Abstract
In this study, we have examined the effect of hesperidin on rats fed on an experimental high-fat diet. Male Wistar rats were given a high-fat diet orally for one month for developing an HFD (High fat- diet) model. Rats were also supplemented with hesperidin (100 mg/kg body weight) for one month. We determined serum LDL (Low-density lipoprotein) oxidation, Paraoxonase-1 (PON-1) activity, and histopathological profile of the liver. Inflammatory cytokines levels were also measured in serum. HFD induced significant changes in LDL oxidation and PON-1 activity. Liver tissue histopathology and gene expression of inflammatory markers (Il-6(Interleukin-6), TNF- alpha (Tumor necrosis factor alpha), NF-KB (Nuclear factor kappa B) show that significant changes occur in the hyperlipidemic model of rats. We also show that hesperidin can effectively improve plasma antioxidant, LDL oxidation, and inflammatory cytokine expression in rats already subjected to hyperlipidemic stress. We conclude that hesperidin may protect the liver from oxidative stress by improving hepatic function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Due to their excessive intake, refined sugars and saturated fats have a significant role in the etiology of hyperlipidemia and disorders like cardiovascular disease, diabetes, and hypertension [1]. Long-term consumption of a high-fat diet can impair lipid metabolism leading to numerous disease conditions such as fat deposition, obesity, and hyperlipidemia. These conditions are commonly manifested as an increase in total cholesterol (TC), triglycerides (TG), and LDL cholesterol (LDL-C), as well as a decrease in high-density lipoprotein (HDL-C) [2].
Triglycerides produce VLDL (Very low-density lipoprotein), which is then transformed into low-density lipoprotein (LDL), which aggregates as tiny droplets in hepatocytes [3]. The excessive accumulation of LDL or the absence of LDLreceptors in hepatocytes can result in oxidized LDL (Ox-LDL), which is then ingested by macrophages and transformed into foam cells. Foam cells then gather to create plaques in the vascular endothelium. These plaques ultimately cause circulatory and cardiovascular problems and widen blood–brain barrier permeability [4].
Another significant factor is the regulatory control of fatty acids metabolism through the transcription of hepatic genes. Targets of fatty acid regulation have been shown to include several transcription factors. This mechanism is accomplished either directly by Fatty acids binding to the transcription factor or indirectly by Fatty acids controlling signaling pathways that control the expression of transcription factors or the phosphorylation, ubiquitination, or proteolytic cleavage of the transcription factor [5]. To activate downstream signaling pathways, Fatty acids interact with a variety of transcriptional factors [5]. Peroxisome proliferator-activated receptor (PPAR) is a ligand-activated transcription factor that serves as a sensor for lipid-regulating proteins among all transcription molecules [6]. In the liver, transcription factors that interact with polyunsaturated fatty acids (PUFAs) may modify the genes involved in their production and promote the uptake of phospholipids, fatty acids, and cholesterol [7]. PUFAs also interact with sterol regulatory element binding proteins (SREBPs). Excessive body adiposity may increase the release of tumor necrosis factor-alpha (TNF-alpha), adipokines, and ROS, which generates chronic inflammation [8].
Recent studies have revealed that statins also induce rhabdomyolysis, liver damage, depression, and other side effects, although they improve blood cholesterol levels by raising HDL levels and decreasing LDL levels in plasma [9].Finding safe and efficient medications derived from natural ingredients for conditions including hypertension, hyperlipidemia, and atherosclerosis is an emerging topic in research [10]. A growing body of research suggests that medicinal herbs rich in flavonoids, phenolics, saponins, and Vitamin C can reduce oxidative stress and may be protective against cardiovascular illnesses and other metabolic disorders [11, 12].
It has been underlined that polyphenols play a role in atherosclerosis-related ischemic heart disease and stroke [13]. The peel of lemons and sweet oranges is rich in hesperidin (C28H34O15) (3, 5, 7-Trihydroxy-Flavone 7-rhamnoglukoside), a citrus-bioflavonoid. Many biological characteristics of hesperidin include antioxidant, anti-inflammatory, hepatoprotective, antibacterial, anti-lipidemic, antifungal, antiviral, and anticancer effects [14].
To confirm the use of hesperidin as a successful intervention approach for hyperlipidemia and CVD at the biochemical and molecular level, the current study aims to assess the effect of hesperidin on a high-fat diet-induced hyperlipidemic rat model.The objective of this study is to determine how a high-fat diet affects the expression of inflammatory cytokines, the risk, and development of NAFLD (Non-alcoholic fatty liver disease), and the preventive effects of hesperidin in reducing this burden.
Materials and Methods
All the chemicals and reagents used for this study were purchased from Sigma (St. Louis, MO, USA) and SRL Chemicals, Mumbai, India.
Experimental Animals and Dose Supplementation
This study was done on male Wistar rats of age 15–18 months, corresponding to 30–40 years of human age [15] weighing 200–275 g. Previously and during the investigation, rodents were housed in enclosures and kept on a 12/12-h light/dark cycle at a temperature of 23 ± 2 °C, with free access to drinking water and standard supplement-rich pellets. The High-fat diet administrated to the rodents was fundamentally made out of 0.5% cholesterol, 3% coconut oil, and 0.25% cholic acid. The hesperidin supplementation was 100 mg/kg bodyweight for the present investigation based on our previously reported experiments [16]. The whole experiment spanned one month. The experimental protocols were approved by the Control and Supervision of Experiments on Animals (CPCSEA) and Institutional Animal Ethics Committee (IAEC), University of Allahabad, India vide IAEC/AU/2017(1)/011..
Rats Groups
Group I
Vehicle control (n = 6), during 30 days, this group was given 1.0 mL of carboxymethylcellulose (CMC; 0.5% dissolved in water) orally.
Group II
Experimental hyperlipidemic (HFD) group (n = 6). During 30 days, this group was given a high-fat diet orally in two evenly split dosages of 0.5 mL each per rat each day (9.00 am and 4.00 pm).
Group III
Hesperidin supplementation group. For 30 days, this group was given hesperidin (100 mg/kg body weight) orally and dissolved in CMC once per day.
Group IV
Hyperlipidemic and hesperidin-supplemented groups, During 30 days, this group was given a high-fat diet orally in two evenly split dosages of 0.5 mL each per rat each day (morning and evening) and this group was also given hesperidin (100 mg/kg body weight) orally and dissolved in CMC once per day.
In this experiment, we only selected male rats because several experimental findings show that female rats respond differently to the same stimuli due to hormonal fluctuations throughout the female cycle leading to error in results [17].
Before sacrifice, all groups of rats were kept under overnight fasting condition. Following the prescribed course of treatment, blood was drawn via heart puncture into heparinized syringes while being given a mild sedative (pentobarbital 50 mg/kg body weight). Blood was centrifuged at 800 g for 10 min at 4 °C to separate the erythrocytes. For additional biochemical analysis, the separated plasma was kept at 80 °C. The buffy coat and the top 15% of packed red blood cells were removed, and the remaining packed red blood cells (PRBCs) were then twice washed in ice-cold phosphate buffer saline (PBS).
Determination of serum triglyceride, and total cholesterol, was performed using reagent kits from Span diagnostic, and measurements were made on an Erba Mannheim Chem.-7 analyzer, India.
Estimation of Total Protein Content in Plasma
Plasma protein was measured by the method of Lowry et, al [18]. Bovine serum albumin was used as a standard.
Measurement of LDL Oxidation
The assay was carried out in accordance with the procedure described by Schnitzer et al. [19]. In the experiment mixture (2 mL) containing 0.72 mM sodium citrate, 90 μM copper chloride, and 40 μl plasma in 10 mM phosphate buffer (pH 7.4), the rate of LDL oxidation was determined. A graph showing the absorbance as a function of time was created after 3000 s of 245 nm absorbance monitoring.
Estimation of Paraoxonase–1 Activity (PON‑1 Activity)
The PON-1 activity was assayed following the method [20] as already described in detail [21]. In brief, the initial rate of substrate hydrolysis was measured in a 3 mL assay mixture (2 mM substrate (phenylacetate), 2 mM CaCl2, and 10 μl of plasma in 100 mM Tris–HCl, (pH 8.0). The absorbance was measured at 270 nm, for three minutes. The results are expressed in U/ml, (1 U of arylesterase hydrolyzes 1 mmol of phenylacetate per minute).
Liver Histology
After centrifuging the tissue homogenate, a small portion of the liver was homogenized in 0.1 M phosphate buffer saline (PBS) (pH 7.4), and the supernatant was utilized for biochemical assays. The liver tissues were placed in 10% formalin for 72 h before being dehydrated in various alcohol concentrations (50–100%) and covered in paraffin wax (melting point 58–60 °C). A typical dehydration sequence for specimens not more than 5 mm thick were as follows: serial treatment with different concentrations of ethanol followed by cleaning. Subsequently, the tissue was stained with hematoxylin and eosin (H&E) to stain liver sections following accepted histological procedures. Images were analyzed using an Olympus microscope (CX21iTR-LED) outfitted with a Magnus UHCCD-USB 21.4-megapixel camera.
Gene Expression Studies
By applying the reverse transcriptase-polymerase chain reaction, gene expression was evaluated following the manufacturer’s directions, total RNA was extracted from liver tissue using the TRIzol reagent (Sigma). RNA integrity was verified using agarose gel electrophoresis in a denaturing environment, and RNA quantity was determined using the Spectro star nano (BMG Labtech,Germany) at 260 nm. The cDNA Synthesis Kit (New England Biolabs) was used according to the manufacturer’s instructions to synthesize C-DNA.
Using gene-specific primers, amplification for Il-6, TNF-alpha, NF-KB, and beta-actin was carried outand visualized under UVChemi Doc MP imaging system (Bio-Rad), PCR products were seen on a 1.5% agarose gel containing ethidium bromide. The densitometry system software provided by (Bio-Rad) was used to analyze the bands.
Statistical Analysis
Data are expressed as mean ± SD for six independent experiments. Using PRISM version 5.01, a one-way ANOVA with Bonferroni’s test was conducted to determine all pairwise wise and group-wise comparisons. A probability (p) value of less than 0.05 was considered statistically significant.
Results
Biochemical Analysis of Total Cholesterol and Triglycerides Level in Serum
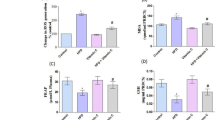
On an Erba Mannheim Chem.–7 analyzer, serum triglyceride and total cholesterol levels were determined using reagent kits from ERBA diagnostics. Figures 1A and B represent the total cholesterol and triglyceride level in all experimental groups of rats. The HFD-treated groups of rats show a significant (p < 0.05) increase in the level of both these parameters whereasHFD + hesperidin-supplemented groups show significant (p < 0.05) reduced levels of total cholesterol and triglyceride level in serum. The data are represented in mg/dl.
A & B High-fat diet-associated lipidemic alterations are measured by triglyceride and total cholesterol levels in each group of rats in figure respectively. Data are provided as the mean SD of six independent experiments, with * denoting a significant (p < 0.05) difference from the control group and # denoting a significant difference from groups supplemented with HFD + HES
LDL-Oxidation and PON-1 Activity
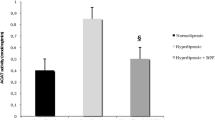
LDL oxidation and PON-1 are important markers for per-oxidative alterations Figs. 2A and Brepresent the LDL-oxidation and PON-1actvity respectively. The HFD-treated groups of rats show a significant (p < 0.05) increase in the level of both these parameters whereasHFD + hesperidin-supplemented groups show significant (p < 0.05) reduced levelsof LDL oxidation and PON-1.* compared to the control group, # compared to HFD + HES group.
Liver Histology Analysis
Figure 3 represents the Liver histology of all groups of rats. Figure 3A represents the control groups,Fig. 3B represents the HFD-treated groups, Fig. 3C represents the hesperidin-supplemented group and Fig. 3D represents the HFD + HES supplemented groups sections of the liver after H&E staining revealed normal histology. Normal liver tissue morphology and there was no congestion in both the control and hesperidin groups. While the HFD-treated group, congestion of CV, and development of inflammatory cells, are observed. Upon treatment of hesperidin in the hyperlipidemic + hesperidin-supplemented group, there was a decrease in inflammation and inflammatory cells in the liver tissue. The blackarrow shows in Fig. 3A normal cells, and the red arrow in Fig. 3B presents CV congestion and infiltration of inflammatory cells.
Liver tissue histology represents the liver tissue histology of different groups of rats after dye with Hematoxylin& Eosin. The tissue section was taken at 100X. A Control Group; B HFD treated Group; C HES supplemented group and D HFD + HES treated group. The BLACK arrow shows normal cells whereas the yellow arrow shows damaged cells, and infiltration of inflammatory cells.
Gene expression Study of Inflammatory Cytokines
Figure 4A–C represents the various inflammatory cytokine IL-6, TNF-alpha, and NF-KB activity respectively.A significant increase (P < 0.05) in the inflammation level in HFD treated group, in all threecytokines IL-6, TNF-alpha, and NF-KB, while after hesperidin supplementation all these cytokine levels decreased significantly(P < 0.05) when compared with their respective control groups. * represents when compared to the control group, and # represents compared tothe HFD + HES group.
A, B, and C Gene expression study. Il-6, TNF-alpha and NFKB gene expression activity is shown in figure. Data are shown as the mean SD of six separate studies. Data are provided as the mean SD of six independent experiments, with * denoting a significant (p < 0.05) difference from the control group and # denoting a significant difference from groups supplemented with HFD + HES
Discussion
Hesperidin is a plant substance having multiple uses including the promotion of iron absorption, wound healing, and the stimulation of several metabolic pathways [14]. Increased dietary fat consumption alters the lipidemic balance, which produces reactive oxygen species and, as a result, causes the growth of white adipose tissue, which secretes pro-inflammatory cytokines, resulting in a chronic state of inflammation [22]. Reactive oxygen species are intimately linked to atherosclerosis and several cardiovascular disorders.
In the present study, we found reduced levels of total cholesterol and triglyceride level in hesperidin-supplemented groups of rats with respect to HFD groups of rats.Hesperidin has been shown to reduce hepatic TG concentration by inhibiting lipogenesis and inducing fatty acid oxidation, as well as by downregulating the production and secretion of very low-density lipoproteins. However, some studies have also reported that there were no significant changes in cholesterol and lipidemic parameters after the hesperidin and naringin supplementation [23, 24].
The term “oxidized LDL” (ox-LDL) refers to a variety of lipid peroxidation-induced modifications of both lipid and apolipoprotein B (apoB) components. Inflammatory and immunologic processes that result in the development of macrophage foam cells, promote atherosclerosis [4]. In our study, we also observed that LDL oxidation was much higher in the HFD groups while it was significantly lower in the Hesperidin-supplemented groups.
Hesperidin exerts its hepatoprotective properties via different mechanisms including elevation in the activities of nuclear factor-like 2/antioxidant response element and hemeoxygenase 1 as well as the levels of enzymatic and non-enzymatic antioxidants [25].Hesperidin also activates AMPK, increasing its phosphorylation, in addition, a rise in Nrf-2 expression was seen in animal tissue. An up-regulation in the Nrf-2 is known to activate various transcription factors [26].Furthermore, hesperidin also increased HDL-C and TG in a mildly dose-dependent manner while considerably lowering high LDL-C and cholesterol. This may have been accomplished by hesperidin’s antioxidant capabilities [27].
The effects of hesperidin on lipid balance in the bodyare attributed to a decrease in the activity of the enzymes hepatic fatty acid synthase, glucose-6-phosphate dehydrogenase, and phosphatidate phosphohydrolase as well as an increase in fecal triglycerides [11, 28]. Also, it has been shown that hesperidin treatment reduced plasma and hepatic cholesterol levels via downregulating the activity of the enzymes acyl CoA: cholesterol acyltransferase (ACAT) and 3-hydroxy-3-methylglutaryl-coenzyme (HMG-CoA) reductase in the liver [29].
PON-1 is an HDL-associated lactonase that possesses antioxidant and anti-atherogenic properties [30].The HDL-linked enzyme Paraoxonase-1 (PON-1) protects LDL and HDL against lipid peroxidation. PON-1 is a protective factor in diseases associated with inflammation and oxidation, such as diabetes mellitus and non-alcoholic fatty liver diseases. Several studies demonstrate how polyphenols can increase PON-1 [20]. The preservation of the enzyme-SH group supports the idea that the plasma redox state plays a significant role in controlling PON-1 activity [21]. Hesperidin’s antioxidant significance is highlighted by our observation that hesperidin supplementation can reverse the effects of hyperlipidemic stress in rats receiving atherogenic supplementation.
Obesity is typically linked to low-grade inflammatory diseases such as dyslipidemia and type 2 diabetes mellitus. A balance between pro-inflammatory and anti-inflammatory cytokines regulates low-grade inflammation. According to a few studies, those who are obese or have type 2 diabetes and consume a lot of dietary fat have elevated levels of NF-KB, IL-6, and TNF-alpha [22, 31]. In line with earlier research, this study also observed that rats fed a high-fat diet had much higher levels of IL-6, TNF-alpha, and NF-KBcompared to rats fed the regular diet.Whereas the hesperidin-treated group showed decreased levels of IL-6, TNF-alpha and NF-KB which can beexplained based on the antioxidant property of hesperidin.
Adipocytokine release is the cause of hepatocyte lipotoxicity because it harms hepatocytes by triggering apoptosis and enlisting pro-inflammatory mediators [31]. While blood levels of HDL cholesterol are a protective factor against CVD and aid to lower the chance of developing NAFLD progression, LDL cholesterol has been linked to atheromas [32]. In the current investigation, we observed damaged hepatic cells and the development of necrotic cells in the rat HFD groups, whereas these alterations were reversed in the hesperidin-supplemented groups.
Glucose/lipid metabolism is regulated by the protein hormone leptin, which also regulates appetite and energy homeostasis [33]. Plasma leptin levels and body fat percentage are related [34], and increased leptin release is linked to larger and more numerous fat cells. Additionally, our results are supported by the leptin gene expression profile in liver tissue. The HFD group of rats expressed more leptin when compared to the control group of rats. Hesperidin possesses anti-hyperlipidemic capabilities as evidenced by the fact that it decreased leptin expression in HFD rats.
Conclusion
The results indicate that hesperidin can successfully enhance liver lipid metabolism by regulating cholesterol and triglyceride levels, decreasing NF-KB expression, and protecting the liver from oxidative stress by enhancing hepatic function. Hesperidin supplementation reduced lipidemic stress by decreasing lipid regeneration and increasing lipid oxidation. The hypolipidemic effects of hesperidin as a functional diet need to be examined in targeted human clinical trials. The conclusions derived from this study have the limitation of a controlled study based on selected parameters. A comprehensive study with more parameters is needed before these findings can be extrapolated on humans.
Data Availability
The information that helps the finding of this study is accessible from the corresponding author upon reasonable request.
References
Siri-Tarino PW, Sun Q, Hu FB, Krauss RM. Saturated fat, carbohydrate, and cardiovascular disease. Am J Clin Nutr. 2010;91:502–9.
Wali JA, Jarzebska N, Raubenheimer D, Simpson SJ, Rodionov RN, O’Sullivan JF. Cardio-metabolic effects of high-fat diets and their underlying mechanisms-a narrative review. Nutrients. 2020;12:1505.
Alves-Bezerra M, Cohen DE. Triglyceride metabolism in the liver. Compr Physiol. 2017;8:1–8.
Levitan I, Volkov S, Subbaiah PV. Oxidized LDL: diversity, patterns of recognition, and pathophysiology. Antioxid Redox Signal. 2010;13:39–75.
Jump DB, Tripathy S, Depner CM. Fatty acid-regulated transcription factors in the liver. Annu Rev Nutr. 2013;33:249–69.
Grygiel-Górniak B. Peroxisome proliferator-activated receptors and their ligands: nutritional and clinical implications–a review. Nutr J. 2014;13:17.
Koundouros N, Poulogiannis G. Reprogramming of fatty acid metabolism in cancer. Br J Cancer. 2020;122:4–22.
Tan BL, Norhaizan ME. Effect of high-fat diets on oxidative stress, cellular inflammatory response and cognitive function. Nutrients. 2019;11:2579.
Safitri N, Alaina MF, Pitaloka DAE, Abdulah R. A narrative review of statin-induced rhabdomyolysis: molecular mechanism, risk factors, and management. Drug Healthc Patient Saf. 2021;13:211–9.
Shaito A, Thuan DTB, Phu HT, Nguyen THD, Hasan H, Halabi S, et al. Herbal medicine for cardiovascular diseases: efficacy, mechanisms, and safety. Front Pharmacol. 2020;11:422.
Tungmunnithum D, Thongboonyou A, Pholboon A, Yangsabai A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: an overview. Medicines. 2018;5:93.
Kumar R, Rizvi SI. Vitamin C improves inflammatory-related redox status in hyperlipidemic rats. Ind J Clin Biochem. 2022. https://doi.org/10.1007/s12291-022-01070-8.
Cheng Y-C, Sheen J-M, Hu WL, Hung Y-C. Polyphenols and oxidative stress in atherosclerosis-related ischemic heart disease and stroke. Oxid Med Cell Longev. 2017;2017:8526438.
Pyrzynska K. Hesperidin: a review on extraction methods, stability and biological activities. Nutrients. 2022;14:2387.
Sengupta P. The laboratory rat: relating its age with human’s. Int J Prev Med. 2013;4:624–30.
Kumar R, Akhtar F, Rizvi SI. Hesperidin attenuates altered redox homeostasis in an experimental hyperlipidaemic model of rat. Clin Exp Pharmacol Physiol. 2020;47:571–82.
Becker JB, Prendergast BJ, Liang JW. Female rats are not more variable than male rats: a meta-analysis of neuroscience studies. Biol Sex Differ. 2016;7:34.
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–75.
Schnitzer E, Pinchuk I, Bor A, Fainaru M, Samuni AM, Lichtenberg D. Lipid oxidation in unfractionated serum and plasma. Chem Phys Lipids. 1998;92:151–70.
Ayub A, Mackness MI, Arrol S, Mackness B, Patel J, Durrington PN. Serum paraoxonase after myocardial infarction. Arterioscler Thromb Vasc Biol. 1999;19:330–5.
Mehdi MM, Rizvi SI. Human plasma paraoxonase 1 (PON1) arylesterase activity during aging: correlation with susceptibility of LDL oxidation. Arch Med Res. 2012;43:438–43.
Ellulu MS, Patimah I, Khazaai H, Rahmat A, Abed Y. Obesity and inflammation: the linking mechanism and the complications. Arch Med Sci. 2017;13:851–63.
Mohammadi M, Ramezani-Jolfaie N, Lorzadeh E, Khoshbakht Y, Salehi-Abargouei A. Hesperidin, a major flavonoid in orange juice, might not affect lipid profile and blood pressure: a systematic review and meta-analysis of randomized controlled clinical trials. Phytother Res. 2019;33:534–45.
Demonty I, Lin Y, Zebregs YEMP, Vermeer MA, van der Knaap HCM, Jäkel M, et al. The citrus flavonoids hesperidin and naringin do not affect serum cholesterol in moderately hypercholesterolemic men and women. J Nutr. 2010;140:1615–20.
Tabeshpour J, Hosseinzadeh H, Hashemzaei M, Karimi G. A review of the hepatoprotective effects of hesperidin, a flavanon glycoside in citrus fruits, against natural and chemical toxicities. Daru. 2020;28:305–17.
Morris G, Walker AJ, Walder K, Berk M, Marx W, Carvalho AF, et al. Increasing Nrf2 activity as a treatment approach in neuropsychiatry. Mol Neurobiol. 2021;58:2158–82.
Aja PM, Ekpono EU, Awoke JN, Famurewa AC, Izekwe FI, Okoro EJ, et al. Hesperidin ameliorates hepatic dysfunction and dyslipidemia in male Wistar rats exposed to cadmium chloride. Toxicol Rep. 2020;7:1331–8.
Jung UJ, Lee M-K, Park YB, Kang MA, Choi M-S. Effect of citrus flavonoids on lipid metabolism and glucose-regulating enzyme mRNA levels in type-2 diabetic mice. Int J Biochem Cell Biol. 2006;38:1134–45.
Grande F, Occhiuzzi MA, Perri MR, Ioele G, Rizzuti B, Statti G, et al. Polyphenols from citrus Tacle® extract endowed with HMGCR inhibitory activity: an antihypercholesterolemia natural remedy. Molecules. 2021;26:5718.
Cakatay U, Kayali R, Uzun H. Relation of plasma protein oxidation parameters and paraoxonase activity in the ageing population. Clin Exp Med. 2008;8:51–7.
Roy PK, Islam J, Lalhlenmawia H. Prospects of potential adipokines as therapeutic agents in obesity-linked atherogenic dyslipidemia and insulin resistance. Egypt Heart J. 2023;75:24.
Vergeer M, Holleboom AG, Kastelein JJP, Kuivenhoven JA. The HDL hypothesis: does high-density lipoprotein protect from atherosclerosis? J Lipid Res. 2010;51:2058–73.
Paz-Filho G, Mastronardi C, Wong M-L, Licinio J. Leptin therapy, insulin sensitivity, and glucose homeostasis. Indian J Endocrinol Metab. 2012;16:S549-555.
Jürimäe T, Sudi K, Jürimäe J, Payerl D, Rüütel K. Relationships between plasma leptin levels and body composition parameters measured by different methods in postmenopausal women: leptin level and body composition in women. Am J Hum Biol. 2003;15:628–36.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors of this manuscript have no conflict of interest.
Ethical Approval
All animal care and exploratory methods conformed with the guidelines of the Control and Supervision of Experiments on Animals (CPCSEA) and Institutional Animal Ethics Committee (IAEC), University of Allahabad, India.
Informed Consent
No human experimentation was undertaken hence informed consent is not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumar, R., Khan, M.I., Ashfaq, F. et al. Hesperidin Supplementation Improves Altered PON -1, LDL Oxidation, Inflammatory Response and Hepatic Function in an Experimental Rat Model of Hyperlipidemia. Ind J Clin Biochem 39, 257–263 (2024). https://doi.org/10.1007/s12291-023-01140-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12291-023-01140-5