Abstract
Hyperlipidemia is one of the leading causes of, atherosclerosis, and cardiovascular disease. In this study, we evaluated the protective role of hesperidin (HES) against lipidemic stress in a hyperlipidemic model of rats. We developed a hyperlipidemic model of the rat through an i.p dose of poloxamer-407, 0.5 g/kg body weight for 3 alternative days in a week for 30 days and rats were supplemented with HES orally (100 mg/kg body weight) once daily. Bodyweight, fasting glucose, insulin, HOMA-IR index, triglyceride, cholesterol, ROS, FRAP, GSH, PMRS, AGE, MDA, PCO, AOPP, PON-1, TNF-α and IL-6, SGPT and SGOT were estimated in blood and plasma, and histopathology was done in liver tissue. Our data show that oxidative stress, inflammatory markers were increased in the P-407 treated group. Liver tissue histology also changes in the hyperlipidemic groups of rats.HES supplementation protects against P-407 induced alterations and maintains the redox homeostasis. Our results provide evidence that HES protects against lipidemic stress and redox imbalance induced by P-407 in rats.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hyperlipidemia constitutes one of the prominent risk factors contributing to the prevalence and severity of cardiovascular diseases (CVD). The earliest symptoms of hyperlipidemia include atherosclerosis. Hyperlipidemia is frequently managed by prescription lipid-lowering medications including statins and fibrates; however, there are side effects and contraindications of their long-term use. It is in this context that the use of natural polyphenols as a therapeutic intervention to manage hyperlipidemia and its complications is being actively investigated (Assini et al. 2013).
The protective and beneficial role of polyphenols has been mainly due to their antioxidant ability and their capacity to regulate various cellular antioxidant defense mechanisms by promoting the synthesis of antioxidant enzymes like SOD, CAT, glutathione S-transferase (GST), and glutathione peroxidase (GPx). (Upadhyay and Dixit 2015; Yan et al. 2020) Under conditions of cellular stress, polyphenols are reported to play a significant role in the activation of various cellular targets which include eNOS and NO, intracellular cytokines like TNFα, IL-6, and IL-8, VCAM-1 and ICAM-1, and adjusting signaling pathways by modifying SIRT1, MAP38 kinase, NFκB, and protecting from ROS (Roohbakhsh et al. 2014; Mahmoud et al. 2017).
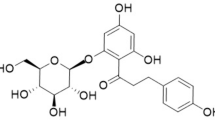
Dietary interventions based on nutrient composition of food and structural interactions represent relevant approaches to regulate postprandial lipemia (Desmarchelier et al. 2019). The role of polyphenols in atherosclerosis-related ischemic heart disease and stroke has been emphasized (Cheng et al. 2017). Hesperidin (C28H34O15) (3, 5, 7-Trihydroxy-Flavone 7-rhamnoglukoside) is a citrus-bioflavonoid abundantly found in the skin of lemons and sweet oranges. Hesperidin displays pleiotropic biological properties including antioxidant, anti-inflammatory, hepatoprotective, antidiabetic, antibacterial, antilipidemic, antifungal, antiviral, and antitumor (Garg et al. 2001; Mahmoud et al. 2019).
The progression of hyperlipidemia and the effectiveness of experimental therapies for hyperlipidemia have been tested on experimental hyperlipidemic animal models (Wout et al. 1992; Johnston and Palmer 1993; Johnston et al. 2017). Hyperlipidemia can be induced in model animals by either a high-fat diet or through chemical means. Poloxamer 407 (P-407) is a nonionic surfactant which has the property to quickly raise plasma lipid and induce long-term hyperlipidemia in rodents (Blonder et al. 1999; Omari-Siaw et al. 2016). Intraperitoneal administration of P-407 to rats has been demonstrated to cause an increase in cholesterol and triglycerides and also cause significant inhibition of plasma lipoprotein lipase (LPL), accompanied by an increase in tissue LPL activity (Johnston 2004). The present study envisages evaluating the effect of hesperidin on a P-407 induced hyperlipidemic rat model to validate the use of hesperidin as an effective intervention strategy for hyperlipidemia and CVD.
Material and method
All the chemicals and reagents such as 2, 4-Dinitrophenylhydrazine (DNPH), 5, 5-Dithiobis nitro benzoic acid (DTNB), and, 4,7-Diphenyl-1,10-phenanthroline disulfonic acid sodium salt (DPI), were purchased from Sigma-Aldrich, USA.
Experimental animals and treatments
This study was done on male Wistar rodents (12–14 months) weighing 250–300 g. Rats were housed in plastic enclosures and kept on a 12/12-h light/dark cycle at a temperature of 23 ± 2 °C, the relative humidity of 50 ± 20%, and ad libitum access to water and standard laboratory nutrient-rich pellets. The sample size has been calculated by power calculation before the study using software G Power 3.0.10. Animal care and exploratory methods conformed with the guidelines of the Control and Supervision of Experiments on Animals (CPCSEA) and Institutional Animal Ethics Committee (IAEC), University of Allahabad, India (# IAEC/AU/2017(1)/011) and also follow guidelines for the Care and Use of Laboratory Animals (1996, published by National Academy Press, 2101 Constitution Ave. NW, Washington, DC 20055, USA). The atherogenic suspension administrated to the rodents was fundamentally made out of a colloidal solution of P-407 that was prepared by dissolving 7 g in 52 mL of cold normal saline (0.13 g/mL), which was stored at 4 °C.
The experimental rats were grouped as below
Group I Normal control group (n = 6). This group received orally 1.0 mL of carboxymethylcellulose (CMC) (0.5% dissolved in water.) and, 0.9% saline per day for 30 days as vehicle control.
Group II Experimental hyperlipidemic group (n = 6). This group received an i.p dose of an atherogenic suspension (poloxamer-407) in 0.5 g/kg body weight for 3 alternative days in a week, at 9 am for 30 days. (Johnston 2004; Johnston et al. 2017).
Group III Hesperidin supplementation group (n = 6). This group received hesperidin orally (100 mg/kg body weight) dissolved in CMC at 9 am for 30 days once a day.
Group IV Hyperlipidemic + hesperidin rats (n = 6). This group received an i.p dose of an atherogenic suspension (poloxamer-407) in 0.5 g/kg body weight for three alternative days in a week followed by hesperidin orally (100 mg/kg body weight) at 9 am for 30 days.
After the treatment protocol, the blood was collected via cardiac puncture in heparinized syringes under light anesthesia (pentobarbital 50 mg/kg body weight). Erythrocytes were isolated by centrifugation of blood at 800 g (10 min, 4 °C). The isolated plasma was stored at − 80 °C for further biochemical evaluation. Removal of the buffy coat and upper 15% of packed red blood cells were done and ice-cold phosphate buffer saline (PBS) was used to wash twice the remaining packed red blood cells (PRBCs).
A limited part of the liver tissue was homogenized in 0.1 M phosphate buffer saline (PBS) (pH 7.4) and the supernatant was used for biochemical tests following centrifugation of the tissue homogenate. In histopathological tests, the liver tissues were put in 10% formalin for 72 h and then dehydrated at various concentrations (50–100%) of alcohol and coated in paraffin wax (melting point 58–60 °C).
Liver Sections (5 µm) were stained with hematoxylin and eosin (H&E) according to standard histological practices and examined under an Olympus microscope (CX21iTR-LED) equipped with Magnus UHCCD-USB 21.4-megapixel camera. Determination of serum triglyceride, total cholesterol, SGOT, and SGPT level was performed using reagent kits from Span diagnostic and ERBA diagnostics, and measurements were made on an Erba Mannheim Chem.-7 analyzer. Fasting glucose level was measured by Roche Accu-chek active blood glucose monitoring system. Fasting insulin level was measured using an ELISA kit supplied by GenxBio, Delhi, India.
Determination of ROS production in erythrocyte
The Spectrofluorimetric analysis for ROS in PRBCs was done by the established protocol (Eruslanov and Kusmartsev 2010) with slight modifications using an oxidation-sensitive probe 2’, 7’-dichlorodihydrofluorescein diacetate (DCFH-DA), described in detail (Eruslanov and Kusmartsev, 2010).
Estimation of erythrocyte MDA content
MDA content was determined following the protocol (Esterbauer and Cheeseman 1990), with slight modification. The quantity of MDA was estimated based on its reaction with thiobarbituric acid. MDA is expressed in terms of nmol/mLPRBCS and ε = 153,000 was used as the extinction coefficient.
Estimation of total protein content in plasma
Plasma protein was measured by the method of Lowry et, al. (Lowry et al. 1951) Bovine serum albumin was used as a standard.
Estimation of protein carbonyl (PCO) level
The level of protein carbonyl in plasma was estimated by the standard procedure (Stadtman and Levine, 2000). Carbonyl content is calculated using an absorption coefficient of 22,000 M−1 cm−1 and value reported in terms of nmol/mg protein.
Evaluation of plasma advanced oxidation protein products (AOPP)
Plasma AOPP was measured by the method (Witko-Sarsat et al. 1996) with slight modification. Chloramine-T solution (0–100 mmol/L) being used as a calibrator was used to calculate the value of oxidized protein products at 340 nm and AOPP concentration is expressed as μmol of chloramine-T equivalent/liter.
Estimation of advanced glycation end products (AGEs)
AGEs were estimated by the method described by Kalousova et al. (Kalousová et al. 2005)In brief, blood serum was diluted 1:50 with PBS, pH 7.4, and fluorescence intensity was recorded at the emission (440 nm) upon excitation at 350 nm. Fluorescence intensity is reported in arbitrary units (AU) and AU/g protein.
The ferric reducing ability of plasma (FRAP)
Plasma total antioxidant potential was estimated by using a well-established protocol for FRAP assay (Benzie and Strain, 1996) FRAP value is expressed as µmol Fe(II)/L of plasma.
Determination of reduced glutathione
Erythrocyte GSH was determined by the method of Beutler (Beutler et al. 1986). This method exploits the ability of thiol reagent, 5, 5′-dithiobis [2-nitrobenzoicacid] (DTNB) to reduce the –SH group to form a yellow anionic product measured at 412 nm. GSH concentration is represented in terms of mg/ml PRBC.
Estimation of paraoxonase–1 activity (PON-1 activity)
The PON-1 activity was performed by the method (Ayub et al. 1999) as already described in detail (Mehdi and Rizvi 2012). The results are expressed in U/ml, (1 U of arylesterase hydrolyzes 1 mmol of phenylacetate per minute).
Plasma membrane redox system (PMRS) activity
Erythrocyte PMRS activity was measured by the method as described (Rizvi et al. 2006) based on the reduction of ferricyanide. The result is represented in mmol ferrocyanide/ml PRBC/30 min.
Estimation of serum pro-inflammatory Cytokines in serum (IL-6 and TNF- α)
Detection of serum cytokine levels was done according to the manufacturing company instructions (Krishgen Biosystem, India) and described earlier (Kumar et al. 2020). The results obtained are reported in terms of Pg/ml.
Statistical analysis
Data are expressed as mean ± SD for six rats (independent experiments performed in duplicates). Differences between the groups were analyzed by one-way ANOVA and determination of intergroup variations was done by post hoc test using Bonferroni’s Multiple Comparison Test. Analyses were done by PRISM 5.01. A value of (p ≤ 0.05 was considered statistically significant).
Results
Role of Poloxamer-407(P-407) and Hesperidin supplementation on intracellular ROS
Intracellular ROS is represented in Fig. 1a. A one-way ANOVA detected a significant effect of P-407, and HES treatment [F (3,8) = 245.1, p ≤ 0.01] Fig. 1a. The post hoc test evaluating the hyperlipidemic group of rats with respect to control group exhibit a significant increase (29.69%) in the intracellular ROS in comparison to the control group, whereas hesperidin supplementation to rats show a significant decrease (25.87%) in the intracellular ROS compared to control. When Hesperidin was supplemented to P-407 rats, there was normalization of ROS compared to control group,and a significant decrease (23.32%) with P-407 treated rat.
Oxidative stress markers a Spectrofluorimetric analysis of the generation of intracellular ROS with fluorescent dye DCFDA in all groups of the rat. Data are represented as mean ± SD of six independent experiments, and the generation of ROS is expressed as % change of normal control.* (p < 0.05) when compared with control. @ (p < 0.05) when compared with hyperlipidemic group. b The role of HFD on the lipid peroxidation level (MDA).* represent significantly increased (p < 0.05) in plasma of the hyperlipidemic group when compared with the control group. @ Significantly decrease (p < 0.05) in the Polo + HES group when compared with the hyperlipidemic group. MDA level is expressed in nmol/mL PRBCs. Protein carbonyl (PCO) & Protein oxidation is measured in terms of AOPP are represented in figure (c, d), * represent significantly increased (p < 0.05) in plasma of hyperlipidemic group when compared with the control group. @ Significantly decrease (p < 0.05) in the Polo + HES group when compared with the hyperlipidemic group. Advanced glycation end products (AGEs) are represented in Fig. 1e * represent significantly increased (p < 0.05) in plasma of Poloxamer treated hyperlipidemic group when compared with the control group. @ Significantly decrease (p < 0.05) in the Polo + HES group when compared with the Polo-treated group. Fluorescence intensity was reported in arbitrary units (AU) and in AU/g protein
Role of P-407and effect of hesperidin on MDA level
A one-way ANOVA detected a significant effect of P-407, and HES treatment [F (3, 8) = 22.23, p ≤ 0.0003]. The post hoc tests show a significant (p ≤ 0.05) increase (19.72%) in the MDA level in the hyperlipidemic group of rats and a significant (p ≤ 0.05) decrease (14.52%) when the hyperlipidemic group of rats is supplemented with HES (Fig. 1b). There was no significant change when normal rats were treated with HES.
Effect of P-407 on PCO & AOPP level
Plasma protein carbonyl and AOPP levels are shown in Fig. 1c, d. A one-way ANOVA detected a significant effect of P-407, and HES treatment on PCO [F (3, 8) = 10.10, p ≤ 0.0043] and for AOPP [F (3, 8) = 245.1, p ≤ 0.0001].The post hoc test show hyperlipidemic group of rats shows a significant increase (21.05% & 24.53%) (p ≤ 0.05) of the PCO and AOPP levels , respectively, concerning control groups. However, HES supplemented rat shows a significant (p ≤ 0.05) decrease in (15.02%) AOPP level and a non-significant decrease in PCO level concerning the hyperlipidemic group.
Effect of Hesperidin supplementation on AGE level in the hyperlipidemic group of rats
The advanced glycation end product is represented in Fig. 1e. A one-way ANOVA detected a significant effect of P-407, and HES treatment [F (3, 8) = 32.13, p ≤ 0.0001]. The result showed that there is a significant (p ≤ 0.05) increase (49.70%) in the AGE level in the hyperlipidemic group of rats and significantly (p ≤ 0.05) decreased level (17.03%) when the group is supplemented with HES.
Role of Poloxamer and effect of Hesperidin supplementation on FRAP level
The reducing antioxidant ability of plasma is measured in the form of FRAP represented in Fig. 2a. A one-way ANOVA was conducted to compare the effect of P-407 and effect of HES treated conditions [F (3, 8) = 141.6, p ≤ 0.0001]. FRAP value shows a significant (p ≤ 0.05) decrease (48.99%) in the experimental hyperlipidemic group of the rats when compared with control, whereas there was also a significant (p ≤ 0.05) increase (50.22%) in FRAP activity after supplementation with HES in the rat concerning the experimental hyperlipidemic group.
Antioxidant biomarkers a The antioxidant ability of plasma is measured in the form of FRAP. * represent significantly decreased (p < 0.05) in FRAP value in the experimental hyperlipidemic group compared with control. @ Significant (p < 0.05) increase in antioxidant potential in the Polo + HES group when compared with the experimental hyperlipidemic group. FRAP is expressed in µmol Fe/L plasma. The values are mean ± SD of six independent experiments. b Antioxidant level measure in erythrocyte in the form of GSH represented in figure (b). * represent Significant (p < 0.05) decrease in the level of GSH in Poloxamer treated hyperlipidemic groups when compared with control.whereas @ shows significant (p < 0.05) increase shown in the GSH level in the HES supplemented group when compared with Poloxamer treated hyperlipidemic group. The value of GSH is expressed in mg/ml PRBCs. (C) Plasma Paraoxonase activity (PON-1) is one of the important biomarkers for the study of LDL oxidation. The value is represented in U/group plasma. High-fat diet treated group show* significantly decreased PON- 1 activity (p < 0.05) with respect to control, while @ Polo + HES group show a significant increase in PON-1 activity when compared with the Poloxamer treated hyperlipidemic group. (D) The plasma membrane redox system (PMRS) is an important function in erythrocyte for maintaining the redox status of plasma. Significant (*p < 0.05) increase in PMRS activity found in the hyperlipidemic group of rats compared to control. @Significant (p < 0.05) decrease in the PMRS activity in the Polo + HES group of rats when compared with the hyperlipidemic group. The activity is expressed as μmol ferrocyanide/ml PRBC/30 min
GSH level
The intracellular GSH level is depicted in Fig. 2b. A one-way ANOVA was conducted to compare the effect of P-407 and effect of HES treated conditions [F (3, 8) = 22.72, p ≤ 0.0001].
Experimental data show a significant (p ≤ 0.05) decrease (41.66%) in the GSH level in the experimental hyperlipidemic group of rats and a significantly (≤ 0.05) increase (74%) when the experimental hyperlipidemic group of rats is supplemented with HES.
PON-1 activity
PON-1 activity is shown in Fig. 2c. A one-way ANOVA was conducted to compare the effect of P-407 and effect of HES treated conditions [F (3, 8) = 54.43, p ≤ 0.0001]. Our result shows that there is a significant (p ≤ 0.05) decrease (37.89%) in PON-1 activity in the hyperlipidemic group of rats when compared with control and it increases (67.50%) significantly (p ≤ 0.05) with HES supplementation.
Role of P-407 and Hesperidin supplementation on PMRS activity
The activity of the plasma membrane redox system is represented in Fig. 2d. A one-way ANOVA was conducted to compare the effect of P-407 and effect of HES treated conditions [F (3, 8) = 7.40, p ≤ 0.0046].PMRS activity is significantly (p ≤ 0.05) increased (55.2%) in the experimental hyperlipidemic group of rats concerning normal control, whereas the HES supplementation in a hyperlipidemic group of rats shows a significant (p ≤ 0.05) decrease (23.7%) in PMRS activity when compared with the hyperlipidemic group.
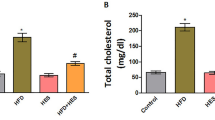
Serum triglyceride and cholesterol level
Figure 3a, b represents the plasma triglyceride and cholesterol level. A one-way ANOVA was conducted to compare the effect of P-407 and the effect of HES conditions for triglyceride [F (3, 8) = 120.4, p ≤ 0.0001] and for cholesterol [F (3, 8) = 176.67, p ≤ 0.0001].
Biochemical parameters Serum triglyceride and Cholesterol levels are represented in figure (a, b). * represent the significant increase (p < 0.05) in the experimental hyperlipidemic group with respect to control and @ shows significantly decreased (p < 0.05) in the Polo + HES supplemented group with respect to the experimental hyperlipidemic group of rats. Value is represented as mg/dl. Liver marker enzyme activity is measured in terms of SGOT and SGPT in figure (c, d) respectively. The Values are represented in terms of U/L.*represent significant (p < 0.05) increased in the level of both the marker in the experimental hyperlipidemic group of rats when compared with the control group while HES supplemented reverse this with respect to experimental hyperlipidemic group. @Show significant (p < 0.05) reduction in the level of SGOT and SGPT level when compared with the experimental hyperlipidemic group
There was a significant (p ≤ 0.05) increase in the level of triglyceride (143%) and cholesterol (131%) in the hyperlipidemic group of the rat concerning the control group, whereas hesperidin supplementation significantly (p ≤ 0.05) decreased the level of both parameters (45.90% & 54.65%), respectively.
Effect of P-407 and protective role of Hesperidin on Liver marker enzyme
Liver function marker enzymes SGOT and SGPT are represented in Fig. 3c, d, respectively. A one-way ANOVA was conducted to compare the effect of P-407 and effect of HES treated conditions for SGOT [F (3, 8) = 15.51, p ≤ 0.0011] and for SGPT [F (3, 8) = 35.71, p ≤ 0.0001]. A significant (p ≤ 0.05) increase (33% &38%) is shown in both the marker enzyme , respectively, concerning the control group, whereas the hyperlipidemic + HES group showed a significant (p ≤ 0.05) decrease (21.37% & 27.57%) in both parameters concerning the experimental hyperlipidemic group of rats.
Cytokine level (IL-6 & TNF- α) in Hyperlipidemic and HES supplemented group of rat
Figure 4a, b represents the cytokine levels (IL-6 & TNF- α), respectively. A one-way ANOVA was conducted to compare the effect of P-407 and effect of HES treated conditions for IL-6 [F (3, 8) = 18.04, p ≤ 0.0006] and for TNF- α [F (3, 8) = 14.61, p ≤ 0.0013].The activity of both cytokinesis significantly (p ≤ 0.05) increased (27% & 50%) in hyperlipidemic groups of rats when compared to control. Whereas a significant (p ≤ 0.05) decrease(17.99% & 21.61%) is seen in the hyperlipidemic + HES supplemented group of rats concerning hyperlipidemic groups of rats.
Serum cytokines level Figure (a, b) represent the cytokines IL-6 and TNF-α levels in serum. The value is expressed as pg/ml serum. *represent the significant (p < 0.05) increase in inflammatory cytokines in Polo-treated hyperlipidemic groups of rats with respect to the control group in both parameters, @show significant (p < 0.05) reduction in the level of cytokine when compared with Polo-treated hyperlipidemic group
Histological analysis
Figure 5a shows that the control tissue and Fig. 5c shows hesperidin supplemented group, sections of the liver after H&E staining revealed normal histology. Hepatocytes have been regular, and there was no central vein (CV) congestion in both control and hesperidin groups. In the Polo-treated group (Fig. 5b), congestion of CV, development of inflammatory cells, and vacuolated cytoplasm are observed. Upon treatment of hesperidin in the hyperlipidemic group Fig. 5d, there was a decrease in inflammation and inflammatory cells in the liver tissue.
Histology of Liver Microscopic Image of liver tissue section was taken at 100X. (Dye: Hematoxylin& Eosin) (a) Control Group; (b) Polo-treated hyperlipidemic group; (c) Hesperidin-treated Group; and (e) Polo with Hesperidin-treated group. The BLACK arrow shows necrotic cells, Blue arrow represents vacuolized hepatocytes, and the asterisk mark presents CV congestion and infiltration of inflammatory cells in polo-treated rat in figure (b). Yellow arrow shows normal CV and red arrow shows decrease in the necrotic cells figure (d)
Effect of Poloxamer -407 and Hesperidin supplementation on biochemical parameters
After 30 days of dosing, there is a significant increase (p ≤ 0.05) in the body weight, fasting glucose, fasting insulin, and HOMA-IR levels in a hyperlipidemic group of rats concerning controls, while HES supplementation shows a significant decrease (p ≤ 0.05) in the body weight, fasting glucose levels, and fasting insulin and HOMA-IR index (Table 1).
Discussion
Oxidative stress plays a key role in the creation of multiple illnesses, some of which are obesity, cardiovascular disease, and age (Liguori et al. 2018). Reactive oxygen species (ROS) are continuously generated in the body by mitochondrial bioenergetics and oxidative metabolism (Zorov et al. 2014). Compelling evidence indicates that the overproduction of ROS induces combined oxidative harm to macromolecules contributing to neuronal death (Le Lay et al. 2014). High consumption of dietary fat contributes to obesity, which induces a permanent state of inflammation via the generation of white adipose tissue that secretes pro-inflammatory factors (Fuster et al. 2016). An increase in reactive oxygen species production is directly associated with atherosclerosis (Kattoor et al. 2017). ROS attacks low- density lipoprotein (LDL) converting it into oxidized LDL. The oxidized LDL stimulates the migration of monocytes to the sub-endothelial space leading to endothelial cell injury. The modified LDL is taken up by macrophages which become foam cells, leading to the formation of atherosclerotic plaque.
The mechanisms involved in the TG-lowering effects of hesperidin have been reported to be the reduction of hepatic TG content through inhibition of lipogenesis and induction of fatty acid oxidation (Xiong et al. 2019), and the downregulation of synthesis and secretion of very-low-density lipoproteins (VLDL) (Mulvihill et al. 2016). We also observe that hesperidin-treated animals show a decreased level of TG and cholesterol. Hesperidin is also reported to stimulate the release of cholecystokinin in entering endocrine STC-1 cells, an appetite-regulating hormone, which is ultimately is used to treat obesity by suppressing appetite and decrease the body weight (Belskaya et al. 2016), this fact is also supported by our result of a decrease in body weight of HFD rats after hesperidin treatment.
Various lines of evidence suggest that lipid peroxidation (LPO) due to an increase in the levels of ROS is mutagenic and cancerous (Dianzani, 1989; Ramana et al. 2014) and have been described in several diseases and illnesses as an underlying mechanism (Aslan et al. 1997). Increased LPO influences the membrane structure by reducing the membrane fluidity and altering the activity of membrane binding enzymes and receptors (Ahmad and Beg, 2014) The primary site of MDA is to bind to the ε-amino group of apo B-which is present in LDL causing an alteration in the topology of LDL, as a result, it cannot bind with its apoB receptor. The increased amount of LDL in circulation causes blockages of arteries (M Mobarak Hossain, 2013; Carquin et al. 2016).
As lipid oxidation of cell membrane increases, the polarity of lipid-phase surface charge and the formation of protein oligomers increase. The molecular mobility of lipids, number of -SH (Thiol) groups and resistance to thermo-denaturation also decrease (Bok et al. 1999). Our observation of a decrease in MDA after hesperidin treatment is thus highly significant. Importantly hesperidin is known to suppress the activity of enzyme HMG-CoA reductase and Acyl CoA: cholesterol O-acyltransferase in rat, and to reduce net apoB secretion in HepG2 cells, which play a significant role in the progression of atherosclerosis (Shelton and Jaiswal, 2013).
ROS targets plasma proteins and leads to carbonyl groups on multiple amino acids under stress conditions. Various molecular pathways for carbonation of proteins have been suggested, some of them triggered by direct ROS attacks and by the radical forming of alkoxides contributing to protein cleavage. (Elavarasan et al. 2012). A high level of PCO has been reported to induce atherogenesis in rats(Chen et al. 2010). Our findings of hesperidin induced decrease in PCO validates the antihyperlipidemic effect of hesperidin. Reports document that HES can neutralize ROS including superoxide anions, hydroxyl radicals, peroxynitrite, and radicals of nitric oxides (Wang et al. 2011) HES also stimulates or up-regulates the Nrf2 and ERK 1/2 gene expression(Kalousová et al. 2005; Chen et al. 2010).
AOPPs and AGEs are important biomarkers of oxidative stress (Kalousová et al. 2005). A high level of plasma AOPP was found in the atherogenic treated rat, which increases oxidative stress, hesperidin supplementation mitigates this effect. AGEs play a causative role in the accumulation of random damage to extracellular proteins. These advanced glycation end-products have potentially harmful effects on biological function and are associated with aging and many degenerative diseases such as diabetic complications, atherosclerosis, and chronic renal failure (Chen et al. 2018).
Paraoxonase 1 (PON-1) is an HDL linked enzyme providing lipid peroxidation defense of LDL and HDL. It has been established that PON1 is a protective factor in inflammation and oxidation-related disorders, such as diabetes mellitus and non-alcoholic fatty liver disorders. A few reports show the role of polyphenols to stimulate PON-1 (Ayub et al. 1999). The maintenance of the enzyme-SH group justifies the importance of plasma redox status as an important factor in the modulation of PON-1 activity (Mehdi and Rizvi 2012). Our observation of hesperidin supplementation reversing the effect of hyperlipidemic stress in atherogenic supplemented rats highlights the antioxidant role of hesperidin.
The plasma membrane redox system (PMRS) is an important function in erythrocyte for maintaining the redox status of plasma in various conditions including aging and diabetes (Rizvi et al. 2006). The PMRS activity is based on the transfer of electrons from intracellular donors that is NADH and/or ascorbate (ASC) to its extracellular acceptors. Previous reports suggest that polyphenols exert their antioxidant effects, in vivo, through a mechanism that is based on the activation of PMRS (Pandey and Rizvi 2009). Our results on the effect of hesperidin on erythrocyte PMRS activity support our earlier findings with resveratrol (Pandey and Rizvi 2009) and tea catechins (Fatima et al. 2013) emphasizing the important role hesperidin may play during the hyperlipidemic condition.
The liver is the main organ of oxidative and detoxifying action. The Polo-treated hyperlipidemic group shows congestion of central vein (CV), development of inflammatory cells, and vacuolated cytoplasm. Supplemented with hesperidin, a decreased congestion in inflammatory cells is observed in liver tissue. These findings confirm that hesperidin has a hepatoprotective effect under hyperlipidemic conditions.
Higher levels of inflammatory cytokines have been recorded under hyperlipidemic conditions (Feingold and Grunfeld 1992). This finding is also confirmed in our results. This process is primarily triggered by the enhanced production of lipid peroxidation. This high degree of lipid peroxidation contributes to redox-sensitive transcription, nuclear factor KB (NK-KB), and inflammatory cytokine secretion: IL-6 and TNF-α. Both the cytokines IL-6 and TNF-α levels are found higher in HFD treated groups of rats when compared with control. Whereas the hesperidin-treated group showed decreased levels of IL-6 and TNF-α, which can be explained based on the antioxidant property of hesperidin against lipid peroxidation(Abuelsaad et al. 2014).
Conclusion for future biology
Based on our observations we hereby provide experimental evidence that P-407 treated hyperlipidemic rats show alterations that mimic chemically induced hyperlipidemia. Hesperidin protects hyperlipidemic alterations, the action of hesperidin is primarily dependent on its antioxidant activity. Our findings validate the use of hesperidin as a therapeutic intervention in conditions of hyperlipidemia.
Data availability statement
The information that helps the finding of this study is accessible from the corresponding author upon reasonable request.
References
Abuelsaad ASA, Allam G, Al-Solumani AAA (2014) Hesperidin Inhibits Inflammatory Response Induced by Aeromonas hydrophila Infection and Alters CD4 +/CD8 + T Cell Ratio. Mediators Inflamm 2014:1–11
Ahmad S, Beg ZH (2014) Mitigating the role of thymoquinone rich fractions from Nigella sativa oil and its constituents, thymoquinone and limonene on lipidemic-oxidative injury in rats. SpringerPlus 3(1):316
Aslan R, Sekeroğlu MR, Tarakçioğlu M, Köylü H (1997) Investigation of malondialdehyde formation and antioxidant enzyme activity in stored blood. Haematologia (Budapest) 28(4):233–237
Assini JM, Mulvihill EE, Huff MW (2013) Citrus flavonoids and lipid metabolism. Curr Opin Lipidol 24(1):34–40
Ayub A, Mackness MI, Arrol S, Mackness B, Patel J, Durrington PN (1999) Serum paraoxonase after myocardial infarction. Arterioscler Thromb Vasc Biol 19(2):330–335
Belskaya LV, Kosenok VK, Massard Z, Zav’yalov AA (2016) Status indicators of lipid peroxidation and endogenous intoxication in lung cancer patients. Annals Russ Med Sci 71(4):313–322
Benzie IFF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 239(1):70–76
Beutler E, Gelbart T, Pegelow C (1986) Erythrocyte glutathione synthetase deficiency leads not only to glutathione but also to glutathione-S-transferase deficiency. J Clin Invest 77(1):38–41
Blonder JM, Baird L, Fulfs JC, Rosenthal GJ (1999) Dose-dependent hyperlipidemia in rabbits following administration of poloxamer 407gel. Life Sci 65(21):261–266
Bok S-H, Lee S-H, Park Y-B, Bae K-H, Son K-H, Jeong T-S, Choi M-S (1999) Plasma and Hepatic Cholesterol and Hepatic Activities of 3-Hydroxy-3-methyl-glutaryl-CoA Reductase and Acyl CoA: cholesterol transferase are lower in rats fed citrus peel extract or a mixture of citrus bioflavonoids. J Nutr Biochem 129(6):1182–1185
Carquin M, D’Auria L, Pollet H, Bongarzone ER, Tyteca D (2016) Recent progress on lipid lateral heterogeneity in plasma membranes: from rafts to submicrometric domains. Prog Lipid Res 62:1–24
Chen M, Ye Y, Ji G, Liu J (2010) Hesperidin upregulates heme oxygenase-1 to attenuate hydrogen peroxide-induced cell damage in hepatic L02 cells. J Agric Food Chem 58(6):3330–3335
Chen J-H, Lin X, Bu C, Zhang X (2018) Role of advanced glycation end products in mobility and considerations in possible dietary and nutritional intervention strategies. Nutr Metab (Lond) 15(1):72
Cheng Y-C, Sheen J-M, Hu WL, Hung Y-C (2017) Polyphenols and oxidative stress in atherosclerosis-related ischemic heart disease and stroke. Oxid Med Cell Longev 2017:1–16
Desmarchelier C, Borel P, Lairon D, Maraninchi M, Valéro R (2019) Effect of nutrient and micronutrient intake on chylomicron production and postprandial lipemia. Nutrients 11(6):1299
Dianzani MU (1989) Lipid peroxidation and cancer: a critical reconsideration. Tumori 75(4):351–357
Elavarasan J, Velusamy P, Ganesan T, Ramakrishnan SK, Rajasekaran D, Periandavan K (2012) Hesperidin-mediated expression of Nrf2 and upregulation of antioxidant status in senescent rat heart: hesperidin and cardiac aging. J Pharm Pharmacol 64(10):1472–1482
Eruslanov E, Kusmartsev S (2010) Identification of ROS Using Oxidized DCFDA and Flow-Cytometry. In: Advanced protocols in oxidative stress II (Armstrong D, Ed.). Humana Press, pp 57–72
Esterbauer H, Cheeseman KH (1990) Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol 186:407–421
Fatima M, Kesharwani RK, Misra K, Rizvi SI (2013) Protective effect of theaflavin on erythrocytes subjected to in vitro oxidative stress. Biochem Res Int, pp 1–7
Feingold KR, Grunfeld C (1992) Role of cytokines in inducing hyperlipidemia. Diabetes 41(2):97–101
Fuster JJ, Ouchi N, Gokce N, Walsh K (2016) Obesity-induced changes in adipose tissue microenvironment and their impact on cardiovascular disease. Circ Res 118(11):1786–1807
Garg A, Garg S, Zaneveld LJD, Singla AK (2001) Chemistry and pharmacology of the citrus bioflavonoid hesperidin. Phytother Res 15(8):655–669
Johnston TP (2004) The P-407-induced murine model of dose-controlled hyperlipidemia and atherosclerosis: a review of findings to date. J Cardiovasc Pharmacol 43(4):595–606
Johnston TP, Palmer WK (1993) Mechanism of poloxamer 407-induced hypertriglyceridemia in the rat. Biochem Pharmacol 46(6):1037–1042
Johnston TP, Korolenko TA, Sahebkar A (2017) P-407-induced mouse model of dose-controlled hyperlipidemia and atherosclerosis: 25 years later. J Cardiovasc Pharmacol 70(5):339–352
Kalousová M, Zima T, Tesař V, Dusilová-Sulková S, Škrha J (2005) Advanced glycoxidation end products in chronic diseases—clinical chemistry and genetic background. Mutat Res 1(2):37–46
Kattoor AJ, Pothineni NVK, Palagiri D, Mehta JL (2017) Oxidative stress in atherosclerosis. Curr Atheroscler Rep 19(11):42
Kumar R, Akhtar F, Rizvi SI (2020) Hesperidin attenuates altered redox homeostasis in an experimental hyperlipidemic model of rat. Clin Exp Pharmacol Physiol 47(4):571–582
Le Lay S, Simard G, Martinez MC, Andriantsitohaina R (2014) Oxidative stress and metabolic pathologies: from an adipocentric point of view. Oxid Med Cell Longev 2014:1–18
Liguori I, Russo G, Curcio F, Bulli G, Aran L, Della-Morte D, Gargiulo G, Testa G, Cacciatore F, Bonaduce D, Abete P (2018) Oxidative stress, aging, and diseases. Clin Interv Aging 13:757–772
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
Mahmoud AM, Mohammed HM, Khadrawy SM, Galaly SR (2017) Hesperidin protects against chemically induced hepatocarcinogenesis via modulation of Nrf2/ARE/HO-1, PPARγ and TGF-β1/Smad3 signaling, and amelioration of oxidative stress and inflammation. Chem Biol Interact 277:146–158
Mahmoud AM, Hernández Bautista RJ, Sandhu MA, Hussein OE (2019) Beneficial effects of citrus flavonoids on cardiovascular and metabolic health. Oxid Med Cell Longev 2019:1–19
Mehdi MM, Rizvi SI (2012) Human plasma paraoxonase 1 (PON1) arylesterase activity during aging: correlation with susceptibility of LDL oxidation. Arch Med Res 43(6):438–443
Mobarak M, Hossain SA (2013) Lipid triad: an important predictor of dyslipidemia related disorders and its therapeutic intervention. J Glycomics Lipidomics 3(1):1. https://doi.org/10.4172/2153-0637.1000e114
Mulvihill EE, Burke AC, Huff MW (2016) Citrus flavonoids as regulators of lipoprotein metabolism and atherosclerosis. Annu Rev Nutr 36(1):275–299
Omari-Siaw E, Zhu Y, Wang H, Peng W, Firempong CK, Wang YW, Cao X, Deng W, Yu J, Xu X (2016) Hypolipidemic potential of perillaldehyde-loaded self-nanoemulsifying delivery system in high-fat diet induced hyperlipidemic mice: formulation, in vitro and in vivo evaluation. Eur J Pharm 85:112–122
Pandey KB, Rizvi SI (2009) Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev 2(5):270–278
Ramana KV, Srivastava S, Singhal SS (2014) Lipid peroxidation products in human health and disease 2014. Oxid Med Cell Longev 2014:162414
Rizvi SI, Jha R, Maurya PK (2006) Erythrocyte plasma membrane redox system in human aging. Rejuvenation Res 9(4):470–474
Roohbakhsh A, Parhiz H, Soltani F, Rezaee R, Iranshahi M (2014) Neuropharmacological properties and pharmacokinetics of the citrus flavonoids hesperidin and hesperetin—A mini-review. Life Sci 113(1–2):1–6
Shelton P, Jaiswal AK (2013) The transcription factor NF-E2-related Factor 2 (Nrf2): a protooncogene? FASEB J 27(2):414–423
Stadtman ER, Levine RL (2000) Protein oxidation. Ann N Y Acad Sci 899:191–208
Upadhyay S, Dixit M (2015) Role of polyphenols and other phytochemicals on molecular signaling. Oxid Med Cell Longev 2015:504253
Wang X, Hasegawa J, Kitamura Y, Wang Z, Matsuda A, Shinoda W, Miura N, Kimura K (2011) Effects of hesperidin on the progression of hypercholesterolemia and fatty liver induced by high-cholesterol diet in rats. J Pharmacol Sci 117(3):129–138
Witko-Sarsat V, Friedlander M, Capeillère-Blandin C, Nguyen-Khoa T, Nguyen AT, Zingraff J, Jungers P, Descamps-Latscha B (1996) Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int 49(5):1304–1313
Wout ZG, Pec EA, Maggiore JA, Williams RH, Palicharla P, Johnston TP (1992) Poloxamer 407-mediated changes in plasma cholesterol and triglycerides following intraperitoneal injection to rats. J Parenter Sci Technol 46(6):192–200
Xiong H, Wang J, Ran Q, Lou G, Peng C, Gan Q, Hu J, Sun J, Yao R, Huang Q (2019) Hesperidin: a therapeutic agent for obesity. Drug Des Devel Ther 13:3855–3866
Yan Z, Zhong Y, Duan Y, Chen Q, Li F (2020) Antioxidant mechanism of tea polyphenols and its impact on health benefits. Anim Nutr 6(2):115–123
Zorov DB, Juhaszova M, Sollott SJ (2014) Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Phys Rev 94(3):909–950
Funding
This work was supported by a research grant to SIR from SERB-DST, Govt of India (EMR/2016/006470). The Department of Biochemistry is supported by the FIST grant of DST, New Delhi, India.
Author information
Authors and Affiliations
Contributions
RK Performing experiments, compiling results, a draft of the manuscript. FA Performing experiments. SIR Conception of the study, analysis of results, and manuscript preparation.
Corresponding author
Ethics declarations
Conflict of interest
The authors of this manuscript have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kumar, R., Akhtar, F. & Rizvi, S.I. Protective effect of hesperidin in Poloxamer-407 induced hyperlipidemic experimental rats. BIOLOGIA FUTURA 72, 201–210 (2021). https://doi.org/10.1007/s42977-020-00053-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42977-020-00053-1