Abstract
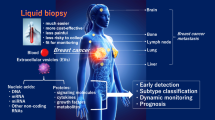
This article examines liquid biopsy using non-coding RNAs and extracellular vesicles in detail. Liquid biopsy is emerging as a prominent non-invasive diagnostic tool in the treatment of breast cancer. We will elucidate the roles of these molecules in early detection, monitoring treatment effectiveness, and prognostic assessment of breast cancer. Additionally, the clinical significance of these molecules will be discussed. We aim to delve into the distinct characteristics of these molecules and their possible roles in breast cancer management, with an anticipation of their contribution to future diagnostic and therapeutic advancements.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With advances in cancer biology and treatment, identifying biomarkers that reflect tumor characteristics is critical, in addition to elucidating basic information, such as histologic type, when devising a treatment strategy. Hormone receptor expression and human epidermal growth factor receptor 2 (HER2) overexpression are critical in navigating therapies against breast cancer. Traditionally, tissue biopsy has been the cornerstone for identifying biomarkers; however, this method is highly invasive [1]. Consequently, there is ongoing research on the clinical implementation of liquid biopsy (LB), which is less invasive, allows multiple testing, and can address the temporal and spatial heterogeneity of tumors [2, 3].
LB can detect tumor cells and tumor-derived substances, primarily in the blood (serum and plasma). The use of LB has recently been extended to include all body fluids, such as saliva, urine, and cerebrospinal fluid. Initially, the focus of LB was on circulating tumor cells, cell-free DNA in blood, and circulating tumor DNA, which originates from tumors. However, with advances in research, the utility of tumor-derived RNAs and extracellular vesicles has gained attention. Liquid biopsy strategies for breast cancer involving non-coding RNAs (ncRNAs) and extracellular vesicles are varied and have been extensively reported. Reviewing these approaches and considering their implications for future clinical strategies and prospects is essential.
This article aims to comprehensively review ncRNAs and extracellular vesicles as LB tools in managing breast cancer.
ncRNAs (Table 1)
ncRNAs refers to all RNAs except messenger RNA (mRNA), which are translated into proteins. Previously considered non-functional and labeled “junk,” ncRNAs influence various stages of gene expression, including transcription, post-transcription, translation, and signal transduction, serving as functional regulatory molecules that orchestrate intracellular processes. Certain ncRNAs are implicated in cancer, functioning as oncogenes or tumor suppressors, and their dysregulation may contribute to cancer development, progression, and metastasis [4].
ncRNAs are categorized by their base length. Notably, RNAs shorter than 300 nucleotides are designated small ncRNAs (sncRNAs), with microRNAs (miRNAs) being the most well-studied sncRNA in the context of cancer [5]. They play significant roles in the initiation, growth, and spread of malignant tumors [4].
miRNAs
Since the discovery of the first miRNAs in 1993, miRNAs and their target genes have been known to create a sophisticated regulatory network integral to various biologic functions, including cell proliferation, differentiation, and apoptosis. These processes are crucial for elucidating cancer development and progression [6].
As of November 1, 2023, 1917 miRNAs have been cataloged in the miRNA database (miRBase [7]). A single miRNA can target multiple mRNAs and the untranslated regions of an mRNA can have several miRNA binding sites, forming a complex network that controls mRNA translation [7]. This complexity is reflected in the multifaceted roles of miRNAs, such as miR-21 and miR-140-5p, in the diagnosis, staging, treatment, survival, and recurrence of cancer [8]. To address these complexities, methodologies leveraging artificial intelligence, machine learning, deep learning, and web tools have been developed and employed, facilitating comprehensive searches of miRNA data using machine learning algorithms [9].
miRNAs are involved in malignant tumor dysregulation through gene amplification, deletion, transcriptional anomalies, and epigenetic modifications. miRNAs functioning as cancer promoters are termed oncomiRs, while miRNAs with tumor-suppressive functions also exist [10]. For instance, miR-761 in breast cancer acts as an oncogene and is involved in the colony formation, migration, invasion, and promotion of lung metastasis. Moreover, miRNAs specific to brain, liver, bone, and lymph node metastases have been identified [10].
miRNAs are pivotal in regulating immune responses, influencing the development and differentiation of immune cells. They modulate interactions between immune and cancer cells within the tumor microenvironment (TME), affecting tumor-promoting and anti-tumor immune responses [11]. miRNAs have promising therapeutic potential as they can inactivate target genes and regulate multiple genes. They may offer a synergistic effect by concurrently targeting a range of oncogenes and cancer-promoting pathways. Therapeutic strategies involving miRNAs include inhibiting cancer-promoting miRNAs and introducing tumor-suppressing miRNAs. These approaches could be potent therapies for various cancer stages and pathologic conditions [12].
Long non-coding RNA
Among the various types of ncRNA, long ncRNAs (lncRNAs) are receiving significant attention in research. These molecules form intricate networks and interact with other nucleic acids and proteins. Their role in mediating intracellular processes, such as transcription, post-transcriptional modifications, and signal transduction, considerably influences cancer survival and progression [13]. As they are more stable than miRNAs in body fluids, lncRNAs have emerged as promising targets for liquid biopsy (LB) [13].
One of the functions of lncRNAs is to act as a “sponge,” absorbing miRNAs and inhibiting their activity. For instance, lncRNA H19 exhibits a “sponge effect” on miRNAs, regulating epithelial-mesenchymal transition and mesenchymal-epithelial transition, which potentially promotes distant metastasis in breast cancer [14]. lncRNAs involved in evading anti-tumor immune responses have also been identified [15].
The lncRNA HOTAIR (HOX transcript antisense intergenic RNA) is particularly noteworthy and has been extensively studied since its association with breast cancer progression was first reported in 2010 [16]. Extracellular vesicles containing HOTAIR found in the plasma of patients with breast cancer are associated with high ErbB2 expression levels and an increased incidence of lymph node metastasis [17]. These findings suggest potential applications for HOTAIR in breast cancer management.
Some lncRNAs affect the cell cycle via the CCND1/CDK4-6 complex [13]. lncRNA TMPO-AS1 plays a significant role in the proliferation of hormone receptor-positive breast cancer and resistance to hormone therapy [18]. LINC00673 is implicated in the EMT (epithelial–mesenchymal transition) [19] and phosphatidylinositol 3-kinase/protein kinase B (PI3K/AKT) signaling pathway [20]. The growth arrest-specific transcript 5 (GAS5) regulates the PI3K/AKT pathway [15, 21]. These and several other lncRNAs are increasingly recognized for their contributions to the growth, progression, and metastasis of breast cancer.
Circular RNA
Circular RNAs (circRNAs) are distinguished by their covalently closed continuous loop structure without a 5′ cap or a 3′ poly(A) tail. This configuration renders them resistant to exoribonuclease degradation and more stable than their linear RNA counterparts. Notably, the plasma half-life of circRNAs is > 48 h, significantly longer than the average 10-h half-life of miRNAs, and they are abundant in body fluids [22, 23]. CircRNAs are implicated in regulating miRNA function, acting as RNA sponges, gene splicing, transcription, RNA-binding protein sponges, and protein/peptide translation. The ability of circRNAs to affect the TME via intercellular signaling has garnered substantial interest, highlighting their importance in oncologic research [23, 24]. An analysis of circRNA expression levels in breast cancer tissues and patient-derived extracellular vesicles revealed > 1000 circRNAs with altered expression levels, highlighting their potential significance in the pathophysiology of breast cancer [25].
Extracellular vesicles and ncRNA (Table 2)
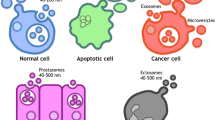
Extracellular vesicles (EVs) can be categorized based on their size and origin: small EVs (50–150 nm) arise from the fusion of multivesicular bodies with the plasma membrane, large EVs (100–1000 nm) bud directly from the plasma membrane, and apoptotic bodies (500–5000 nm) form during cell death. Once regarded merely as cellular waste-disposal mechanisms, EVs are now recognized for their crucial functions and are the subject of extensive research [26]. According to the Minimal Information for Studies of Extracellular Vesicles (MISEV) 2018 guidelines, EV subtypes are classified by physical characteristics such as size, density, and biochemical composition [27]. EVs are found in all body fluids, including urine, breast milk, saliva, cerebrospinal fluid, and blood [28]. Both normal and tumor cells intensify EV secretion, particularly under stress conditions such as hypoxia or heat shock, with tumor cells showing a marked increase [29].
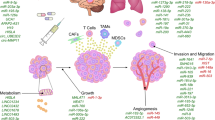
EVs encapsulate and convey RNA, proteins, and lipids between cells, transmitting biologic information within normal cellular contexts, among tumor cells, and within the TME [30]. Moreover, EVs package cytokines such as interleukin, transforming growth factor-β (TGF-β), and tumor necrosis factor-α, which can modulate the physiologic and pathologic activities of target cells [31]. The migration and invasion of cancer cells are essential for metastasis. EVs secreted by invasive breast cancer cells can influence neighboring normal epithelial cells and other breast cancer cells, promoting proliferation, migration, and invasion. These EV components may suppress apoptosis and autophagy [32]. They also contribute to metastatic processes such as abnormal angiogenesis, the degradation of the vascular wall for intravascular invasion, and the alteration of the pre-metastatic niche for targeted organ invasion [32].
Extracellular-vesicle-mediated miRNAs (EV-miRNAs) transport propagates drug resistance [33]. This signaling is bidirectional; breast cancer cells can transform normal fibroblasts into cancer-associated fibroblasts (CAFs) by transferring miRNAs via EVs [34]. Conversely, CAFs may enhance the proliferation of breast cancer cells by introducing miRNAs into the cells through EVs [35]. Hence, extracellular-vesicle-mediated signaling is pivotal in regulating the EMT, proliferation and motility of malignant cells, metastatic processes, angiogenesis stimulation, immune system evasion, cell growth, chemotherapy resistance, and modulation of the TME [28, 29, 36].
EVs containing tumor-derived material are potential LB candidates for disease diagnosis, prognosis, and therapeutic management [29, 37]. EVs are also implicated in the pathogenesis of brain metastases in breast cancer. The detection of characteristic EVs-miRNAs in patients with breast cancer experiencing brain metastases [38] and the evidence that EVs can disrupt tight junctions in brain microvascular endothelial cells, facilitating passage across the blood–brain barrier, underscore their role in establishing metastasis [39].
While EVs have been primarily studied as carriers of tumor-derived miRNAs, they themselves can be analyzed to diagnose cancer using specific markers [40], including heat shock protein 70 (HSP70), which is abundantly expressed on the membranes of tumor-derived EVs. HSP70 is a biomarker for breast cancer diagnosis, staging, prognosis, and therapeutic response [41].
Using ncRNAs and extracellular vesicles in breast cancer therapies (Table 3)
Early diagnosis
In addition to imaging, LB is a promising approach for early breast cancer diagnosis. Shimomura et al. [42] demonstrated that combining five miRNAs (miR-1246, miR-1307-3p, miR-4634, miR-6861-5p, and miR-6875-5p) can accurately detect breast cancer, yielding a sensitivity of 97.3%, specificity of 82.9%, and an overall accuracy of 89.7%. Notably, the sensitivity for Stage 0 and Stage I early-stage breast cancer is particularly high. Similar studies have been reported, as well as research focusing on miRNAs within exosomes [43, 44]. Studies have also been conducted with biofluids other than blood, specifically focusing on urine, saliva, and tears [45,46,47]. Further, ncRNAs other than miRNAs have also been explored. Particularly, 2300-bp lncRNA H19 is crucial in genomic imprinting and is linked with various malignancies, including breast cancer; it has potential as a biomarker in early breast cancer diagnosis [48, 49].
A 2021 meta-analysis on breast cancer diagnosis using circRNAs reported nine tumor-promoting (circ_0008673, circIFI30, circSEPT9, circAGFG1, circ_0001785, circ_0108942, circ_006054, circ_100219, and circ_406697) and twelve tumor-suppressing circRNAs (circVRK1, circ_0104824, circ_0043278, circ_0001073, circ_0068033, circAHNAK1, circTADA2A‐E6, circTADA2A‐E5/E6, circ_0068033, circ_103110, circ_104689, circ_104821). The overall sensitivity and specificity are 77% and 71%, respectively [50].
Yu et al. [51] showed that three circRNAs (hsa_circ_0001785, hsa_circ_0108942, and hsa_circ_0068033) aid early breast cancer diagnosis with a sensitivity of 97.1%, specificity of 90.2%, and AUC (area under the curve) of 0.974. Combining the most accurate circRNA, hsa_circ_0001785, with a tumor marker (CEA/CA15-3) resulted in a sensitivity of 75.8%, specificity of 90.4%, and an AUC of 0.839 (95% confidence interval 0.572–0.773), indicating high accuracy [52].
Staging and subtype evaluation
While imaging studies such as mammography, ultrasonography, contrast-enhanced mammography, magnetic-resonance imaging, and contrast-enhanced computed tomography are traditionally utilized for staging breast cancer using the TNM classification, implementing LB may potentially enhance the precision of this approach. Hosseinpour et al. [53] identified miRNAs with characteristic expression levels corresponding to each cancer stage, from Stage I to Stage IV. Specifically, the expression levels of hsa-miR-592, hsa-miR-449a, and hsa-miR-1269a are characteristic in Stage I, hsa-miR-3662, Hsa-miR-429, and hsa-miR-1269a in Stage II, hsa-miR-3662 in Stage III, and hsa-miR-429, has-miR-23c, and hsa-miR-449a in Stage IV.
Numerous potential indicators of metastatic recurrence are documented. Sueta et al. [54] distinguished differences in serum miRNA expression between patients with metastatic and non-metastatic breast cancer, revealing variations in 11 distinct miRNAs: miR-338-3p, miR-340-5p, and miR-124-3p were significantly upregulated, and miR-29b-3p, miR-20b-5p, miR-17-5p, miR-130a-3p, miR-18a-5p, miR-195-5p, miR-486-5p, and miR-93-5p were downregulated. Additionally, the expression of miR-93-5p during breast cancer diagnosis may serve as a prognostic marker for recurrence. lncRNA H19 is overexpressed in lymph nodes and distant metastases but decreases post-surgery [48]. Continuous monitoring of lncRNA expression could potentially forecast cancer progression.
The minimally invasive and real-time assessment of miRNA subtypes through LB is ongoing. Souza et al. [55] identified 19, 8, 10, 4, and 1 miRNAs associated with the luminal A subtype, luminal B, luminal HER2, HER2-enriched, and TNBC (triple negative breast cancer), respectively (see Table 3 for details on the combination of subtypes and miRNA expression). Additionally, in patient samples from the GeparSixto study, miRNAs with differing expression levels in TNBC and HER2-positive breast cancer were demonstrated (miR-27a, miR-27b, miR-335, miR-365, miR-376c, miR-382, miR-422a, miR-433, and miR-628) [56].
One limitation of LB-based cancer diagnostics, including miRNA assessment, is their lack of reproducibility. Meta-analyses [57] have been performed to evaluate the sensitivity and specificity of miRNA-based diagnostics for breast cancer. From 658 screened studies, seven were included in the meta-analysis. The results indicated a combined sensitivity of 67%, specificity of 81%, and diagnostic odds ratio of 10.2. Although the sensitivity of LB was not consistently high, it may be beneficial in combination with mammography and breast ultrasound.
Prognostic biomarkers
Hormone receptor-positive HER2-negative breast cancer generally has a favorable prognosis. However, identifying the subgroups that require chemotherapy and predicting late recurrences remain challenging. Additionally, patients with TNBC have a high risk of recurrence despite perioperative chemotherapy. Hence, accurately predicting these outcomes has significant clinical relevance.
Mitobe et al. [18] demonstrated that high expression levels of lncRNA TMPO-AS1, which correlates with the proliferation marker, Ki67, and proliferating cell nuclear antigen, indicate a worse prognosis in terms of disease-free survival and overall survival (OS). The study findings suggest poor outcomes for patients with elevated TMPO-AS1 expression levels. Moreover, miRNAs associated with trastuzumab resistance correlate with event-free survival in patients with early-stage breast cancer and progression-free survival in those with metastatic recurrent breast cancer [58].
Prognostic models for breast cancer utilizing EVs have been recently proposed; Long et al. [59] suggested a combination of 10 RNAs and proteins within EVs (HLA-DQB2, COL17A1, miR-324-5p, P2RX1, miR-99a-5p, SLC1A5, LINC01055, AURKA, RTCA, and C3), which provide prognostic insights. A test panel comprising three genome instability (GI)-related miRNAs (miR-421, miR-128–1, and miR-128–2) in EVs has shown predictive capability for cases with poor OS [60]. GI is a driving force behind both inter- and intra-tumor heterogeneity and is crucial for the survival, proliferation, and metastasis of cancer cells. Hence, LB assessments may enhance prognostic precision, complementing traditional clinicopathological risk evaluations.
Biomarkers for predicting treatment response
Although chemotherapy resistance occurs through various mechanisms, miRNA-related chemotherapy resistance is associated with changes in mRNA expression induced by miRNAs [61]. These miRNAs also affect sensitivity to radiotherapy [62].
Response-guided therapy is an approach where adjuvant chemotherapy is tailored according to the response to NACT (Neoadjuvant chemotherapy). Predicting the pathologic complete response (pCR) is now considered crucial in managing early-stage breast cancer, highlighting the significance of NACT and pCR as key determinants of chemotherapy strategies. Han et al. [63] found that miR-1275 levels measured before and after NACT containing anthracyclines and taxanes could predict treatment resistance. This finding was corroborated by discovering that changes in miR-1275 expression indicate resistance. Several reports have indicated the potential of using miRNAs for efficacy prediction [64, 65].
Efforts have been made to predict the efficacy of trastuzumab in HER2-positive breast cancer. Data from the NeoALTTO trial has identified miRNAs that may predict the efficacy of trastuzumab, wherein pCR can be anticipated by assessing changes in two specific miRNAs (ct-miR-148a-3p and ct-miR-374a-5p) after trastuzumab treatment [66]. Zhang et al. [58] reported significantly increased levels of miR-1246 and miR-155 in patients resistant to trastuzumab.
The potential of RNAs within EVs to predict NACT efficacy has been explored; Sadovska et al. [67] analyzed miRNA and lncRNA expression levels pre- and post-treatment in patients with Stages II–III breast cancer and identified ten nucleic acids (miR-12113, miR-190b-5p, miR-34b-5p, miR-152-5p, miR-132-5p, lnc-PARP806, lnc-DPH7-1, lnc-KLF17-1, lnc-ALX1-2, and SNORD111) as predictors of drug therapy success. In a synthesis of studies on miRNAs and radiosensitivity [62], ten miRNAs (miR-7, miR-27a, miR-155, miR-205, miR-211, miR-21, miR-33a, miR-139-5p, and miR-210) have been associated with radiosensitivity and the outcome after RT(Radio therapy), potentially modulating it through key pathways such as PI3K/AKT/mTOR, RAS/MEK/ERK, and ATM/ATR, particularly in the context of DNA double-strand break repair.
Therapeutic applications
Since the discovery of miRNA silencing by small interfering RNAs (siRNAs) in the 1990s, numerous studies on RNA interference have been conducted, with great expectations for its application in cancer treatment. Mitobe et al. [68] introduced siRNAs targeting TMPO-AS1 (siTMPO-AS1) into a TNBC cell line, inhibiting cell cycle progression and apoptosis and impairing cell migration and metastasis via the TGF-β pathway. Similarly, deactivating the lncRNA OLBC15, implicated in tumor cell proliferation, migration, and metastasis, considerably reduces the survival of TNBC cell lines [21]. These findings provide optimistic prospects for developing novel cancer therapies utilizing siRNAs. However, many challenges must be overcome and further advancements in research are anticipated [62].
Several therapeutic strategies involving EVs have been explored [69]. Research into a drug-delivery system that encases therapeutic agents, nucleic acids, and proteins within EVs for transport to tumor cells is in progress [70], along with investigations into cancer vaccines employing EVs from immune cells with anti-tumor properties [71].
In conclusion, this review was conducted on breast cancer diagnostics and treatment using ncRNAs and exosomes. It is strongly anticipated that the use of ncRNAs and exosomes will significantly advance the field of breast cancer diagnostics and treatment.
References
Shyamala K, Girish HC, Murgod S. Risk of tumor cell seeding through biopsy and aspiration cytology. J Int Soc Prev Community Dent. 2014;4:5–11.
Alix-Panabières C, Pantel K. Liquid biopsy: from Discovery to clinical application. Cancer Discov. 2021;11:858–73.
Nikanjam M, Kato S, Kurzrock R. Liquid biopsy: current technology and clinical applications. J Hematol Oncol. 2022;15:131.
Zhang Z, Zhang J, Diao L, Han L. Small non-coding RNAs in human cancer: function, clinical utility, and characterization. Oncogene. 2021;40:1570–7.
Peng Y, Croce CM. The role of microRNAs in human cancer. Signal Transduct Target Ther. 2016;1:15004.
Miska EA. How microRNAs control cell division, differentiation and death. Curr Opin Genet Dev. 2005;15:563–8.
miRBase. 2023.
Ling L, Aldoghachi AF, Chong ZX, Ho WY, Yeap SK, Chin RJ, et al. Addressing the clinical feasibility of adopting circulating miRNA for breast cancer detection, monitoring and management with artificial intelligence and machine learning platforms. Int J Mol Sci. 2022;23:15382.
Li R, Qu H, Wang S, Chater JM, Wang X, Cui Y, et al. CancerMIRNome: an interactive analysis and visualization database for miRNome profiles of human cancer. Nucleic Acids Res. 2022;50:D1139–46.
Baldasici O, Pileczki V, Cruceriu D, Gavrilas LI, Tudoran O, Balacescu L, et al. Breast cancer-delivered exosomal miRNA as liquid biopsy biomarkers for metastasis prediction: a focus on translational research with clinical applicability. Int J Mol Sci. 2022;23:9371.
Cortez MA, Anfossi S, Ramapriyan R, Menon H, Atalar SC, Aliru M, et al. Role of miRNAs in immune responses and immunotherapy in cancer. Genes Chromosomes Cancer. 2019;58:244–53.
Chen Y, Gao DY, Huang L. In vivo delivery of miRNAs for cancer therapy: challenges and strategies. Adv Drug Deliv Rev. 2015;81:128–41.
Zangouei AS, Zangoue M, Taghehchian N, Zangooie A, Rahimi HR, Saburi E, et al. Cell cycle related long non-coding RNAs as the critical regulators of breast cancer progression and metastasis. Biol Res. 2023;56:1.
Zhou W, Ye XL, Xu J, Cao MG, Fang ZY, Li LY, et al. The lncRNA H19 mediates breast cancer cell plasticity during EMT and MET plasticity by differentially sponging miR-200b/c and let-7b. Sci Signal. 2017;10:eaak9557.
Hu Q, Ye Y, Chan LC, Li Y, Liang K, Lin A, et al. Oncogenic lncRNA downregulates cancer cell antigen presentation and intrinsic tumor suppression. Nat Immunol. 2019;20:835–51.
Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–6.
Wang YL, Liu LC, Hung Y, Chen CJ, Lin YZ, Wu WR, Wang SC. Long non-coding RNA HOTAIR in circulatory exosomes is correlated with ErbB2/HER2 positivity in breast cancer. Breast. 2019;46:64–9.
Mitobe Y, Ikeda K, Suzuki T, Takagi K, Kawabata H, Horie-Inoue K, Inoue S. ESR1-stabilizing long noncoding RNA TMPO-AS1 promotes hormone-refractory breast cancer progression. Mol Cell Biol. 2019;39:e00261-19.
Xia E, Shen Y, Bhandari A, Zhou X, Wang Y, Yang F, Wang O. Long non-coding RNA LINC00673 promotes breast cancer proliferation and metastasis through regulating B7–H6 and epithelial-mesenchymal transition. Am J Cancer Res. 2018;8:1273–87.
Li S, Zhou J, Wang Z, Wang P, Gao X, Wang Y. Long noncoding RNA GAS5 suppresses triple negative breast cancer progression through inhibition of proliferation and invasion by competitively binding miR-196a-5p. Biomed Pharmacother. 2018;104:451–7.
Deng C, Zhang B, Zhang Y, Xu X, Xiong D, Chen X, Wu J. A long non-coding RNA OLBC15 promotes triple-negative breast cancer progression via enhancing ZNF326 degradation. J Clin Lab Anal. 2020;34: e23304.
Jeck WR, Sharpless NE. Detecting and characterizing circular RNAs. Nat Biotechnol. 2014;32:453–61.
Li Y, Zheng Q, Bao C, Li S, Guo W, Zhao J, et al. Circular RNA is enriched and stable in exosomes: a promising biomarker for cancer diagnosis. Cell Res. 2015;25:981–4.
Tang X, Ren H, Guo M, Qian J, Yang Y, Gu C. Review on circular RNAs and new insights into their roles in cancer. Comput Struct Biotechnol J. 2021;19:910–28.
Wang J, Zhang Q, Zhou S, Xu H, Wang D, Feng J, et al. Circular RNA expression in exosomes derived from breast cancer cells and patients. Epigenomics. 2019;11:411–21.
Chen X, Liang H, Zhang J, Zen K, Zhang CY. Horizontal transfer of microRNAs: molecular mechanisms and clinical applications. Protein Cell. 2012;3:28–37.
Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750.
Lee Y, Ni J, Beretov J, Wasinger VC, Graham P, Li Y. Recent advances of small extracellular vesicle biomarkers in breast cancer diagnosis and prognosis. Mol Cancer. 2023;22:33.
Shefer A, Yalovaya A, Tamkovich S. Exosomes in breast cancer: involvement in tumor dissemination and prospects for liquid biopsy. Int J Mol Sci. 2022;23:8845.
Xu R, Rai A, Chen M, Suwakulsiri W, Greening DW, Simpson RJ. Extracellular vesicles in cancer—implications for future improvements in cancer care. Nat Rev Clin Oncol. 2018;15:617–38.
Jung HH, Kim JY, Cho EY, Oh JM, Lee JE, Kim SW, et al. Elevated level of nerve growth factor (NGF) in Serum-derived exosomes predicts poor survival in patients with breast cancer undergoing neoadjuvant chemotherapy. Cancers. 2021;13:5260.
Tan Y, Luo X, Lv W, Hu W, Zhao C, Xiong M, et al. Tumor-derived exosomal components: the multifaceted roles and mechanisms in breast cancer metastasis. Cell Death Dis. 2021;12:547.
Chen WX, Cai YQ, Lv MM, Chen L, Zhong SL, Ma TF, et al. Exosomes from docetaxel-resistant breast cancer cells alter chemosensitivity by delivering microRNAs. Tumour Biol. 2014;35:9649–59.
Yang SS, Ma S, Dou H, Liu F, Zhang SY, Jiang C, et al. Breast cancer-derived exosomes regulate cell invasion and metastasis in breast cancer via miR-146a to activate cancer associated fibroblasts in tumor microenvironment. Exp Cell Res. 2020;391: 111983.
Chen B, Sang Y, Song X, Zhang D, Wang L, Zhao W, et al. Exosomal miR-500a-5p derived from cancer-associated fibroblasts promotes breast cancer cell proliferation and metastasis through targeting USP28. Theranostics. 2021;11:3932–47.
Encarnación-Medina J, Godoy L, Matta J, Ortiz-Sánchez C. Identification of exo-miRNAs: a summary of the efforts in translational studies involving triple-negative breast cancer. Cells. 2023;12:1339.
Yu W, Hurley J, Roberts D, Chakrabortty SK, Enderle D, Noerholm M, et al. Exosome-based liquid biopsies in cancer: opportunities and challenges. Ann Oncol. 2021;32:466–77.
Curtaz CJ, Reifschläger L, Strähle L, Feldheim J, Feldheim JJ, Schmitt C, et al. Analysis of microRNAs in exosomes of breast cancer patients in search of molecular prognostic factors in brain metastases. Int J Mol Sci. 2022;23:3683.
Luo T, Kang Y, Liu Y, Li J, Li J. Small extracellular vesicles in breast cancer brain metastasis and the prospect of clinical application. Front Bioeng Biotechnol. 2023;11:1162089.
Hoshino A, Kim HS, Bojmar L, Gyan KE, Cioffi M, Hernandez J, et al. Extracellular vesicle and particle biomarkers define multiple human cancers. Cell. 2020;182:1044-1061.e18.
Chanteloup G, Cordonnier M, Isambert N, Bertaut A, Hervieu A, Hennequin A, et al. Monitoring HSP70 exosomes in cancer patients’ follow up: a clinical prospective pilot study. J Extracell Vesicles. 2020;9:1766192.
Shimomura A, Shiino S, Kawauchi J, Takizawa S, Sakamoto H, Matsuzaki J, et al. Novel combination of serum microRNA for detecting breast cancer in the early stage. Cancer Sci. 2016;107:326–34.
Hannafon BN, Trigoso YD, Calloway CL, Zhao YD, Lum DH, Welm AL, et al. Plasma exosome microRNAs are indicative of breast cancer. Breast Cancer Res. 2016;18:90.
Yoshikawa M, Iinuma H, Umemoto Y, Yanagisawa T, Matsumoto A, Jinno H. Exosome-encapsulated microRNA-223-3p as a minimally invasive biomarker for the early detection of invasive breast cancer. Oncol Lett. 2018;15:9584–92.
Hirschfeld M, Rucker G, Weiss D, Berner K, Ritter A, Jager M, Erbes T. Urinary Exosomal microRNAs as potential non-invasive biomarkers in breast cancer detection. Mol Diagn Ther. 2020;24:215–32.
Zhang L, Xiao H, Karlan S, Zhou H, Gross J, Elashoff D, et al. Discovery and preclinical validation of salivary transcriptomic and proteomic biomarkers for the non-invasive detection of breast cancer. PLoS ONE. 2010;5: e15573.
Inubushi S, Kawaguchi H, Mizumoto S, Kunihisa T, Baba M, Kitayama Y, et al. Oncogenic miRNAs identified in tear exosomes from metastatic breast cancer patients. Anticancer Res. 2020;40:3091–6.
Zhong G, Wang K, Li J, Xiao S, Wei W, Liu J. Determination of serum exosomal H19 as a noninvasive biomarker for breast cancer diagnosis. Onco Targets Ther. 2020;13:2563–71.
Zhang K, Luo Z, Zhang Y, Zhang L, Wu L, Liu L, et al. Circulating lncRNA H19 in plasma as a novel biomarker for breast cancer. Cancer Biomark. 2016;17:187–94.
Chu M, Fang Y, Jin Y. CircRNAs as promising biomarker in diagnosis of breast cancer: an updated meta-analysis. J Clin Lab Anal. 2021;35: e23934.
Yu Y, Zheng W, Ji C, Wang X, Chen M, Hua K, et al. Tumor-derived circRNAs as circulating biomarkers for breast cancer. Front Pharmacol. 2022;13: 811856.
Yin WB, Yan MG, Fang X, Guo JJ, Xiong W, Zhang RP. Circulating circular RNA hsa_circ_0001785 acts as a diagnostic biomarker for breast cancer detection. Clin Chim Acta. 2018;487:363–8.
Hosseinpour Z, Rezaei Tavirani M, Akbari ME. Stage analysis of breast cancer metabolomics: a system biology approach. Asian Pac J Cancer Prev. 2023;24:1571–82.
Sueta A, Yamamoto Y, Tomiguchi M, Takeshita T, Yamamoto-Ibusuki M, Iwase H. Differential expression of exosomal miRNAs between breast cancer patients with and without recurrence. Oncotarget. 2017;8:69934–44.
Souza KCB, Evangelista AF, Leal LF, Souza CP, Vieira RA, Causin RL, et al. Identification of cell-free circulating microRNAs for the detection of early breast cancer and molecular subtyping. J Oncol. 2019;2019:8393769.
Stevic I, Muller V, Weber K, Fasching PA, Karn T, Marme F, et al. Specific microRNA signatures in exosomes of triple-negative and HER2-positive breast cancer patients undergoing neoadjuvant therapy within the GeparSixto trial. BMC Med. 2018;16:179.
Hong F, Li N, Feng Z, Zheng Y, Zhu C, Zhang F. Exosomal microRNAs as novel diagnostic biomarkers in breast cancer: a systematic evaluation and meta-analysis. Asian J Surg. 2023;46:4727–36.
Zhang Z, Zhang L, Yu G, Sun Z, Wang T, Tian X, et al. Exosomal miR-1246 and miR-155 as predictive and prognostic biomarkers for trastuzumab-based therapy resistance in HER2-positive breast cancer. Cancer Chemother Pharmacol. 2020;86:761–72.
Long F, Ma H, Hao Y, Tian L, Li Y, Li B, et al. A novel exosome-derived prognostic signature and risk stratification for breast cancer based on multi-omics and systematic biological heterogeneity. Comput Struct Biotechnol J. 2023;21:3010–23.
Bao S, Hu T, Liu J, Su J, Sun J, Ming Y, et al. Genomic instability-derived plasma extracellular vesicle-microRNA signature as a minimally invasive predictor of risk and unfavorable prognosis in breast cancer. J Nanobiotechnol. 2021;19:22.
Hammond SM. An overview of microRNAs. Adv Drug Deliv Rev. 2015;87:3–14.
To NH, Nguyen HQ, Thiolat A, Liu B, Cohen J, Radosevic-Robin N, Belkacemi Y. Radiation therapy for triple-negative breast cancer: emerging role of microRNAs as biomarkers and radiosensitivity modifiers. A systematic review. Breast Cancer Res Treat. 2022;193:265–79.
Han X, Li M, Xu J, Fu J, Wang X, Wang J, et al. miR-1275 targets MDK/AKT signaling to inhibit breast cancer chemoresistance by lessening the properties of cancer stem cells. Int J Biol Sci. 2023;19:89–103.
Todorova VK, Byrum SD, Gies AJ, Haynie C, Smith H, Reyna NS, Makhoul I. Circulating exosomal microRNAs as predictive biomarkers of neoadjuvant chemotherapy response in breast cancer. Curr Oncol. 2022;29:613–30.
Salvador-Coloma C, Santaballa A, Sanmartin E, Calvo D, Garcia A, Hervas D, et al. Immunosuppressive profiles in liquid biopsy at diagnosis predict response to neoadjuvant chemotherapy in triple-negative breast cancer. Eur J Cancer. 2020;139:119–34.
Di Cosimo S, Appierto V, Pizzamiglio S, Silvestri M, Baselga J, Piccart M, et al. Early modulation of circulating microRNAs levels in HER2-positive breast cancer patients treated with trastuzumab-based neoadjuvant therapy. Int J Mol Sci. 2020;21:1386.
Sadovska L, Zayakin P, Eglītis K, Endzeliņš E, Radoviča-Spalviņa I, Avotiņa E, et al. Comprehensive characterization of RNA cargo of extracellular vesicles in breast cancer patients undergoing neoadjuvant chemotherapy. Front Oncol. 2022;12:1005812.
Mitobe Y, Ikeda K, Sato W, Kodama Y, Naito M, Gotoh N, et al. Proliferation-associated long noncoding RNA, TMPO-AS1, is a potential therapeutic target for triple-negative breast cancer. Cancer Sci. 2020;111:2440–50.
Rezaie J, Feghhi M, Etemadi T. A review on exosomes application in clinical trials: perspective, questions, and challenges. Cell Commun Signal. 2022;20:145.
Bunggulawa EJ, Wang W, Yin T, Wang N, Durkan C, Wang Y, Wang G. Recent advancements in the use of exosomes as drug delivery systems. J Nanobiotechnol. 2018;16:81.
Xu Z, Zeng S, Gong Z, Yan Y. Exosome-based immunotherapy: a promising approach for cancer treatment. Mol Cancer. 2020;19:160.
Acknowledgements
The authors acknowledge Ms. Masayo Kawamura for her kind assistance and administrative support.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. The material preparation, data collection, and analysis were performed by KH. The first draft of the manuscript was written by KH and all authors commented on previous versions. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Dr. Shimomura reports grants from Chugai Pharmaceutical, Taiho Pharmaceutical, AstraZeneca, Mochida Pharmaceutical, Daiichi Sankyo, Eisai, and Gilead Sciences outside the submitted work, and lecture fees from Chugai Pharmaceutical, Daiichi Sankyo, Eli-Lilly, Kyowa Kirin, AstraZeneca, MSD, Gilead Sciences, Pfizer, Exact Science, and Nihon Medi-Physics outside the submitted work. The other authors declare no potential conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Hashimoto, K., Ochiya, T. & Shimomura, A. Liquid biopsy using non-coding RNAs and extracellular vesicles for breast cancer management. Breast Cancer (2024). https://doi.org/10.1007/s12282-024-01562-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12282-024-01562-w