Abstract
A micropropagation system for Bauhinia racemosa Lam. was developed involving axillary shoot proliferation and ex vitro rooting using nodal explants obtained from mature tree. MS medium with 3.0 mg l−1 BA (6-benzyladenine) was optimum for shoot bud induction. For shoot multiplication, mother explants were transferred repeatedly on medium containing low concentration of BA (0.75 mg l−1). Number of shoots was increased up to two passages and decreased thereafter. Shoot multiplication was further enhanced on MS medium containing 0.25 mg l−1 each of BA and Kin (Kinetin) with 0.1 mg l−1 of NAA (α-naphthalene acetic acid). Addition of 0.004 mg l−1 TDZ (thidiazuron) increased the rate of shoot multiplication and 21.81 ± 1.26 shoots per culture vessel were obtained. In vitro regenerated shoots were rooted under ex vitro conditions treated with 400 mg l−1 IBA (indole-3-butyric acid) for 7 min on sterile soilrite. After successful hardening in greenhouse, ex vitro rooted plants were transferred to the field conditions with ≈85% of survival rate. Micromorphological changes were observed on leaf surface i.e. development of vein density and trichomes and stomatal appearance, when plants were subjected to environmental conditions. This is the first report on in vitro regeneration of B. racemosa from mature tree.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bauhinia racemosa Lam., a medicinal tree in family of Caesalpiniaceae, is a native of north east India and geographically distributed in many parts of Asia (Bandhopadhyay 1997). In India, it is commonly known as ‘jhinjha’ or ‘apta’ and grown mainly for medicinal as well as religious purposes. Pharmacological studies with various plant parts revealed anti HIV (Rashed et al. 2013), antihyperglycemic (Prusty et al. 2012), antitumor (Gupta et al. 2004a) and hepatoprotective (Gupta et al. 2004b) properties of the tree. Several active compounds i.e. De-O-methylracemosol (Prabhakar et al. 1994), Pacharin (Anjaneyulu et al. 1984) and Racemosol (Anjaneyulu et al. 1986) have been isolated from the tree. Tree has several ethnobotanical uses and leaves are good source of fodder (Singh and Pandey 1998). Natural propagation of B. racemosa through seeds is limited due to seed coat imposed dormancy resulting in low seed germination (Prasad and Nautiyal 1996). Khan et al. (2015) reported only 40% germination even after scarification with concentrated H2SO4. Furthermore, seedling mortality caused by drying of the radical is another limiting factor ensuing decrease in natural population of this leguminous tree (Ravindranath et al. 2003). Despite being highly medicinal and pharmacologically important tree, there are no alternative methods for large scale propagation of this legume species. Therefore, alternative means of propagation of B. racemosa are necessary.
The technique of Plant tissue culture contributes for clonal propagation of superior genotypes or ‘plus tree’ (Valverde-Cerdas et al. 1997) which can be used for afforestation, biomass production and elite germplasm conservation (Goyal et al. 2012). Micropropagation of woody trees has several advantages over conventional methods of propagation i.e. production of disease-free plants, rapid release of improved cultivars, conservation and easy exchange of germplasm (Asthana et al. 2011). However, culture contamination, in vitro recalcitrance, exudation of phenolics and slow growth response are the problems arise during in vitro propagation of woody trees (Rai et al. 2010). Rooting of microshoots in woody trees is considered to be difficult due to poor development and low frequency of rooting (Klerk 2002). Microshoots can be rooted by in vitro as well as ex vitro methods; however, ex vitro rooted plants have better-developed root system and more chances of survival than the in vitro rooted plants (Borkowska 2001). Furthermore, ex vitro rooting is economically sound and less time consuming due to simultaneous occurrence of rooting and acclimatization process. Success of micropropagation greatly affected by altered conditions during transfer from in vitro to field environment which are stressful for micropropagated plants. Micropropagated plants are subjected to developmental changes during hardening and subsequent field transfer (Kevers et al. 2004). Study of these modifications and changes could help for better establishment and survival of plants ex vitro. Several reports on micromorphological and anatomical studies of changes during transfer of plants from in vitro to hardening stage have been published in recent years (Lodha et al. 2015; Shekhawat and Manokari 2016a).
Earlier, in vitro regeneration of B. racemosa was reported by Rajanna et al. (2011) employing cotelydonary nodes as explants. To date, there is not any report on micropropagation of B. racemosa using nodal explants derived from mature tree. Thus, prime objective of the present study was to develop an efficient regeneration method for B. racemosa using nodal explants from mature tree. In order to reduce the protocol cost and to save the time, optimum conditions for ex vitro rooting have also been standardized. Efforts have also been made for studying comparative micromorphological changes occurring on leaf epidermis during hardening.
Materials and methods
Explants selection and surface sterilization
After extensive field surveys conducted for the selection of elite germplasm, a morphologically healthy and sexually mature tree (about 20 years old) growing in Jodhpur (26°27′13″N and 73°03′70″E), was selected as source of explants. The chosen tree was subjected to lopping during winter season (January). Newly sprouted and actively growing young branches (with 12–15 nodes) were harvested in the subsequent spring (April–May) and foliar parts were removed. Branches were cut into segments of 3–4 cm length, with 1–2 nodes per segment. These nodal shoot segments were selected as explants for in vitro culture establishment. Further experiments were performed under aseptic conditions of laminar air flow hood. Explants were rinsed with autoclaved distilled water for 3–4 times and treated with 0.1% (w/v) Bavistin (Carbendazim powder from BASF India Limited), a broad spectrum systemic fungicide, for 12–15 min followed by surface sterilization with 0.1% (w/v) HgCl2 (Ases Chemical Works, Jodhpur, India) for 4–5 min. Surface sterilized explants were then soaked in chilled antioxidant solution (sterilized) of 0.1% ascorbic acid and 0.05% citric acid for 10 min. After each treatment, explants were thoroughly washed with sterilized water for removal of disinfectant traces.
Nutrient medium, culture establishment and culture conditions
Murashige and Skoog (1962) medium supplemented with additives (50 mg l−1 ascorbic acid and 25 mg l−1 each of adenine sulphate, citric acid and l-arginine) and 3% (w/v) sucrose was used as a basal medium for shoot bud induction and multiplication. 0.8% Agar (Bacteriological grade, Qualigens Fine Chemicals, Mumbai, India) was used as gelling agent. pH of the medium was adjusted to 5.8 ± 0.02 using 0.5 N KOH or HCl and medium was autoclaved at 15 psi pressure and 121 °C temperature for 15 min. For shoot bud induction, surface sterilized nodal shoot segments were vertically inoculated on culture medium supplemented with various concentrations (0.0, 1.0, 2.0, 3.0, 4.0 mg l−1) of cytokinins (BA or Kin) in test tubes. Cultures were initially incubated in diffused light conditions (20–25 μmol m−2 s−1 PFD) for 48 h followed by transfer at light intensity of 40–50 µmol m−2 s−1 PFD provided by cool and white fluorescent tubes (Philips, India). Temperature of culture room was maintained at 26 ± 2 °C with 55–60% relative humidity (RH).
Shoot multiplication
Regeneration capacity of mother explants was evaluated by successive transfer on culture media containing various concentrations (0.0, 0.25, 0.5, 0.75, 1.0 mg l−1) of cytokinins (BA or Kin) up to four passages. For further shoot multiplication, in vitro regenerated shoots were sectored in clumps and sub-cultured on media containing different concentrations of BA (0.25, 0.5, 0.75 mg l−1) or Kin (0.25, 0.5, 0.75 mg l−1) alone or in combination with TDZ (0.004 or 0.008 mg l−1) and NAA (0.1 mg l−1). Established shoot cultures of B. racemosa were regularly sub-cultured after an interval of 30–35 days.
Ex vitro rooting and hardening of plantlets
In order to reduce the protocol cost and time, in vitro regenerated shoots were rooted by ex vitro rooting. For this, in vitro raised shoots (4–5 cm) were harvested and their basal ends (4–5 mm) were dipped in different concentrations (0.0, 200, 400, 600 mg l−1) of auxins (IBA and/or NOA) alone or in combinations for 7 min. Auxin solutions were prepared by dissolving in 1 N NaOH. Individual shoots without any auxin treatment were served as control. Auxin treated shoots were transplanted to bottles containing (1/3 of height) autoclaved soilrite® (a mixture of irish peat moss, exfoliated vermiculite and horticulture grade expanded perlite in equal ratio from Kel Perlite, Bangalore, India) and capped with polycarbonated caps. These were irrigated with quarter strength solution of MS basal salt (10–12 ml per bottle), with an interval of one week. Bottles were placed in the green house near the pad section (region of high relative humidity i.e. RH 80–90% and 28 ± 2 °C). Caps of bottles were gradually loosened during acclimation process under ex vitro condition. Simultaneously, bottles were gradually shifted towards the fan section (RH 40–50% and 32 ± 2 °C) of green house for further hardening and kept for next 3–4 weeks near the fan section. After hardening, cap of bottles were removed completely. After successful hardening of plantlets, these were transferred to polybags containing a mixture of soilrite and field soil (1:1) and kept in green house for next 4 weeks. Finally, the hardened plants were transferred to the nursery.
Comparative foliar micromorphological studies of B. racemosa
Experiments were conducted for the micromorphological study of development of veins (vein density and venation pattern), trichomes and stomata in the leaves of in vitro raised shoots (after 4th sub-culturing) and plantlets undergoing hardening (after 6–7 weeks of rooting). Plants were randomly selected from both stages and 3rd to 7th leaves from the bases were removed. Leaves were rinsed with distilled water and macerated in 5% NaOH for 24 h at room temperature. Thereafter, the leaves were decolorized by soaking in 70% ethanol for 48 h. After removal of chlorophyll, leaves were stained with 1% (w/v) safranin (Loba chemie, India) for 3–4 min and rinsed with water for removal of excess stain followed by mounting in distilled water. Photomicrographs were captured with digital camera mounted on the microscope (Olympus CH20i, Japan).
Collection of data and statistical analysis
Randomized block design was used for experimental setup with 15 replicates per treatment and each experiment was repeated thrice. Results are shown in Mean ± SD. The data was analyzed using one way analysis of variance (ANOVA) and the significant differences between means were assayed by Duncan Multiple Range Test (DMRT) at P < 0.05 by SPSS ver. 17 (SPSS Inc., USA).
Results and discussion
In the present study, axenic culture of B. racemosa was established from explants obtained from mature tree. Nodal segments derived from rejuvenated shoots of lopped tree were selected as explants for culture establishment. Due to lopping of trees, older branches get removed which results into sprouting of reinvigorated shoots. Maturation and physiological stages of initial explant greatly influence the cloning of woody plant species (Montenius 1987). The excessive browning of explants and darkening of the medium due to phenolic exudation from cut end of explants is one of the major problems when explants taken from woody plant species of desert (Rathore et al. 1992; Shekhawat et al. 1993). In the present investigation, the problem of phenolic exudation was checked by soaking the explants in autoclaved and chilled solution of antioxidants (0.1% ascorbic acid and 0.05% citric acid for 10 min). Applicability of antioxidant treatment to overcome the problem of phenolic exudation has also been reported in several tree species (Shekhawat et al. 1993; Phulwaria et al. 2012; Vibha et al. 2014).
Effect of cytokinins on axillary bud induction
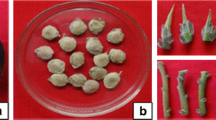
Bud break of nodal explants was exhibited only on MS medium having cytokinins. Shoot induction was not observed on MS basal medium (without PGRs) which shows that addition of cytokinins is mandatory for culture initiation in B. racemosa. Although both cytokinins independently promoted the shoot induction, but medium containing BA (3.0 mg l−1) responded maximum in terms of percent shoot response (90.4%), shoots number (2.89 ± 0.42) and shoot length (3.16 ± 0.35) (Fig. 1b; Table 1). Shoot induction was observed after 18–20 days of culture on medium containing BA. Kin was found to be less efficient cytokinin in comparison to BA for shoot induction and multiplication. Efficacy of BA over kinetin for nodal bud break may be attributed to its easy metabolism by plant tissues than other cytokinins (Rai et al. 2010), easy permeability, less resistance for cytokinin oxidase and natural hormone induction within the tissue (Lodha et al. 2015). Similar results have also been observed in Pithocellobium dulce (Goyal et al. 2012), Moringa peregrine (Khateeb et al. 2013), Dalbergia sisso (Vibha et al. 2014).
In vitro propagation and ex vitro rooting of B. racemosa. a Young branches with axillary nodes. b Induction of axillary shoots on MS + 3 mg l−1 of BA. c Repeated transfer of mother explants on MS + 0.75 mg l−1 of BA. d, e Shoot multiplication and subculturing on MS medium supplemented with BA (0.25 mg l−1), Kin (0.25 mg l−1), NAA (0.1 mg l−1) and TDZ (0.004 mg l−1). f Ex vitro rooting in shoots pulse treated with IBA (400 mg l−1) for 7 min. g Acclimatization of ex vitro rooted plantlets under greenhouse conditions. h Transfer of a successfully hardened plant into polybag
Effect of plant growth regulators on shoot multiplication
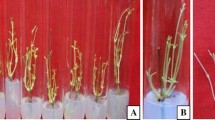
For bud break, explants are usually exposed to high cytokinin concentrations causing its accumulation in mother tissue which results into inhibition of shoot regeneration and growth of shoots (Malik et al. 2005). Therefore, explants were transferred to comparatively lower concentrations of cytokinins for further multiplication. Maximum numbers of shoots (5.32 ± 0.50) were regenerated on medium containing low concentration of BA (0.75 mg l−1) (Fig. 1c; Table 2). In comparison to BA, effect of lower concentrations of Kin found to be less significant on shoot multiplication. For further shoot multiplication, mother explants were transferred repeatedly on same medium up to four passages and it was observed that number of shoots was increased up to two passages and dwindled thereafter (Fig. 2). Repeated transfer is the most preferred method to further evaluate the morphogenetic activity of mother explants. Number of shoots increases due to suppression of apical dominance during reculturing which stimulate the basal dormant meristems to emerge new shoots (Tripathi and Kumari 2010; Shekhawat and Shekhawat 2011).
The shoot regeneration was enhanced when microshoots sectored in clumps of 4–5 shoots and sub-cultured on medium with different auxins and cytokinins. The combination of BA and Kin (0.25 mg l−1 of each) with 0.1 mg l−1 of NAA was optimum for shoot regeneration. The influence of lower concentrations of auxin with cytokinins on the emergence of new shoots and their growth, may be due to auxin –cytokinin crosstalk which stimulates the new bud primordia followed by bud formation and multiple shoot emergence in cultures (Zhang et al. 2015). Addition of 0.004 mg l−1 thidiazuron (TDZ) with above combination increased the shoot multiplication and observed maximum 21.81 ± 1.26 shoots per culture vessel with 6.95 ± 0.91 cm average shoot length (Fig. 1d, e; Table 3). Further increase in the concentration of TDZ from 0.004 mg l−1 induced the callus at the base of shoot clumps and resulted in regeneration of less number and stunted growth of shoots. TDZ is one of the most active cytokinin like substances which stimulates in vitro shoot proliferation particularly in many recalcitrant woody plants when added in very low concentrations (Huetteman and Preece 1993). Its function may be attributed by mimicking auxin and cytokinin effects on growth of cultures, by modulating the endogenous PGRs (either directly or induced by stress), modification in cell membrane, energy levels and uptake of nutrients (Murthy et al. 1998).
Ex vitro rooting of microshoots and hardening
Ex vitro rooting method was used in order to achieve rooting and hardening simultaneously. Both auxins (IBA and NOA) independently and in combination induced the ex vitro rooting in microshoots. However, maximum rooting response (91.06%) with highest number of roots per shoot (5.47 ± 0.64) and root length (4.22 ± 0.51), was observed on IBA 400 mg l−1 for 7 min (Fig. 1f; Table 4). IBA alone was found optimum for ex vitro rooting followed by IBA + NOA (200 mg l−1 of each) with 79.96% response and NOA (400 mg l−1) with 46.63% response. Efficacy of IBA might be due to its easier uptake and permeability, comparatively more steadiness and successive gene activation (Ludwig-Muller 2000; Vengadesan and Pijut 2009). Ex vitro rooting of microshoots have been induced in many of the difficult to root woody plant species (Benmahioul et al. 2012, Vibha et al. 2014). Ex vitro rooting is cost and time effective as well as simpler due to elimination of rooting under sterile condition (Preece and Shutter 1991; Pospisilova et al. 1999; Yan et al. 2010). According to Arya et al. (2003) chances of root damage during transplantation to soil are lesser in case of ex vitro rooting. Moreover, plantlets rooted by such method are more vigorous to cope with environmental stresses during the process of hardening (Vengadesan and Pijut 2009).
By gradual lowering of air humidity and increase in temperature from pad section to fan section, plantlets were methodically hardened in green house (Fig. 1g). The pad section is made up of cellulose pads lying the wall and are kept constantly wet, the air is sucked by them from outside which remain fully saturated initially but gradually loses moisture content thereby, maintaining a humidity gradient from pad to fan section. Loosening of caps of bottles slowly decreased the relative humidity in the very vicinity of plantlets thus assisted in hardening. Micro-irrigation of rooted plantlets with quarter strength of MS, provide essential nutrients to acclimatizing plant thus increasing chances of survival. Thereafter, plants were transplanted in polybags (Fig. 1h) and kept in green house for 1 month. Finally, the plants were shifted from green house to the nursery (similar to natural environmental conditions) with ≈85% of survival rate. Plantlets rooted by ex vitro method have lateral roots similar to natural root system with more root length which leads to higher survival rate during transplanting (Yan et al. 2010). No detectable morphological variation was found in hardened plants when compared to the donor plant, although in vitro raised plants exhibited significant changes during acclimatization.
Foliar micromorphological studies of Bauhinia racemosa
Micromorphological changes were studied in leaves of plantlets undergoing hardening. Leaves from both environments (in vitro and hardening stage) were similar in external morphology. However, significant differences were found during microscopic studies. Veins were observed under development with lesser vein density during in vitro conditions (Fig. 3a) in comparison to plants undergoing hardening (Fig. 3b). Leaves under in vitro conditions showed developing trichomes with smaller size (Fig. 3c) against the larger trichomes of hardening phase (Fig. 3d). Stomata of in vitro developed leaves were found to be non functional as they were always open and more frequent (Fig. 3e) while in the leaves of hardening stage, stomata were lesser in number and opened less frequently (Fig. 3f). Increased density of veins during the hardening shows the gradual acclimatization of plants which is a prerequisite for survival and successful establishment in field conditions. Poor functioning of stomata and altered morpho-anatomical features of in vitro raised plants leads to inadequate photosynthetic ability to get positive carbon balance (Pospisilova et al. 2000). Recently, the changes in vein density, trichomes development and stomatal functioning have also been reported in a number of plants (Lodha et al. 2015; Shekhawat and Manokari 2016b). Our study is in accordance with the fact that after transfer from in vitro cultures to the green house, plantlets underwent substantial changes in leaf morphology and anatomy especially epidermal characters (Pospisilova et al. 1999).
In conclusion, an in vitro propagation protocol discussed here can be applied for large-scale production of plants of B. racemosa using mature-tree-derived nodal explants. The ex vitro rooting along with simultaneous hardening made the protocol more economical and shortened the in vitro regeneration protocol of B. racemosa. Micromorphological studies explained the responses of plants towards natural environmental conditions.
Abbreviations
- BA:
-
6-Benzyladenine
- IBA:
-
Indole-3-butyric acid
- Kin:
-
Kinetin (N6-furfuryladenine)
- MS:
-
Murashige and Skoog (1962) medium
- NAA:
-
α-Naphthalene acetic acid
- NOA:
-
2-Naphthoxy acetic acid
- PFD:
-
Photon flux density
- PGRs:
-
Plant growth regulators
- RH:
-
Relative humidity
- TDZ:
-
Thidiazuron
References
Anjaneyulu ASR, Reddy AVR, Reddy DSK, Ward RS, Adhikesavalu D, Cameron TS (1984) Pacharin: a new dibenzo(2,3-6,7)oxepin derivative from Bauhinia racemosa lamk. Tetrahedron 40:4245–4252
Anjaneyulu ASR, Reddy AVR, Reddy DSK, Cameron TS, Roe SP (1986) Racemosol: a novel tetracyclic phenol from Bauhinia racemosa lamk. Tetrahedron 42:2417–2420
Arya V, Shekhawat NS, Singh RP (2003) Micropropagation of Leptadenia reticulata—a medicinal plant. In Vitro Cell Dev Biol Plant 39:180–185
Asthana P, Jaiswal VS, Jaiswal U (2011) Micropropagation of Sapindus trifoliatus L. and assessment of genetic fidelity of micropropagated plants using RAPD analysis. Acta Physiol Plant 33:1821–1822
Bandhopadhyay S (1997) Notes on the distribution of Bauhinia racemosa Lam. (Leguminosae: caesalpinioideae) in India. J Econ Tax Bot 21:662
Benmahioul B, Dorion N, Kaid-Harche M, Daguin F (2012) Micropropagation and ex vitro rooting of Pistachio (Pistacia vera L.). Plant Cell Tissue Organ Cult 41:71–73
Borkowska B (2001) Morphological and physiological characteristics of micropropagated strawberry plants rooted in vitro or ex vitro. Sci Hortic 89:195–206
Goyal P, Kachhawa S, Kothari SL (2012) Micropropagation of Pithecellobium dulce (Roxb.) Benth-a multipurpose leguminous tree and assessment of genetic fidelity of micropropagated plants using molecular markers. Physiol Mol Biol Plants 18:169–176
Gupta M, Mazumdar UK, Kumar RS, Kumar TS (2004a) Antitumor activity and antioxidant role of Bauhinia racemosa against Ehrlich ascites carcinoma in Swiss albino mice. Acta Pharmacol Sin 25:1070–1076
Gupta M, Mazumdar UK, Kumar TS, Gomathi P, Kumar RS (2004b) Antioxidant and hepatoprotective effects of Bauhinia racemosa against paracetamol and carbon tetrachloride induced liver damage in rats. Iran J Pharmacol Ther 3:12–20
Huetteman CA, Preece JE (1993) Thidiazuron: a potent cytokinin for woody plant tissue culture. Plant Cell Tissue Organ Cult 33:105–119
Kevers C, Franck T, Strasser RJ, Dommes J, Gaspar T (2004) Hyperhydricity of micropropagated shoots; a typically stress induced change of physiological state. Plant Cell Tissue Organ Cult 77:181–191
Khan D, Zaki MJ, Anis M (2015) Seedling characteristics of jhinjera (Bauhinia racemosa Lamk.). Int J Biol Biotech 12:143–154
Khateeb WA, Bahar E, Lahham J, Schroeder D, Hussein E (2013) Regeneration and assessment of genetic fidelity of the endangered tree Moringa peregrina (Forsk.) Fiori using inter simple sequence repeat (ISSR). Physiol Mol Biol Plant 19:157–164
Klerk GJD (2002) Rooting of microcuttings: theory and practice. In Vitro Cell Dev Biol Plant 38:415–422
Lodha D, Patel A, Shekhawat NS (2015) A high-frequency in vitro multiplication, micromorphological studies and ex vitro rooting of Cadaba fruticosa (L.) Druce (Bahuguni): a multipurpose endangered medicinal shrub. Physiol Mol Biol Plants 21:407–415
Ludwig-Muller J (2000) Indole-3-butyric acid in plant growth and development. Plant Growth Regul 32:219–230
Malik SK, Chaudhury R, Kalia RK (2005) Rapid in vitro multiplication and conservation of Garcinia indica: a tropical medicinal tree species. Sci Hortic 106:539–553
Montenius O (1987) In vitro meristem culture of juvenile and mature Sequoiandendron giagantium. Tree Physiol 3:265–272
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Murthy BNS, Murch SJ, Saxena PK (1998) Thidiazuron: a potent regulator of in vitro plant morphogenesis. In Vitro Cell Dev Biol Plant 34:267–275
Phulwaria M, Rai MK, Harish Gupta AK, Ram K, Shekhawat NS (2012) An improved micropropagation of Terminalia bellirica from nodal explants of mature tree. Acta Physiol Plant 34:299–305
Pospisilova J, Ticha I, Kadleck P, Haisel D, Plzakova S (1999) Acclimatization of micropropagated plants to ex vitro conditions. Biol Plant 42:481–497
Pospisilova J, Synkova H, Rulcova J (2000) Cytokinins and water stress. Biol Plant 43:321–328
Prabhakar P, Ghandhidasan R, Raman PV, Krishnasamy NR, Nanduri S (1994) De-o-methylracemosol: a tetracyclic 2,2-dimethylchroman from the roots of Bauhinia racemosa. Phytochemistry 36:817–818
Prasad P, Nautiyal AR (1996) Physiology of germination in Bauhinia: involvement of seed coat in inhibition of germination in B. racemosa Lam. seeds. Seed Sci Technol 24:305–308
Preece JE, Shutter EG (1991) Acclimatization of micropropagated plants to the greenhouse and field. In: Debergh PC, Zimmerman RH (eds) Micropropagation: technology and application. Kluwer Academic Publishers, Dordrecht, pp 71–94
Prusty KB, Rao JV, Subudhi SK, Reddy PA, Kumar JR (2012) Anti hyperglycemic activity of extracts of leaves of Bauhinia Racemosa Lamk (family-Caesalpiniaceae) on normal and alloxan induced diabetic rats. Int J Pharm Res Allied Sci 1:94–99
Rai MK, Asthana P, Jaiswal VS, Jaiswal U (2010) Biotechnological advances in guava (Psidium guajava L.): recent developments and prospects for further research. Trees Struct Funct 24:1–12
Rajanna LN, Sharanabasappa G, Seetharam YN, Aravind B, Mallikharjuna PB (2011) In vitro regeneration of cotyledonary node explant of Bauhinia racemosa. Bot Res Intl 4:75–80
Rashed K, Luo M, Zhang L, Zheng Y (2013) Anti-HIV-1 potential of Bauhinia racemosa Lam. (caesalpiniaceae) and phytochemical profile. Topcls J Herb Med 2:95–102
Rathore TS, Deora NS, Shekhawat NS (1992) Cloning of Maytenus emarginata (Willd.) Ding Hou—a tree of the Indian Desert, through tissue culture. Plant Cell Rep 11:449–451
Ravindranath NH, Bhat DM, Swamy VS (2003) Nursery manual for forest tree species. University Press, Hyderabad
Shekhawat MS, Manokari M (2016a) Optimization of in vitro and ex vitro regeneration and micromorphological studies in Basella alba L. Physiol Mol Biol Plants 22:605–612
Shekhawat MS, Manokari M (2016b) In vitro propagation, micromorphological studies and ex vitro rooting of cannon ball tree (Couroupita guianensis aubl.): a multipurpose threatened species. Physiol Mol Biol Plants 22:131–142
Shekhawat MS, Shekhawat NS (2011) Micropropagation of Arnebia hispidissima (Lehm) DC. and production of alkannin from callus and cell suspension culture. Acta Physiol Plant 33:1445–1450
Shekhawat NS, Rathore TS, Singh RP, Deora NS, Rao SR (1993) Factors affecting in vitro cloning of Prosopis cineraria. Plant Growth Regul 12:273–280
Singh V, Pandey RP (1998) Ethnobotany of Rajasthan. Scientific Publisher, Jodhpur
Tripathi M, Kumari N (2010) Micropropagation of a tropical fruit tree Spondias mangifera Willd. through direct organogenesis. Acta Physiol Plant 32:1011–1015
Valverde-Cerdas L, Dufour M, Villalobos VM (1997) In vitro propagation of Pithecellobium saman (Rain tree). In Vitro Cell Dev Biol Plant 33:8–42
Vengadesan G, Pijut PM (2009) In vitro propagation of northern red oak (Quercus rubra L.). In Vitro Cell Dev Biol Plant 45:474–482
Vibha JB, Shekhawat NS, Mehandru P, Dinesh R (2014) Rapid multiplication of Dalbergia sissoo Roxb.: a timber yielding tree legume through axillary shoot proliferation and ex vitro rooting. Physiol Mol Biol Plant 20:81–87
Yan H, Liang C, Yang L, Li Y (2010) In vitro and ex vitro rooting of Siratia grosvenori a traditional medicine plant. Acta Physiol Plant 32:115–120
Zhang A, Wang H, Shao Q, Xu M, Zhang W, Li M (2015) Large scale in vitro propagation of Anoectochilus roxburghii for commercial application: pharmaceutically important and ornamental plant. Ind Crop Prod 70:158–162
Acknowledgement
Udit Sharma gratefully acknowledges to University Grants Commission (UGC), New Delhi for the financial assistance in the form of Junior Research Fellowship (JRF). Vinod Kataria is thankful to the UGC, New Delhi for providing assistance in the form of CAS (Centre of Advance Study) to the Department of Botany, Jai Narain Vyas University, Jodhpur.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Sharma, U., Kataria, V. & Shekhawat, N.S. In vitro propagation, ex vitro rooting and leaf micromorphology of Bauhinia racemosa Lam.: a leguminous tree with medicinal values. Physiol Mol Biol Plants 23, 969–977 (2017). https://doi.org/10.1007/s12298-017-0459-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-017-0459-2