant length in ‘Fino 77’ (P < 0.001), 1 mg l−1 BA giving the longest explants (Fig. 1B). These results are in disagreement with those of Kotsias and Roussos (2001), these authors observed that in explants of lemon seedlings the greatest shoot length was obtained with 2 mg l−1 BA, without GA.
Productivity was affected significantly by genotype, the BA and GA concentrations (P < 0.001 for all the parameters) and all the interactions (P < 0.001 for all), being highest with 2 mg l−1 BA and 2 mg l−1 GA (Fig. 1C). Given the important interaction of BA × GA, each GA concentration was studied separately. When the GA concentration was 1 or 2 mg l−1, the productivity was lowest with 3 mg l−1 BA, except for ‘Eureka’ which had its lowest productivity with 1 mg l−1 BA and 2 mg l−1 GA. Significant effects of the BA concentration were not observed with 1 mg l−1 GA for ‘Eureka’, ‘Fino 46’, ‘Fino 49’ or ‘Lisbon’ and with 2 mg l−1 GA for ‘Fino 46’ or ‘Lisbon’. When the GA concentration was 0 mg l−1, the productivity was independent of BA (Fig. 1C).
6-Benzyladenine, at several concentrations, has been the most-commonly-used plant growth regulator for proliferation of citrus shoots (Carimi and De Pasquale 2003). In our study, when BA was the only phytohormone used, proliferation was lowest and the shoots were short with large leaves (Figs. 1A, B, 2A). These results are in agreement with those found by Wochok and Sluis (1980) in shoot cultures of the salt-tolerant plant Atriplex canescens, which remained stunted when BA was the only phytohormone used. The treatment with GA had the effect of first promoting the multiplication of adventitious shoots, and then stimulating their elongation. In lemon, the best results regarding productivity were obtained with 2 mg l−1 GA.
In most plants, the use of gibberellins in shoot culture media is detrimental, producing elongated shoots with narrow leaves. In tree species of the Myrtaceae, adding GA to proliferation-stage cultures caused too much elongation and the formation of narrow leaves (De Fossard and De Fossard 1988). Sometimes, at the proliferation stage, GA can enhance growth and/or increase the rate of shoot proliferation (Moshkov et al. 2008), so the addition of GA with BA caused high-frequency bud breaks and shoot multiplication in apical shoot buds and nodal explants of Morus cathyana (Pattnaik and Chand 1997), and Kotsias and Roussos (2001) observed that, in node culture of lemon seedlings, a low concentration of GA enhanced shoot production and the shoot elongation during the establishment and proliferation stages. In our work, the addition of GA to the proliferation medium was essential for optimal proliferation, although sometimes explants with chlorosis, hyperhydricity and narrow leaves were observed in combinations of high concentrations of BA and GA (Fig. 2B). In these cases, a lower concentration of BA and/or GA was necessary, even though it resulted in decreased productivity.
The benefits of using GA at the proliferation stage often vary between closely-related plant genotypes. Thus, GA was antagonistic to shoot proliferation in ‘McIntosh’ apple shoot cultures (Lane 1978), but was essential for the ‘Ottawa 3’ apple rootstock, where, without the addition of 5 mg l−1 GA, shoots had short internodes and small, deformed leaves. In Citrus sinensis, the addition of GA to the basal medium completely suppressed shoots bud formation (Maggon and Singh 1995), but in node culture of C. limon seedlings it enhanced the multiplication (Kotsias and Roussos 2001). In our study, for the culture nodes from mature lemon plants, GA was indispensable for all cultivars studied and the effect of this hormone in the proliferation phase was more significant than that of BA.
Marín and Duran-Vila (1991) reported that the optimal cytokinin concentration for shoot proliferation varies considerably among Citrus species, but little variation was observed among cultivars belonging to the same species. These results are in agreement with ours for lemon: few differences in the BA or GA concentrations necessary for an optimal multiplication rate were observed among the six cultivars studied and the best results for productivity were obtained with 2 mg l−1 BA and 2 mg l−1 GA for all cultivars.
Effect of different auxins and their concentrations on rooting of shoots
The presence of an auxin in the culture medium is generally necessary to promote rooting in Citrus. Usually, the incubation of adventitious shoots on an NAA (naphthalene-acetic acid) or IBA-supplemented medium induces the formation of roots in many different Citrus species (Carimi and De Pasquale 2003).
In lemon, all combinations of auxins produced high percentages of rooted shoots. The rooting percentage was affected significantly by the IBA and IAA concentrations (P < 0.001 for both), the cultivar (P < 0.001) and the IBA × IAA interaction (P < 0.01). The rest of the interactions were not significant and were eliminated from the model. The best results were produced by 3 mg l−1 IBA, alone or in combination with 1 mg l−1 IAA (Fig. 3). ‘Messina’ was the cultivar with the best rooting (94.77%, averaged over all treatments) and the lowest rooting was in ‘Fino 49’ (54.55%, averaged over all treatments). These rooting percentages are very high and superior to the values obtained by Kotsias and Roussos (2001) in lemon seedling explants. These authors reached 48% rooting when 4 mg l−1 IBA was added to the medium.
The ANOVA showed that the root number was affected by the IAA concentration (P < 0.001), the genotype (P < 0.001) and the interactions of IBA × IAA and genotype × IAA (P < 0.001 for both). Considering all cultivars, the number of roots was higher with 1 mg l−1 IBA and 1 mg l−1 IAA (Fig. 3). The highest root number was in ‘Messina’ (2.21 roots per explant, averaged over all treatments, and 4.21 with 1 mg l−1 IAA) and the lowest was in ‘Fino 49’ (1.74 roots per explant, averaged over all treatments, and 1.98 with 1 mg l−1 IAA).
The average root length was affected significantly by the IBA and IAA concentrations (P < 0.001 for both), the genotype (P < 0.001) and all interactions (P < 0.001 for all) except the IBA × IAA interaction. Root length was higher when 3 mg l−1 IBA and 0 mg l−1 IAA were used. In this rooting medium, the explants had a better appearance, with greener and larger leaves (Fig. 4). The longest roots were in ‘Fino 77’ (30.35 mm, averaged over all treatments) and the shortest were in ‘Eureka’ (25.52 mm, averaged over all treatments). These results differ from those obtained by Kotsias and Roussos (2001), who obtained the longest roots with 1 mg l−1 IBA.
Kitto and Young (1981) observed, in ‘Carrizo citrange’, that NAA enhanced rooting, compared with IBA or IAA. These findings are in agreement with those of Begum et al. (2004) in Pummelo. However, the preference of a cultivar for a specific auxin has been reported (Omura and Hidaka 1992; Goh et al. 1995) and, in lemon seedling explants, IBA seemed to be more efficient in inducing rhizogenesis than NAA (Kotsias and Roussos 2001). In lemon adult explants, IBA alone or in combination with IAA, produced very high percentages of rooted explants.
On the other hand, in Citrus aurantifolia Swing, the highest rooting percentage was obtained on a medium with 1 mg l−1 NAA or 0.5 mg l−1 NAA and 2 mg l−1 IBA (Abdulaziz and Al Bahrany 2002). These authors observed that the number of roots formed per shoot increased in response to increasing NAA and IBA concentrations and that the addition of IBA alone significantly improved root elongation. These results are in agreement with our finding that for lemon, root length was higher when IBA alone was used in the culture medium, although the number of roots was higher when IBA was used in combination with IAA.
Rooting from adult explants has limited success. Explants of seedling and adult ‘Pineapple’ sweet orange could be propagated, but no rooted plantlets were obtained from adult explants and all attempts to recover rooted plants by modifying the composition of the rooting medium failed (Marín and Duran-Vila 1991). In our study, explants from mature lemon plants produced high rooting percentage.
For most plants, the use of gibberellins in shoot culture media is detrimental, since shoots treated with GA may be difficult to root (Moshkov et al. 2008). In lemon, the use of GA in the culture medium had no effect on the rooting.
The percentage of success during the acclimatisation was close to 100% for all cultivars, and the plantlets exhibited normal growth in soil, under greenhouse conditions.
Conclusions
In most reports on Citrus micropropagation, juvenile explants derived from seedling tissues have been used. Studies on the micropropagation of lemon are very scarce and seedling or moderately-mature tissues have been selected like mother plant. We have established an efficient and simple protocol for the micropropagation of lemon, by culturing nodes from mature plants. The protocol was optimised by manipulating the concentrations of plant growth regulators. In the proliferation stage, combinations of BA and GA were indispensable for explant multiplication, and GA had a great influence. In the rooting stage, IBA and IAA combinations produced the best results and nearly 100% of explants were rooted, depending on the cultivar. The micropropagated explants could be used as an ideal source of aseptic and homogeneous material in different transformation or mutagenesis experiments, and the protocol of micropropagation described in this study can be easily applied for the effective propagation of genetically-modified plants.
Abbreviations
- BA:
-
6-Benzyladenine
- DKW medium:
-
Driver and Kuniyuki medium
- GA:
-
Gibberellic acid
- IAA:
-
Indole acetic acid
- IBA:
-
Indole butyric acid
- LSD:
-
Least significant difference test
- MS medium:
-
Murashige and Skoog medium
- NAA:
-
Naphthalene acetic acid
- RH:
-
Relative humidity
References
Abdulaziz M, Al Bahrany AM (2002) Effect of phytohormones on in vitro shoot multiplication and rooting of lime Citrus aurantifolia (Christm.) Swing. Sci Hortic 95:285–295
Al Khayri JM, Al Bahrany AM (2001) In vitro micropropagation of Citrus aurantifolia (lime). Curr Sci 81:1242–1246
Altman A, Goren R (1974) Growth and dormancy cycle in citrus bud culture and their hormonal control. Physiol Plant 30:240–245
Barlass M, Skene KGM (1982) In vitro plantlet formation from Citrus species and hybrids. Sci Hortic 17:333–341
Barlass M, Skene KGM (1986) Citrus (Citrus species). In: Bajaj YPS (ed) Biotechnology in agriculture and forestry. Springer, Berlin, pp 207–219
Begum F, Amin MN, Islam S, Azad MAK (2004) A comparative study of axillary shoot proliferation from the nodal explants of three varieties pummelo (Citrus grandis L. Osb.). Biotechnology 3:46–62
Bonga JM (1982) Vegetative propragation in relation to juvenility, maturity and rejuvenation. In: Bonga JM, Durzan DJ (eds) Tissue culture in forestry. Martinus Nijhoff/Dr. W. Junk publishers, Dordrecht, Boston, Lancaster. pp 387–412
Carimi F, De Pasquale F (2003) Micropropagation of Citrus. In: Jain SM, Ishii K (eds) Micropropagation of woody trees and fruits. Kluwer, Netherlands, pp 589–619
Chaturvedi HC, Singh SK, Sharma AK, Agnihotri S (2001) Citrus tissue culture employing vegetative explants. Indian J Exp Biol 39:1080–1095
De Fossard RA, De Fossard H (1988) Micropropagation of some members of the Myrtaceae. Acta Hortic 227:346–351
Drew RA (1988) Rapid clonal propagation of papaya in vitro from mature field grow trees. HortScience 23:609–611
Driver JA, Kuniyuki AH (1984) In vitro propagation of Paradox walnut rootstock. HortScience 19:507–509
George EF (ed) (1993) Plant propagation by tissue culture (Part 1): the technology. Exegetics Ltd, London, pp 231–272
Gmitter FG, Grosser JW, Moore GA (1992) Citrus. In: Litz RE, Hammerschlag F (eds) Biotechnology of perennial fruit crops. CAB International, Oxon, pp 335–369
Goh CJ, Sim GE, Morales CL, Loh CS (1995) Plantlet regeneration through different morphogenic pathways in pommelo tissue culture. Plant Cell Tissue Organ Cult 43:301–303
Hassanein AM, Azooz MM (2003) Propagation of Citrus reticulata via in vitro seed germination and shoot cuttings. Biol Plant 47:173–177
Kitto SL, Young MJ (1981) In vitro propagation of Carrizo citrange. HortScience 16:305–306
Kotsias D, Roussos PA (2001) An investigation on the effect of different plant growth regulating compounds in in vitro shoot tip and node culture of lemon seedlings. Sci Hortic 89:115–128
Lane WD (1978) Regeneration of apple plants from shoot meristem tips. Plant Sci Lett 13:281–285
Maggon R, Singh BD (1995) Promotion of adventitious bud regeneration by ABA in combination with BAP in epicotyl and hypocotyl explants of sweet orange (Citrus sinensis L. Osbeck). Sci Hortic 63:123–128
Marín ML, Duran-Vila N (1991) Conservation of citrus germplasm in vitro. J Am Soc Hortic Sci 116:740–746
Moshkov IE, Novikova GV, Hall MA, George EF (2008) Plant growth regulators III: gibberellins, ethylene, abscisic acid, their analogues and inhibitors; miscellaneous compounds. In: George EF, Hall MA, De Klerk GJ (eds) Plant propagation by tissue culture. Springer, The Netherlands, pp 227–282
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Omura M, Hidaka T (1992) Shoot tip culture of Citrus. I. Longevity of cultured shoots. Bull Fruit Tree Res Stn 22:37–47
Pattnaik SK, Chand PK (1997) Rapid clonal propagation of three mulberries, Morus cathayana Hemsl, M. ihou Koiz, M. serrata Roxb, through in vitro culture of apical shoot buds and nodal explants from mature trees. Plant Cell Rep 16:503–508
Pérez-Molphe E, Ochoa-Alejo N (1997) In vitro plant regeneration of Mexican lime and mandarin by direct organogenesis. HortScience 32:931–934
Pérez-Tornero O, Porras I (2008) Assessment of polyembryony in lemon: rescue and in vitro culture of immature embryos. Plant Cell Tissue Organ Cult 93(2):173–180
Randall PN (1994) Growth of embryogenic sweet orange callus on media varying in the ratio of nitrate to ammonium nitrogen. Plant Cell Tissue Organ Cult 39:1–5
Sim GE, Goh CJ, Loh CS (1989) Micropropagation of Citrus mitis Blanco—multiple bud formation from shoot and root explants in the presence of 6-benzylaminopurine. Plant Sci 59:203–210
Singh S, Ray BK, Bhattacharya S, Deka PC (1994) In vitro propagation of Citrus reticulata Blanco and Citrus limon Burm F. HortScience 29:214–216
Wochok ZS, Sluis CJ (1980) Gibberellic acid promotes Atriplex shoot multiplication and elongation. Plant Sci Lett 17:363–369
Acknowledgments
We wish to thank Eva Mª Arques and Fernando Córdoba for their excellent assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pérez-Tornero, O., Tallón, C.I. & Porras, I. An efficient protocol for micropropagation of lemon (Citrus limon) from mature nodal segments. Plant Cell Tiss Organ Cult 100, 263–271 (2010). https://doi.org/10.1007/s11240-009-9643-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-009-9643-6


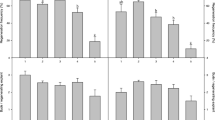





 ‘Eureka’,
‘Eureka’,  ‘Fino 46’,
‘Fino 46’,  ‘Fino 49’,
‘Fino 49’,  ‘Fino 77’,
‘Fino 77’,  ‘Lisbon’,
‘Lisbon’,  ‘Messina’. Bars represent standard error of mean
‘Messina’. Bars represent standard error of mean