Abstract
Excessive dietary fat is mainly responsible for metabolic diseases including atherosclerosis and cardiovascular disease. We have evaluated the role of Vitamin C in an experimental hyperlipidemic model of rats (male Wistar rat 12–16 months). The hyperlipidemic model of the rat was created by treatment with an atherogenic suspension: cholesterol, cholic acid, and coconut oil, for 30 days once daily, and supplemented with Vitamin C (Ascorbic acid) doses of 0.5 g/kg body weight (orally) for the 30 days once daily. Bodyweight, fasting glucose, triglyceride, cholesterol, ROS (Reactive oxygen species), MDA (Malondialdehyde), FRAP (Ferric reducing the ability of plasma), GSH (Reduced glutathione), PCO (Protein carbonyl), PON-1(Paraoxonase-1), AGE (Advanced glycation end product), PMRS (Plasma membrane reduced system), and inflammatory cytokines (TNF-α and IL-6) were estimated in blood and plasma. Our result shows that oxidative stress, and inflammatory markers, were increased in the HFD-treated group of rats. Vitamin C supplementation protected against lipidemic and, oxidative stress. We conclude that Vitamin C may be useful in maintaining cellular redox balance and protecting against lipidemic stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hyperlipidemia is one of the main causes of cardiovascular disease, atherosclerosis, and other metabolic disorders [1]. There is ample scientific evidence to document an important link between complex oxidation reactions and the development of atherosclerosis [2]. Oxidative stress is known to result in inflammatory neutrophil infiltration, increased protease secretion, and a large number of oxidative intermediates being formed which play an important role in cardiovascular disease pathogenesis [3]. Increased oxidative stress also contributes to the subsequent formation and progression of atherosclerotic plaques [4]. In particular, oxidized low-density lipoprotein (LDL) induces inflammation and favors atherosclerosis by encouraging the development of cytokines.
A hot subject in research is finding safe and effective drugs extracted from natural products for diseases such as hypertension, hyperlipidemia, and atherosclerosis [5]. Accumulating evidence indicates that medicinal plants that have an abundance of flavonoids, phenolics, and saponins, as well as Vitamin C, can reduce oxidative stress [6] and have the potential to protect against cardiovascular diseases and other metabolic disorders.
Vitamin C protects against oxidative stress-induced cellular damage by scavenging reactive oxygen species and recycling the eNOS cofactor, tetrahydrobiopterin, which is relevant to arterial elasticity and blood pressure regulation. Various studies document the antioxidant, anticancerous, and antiaging properties of Vitamin C [7] but the anti-hyperlipidemic property of Vitamin C has not been fully explored. The present study envisages evaluating the effect of Vitamin C on a high-fat diet-induced hyperlipidemic rat model to validate the use of this vitamin as an effective intervention strategy for hyperlipidemia and CVD.
Materials and Methods
All the chemicals and reagents used in this study were procured from Sigma, St. Louis, USA, and Merck, Germany. Ascorbic acid and Cholic acid were purchased from SRL Chemicals (India).
Laboratory Animals, Treatment, and Groups
This study was performed on male Wistar rats with a bodyweight of 220 ± 30 g. All rats were fed a standard laboratory pellet diet and had free access to drinking water. The research was performed using standard lab protocols accepted by the University of Allahabad Ethical Committee, India.
Experimental Hyperlipidemic Model in Rats
The atherogenic suspension administrated to rats was made in the lab by the method published [8]. Vitamin C was supplemented to rats in doses of 0.5 g/kg body weight [9] during the study.
The following were the experimental group of rats:
Group I
Control group of rats (n = 6) (without any treatment) having-free access to drinking water and lab feed.
Group II
Experimental hyperlipidemic group of rats (n = 6). This group of rats received orally 1.0 ml of atherogenic suspension every day (morning 0.5 ml and evening 0.5 ml) for 30 days.
Group III
This group of rats (n = 6) were supplemented with Vitamin C (freshly prepared) orally (0.5 g/kg BW) and dissolved in water for 30 days. The selection of dose was based on previous reports [10].
Group IV
This group of rats (n = 6) received orally 1.0 ml of atherogenic suspension and Vitamin C orally (0.5 g/kg BW) dissolved in water for 30 days.
It needs to be clarified that there are substantial differences between rodents and human timescales in normal biological processes. However, these differences only become important when the results need to be translated into clinical practice [11]. Our protocol of 30 days refers only to time period with reference to human timescale.
Isolation of Blood, Plasma, Serum, and RBC
After the 30 days of treatment with either atherogenic suspension/ Vitamin C or both, blood samples were obtained by cardiac puncture and collected under light anesthesia in heparinized syringes (pentobarbital 50 mg/kg body weight). Erythrocyte, plasma, and serum were isolated by centrifugation, and plasma and serum were stored at 80 ° C, for further use. For biochemical analysis, red blood cells (RBCs) were then washed three times with cold phosphate buffer saline (PBS).
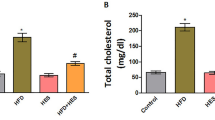
The study of serum triglyceride and total cholesterol was performed utilizing reagent packs from ERBA diagnostics (Transasia India) on an Erba Mannheim Chem. − 7 analyzer. The fasting glucose level was estimated by the Roche Accu-chek blood glucose checking unit.
Intracellular ROS in PRBCs
Oxidative stress was measured by using DCFH-DA fluorescence dye by following the protocol [12] on a Cary Eclipse fluorescence spectrophotometer (Agilent Technologies). 485 nm set as excitation wavelength and 528 nm emission wavelength. The control group value of ROS is set as 100%.
Estimation of Lipid Peroxidation in the Terms of MDA
MDA was assayed by protocol [13]. In brief 0.2 ml PRBCs, 1 ml of 10% trichloroacetic acid, and 2 ml of 0.67% thiobarbituric acid mixed well and kept in a boiling water bath for 20 min and subsequently cooled Centrifuged at 1000 g and the supernatant collected, read at 532 nm. Extinction coefficient (1.56 × 10 5 M− 1 cm− 1) was used for the calculation and reported as nmol/ml of PRBCs.
Estimation of Total Antioxidant Activity in the Terms of FRAP
The plasma FRAP value was measured by a standard method [14]. In short, 100 µl of plasma sample and 900 µl of FRAP reagent containing 300mM acetate buffer, pH 3.6, 20mM ferric chloride, and 10mM 2,4,6-tripyridyl-s-triazine in 40mM hydrochloric acid in the ratio of 10:1:1 was mixed well and absorbance was taken at 593 nm at 30 s interval for 5 min.
Estimation of Glutathione (GSH) in Erythrocytes
Erythrocyte GSH value was measured by using the established protocol [15]. In brief, the assay is based on the reduction of 5,5,-dithiobis, 2-nitrobenzoic acid by sulphdryl group (SH). The concentration of GSH is reported in mg/mL PRBCs. The absorbance was read at 412 nm and the value of GSH is reported in terms of mg/ml PRBCs.
Total Protein Content in Plasma
Plasma protein was evaluated by the method of Lowry et al. [16].
Plasma Protein Carbonyls (PCO)
Protein carbonyl in plasma was estimated by following the method given [17]. Protein carbonyls react with 2,4-dinitrophenylhydrazine (DNPH) and produce hydrazones, these hydra zones are detected spectrophotometrically at 370 nm. The carbonyl content is reported in nmol/mg protein by using an absorption coefficient of 22,000 M− 1 cm− 1.
Paraoxonase − 1 Activity (PON-1 Activity)
The PON-1 activity was measured by the protocol given by [18]. The value was reported in U/ml, 1 U of arylesterase hydrolyses 1 mmol of phenylacetate per minute.
Estimation of advanced glycation end products (AGEs)
Advanced glycation end products were estimated by using a spectrofluorometer, following the method of [19]. In brief, serum was diluted at 1:50 with PBS, pH 7.4. Emission (440 nm) and excitation at 350 nm were used for the assay. The result was reported in arbitrary units (AU) and AU/g protein.
Erythrocyte PMRS activity
The erythrocyte PMRS activity was assayed by the method described earlier [20]. PRBCs were suspended in PBS (containing 5 mM/L glucose and 1 mM/L potassium ferricyanide) up to a final volume of 2.0 ml. The suspension was incubated at 37 °C for 30 min and later centrifuged at 4 °C at 800 g. The absorbance was measured at 535 nm. The coefficient (= 20,500 M− 1 cm-1) was used to assess the result and the results are reported in the terms of µmol ferrocyanide/ml PRBCs/30 min.
Estimation of Cytokine levels (IL-6 and TNF – alpha) in the serum
Cytokine levels were estimated following the instructions provided in the manufacturer’s manual (Krishgen BioSystem, India), as described in detail [8]. Briefly, antibody-coated plates (either TNF-α or IL-6) were loaded with standard (known concentration) and experimental samples and kept for incubation at 37 °C for 1 h. After incubation and washing (at least five times), a specific detection antibody was added for 30 min at 37 °C. After the incubation, a secondary antibody (streptavidin-conjugated HRP) was added. After 30 min of incubation, the stopping reagent was added and the blue color generated was read at 450 nm by using an ELISA microplate reader, Spectrostar Nano (BMG-Lab Tech). The results are reported in Pg/mL.
Statistical Analysis
Data were analyzed by one-way ANOVA and compared between all experimental groups using the software PRISM 5.01. All data are expressed as mean ± SD for six independent experiments. A value of p < 0.05 was considered statistically significant.
Results
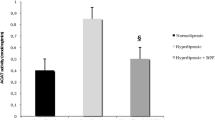
ROS Level in HFD Treated and Vitamin C Supplemented Group of Rats
Intracellular ROS determination is represented in Fig. 1 A. The ROS level was found to be significantly (p < 0.05) increased (144.17%) in the HFD treated group with respect to the control group. while a significant (p < 0.05) decrease (42.54%) was found in the ROS level of the HFD + Vitamin C group with respect to the HFD group of the rat. A one-way ANOVA was conducted to compare the effect of Vitamin C on the HFD + Vitamin C treated group of rats.
(A) Intracellular ROS was analyzed with fluorescent dye DCFDA in all groups of rats with Spectrofluorimetric. Data are shown as mean ± SD of six independent experiments, and the generation of ROS is expressed as % change of normal control.* (p < 0.05) when compared with normal control. # (p < 0.05) represent when compared with HFD group of the rat
(B) Lipid peroxidation is represented in terms of MDA in all groups.* represent significantly increased (p < 0.05) in MDA level of the HFD group when compared with the control group. # represents a significant decrease (p < 0.05) in the HFD + Vitamin C group when compared with the HFD group
FRAP and GSH values are represented in Fig. 1(C &D). Data are shown as mean ± SD of six independent experiments, * (p < 0.05) when compared with normal control. # (p < 0.05) represent when compared with HFD group of the rat
Lipid Peroxidation (MDA) Level on HFD Treated and Vitamin C Supplementation Group of Rats
The lipid peroxidation measured in the form of MDA is represented in Fig. 1B. Our finding shows that it is a significant (p < 0.05) increase (32.28%) in the MDA level in the HFD group of rats with respect to the control group and is significantly (p < 0.05) decreased (21.56%) when the HFD group of rats is supplemented with Vitamin C and compared with HFD group of rats.
The Impact of HFD Treatment on Ferric Reducing the Antioxidant Ability of Plasma Value is Represented in the Form of FRAP in Fig. 1 C
Data shows a significant (p < 0.05) decrease (38.00%) in the FRAP value in the HFD treated group, after the supplementation of Vitamin C the HFD group of rats show a significant (p < 0.05) increase (40.57%) in the FRAP value with respect to HFD treated group.
Figure 1D Represents the Intracellular GSH Level
Our finding shows a significant (p < 0.05) decrease (57.44%) in the GSH level in the HFD group of rats with respect to the control group and a significant (p < 0.05) increase (63.33%) when the HFD group of rats is supplemented with Vitamin C and compared with HFD group of rats. A one-way ANOVA was conducted to compare the effect of Vitamin C on the HFD + Vitamin C treated group of rats.
PCO and PON-1 level in HFD treated and Vitamin supplementation group of rat
PCO and PON-1 levels are represented in Fig. 2 A & 2B respectively. Our result shows that there is a significant (p < 0.05) increase in both the value in HFD treated group of rats respectively with respect to the control group, while after the treatment with Vitamin C significantly (p < 0.05) decreased both values when the compared with HFD group of rats.
Protein carbonyl (PCO) & PON-1 are represented in Fig. 2(A& B).* represent (p < 0.05) when compared with normal control group of rat. # (p < 0.05) represent when compared with HFD group of rat. (C & D) represent the AGE and PMRS level in all experimental group of the rat. Data are shown as mean ± SD of six independent experiments.* (p < 0.05) when compared with normal control. # (p < 0.05) represent when compared with HFD group of the rat
AGE Level on High-fat Diet Treated and Vitamin C Supplementation Group of Rat
The advanced glycation end product is shown in the form of AGE depicted in Fig. 2 C. Our data showed a significant (p < 0.05) increase (144.77%) in the AGE level in the HFD group of rats with respect to the control group, and a significantly (p < 0.05) decrease (31.14%) level in Vitamin C supplemented group of rat when compared with HFD group of the rat.
Role of HFD and Vitamin C Supplementation on PMRS Level
PMRS activity is represented in Fig. 2D. PMRS activity is significantly (p < 0.05) increased (227.19%) in the HFD group of rats, whereas HFD with supplementation with Vitamin C shows a significant (p < 0.05) decrease (37.80%) in PMRS activity when compared with HFD group.
Cytokine Level After the Treatment of HFD and Vitamin C Supplementation
Inflammatory Cytokine levels are shown in Fig. 3 A & 3B respectively. The activity of both cytokines (Il-6 & TNF- α) is significantly (p < 0.05) increased (41.32% &38.97% ) in HFD groups of rats with respect to control. A significant (p < 0.05) reduction (25.64%,28.05%) is seen in both parameters in the HFD + Vitamin C group of rats when compared with HFD groups of rats.
Discussion
Vitamin C is a multi-function vitamin with a powerful antioxidative property, wound healing effect, cooperation with iron absorption, and activation of hemoglobin. Ascorbic acid is also a cofactor for collagen biosynthesis, carnitine, catecholamines, and some neurotransmitters [21]. High dietary fat intake leads to altered lipidemic balance, leading to the production of reactive oxygen species and thereby inducing a permanent state of inflammation through the development of white adipose tissue that secretes pro-inflammatory factors [22]. Increased development of reactive oxygen species is directly related to atherosclerosis and various cardiovascular diseases. Dyslipidemia-induced impaired redox status is commonly seen in women with menopause. Although menopause is an inevitable part of getting older it may also speed up the aging process [23, 24].
Malondialdehyde (MDA) influences the exchange of ions from the cell membranes, allowing membrane-located compounds to cross-link, increased lipid peroxidation may alter membrane fluidity, and affect the activity of enzymes and receptors which are bound with the membrane [25]. The lipid-phase surface charge polarity and the formation of protein oligomers increase as lipid oxidation of the cell membrane increases. The molecular mobility of lipids, the number of groups of -SH (Thiol), and the resistance to thermal denaturation also may reduce. Our observation of a decrease in MDA after Vitamin C treatment is explained due to its ability to protect against lipid peroxidation by acting as a scavenger of ROS and by one-electron reduction of lipid hydroperoxyl radicals via the vitamin E redox cycle [26].
ROS targets plasma proteins and, under stress, conditions contribute to carbonyl groups on multiple amino acids. Different molecular pathways have been suggested for protein carbonation, some of them caused by direct ROS attacks and by the radical formation of alkoxides that lead to protein cleavage. According to a previously published research article high PCO has been recorded in atherogenic rats [27]. The anti-hyperlipidemic effect of vitamin C is validated by our vitamin C-induced decrease in PCO findings. Reports document that there are several possible mechanistic explanations for the observed beneficial effects of systemically administered vitamin C on endothelial function [28]. As stated above, one explanation is that vitamin C eliminates intracellular superoxide that would otherwise deactivate NO by forming peroxynitrite, and can neutralize ROS including superoxide anions, hydroxyl radicals, peroxynitrite, and radicals of nitric oxides [7].
AOPPs and AGEs are important oxidative stress biomarkers [29]. In the atherogenic treated rat which is characterized by increased oxidative stress, a high level of plasma AOPP was found. Vitamin C supplementation mitigates this effect. In accumulating random damage to extracellular proteins, AGEs play a causative function. These advanced end products of glycation have potentially adverse effects on biological function and are related to aging and many degenerative diseases, such as diabetic complications, atherosclerosis, and chronic renal failure [30].
Paraoxonase 1 (PON-1) is an enzyme linked to HDL that provides LDL and HDL for lipid peroxidation protection. It has been identified that PON-1 is a protective factor in disorders associated with inflammation and oxidation, such as diabetes mellitus and non-alcoholic liver fat disorders. The role of polyphenols in stimulating PON-1 is shown in a few studies [18]. Enzyme-SH group maintenance justifies the significance of plasma redox status as an important factor in the modulation of the behavior of PON-1 [31]. The antioxidant function of Vitamin C is illustrated by our observation of vitamin C supplementation reversing the impact of hyperlipidemic stress in atherogenic supplemented rats.
In erythrocytes, the plasma membrane redox system (PMRS) is an essential mechanism for preserving plasma redox status in different situations, including aging and diabetes [32]. The behavior of the PMRS is focused on the transfer of electrons to its extracellular acceptors from intracellular donors NADH and/or ascorbate (ASC). Previous reports indicate that in vivo polyphenols exert their antioxidant effects through a process based on PMRS activation [20]. Our results on the effect of Vitamin C on erythrocyte PMRS activity support our earlier findings with resveratrol [33] and tea catechins [34] emphasizing the important role Vitamin C may play during the hyperlipidemic condition.
In hyperlipidemic conditions we observe higher levels of inflammatory cytokines, this has also been reported in our previous findings [8]. In the hyperlipidemic condition, a high degree of lipid peroxidation occurs and leads to the activation of nuclear factor kB (NF-kB), a redox-sensitive transcription factor that plays an important role in the inflammatory cytokine secretion. Inflammation in the vasculature caused by reactive oxygen species (ROS) is a key connection between cardiovascular disease and metabolic syndrome. Atherosclerosis is characterized by chronic inflammation, which is a harmful characteristic. Endothelial dysfunction activates innate immunological inflammatory pathways upstream of the nuclear transcription factor NF-kB. As a result, ROS-induced inflammation contributes to endothelial dysfunction and promotes inflammation [35]. Both cytokines IL-6 and TNF-alpha levels are found to be higher in rat groups treated with HFD compared to control. Whereas the group treated with vitamin C showed decreased IL-6 and TNF-alpha levels, which can be explained by the antioxidant properties of vitamin C against lipid peroxidation. Based on our observations we hereby provide experimental evidence explaining that hyperlipidemic rats show alterations in lipidemic and oxidative stress levels in the intracellular environment. Vitamin C protects against hyperlipidemic alterations, redox imbalance and reduced level of inflammatory cytokines such as IL-6 and TNF-alpha which represent the antioxidant, anti-inflammatory and antilipidemic activity of Vitamin C. Our findings validate the use of Vitamin C as a therapeutic intervention in conditions of hyperlipidemia and to reduce the intracellular inflammation condition.
Data Availability
The information that helps the finding of this study is accessible from the corresponding author SIR upon reasonable request.
References
Nelson RH. Hyperlipidemia as a risk factor for cardiovascular disease. Prim Care. 2013 Mar;40(1):195–211.
Lepedda AJ, Formato M. Oxidative Modifications in Advanced Atherosclerotic Plaques: A Focus on In Situ Protein Sulfhydryl Group Oxidation. Oxidative Medicine and Cellular Longevity. Jan. 2020;9:2020:1–7.
Toledo-Ibelles P, Mas-Oliva J. Antioxidants in the Fight Against Atherosclerosis: Is This a Dead End? Curr Atheroscler Rep. 2018 May 21;20(7):36.
Yang X, Li Y, Li Y, Ren X, Zhang X, Hu D, et al. Oxidative Stress-Mediated Atherosclerosis: Mechanisms and Therapies. Front Physiol. 2017;8:600.
Shaito A, Thuan DTB, Phu HT, Nguyen THD, Hasan H, Halabi S, et al. Herbal Medicine for Cardiovascular Diseases: Efficacy, Mechanisms, and Safety. Front Pharmacol. 2020;11:422.
Tungmunnithum D, Thongboonyou A, Pholboon A, Yangsabai A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines (Basel). 2018 Aug 25;5(3).
Chambial S, Dwivedi S, Shukla KK, John PJ, Sharma P. Vitamin C in disease prevention and cure: an overview. Indian J Clin Biochem. 2013 Oct;28(4):314–28.
Kumar R, Akhtar F, Rizvi SI. Hesperidin attenuates altered redox homeostasis in an experimental hyperlipidemic model of rat. Clin Exp Pharmacol Physiol. 2019 Dec 2.
Lihm H, Kim H, Chang H, Yoon M, Lee K, Choi J. Vitamin C modulates lead excretion in rats. Anat Cell Biol. 2013;46(4):239.
Adeneye A, Olagunju J. Protective effect of oral ascorbic acid (vitamin c) against acetaminophen- induced hepatic injury in rats. African Journal of Biomedical Research. 2010; 11(2).
Agoston DV. How to Translate Time? The Temporal Aspect of Human and Rodent Biology. Front Neurol. 2017. https://doi.org/10.3389/fneur.2017.00092.
Keller A, Mohamed A, Dröse S, Brandt U, Fleming I, Brandes RP. Analysis of Dichlorodihydrofluorescein and Dihydrocalcein as Probes for the Detection of Intracellular Reactive Oxygen Species. Free Radic Res. 2004 Dec;38(12):1257–67.
Esterbauer H, Cheeseman KH. Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Meth Enzymol. 1990;186:407–21.
Benzie IFF, Strain JJ. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal Biochem. 1996 Jul;239(1):70–6.
Beutler E, Gelbart T, Pegelow C. Erythrocyte glutathione synthetase deficiency leads not only to glutathione but also to glutathione-S-transferase deficiency. Journal of Clinical Investigation. 1986 Jan 1;77(1):38–41.
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–75.
Stadtman ER, Levine RL. Protein oxidation. Ann N Y Acad Sci. 2000;899:191–208.
Ayub A, Mackness MI, Arrol S, Mackness B, Patel J, Durrington PN. Serum Paraoxonase After Myocardial Infarction. Arteriosclerosis, Thrombosis, and Vascular Biology. 1999 Feb;19(2):330–5.
Kalousová M, Skrha J, Zima T. Advanced glycation end-products and advanced oxidation protein products in patients with diabetes mellitus. Physiol Res. 2002;51(6):597–604.
Rizvi SI, Pandey KB. Activation of the erythrocyte plasma membrane redox system by resveratrol: a possible mechanism for antioxidant properties. Pharmacol Rep. 2010 Aug;62(4):726–32.
Traber MG, Stevens JF. Vitamins C. and E: Beneficial effects from a mechanistic perspective. Free Radic Biol Med. 2011 Sep;51(5):1000–13.
Duan Y, Zeng L, Zheng C, Song B, Li F, Kong X, et al. Inflammatory Links Between High Fat Diets and Diseases. Front Immunol. 2018;9:2649.
Telci A, Cakatay U, Akhan SE, Bilgin ME, Turfanda A, Sivas A. Postmenopausal hormone replacement therapy use decreases oxidative protein damage. Gynecol Obstet Invest. 2002;54(2):88–93.
Akçay T, Dinçer Y, Kayali R, Colgar U, Oral E, Cakatay U. Effects of hormone replacement therapy on lipid peroxides and oxidation system in postmenopausal women. J Toxicol Environ Health A. 2000 Jan;14(1):1–5. 59(.
Ayala A, Muñoz MF, Argüelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev. 2014;2014:360438.
Traber MG, Stevens JF. Vitamins C and E: beneficial effects from a mechanistic perspective. Free Radic Biol Med. 2011 Sep;51(5)(1):1000–13.
Pirinccioglu AG, Gökalp D, Pirinccioglu M, Kizil G, Kizil M. Malondialdehyde (MDA) and protein carbonyl (PCO) levels as biomarkers of oxidative stress in subjects with familial hypercholesterolemia. Clin Biochem. 2010 Oct;43(15):1220–4.
Harrison FE, May JM. Vitamin C function in the brain: vital role of the ascorbate transporter SVCT2. Free Radic Biol Med. 2009 Mar;15(6):719–30. 46(.
Marrocco I, Altieri F, Peluso I. Measurement and Clinical Significance of Biomarkers of Oxidative Stress in Humans. Oxidative Med Cell Longev. 2017;2017:1–32.
Chaudhuri J, Bains Y, Guha S, Kahn A, Hall D, Bose N, et al. The Role of Advanced Glycation End Products in Aging and Metabolic Diseases: Bridging Association and Causality. Cell Metab. 2018 Sep 4;28(3):337–52.
Mehdi MM, Rizvi SI. Human Plasma Paraoxonase 1 (PON1) Arylesterase Activity During Aging: Correlation with Susceptibility of LDL Oxidation. Archives of Medical Research. 2012 Aug;43(6):438–43.
Rizvi SI, Jha R, Maurya PK. Erythrocyte plasma membrane redox system in human aging. Rejuvenation Res. 2006;9(4):470–4.
Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev. 2009 Dec;2(5):270–8.
Fatima M, Kesharwani RK, Misra K, Rizvi SI. Protective Effect of Theaflavin on Erythrocytes Subjected to In Vitro Oxidative Stress. Biochem Res Int. 2013;2013:1–7.
Koh KK, Han SH, Quon MJ. Inflammatory Markers and the Metabolic Syndrome. J Am Coll Cardiol. 2005 Dec;46(11):1978–85.
Funding
Financial funding from the Department of Biotechnology, Govt of India under the RRFSP is gratefully acknowledged. The Department of Biochemistry is supported by the FIST grant of DST, New Delhi, and SAP DRS from UGC, New Delhi, India.
Author information
Authors and Affiliations
Contributions
Raushan Kumar: Performing experiments, compiling results, a draft of the manuscript. S I Rizvi: Conception of the study, analysis of results, and manuscript preparation.
Corresponding author
Ethics declarations
Ethical Approval
All animal care and exploratory methods conformed with the guidelines of the Control and Supervision of Experiments on Animals (CPCSEA) and Institutional Animal Ethics Committee (IAEC), University of Allahabad, India.
Conflict of Interest
The authors of this manuscript have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumar, R., Rizvi, S.I. Vitamin C Improves Inflammatory-related Redox Status in Hyperlipidemic Rats. Ind J Clin Biochem 38, 512–518 (2023). https://doi.org/10.1007/s12291-022-01070-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12291-022-01070-8