Abstract
Breast cancer (BC) bone metastasis is primarily osteolytic and has limited therapeutic options. Metastasized BC cells prime the secondary environment in bone by forming a tumor niche, which favors their homing and colonization. The tumor microenvironment (TME) is primarily generated by the cancer cells. Bone TME is an intricate network of multiple cells, including altered bone, tumor, stromal, and immune cells. Recent findings highlight the significance of small non-coding microRNAs (miRNAs) in influencing TME during tumor metastasis. MiRNAs from TME-resident cells facilitate the interaction between the tumor and its microenvironment, thereby regulating the biological processes of tumors. These miRNAs can serve as oncogenes or tumor suppressors. Hence, both miRNA inhibitors and mimics are extensively utilized in pre-clinical trials for modulating the phenotypes of tumor cells and associated stromal cells. This review briefly summarizes the recent developments on the functional role of miRNAs secreted directly or indirectly from the TME-resident cells in facilitating tumor growth, progression, and metastasis. This information would be beneficial in developing novel targeted therapies for BC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer (BC) remains the predominantly diagnosed cancer and the second most cause of mortality among women worldwide [1]. Breast carcinogenesis is a multi-step cascade involving several cell types, and its prevention remains strenuous. The distant metastatic spread of BC, which renders it incurable, to the bones, followed by the lung, liver, and brain, is chiefly responsible for the disease’s associated mortality [2]. Nearly, 70% of BC cells metastasize to bones, and reports suggested that the estrogen receptor positive (ER+) BC cells show a specific preference for bones. Estrogen was observed to modulate the bone microenvironment (BME), which favors the homing and colonization of the metastasized BC cells [3]. Bone metastasis is increasingly reported in metastatic BC patients, adversely affecting their survival and quality of life. BC bone metastasis occurs as a result of increased osteoclastic resorption, leading to degradation of bone. In metastatic disease, the clinical management of bone metastases is still crucial [4].
The tumor microenvironment (TME) of a developing tumor is an integrated system composed of proliferating cancer cells, tumor stroma, infiltrating immune cells, blood vessels, and several host tissue-associated cells. The emerging TME is a distinct and developing entity that arises during tumor development [5]. The interactions of the cancer cells with the cellular and non-cellular constituents of the TME are essential to promote tumor heterogeneity, drug resistance, clonal evolution, etc., and result in cancer progression and subsequent metastasis. Tumor cells in the TME facilitate the cellular interactions and hijack the non-malignant cells, thereby pressuring the stromal cells to lose their characteristic function and acquire tumor-promoting phenotypes [6]. For instance, tumor-associated macrophages (TAMs) are generally derived from the precursor cells recruited by the cancer cells via releasing chemokines such as CCL-2 and CCL-5 and cytokines like CSF-1 [7, 8]. These TAMs secrete factors, including transforming growth factor-beta (TGF-β), tumor necrosis factor-alpha (TNF-α), and vascular endothelial growth factor (VEGF), which aid in the recruitment of macrophages and tumor cells into the target site and promote the formation of a metastatic niche. Thus, in addition to influencing the TME, TAMs also affect the macrophages of the whole body and facilitate tumor progression [9].
Upon reaching the bone, disseminated BC cells undergo dormancy initially but eventually grow and confiscate the BME. Evidence suggested that BC cells, once they enter BME escape immune surveillance using chemokine receptors like CXCR4 and lodge to specific niches in the bone marrow rich in respective ligands (CXCL12) [10]. These tumor cells then secrete multiple factors and severely modify the BME resulting in the formation of metastatic lesions, which perturbs the delicate balance and dynamics of the BME. Various cells in the BME, including osteoclasts, osteoblasts, osteocytes, adipocytes, and nerve cells, are reported to critically regulate the bone metastasis of BC cells via facilitating the crosstalk between metastatic BC cells and BME [11]. While the role of osteoclasts in establishing osteolytic lesions is well established, the involvement of osteoblasts in bone metastasis has been increasingly investigated in recent times. Reports suggested the presence of a subpopulation of osteoblasts in the BME that was immensely altered by metastatic BC cells and exhibited altered functional characteristics. The tumor "educated" osteoblasts do not differentiate but they secrete cytokines that aid in tumor cell maintenance, thus demonstrating a diverted role from their regular function of new matrix deposition [12]. Therefore, understanding the crucial role of the cellular and non-cellular constituents of the BME in driving bone metastasis remains critical in developing better therapeutics to treat tumor-induced bone diseases [13].
Non-coding RNAs (ncRNAs) constitute a significant segment of the human genome (~ 97%) and have been extensively reported to regulate both physiology and pathology [14]. Increasing evidence emphasizes the biological importance of ncRNAs in regulating multiple steps involved in cancer pathogenesis. Studies postulated an association between the activity of ncRNAs and the dysregulation of several genes involved in BC pathogenesis. Further, many ncRNAs were also abnormally expressed in BC cells and tissues, which were identified to promote proliferation, extracellular matrix (ECM) degradation, and increased epithelial to mesenchymal transition (EMT) [15]. In general, the ncRNAs are classified into short (19–24 nucleotides) and long ncRNAs (> 200 nucleotides). Among these, miRNAs and lncRNAs are extensively reported in BC [16, 17]. Multiple findings suggested that miRNAs serve as critical regulators of intra- and intercellular signaling in BC and could modulate cells’ proliferation, apoptosis, invasion, migration, etc. [18]. Specifically, in TME, miRNAs were identified to mediate the crosstalk between the cancer cells and the TME components, thereby influencing tumor growth and progression. These ncRNAs were packed in exosomes and released in the TME, via which they were identified to mediate the communication between the cancer cells and other cells in the TME [19]. In this review, we have emphasized the vital role of TME and its cellular components in mediating bone metastasis of BC. Further, the functional significance of secreted miRNAs in regulating the tumor and its TME during BC bone metastasis is discussed, which might reveal the therapeutic potential of these miRNAs.
Role of tumor microenvironment (TME) in regulating bone metastatic breast cancer
The process of bone metastasis is a systematic multi-step cascade involving bone marrow colonization, dormancy/survival of circulating tumor cells (CTCs) in niches, and reactivation and development of the dormant cells into proliferating aggressive metastases. Owing to the challenges these cells encounter, tumor growth efficiency at the secondary site is less efficient compared to the primary site [20]. The CTCs initially undergo dormancy and house in the vascular and endosteal niches in the bone marrow, which offers a supportive environment for their survival. These dormant cells then get activated years later, and proliferate and modify the functions of the bone cells, thereby promoting skeletal destruction [21]. Bone provides a suitable soil for the CTCs (seeds) as it serves as a reservoir of soluble factors, including interleukins (ILs), calcium (Ca2+), and TGF-β owing to its potential to undergo continuous remodeling throughout life. Further, low pH, increased extracellular calcium concentration, and hypoxia aid in the engraftment of the tumor and its subsequent growth in the bones [22].
The tumor stroma in bone is a complex network comprised of cellular elements such as endothelial cells, bone cells (osteoblasts, osteocytes, osteoclasts, etc.), other stromal cells (fibroblasts, adipocytes), blood and lymphatic vessels, immune cells, and cancer-associated fibroblasts (CAFs), and non-cellular elements such as extracellular matrix (ECM), etc. [23, 24]. Increasing evidence suggested that the tumors comprise tumor parenchyma and supporting stroma, and the crosstalk between these distinct but reciprocal components was found to support tumor growth. Findings from multiple investigations demonstrated that the stromal cells in the TME play critical roles in the initiation, progression, and metastasis of tumors. Especially in BC, CAFs are the most abundant cell type in tumor stroma [25, 26]. These CAFs are reported to produce several soluble factors, including growth factors and cytokines, and alter the associated tumor stroma, promoting tumor growth and invasion. For instance, a study using xenograft models revealed that the CAFs from the primary human BCs could significantly encourage tumor growth and angiogenesis [27]. Interestingly, the composition and expression of the ECM components were identified to differ among different molecular subtypes of BC [28]. For example, triple negative BC (TNBC) and, to some extent, Her2 tumors exhibited increased collagen deposition and promoted CAF invasion [29]. Tumor-associated macrophages (TAMs) represent a subset of tumor-induced differentiated myeloid cells that reside within the TME [30]. The tumor cells in the TME secrete cytokines and drive an altered function of these TAMs, exhibiting anti-inflammatory and tumorigenic properties, and hence TAMs predominantly function in TME remodeling [31, 32]. Tumor-associated endothelial cells (TAEs) present in the TME exhibited a pro-angiogenic nature that is greatly influenced by the hedgehog signaling [33]. The TAEs were reported to regulate neutrophil infiltration, stem cell renewal, drug resistance, invasion, and metastasis, thereby mediating tumor progression and metastasis [34].
Tumor-associated osteoblasts and osteoclasts are the primary regulatory agents of TME in the metastasized bone. Generally, bone metastatic BC patients develop lesions in the bone that are osteoblastic, osteolytic, or a mix of both. Most of the lesions reported are osteolytic, where bone resorption is higher than bone formation [35]. Though the precise mechanism behind the osteoblastic lesion remains obscure, Runx2 was reported to pose a critical factor in regulating this event [36]. Studies demonstrate that metastatic BC cells educate a subset of osteoblasts in the BME and modify their characteristics in the advanced stages of the disease. Further, these tumors “educated” osteoblasts (EOs) were observed to secrete a definitive set of cytokines, including VEGF, MCP-1, GRO-α, IL-8, and IL-6, which aid in the colonization of metastatic BC cells at late stages of the malignancy [12, 37, 38]. Further, recent research suggested that these EOs affect the development of osteoclasts in the metastatic niche, resulting in reduced pre-osteoclast fusion and bone resorption during bone metastasis of BC [12]. In contrast to osteoblasts, the role of osteoclasts in regulating TME during BC bone metastasis is well investigated. The osteoclasts mediate the formation of osteolytic lesions in response to multiple osteoclastogenic factors (IL-1, IL-6, IL-11, PDGF, MIP1α, RANKL, TNF, PTHrP, and M-CSF) secreted by the tumor cells. These factors induce the growth of metastatic BC in the bone and establish the heinous cycle of tumor adhesion and proliferation followed by skeletal destruction [39]. Figure 1 illustrates the immune cells present in the breast microenvironment.
Composition of breast TME in metastasized bone. Breast TME is a complex system comprising BC cells, immune cells, stromal cells, tumor altered bone-resident cells and secreted factors. CAF: Cancer-associated fibroblasts; TAM: Tumor-associated macrophage; TAE: Tumor-associated endothelial cell; TAOB: Tumor-associated osteoblast; TAOC: Tumor-associated osteoclast; TADC: Tumor-associated dendritic cell; Treg: Regulatory T cell; PMN: Polymorphonuclear neutrophil; MDSC: Myeloid-derived suppressor cell
Apart from the above discussed, myeloid-derived suppressor cells (MDSCs), tumor-associated dendritic cells (TADCs), tumor-associated neutrophils (TANs), and regulatory T cells (Tregs) are also reported to regulate the BC pathogenesis and bone metastasis [40]. Table 1 details the list of stromal cells in the breast TME and their respective functions in regulating TME.
Role of miRNAs secreted by TME-resident cells in regulating BC bone metastasis
The bone microenvironment (BME) is a fertile reservoir of growth and signaling factors, making it an ideal site for the metastasis of many solid tumors, including BC. Bone offers a niche to the metastasized BC cells, where they stay dormant and escape from the administered chemotherapeutic regiments. The BME is not inactive but instead attracts and responds to the infiltrating tumor cells [52]. The successful colonization and progression of BC cells in bone rely on the interaction between the metastasized cells and the resident cells in the bone TME, which forms a vicious cycle. Besides the interaction between these cells via direct cell–cell contacts, cytokines such as TGF-β, VEGF, ILs, and PTHrP were also identified to mediate cell–cell communication, thus playing a crucial role in establishing a more robust network and regulate pathology [53]. NcRNA comprise a large class of regulatory molecules that are identified to regulate the multiple-step pathogenesis cascade in human cancers, including BC. Regulatory ncRNAs are further classified into short and long ncRNAs and are expressed in a cell-specific and tissue-specific manner [54]. Increasing evidence suggested that these ncRNAs mediate intracellular and intercellular signaling during pathology [55]. Though miRNAs (short ncRNAs) constitute only a tiny fraction of the total ncRNAs, most ncRNA research has focused on miRNAs [14]. MiRNAs are small ncRNAs well studied for their post-transcriptional regulation of target mRNAs. Dysregulation of miRNA expression results in altered target gene expression, which was observed to contribute to the evolution of cancer phenotypes. Numerous studies have reported dysregulated miRNA expression as a hallmark of human malignancies, including cancers [56]. Many miRNAs regulate breast carcinogenesis by directing autophagy, apoptosis, EMT, therapeutic resistance, etc. [57]. For instance, miRNAs including let-7, miR-21, miR-155-5p, miR-128, miR-200 family, miR-326, and miR-451 were identified to modulate the response of BC cells to chemotherapy [58]. As discussed above, several host cells in the TME of bone including osteoblasts, osteoclasts, CAFs, and TAMs [24] influence the process of colonization and progression of metastasized BC cells in bone. Apart from directly regulating BC cells, miRNAs affect the cells in the TME and indirectly aid in tumor establishment at the secondary site. In the following section, we have discussed the regulatory role of miRNAs in influencing the resident cells in TME during bone metastasis of BC.
Bone marrow stroma-secreted miRNAs and bone metastasis of BC
Bone marrow serves as a reservoir for the sustained production of almost all blood cell lineages throughout an individual’s life. Bone marrow stroma primarily comprises hematopoietic and non-hematopoietic cells, including mesenchymal, neural, and endothelial cells. Together, bone marrow stroma has two principal functions; first participates in bone metabolism and remodeling, and second, in varied stages of blood cell development and generation, thus posing as the master regulator [59]. Besides the direct regulation, miRNAs expressed by the host cells in the TME might influence bone metastasis via the transfer of these miRNAs to BC cells. These miRNAs were observed to be delivered to the BC cells via exosomal or gap junction-mediated transfer. Specifically, the gap junction-mediated exchange of miRNAs between the bone marrow stroma and BC cells was observed to be a critical factor that contributes to the tumor cell dormancy via conferring dormant phenotypes in the bone marrow [60]. Figure 2 depicts the miRNA-mediated interaction between the BC stromal cells and their influence on BC bone metastasis. For instance, a study reported transferring miR-130a and miR-206 from bone marrow stroma to BC cells via gap junctions. These miRNAs were observed to target Tac1 mRNA (Tachykinin) and other cytokines in BC cells. The expression of the TAC1 gene has been linked with stress and was observed to facilitate the entry of BC cells into bone marrow and promote BC proliferation. Thus, these findings suggested the tumor suppressor role of miR-130a and miR-206 in bone marrow stroma by preventing the entry of BC cells [61,62,63].
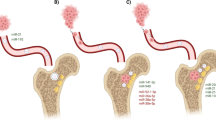
miRNA-mediated crosstalk between the bone marrow stroma and BC cells. Distinct stromal cells in the bone marrow, such as osteoprogenitors, hematopoietic progenitors, mesenchymal stem cells, etc., communicate with the metastasized BC cells by direct transfer of miRNAs via gap junctions or indirect transfer by exosomes or extracellular vesicles (EV), respectively. Transfer of miRNAs exerts an oncogenic or tumor-suppressive role depending on their target gene in the BC cell
Similarly, another study identified that CXCL12-targeting miRNAs, including miR-223, miR-222, miR-197, and miR-127, were transported to BC cells from bone marrow stroma via gap junctions, resulting in decreased CXCL12 levels and reduced proliferation of BC cells [64] (Fig. 2A). Besides gap junction-mediated transfer, miRNAs can also be transferred via extracellular vesicles (EVs) from bone marrow to BC cells. For instance, BC cells that acquired anti-proliferative miRNAs, miR-127, miR-222, and miR-223 via bone marrow stromal cells-derived EVs were observed to enter the quiescent phase (G0 phase) of the cell cycle, thus inducing dormancy in cancer cells [65, 66]. Similarly, an increased expression of miR-23b in exosomes derived from bone marrow mesenchymal stem cells (BMMSCs) was observed to stimulate dormant phenotypes in BC cells via targeting MARCKS (myristoylated alanine-rich C-kinase substrate), which codes for a protein that enhances cell cycle and motility. Further, there was an increased expression of miR-23b in metastatic BC cells in the bone marrow of BC patients and subsequently decreased expression of MARCKS, suggesting that exosomal transfer of this miRNA might facilitate BC cell dormancy in a metastatic niche [67] (Fig. 2B). A study by Vallabhaneni et al. reported on the EV-mediated transfer of miR-21 and miR-34a from human MSCs to BC cells, which were identified to regulate BC proliferation and metastasis in vitro and in vivo. While miR-21 was reported to promote proliferation in BC, miR-34a exhibits an anti-proliferative effect. Though they show contradicting functions, together they exhibited a proliferative role in BC cells [68] (Fig. 2B). Another study by Shu et al. suggested a reduced expression of miR-150-5p in THP-1 monocytes. Their findings suggested that miR-150-5p could target Notch3 and reduce the expression of CCR2 in monocytes, thereby promoting BC metastasis (70). Taken together, these findings highlight the importance of bone marrow stroma collectively in regulating BC progression and further metastasis, which might provide insights on novel therapeutic strategies for treating BC.
TME-resident immune cells secreted miRNAs and bone metastasis of BC
Different types of immune cells, both from innate and adaptive immunity, exhibit association with the TME. The immune response to the tumor is determined by the delicate balance between the immune systems and cancer immunoediting [70]. Though the immune cells fight to eliminate cancer, alterations either in these malignant cells or host immune cells hinder the identification of cancer cells, thus allowing them to evade the immune response [71]. The crosstalk between the host immune cells and tumor cells remains crucial in this setting. Reports suggested the involvement of miRNAs in functioning as a link between cancer and immune response via influencing the immune cells recruitment and activation in the TME and modulating cancer-related signaling cascades in the immune cells, resulting in cancer pathogenesis [72]. Several miRNAs were reported to regulate the interaction between the TME-resident immune cells and BC cells. Herein, we spotlight the functional interaction of TME-resident immune cells during BC development and bone metastasis.
Tumor-associated macrophages
TAMs represent one of the significant populations of immune cells in the TME that exhibits distinguished functional and phenotypic heterogeneity [73]. They exhibit distinguished phenotypic and functional heterogeneity. TAMs were reported to acquire an M2-like phenotype and exert a pro-tumoral and immune suppressive function via co-inhabiting with the tumor cells [74, 75]. Extensive clinical and experimental evidence suggested the tumor-promoting role of TAMs in multiple cancers, including BC [76]. For example, a study reported a downregulated expression of miR-19a-5p in M2 phenotype RAW264.7 macrophages cultured in a conditioned medium of 4T1 mouse mammary tumor cells. They identified that miR-19a-5p reduced the induction of M2 macrophage polarization, a hallmark of cancer [77], via targeting Fra-1, a proto-oncogene and regulated the expression of its downstream signaling molecules, including STAT3, pSTAT3, and VEGF both in vitro and in vivo. Their results suggested a decrease in invasion and migration potential of 4T1 cells in vivo upon intratumor injection of miR-19a-5p, highlighting the potential role of this miRNA in facilitating the crosstalk between tumor cells and macrophage polarization during BC [78]. Another study by Hong et al. identified that miR-204-5p regulates the expression of critical cytokines and genes associated with the immune pathway, thus facilitating TME remodeling and reprogramming via shifting myeloid and lymphoid cell populations during BC. Overexpression of this miRNA had a significant correlation with a reduction in the number of macrophages, MDSCs, and NK cells and an increased number of Tregs, CD4+, and CD8+ T cells in the TME, suggesting its essential role in regulating the crosstalk, between tumor and immune cells in the TME [79].
In contrast, the miR-23a/27a/24-2 cluster was identified to modulate macrophage polarization and thus contribute to BC progression. They identified a decreased expression of miR-23a/27a/24-2 cluster in TAMs of BC patients. Further, their findings suggested the existence of a double feedback loop, including the miR-23a-27a-24-2 cluster and critical regulators of pro- (M1) and anti-inflammatory (M2) phenotypes of TAMs, and eventually promoted tumor progression [80]. Another study found that miR-155 modulates the antitumor response in varied immune subpopulations in BC. Knockdown of miR-155 in myeloid cells in a spontaneous BC mice model substantially promoted the tumor growth via impairing TAM activation by reducing the expression of activation markers and increasing the generation of pro-tumor cytokines, thus inducing M2-polarization of TAMs and resulting in an immune suppressive microenvironment [81]. A study identified overexpression of miR-223 in IL-4-activated macrophages, and miR-223 was observed to be transferred to BC cells via exosomes. The silencing of this miRNA in IL-4+ macrophages was observed to decrease BC invasion in vitro. Their findings suggested that miR-223 enhances the invasiveness of BC cells via the Mef2c-β-catenin pathway, thus serving as an oncogene [82] (Fig. 3A). Together, these studies suggested the importance of TAM-secreted miRNAs in colonization and progression of BC in bone. A better understanding of the interaction between the TAMs and BC cells via miRNAs will assist in developing novel therapeutics to combat BC.
TME-resident cells secreted miRNAs and their role in regulating BC progression and metastasis. Bone TME comprises many cells, such as TAMs, CAFs, TADCs, TAEs, and TAOBs, that secrete miRNAs and regulate BC invasion, homing, and progression in the bone. Differential expression of miRNAs could influence the differentiation, activation, and function of TME-resident cells and regulate BC pathogenesis and metastasis. A TAMs, B CAFs, C MDSCs, D TADCs, E TAOBs, and F TAOCs
Cancer-associated fibroblasts (CAFs)
CAFs are the predominant components of the TME. Besides offering physical support for tumor cells, they exhibit a context-dependent role via stimulating and hindering tumorigenesis. Numerous studies have suggested CAFs as critical regulators of antitumor response, thus highlighting their candidature in cancer immunotherapy [83, 84]. A study reported on the role of tumor-inducing role of hyperactive MAPK signaling (hMAPK)-stimulated miRNAs in regulating BC pathogenesis. Their findings suggested that miR-221/miR-222 was overexpressed in conditional medium (CM) from CAFs, which in turn was identified to repress ER expression in BCs. Interestingly, this miRNA-facilitated ER repression was observed to be specific to CM from basal BC-obtained CAFs [85]. Supporting this, another study reported on the upregulated expression of miR-222 in CAFs, compared to normal fibroblasts (NFs) in breast tissues. This miR-222 was observed to directly target Lamin B Receptor (LBR), and its overexpression or LBR silencing was observed to induce CAF characteristics in NFs. Further, CM from these CAFs was identified to promote BC cell migration and invasion, thus suggesting the oncogenic role of miR-222 [86]. Likewise, Donnarumma et al. reported the exosome-mediated transfer of miRNAs from CAFs to BC cells and suggested their oncogenic role in regulating the aggressive phenotype of BC. They observed an increased expression of miR-21, − 143, and − 378e in CAF-derived exosomes, which in turn was identified to increase the aggressive potential of BC cells via promoting stemness characteristics, EMT, and anchorage-independent growth [87].
In contrast, Ansari et al. identified a reduced expression of miR-146b-5p in CAFs from BC patients compared to their counterparts in normal tissues from the ipsilateral breast. Their findings suggested that increasing the endogenous level of this miRNA in active stromal fibroblasts might suppress the pro-EMT and metastatic effects in BC cells [88]. Similarly, another study reported the downregulated expression of the miR-200s family (miR-200a, miR-200b, miR-200c, and miR-141) in activated CAFs compared to NFs. They found that NFs with reduced expression of the miR-200s family exhibited traits of activated CAFs, such as promoted migration and invasion. Their results suggested a reduced invasion and migration of BC cells upon co-culture with miR-200s overexpressing CAFs compared to control CAFs. Together, their findings indicated the usefulness of miR-200s in hindering the transformation of NFs to CAFs in BC therapeutics [89] (Fig. 3B). Recently, anti-CAF immunotherapies are gaining considerable interest in developing therapeutics for cancer. However, more studies on deciphering the exact role of CAF-secreted miRNAs in regulating bone metastasis are required to develop novel and effective anti-CAF immunotherapies to treat solid tumors, including BC. Figure 3 illustrates the functional significance of TME-resident cell-secreted miRNAs in regulating BC progression and bone metastasis.
Myeloid-derived suppressor cells (MDSCs)
MDSCs are a heterogeneous group of immature myeloid cells that could modulate immune responses. Specifically in cancers, MDSCs are aberrantly generated and recruited to TME to assist in the formation of an immune suppressive TME that promotes tumor immune evasion [90]. Recently, studies have focused on the role of MDSCs and miRNAs in regulating TME. For instance, Deng et al. reported on the role of MDSC-derived miR-126 in regulating breast tumorigenesis in response to doxorubicin (DOX) treatment. Their results suggested that DOX treatment activated MDSCs and IL-13+ Th2 cells. DOX-induced MDSCs were observed to stimulate BC metastasis via miR-126-facilitated stimulation of IL-13+ Th2 cells. Further, IL-13 secreted from the activated IL-13+ Th2 cells stimulates the generation of DOX-MDSC and MDSC-derived exosomal release of miR-126a, thus forming a positive feedback loop, indicating the role of this miRNA in conferring chemoresistance in BC cells (Fig. 3C) [91]. Thus, the exosomal release of miRNAs via exosomes would facilitate the interaction between MDSCs and tumor cells. However, minimal studies reported the role of MDSC-derived miRNA in modulating TME. More experimental and clinical findings are necessary to understand the underlying mechanism for developing MDSC-based therapeutics.
Tumor-associated dendritic cells (TADCs)
Dendritic cells (DCs) are antigen-presenting cells (APCs) that exclusively function in the activation of naive T cells via presenting antigens to them. While the DCs function by mediating antitumor response, cancer cells secrete factors that hinder the differentiation of DCs and their potential to activate immune response [92, 93]. Studies have reported that DCs interact with and alter the TME via releasing miRNAs [94, 95]. In a study, overexpression of miR-22 was observed to inhibit the antitumor effect exhibited by DCs. MiR-22 serves as an endogenous tumor-promoting factor of DCs that could target p38, further downregulate the expression of IL-6, and subsequently impede the development of Th17 in TME. These results collectively suggested the positive influence of miR-22 inhibitors to enhance the function of DCs in cancer immunotherapy [96].
In contrast, a recent study identified a downregulated expression of miR-5119 in splenic DCs from BC-bearing mice in vivo. DCs engineered to overexpress miR-5119 were observed to target and reduce the expression of PD-L1 and inhibited the exhaustion of T cells in mice bearing BC homografts and restored the function of exhausted CD8+ T cells in vitro and in vivo. Their findings suggested the possibility of utilizing miR-5119 overexpressing DCs as an immunotherapy for the treatment of BC [97] (Fig. 3D). These studies demonstrated the regulatory function of DC-secreted miRNAs in influencing bone metastasis of BC, highlighting the candidature of miRNA/DC-based immunotherapy as a novel therapeutic for BC treatment. Nevertheless, more experimental and clinical findings are required for clinical applications.
Tumor-associated endothelial cells (TAEs)
TAEs are one of the essential components of TME that exhibits multifaceted functions directly impacting immune response to tumors. TAEs were observed to build a barrier against immune-stimulatory cells, thus impeding anti-cancer immunity via endothelial energy [98]. These cells depict a broad range of intra-and inter-tumoral heterogeneity associated with tumor progression and metastasis [99]. Studies have reported on the role of miRNAs in regulating tumor angiogenesis via the regulation of tumor cells, TAEs, and other components of TME [100]. For example, a study identified the expression of miR-126 and its host gene EGFL7 specifically in human umbilical vascular endothelial cells (HUVECs) via northern blot and RT-qPCR analyses. In contrast, the expression of miR-126 was downregulated in human breast tumors. Further, this miRNA was observed to target VEGFA and PIK3R2, and its overexpression reduced the VEGF/PI3K/AKT signaling cascade activity in BC cells [101]. This research finding suggested the role of miRNA in facilitating the interaction between TAEs and BC cells during breast tumorigenesis.
Regulatory T cells (Tregs)
Tregs constitute an important subclass of T lymphocytes that play a critical role in regulating immune responses and have the potential to suppress inflammatory responses [102]. In BC patients, an increased number of Tregs was associated with poor prognosis [103]. Studies have identified the role of miRNAs in regulating the T cell polarization towards Tregs, highlighting the importance of miRNAs in influencing the function of Tregs during BC [104, 105]. A study reported on the role of miR-448 in hindering the immune escape of BC via targeting Indoleamine 2, 3-dioxygenase (IDO), which impeded the T cell immunity via facilitating the differentiation and maturation of Treg. They identified a negative correlation between the expression of a long ncRNA (lncRNA) SNHG1 and miR-448 in CD4+ tumor-infiltrating lymphocytes (TILs). LncRNA SNHG1 was observed to sponge miR-448, preventing it from binding to its target IDO. Further, silencing of SNHG1 was observed to inhibit the differentiation of Treg cells via increasing the expression of miR-448 and reducing IDO level, thereby hindering the immune escape of BC cells [106]. Another study by Li et al. suggested a downregulated expression of miR-568 during Treg activation. Overexpression of miR-568 was observed to target NFAT5 and inhibited the generation of IL-10 and TGF-β, thus reducing the proliferation of Treg cells. Their findings suggested the possibility of utilizing miR-568 as a candidate to prevent immune evasion of cancers [107].
In an analogous study, miR-126 was overexpressed in mouse and human Tregs. The silencing of miR-126 was identified to increase the expression of its target, p85β. It subsequently modified the activation of the PI3K/Akt cascade, which remains critical for the decreased induction and immune suppressive functions of Tregs [108]. Similarly, upregulated expression of miR-21 was reported in the Tregs of BC patient tissues. miR-21 was observed to directly target PTEN (phosphatase and tensin homolog deleted on chromosome ten) and subsequently modify the activating the Akt pathway, thus favoring the proliferation of Tregs [109]. These findings suggested the role of miR-126 and miR-21 in regulating the induction and suppressive functions of Tregs via modulating PI3K/Akt cascade, thus highlighting the candidature of this miRNA in cancer therapeutics. A deeper understanding of miRNAs secreted by Tregs in influencing TME during BC might aid in developing novel Treg-based immunotherapies against BC in the future.
Mesenchymal stem cells (MSCs)
MSCs are multipotent cells that migrate to tumor niches, facilitating tumor development and progression [110]. MSCs get recruited to the TME in response to tumor stimuli, where they differentiate into CAFs, thereby supporting tumor progression [111]. In a study, MSC-derived exosomes enriched with miR-16, which could target VEGF, partially induced an anti-angiogenic effect in BC [112]. A study found an upregulated expression of miR-100 in MSC-secreted exosomes, and its transfer to BC cells resulted in a decreased expression of vascular endothelial growth factor (VEGF) via the mTOR/HIF-1α signaling axis. Further, their results suggested that MSC-exosome-mediated downregulation of VEGF could negatively influence the vascular behavior of endothelial cells in vitro [113]. However, more experimental and clinical findings are needed to decipher the complete regulatory mechanism.
Bone cells secreted miRNAs and bone metastasis of BC
Tumor-associated osteoblasts (TAOBs)
MSC-derived osteoblasts, which generate hydroxyapatite crystals, help to mineralize the matrix by acting as bone-forming cells. The homeostatic systems are hijacked by tumor cells that have spread throughout BME, homing to OBs and upsetting bone homeostasis [114]. Specifically, osteolytic BCs with increased expression of pERK1/2 were observed to mediate osteoblastic ERK1/2 activation at the BC–bone interface and impede bone homeostasis [115]. Multiple studies have demonstrated the importance of miRNAs in regulating the function of OBs in the TME [116]. In a study, miR-218 was identified to promote osteoblast differentiation via targeting Wnt signaling inhibitors, including DKK2, SFRP2, and SOST. Further, their results suggested the oncogenic role of miR-218 in BC cells via attenuating Wnt signaling inhibition. Their data together indicated that miR-218 regulates a Wnt signaling loop positively, which promotes osteoblast differentiation and simultaneously stimulates the expression of osteogenic genes in BC genes, thus favoring the homing of BC cells in bone [117]. Another study identified an increased expression of miR-320a and miR-193b in TAOB-derived exosomes compared to normal OBs. In vivo, mice co-administered with TAOBs and human BC cells showed slower tumor growth and reduced tumor size compared to mice administered with normal OBs and human BC cells or BC cells alone (Fig. 3E). Thus, their findings suggested that TAOBs serve as a reservoir of essential factors including miRNAs, in BC bone metastasis [118]. Together, these studies suggested the critical role of TAOB-secreted miRNAs in influencing BC bone metastasis.
Tumor-associated osteoclasts (TAOCs)
Bone metastasis of BC is osteolytic in nature, and hence the role of tumor altered osteoclasts (OCs) in mediating bone metastatic BC is increasingly investigated till date. Metastatic BC cells typically trigger increased OC-mediated bone resorption, causing osteolytic lesions and increased bone damage [119]. A study reported on the role of OC-secreted miR-214-3p in regulating bone metastasis of BC. They identified an increased expression of miR-214-3p in OCs and observed that a gradual increase in the expression of this miRNA is associated with a reduction in the levels of TRAF3 protein in RAW 264.7 cells during RANKL-stimulated osteoclastogenesis. Their findings suggested that miR-214-3p directly targets TRAF3 and ultimately promotes osteolytic bone metastasis of BC. Further, they identified an upregulated expression of miR-214-3p in bone fracture specimens from osteolytic bone metastatic BC patients when compared to BC patients without osteolytic bone metastasis and yet more increased expression of this miRNA when compared to cancer-free bone fracture specimens [120] (Fig. 3F). In addition, their earlier findings highlighted the exosome-mediated secretion of miR-214-3p to the TME in the bone [121], via which miR-214-3p might facilitate osteolytic bone metastasis via increasing BC cell dissemination in vivo [122]. Table 2 provides the detail on the role of the TME-resident immune cells secreted miRNAs on tumorigenesis.
Concluding remarks and future perspectives
BC is one of the leading causes of mortality among females globally. Skeletal metastasis is the most serious condition in BC patients with no curative remedies available. Till date, increasing studies have reported on the role of tumor in regulating TME during bone metastasis. However, studies focusing on the function of TME-resident cells in regulating tumor remain limited. Multiple findings from past decade report on the role of miRNAs in modulating various intricate cancerous networks, highlighting their importance in regulating malignancy. Besides their direct influence on BC, these miRNAs were also observed to be dysregulated in multiple cells of the TME, where they might exhibit opposing functions. More studies on understanding the complex crosstalk between the TME-resident cells and tumor cells might help us in advancing the candidature of miRNAs toward therapeutics. Hence, it is essential to comprehensively delineate the complex function of a potential miRNA to evaluate its regulatory role in TME and acquire insights regarding the cell and context-dependent role of miRNAs. Understanding the molecular function of TME-secreted miRNAs in influencing tumor progression remains indispensable for developing effective therapies against BC. Though many miRNAs were reported to regulate BC, their efficacy in clinical settings remains to be determined. Several factors limit the utility of miRNAs in clinical settings. Significant challenges associated with translating miRNAs to therapeutics include high heterogeneity of methodologies, lack of controls, poor experimental design, reduced sample size, and lack of reproducibility. Developing approaches for specific targeting of dysregulated miRNAs in TME-resident cells might prove helpful in reviving their antitumor function and augment therapeutic response. Combinatorial strategies with miRNA therapeutics have been demonstrated to improve treatment efficacy and reduce therapeutic resistance in pre-clinical trials, thus opening newer avenues for therapies against BC.
Abbreviations
- BC:
-
Breast cancer
- BME:
-
Bone microenvironment
- TME:
-
Tumor microenvironment
- TAM:
-
Tumor-associated macrophages
- TNF-α:
-
Tumor necrosis factor-alpha
- ECM:
-
Extracellular matrix
- EMT:
-
Epithelial mesenchymal transition
- CTC:
-
Circulating tumor cell
- IL:
-
Interleukin
- CAF:
-
Cancer-associated fibroblast
- MARCKS:
-
Myristoylated alanine-rich C-kinase substrate
References
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics. CA: A Cancer J Clin. 2022;72(1):7–33.
Jiang X, Chen G, Sun L, Liu C, Zhang Y, Liu M, Liu C. Characteristics and survival in bone metastatic breast cancer patients with different hormone receptor status: A population-based cohort study. Front Oncol. 2022;12: 977226.
Farach-Carson MC, Lin SH, Nalty T, Satcher RL. Sex Differences and Bone Metastases of Breast, Lung, and Prostate Cancers: Do Bone Homing Cancers Favor Feminized Bone Marrow? Front Oncol. 2017;7:163.
Shao H, Varamini P. Breast Cancer Bone Metastasis: A Narrative Review of Emerging Targeted Drug Delivery Systems. Cells. 2022;11(3):388.
Xiao Y, Yu D. Tumor microenvironment as a therapeutic target in cancer. Pharmacol Ther. 2021;221: 107753.
Baghban R, Roshangar L, Jahanban-Esfahlan R, Seidi K, Ebrahimi-Kalan A, Jaymand M, Kolahian S, Javaheri T, Zare P. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun Signal. 2020;18(1):59.
Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14(7):399–416.
Pan Y, Yu Y, Wang X, Zhang T. Tumor-associated macrophages in tumor immunity. Front Immunol. 2020;11: 583084.
Feng Y, Ye Z, Song F, He Y, Liu J. The Role of TAMs in Tumor Microenvironment and New Research Progress. Stem Cells Int. 2022;15:5775696.
Wilson C, Brown H, Holen I. The endocrine influence on the bone microenvironment in early breast cancer. Endocr Relat Cancer. 2016;23(12):R567–76.
Zarrer J, Haider MT, Smit DJ, Taipaleenmäki H. Pathological Crosstalk between Metastatic Breast Cancer Cells and the Bone Microenvironment. Biomolecules. 2020;10(2):337.
Kolb AD, Shupp AB, Mukhopadhyay D, Marini FC, Bussard KM. Osteoblasts are “educated” by crosstalk with metastatic breast cancer cells in the bone tumor microenvironment. Breast Cancer Res : BCR. 2019;21(1):31.
Haider MT, Smit DJ, Taipaleenmäki H. The Endosteal Niche in Breast Cancer Bone Metastasis. Front Oncol. 2020;10:335.
Wang WT, Han C, Sun YM, Chen TQ, Chen YQ. Noncoding RNAs in cancer therapy resistance and targeted drug development. J Hematol Oncol. 2019;12(1):55.
Dvorská D, Braný D, Ňachajová M, Halašová E, Danková Z. Breast Cancer and the Other Non-Coding RNAs. Int J Mol Sci. 2021;22(6):3280.
Prabhu KS, Raza A, Karedath T, Raza SS, Fathima H, Ahmed EI, Kuttikrishnan S, Therachiyil L, Kulinski M, Dermime S, Junejo K, Steinhoff M, Uddin S. Non-Coding RNAs as Regulators and Markers for Targeting of Breast Cancer and Cancer Stem Cells. Cancers. 2020;12(2):351.
Crudele F, Bianchi N, Reali E, Galasso M, Agnoletto C, Volinia S. The network of non-coding RNAs and their molecular targets in breast cancer. Mol Cancer. 2020;19(1):61.
Najminejad H, Farhadihosseinabadi B, Dabaghian M, Dezhkam A, Rigi Yousofabadi E, Najminejad R, Abdollahpour-Alitappeh M, Karimi MH, Bagheri N, Mahi-Birjand M, Ghasemi N, Mazaheri M, Kalantar SM, Seifalian A, Sheikhha MH. Key Regulatory miRNAs and their Interplay with Mechanosensing and Mechanotransduction Signaling Pathways in Breast Cancer Progression. Mol Cancer Res: MCR. 2020;18(8):1113–28.
Mansoori B, Baradaran B, Nazari A, Gaballu FA, Cho WC, Mansoori B. MicroRNAs in the cancer cell-to-cell communication: An insight into biological vehicles. Biomed Pharmacother Biomed Pharmacother. 2022;153:113449.
Dai R, Liu M, Xiang X, Xi Z, Xu H. Osteoblasts and osteoclasts: an important switch of tumour cell dormancy during bone metastasis. J Exp Clin Cancer Res: CR. 2022;41(1):316.
Clézardin P, Coleman R, Puppo M, Ottewell P, Bonnelye E, Paycha F, Confavreux CB, Holen I. Bone metastasis: mechanisms, therapies, and biomarkers. Physiol Rev. 2021;101(3):797–855.
Taverna S, Giusti I, D’Ascenzo S, Pizzorno L, Dolo V. Breast Cancer Derived Extracellular Vesicles in Bone Metastasis Induction and Their Clinical Implications as Biomarkers. Int J Mol Sci. 2020;21(10):3573.
Liu T, Han C, Wang S, Fang P, Ma Z, Xu L, Yin R. Cancer-associated fibroblasts: an emerging target of anti-cancer immunotherapy. J Hematol Oncol. 2019;12(1):86.
Yin Z, Dong C, Jiang K, Xu Z, Li R, Guo K, Shao S, Wang L. Heterogeneity of cancer-associated fibroblasts and roles in the progression, prognosis, and therapy of hepatocellular carcinoma. J Hematol Oncol. 2019;12:101.
Mun JY, Leem SH, Lee JH, Kim HS. Dual Relationship Between Stromal Cells and Immune Cells in the Tumor Microenvironment. Front Immunol. 2022;13: 864739.
Hu D, Li Z, Zheng B, Lin X, Pan Y, Gong P, Zhuo W, Hu Y, Chen C, Chen L, Zhou J, Wang L. Cancer-associated fibroblasts in breast cancer: Challenges and opportunities. Cancer Commun. 2022;42(5):401–34.
Li Y, Wang C, Huang T, Yu X, Tian B. The role of cancer-associated fibroblasts in breast cancer metastasis. Front Oncol. 2023;13:1194835.
Tan Q, Xu L, Zhang J, Ning L, Jiang Y, He T, Luo J, Chen J, L v, Q., Yang, X., & Xie, H. Breast cancer cells interact with tumor-derived extracellular matrix in a molecular subtype-specific manner. Biomater Adv. 2023;146:213301.
Takai K, Le A, Weaver VM, Werb Z. Targeting the cancer-associated fibroblasts as a treatment in triple-negative breast cancer. Oncotarget. 2016;7(50):82889–901.
Wang N, Liu W, Zheng Y, Wang S, Yang B, Li M, Song J, Zhang F, Zhang X, Wang Q, Wang Z. CXCL1 derived from tumor-associated macrophages promotes breast cancer metastasis via activating NF-κB/SOX4 signaling. Cell Death Dis. 2018;9(9):880.
Yang Q, Guo N, Zhou Y, Chen J, Wei Q, Han M. The role of tumor-associated macrophages (TAMs) in tumor progression and relevant advance in targeted therapy. Acta pharmaceuticaSinica B. 2020;10(11):2156–70.
Wu K, Lin K, Li X, Yuan X, Xu P, Ni P, Xu D. Redefining Tumor-Associated Macrophage Subpopulations and Functions in the Tumor Microenvironment. Front Immunol. 2020;11:1731.
Riobo-Del Galdo NA, Lara Montero Á, Wertheimer EV. Role of Hedgehog Signaling in Breast Cancer: Pathogenesis and Therapeutics. Cells. 2019;8(4):375.
Hida K, Maishi N, Annan DA, Hida Y. Contribution of Tumor Endothelial Cells in Cancer Progression. Int J Mol Sci. 2018;19(5):1272.
Venetis K, Piciotti R, Sajjadi E, Invernizzi M, Morganti S, Criscitiello C, Fusco N. Breast Cancer with Bone Metastasis: Molecular Insights and Clinical Management. Cells. 2021;10(6):1377.
Akech J, Wixted JJ, Bedard K, van der Deen M, Hussain S, Guise TA, van Wijnen AJ, Stein JL, Languino LR, Altieri DC, Pratap J, Keller E, Stein GS, Lian JB. Runx2 association with progression of prostate cancer in patients: mechanisms mediating bone osteolysis and osteoblastic metastatic lesions. Oncogene. 2010;29(6):811–21.
Shupp AB, Kolb AD, Mukhopadhyay D, Bussard KM. Cancer Metastases to Bone: Concepts, Mechanisms, and Interactions with Bone Osteoblasts. Cancers. 2018;10(6):182.
Göbel A, Dell’Endice S, Jaschke N, Pählig S, Shahid A, Hofbauer LC, Rachner TD. The Role of Inflammation in Breast and Prostate Cancer Metastasis to Bone. Int J Mol Sci. 2021;22(10):5078.
Xiang L, Gilkes DM. The Contribution of the Immune System in Bone Metastasis Pathogenesis. Int J Mol Sci. 2019;20(4):999.
Malla RR, Kiran P. Tumor microenvironment pathways: Cross regulation in breast cancer metastasis. Genes Dis. 2020;9(2):310–24.
Shinde AV, Humeres C, Frangogiannis NG. (2017) The role of α-smooth muscle actin in fibroblast-mediated matrix contraction and remodeling. Biochim Biophys Acta. 1863;1:298–309.
Erdogan B, Webb DJ. Cancer-associated fibroblasts modulate growth factor signaling and extracellular matrix remodeling to regulate tumor metastasis. Biochem Soc Trans. 2017;45(1):229–36.
Takahashi H, Sakakura K, Kudo T, Toyoda M, Kaira K, Oyama T, Chikamatsu K. Cancer-associated fibroblasts promote an immunosuppressive microenvironment through the induction and accumulation of protumoral macrophages. Oncotarget. 2017;8(5):8633–47.
Wei C, Yang C, Wang S, Shi D, Zhang C, Lin X, Liu Q, Dou R, Xiong B. Crosstalk between cancer cells and tumor associated macrophages is required for mesenchymal circulating tumor cell-mediated colorectal cancer metastasis. Mol Cancer. 2019;18(1):64.
Jiang M, Chen J, Zhang W, Zhang R, Ye Y, Liu P, Yu W, Wei F, Ren X, Yu J. Interleukin-6 Trans-Signaling Pathway Promotes Immunosuppressive Myeloid-Derived Suppressor Cells via Suppression of Suppressor of Cytokine Signaling 3 in Breast Cancer. Front Immunol. 2017;8:1840.
Li F, Zhao Y, Wei L, Li S, Liu J. Tumor-infiltrating Treg, MDSC, and IDO expression associated with outcomes of neoadjuvant chemotherapy of breast cancer. Cancer Biol Ther. 2018;19(8):695–705.
Cole K, Pravoverov K, Talmadge JE. Role of myeloid-derived suppressor cells in metastasis. Cancer Metastasis Rev. 2021;40(2):391–411.
Russo E, Laffranchi M, Tomaipitinca L, Del Prete A, Santoni A, Sozzani S, Bernardini G. NK Cell Anti-Tumor Surveillance in a Myeloid Cell-Shaped Environment. Front Immunol. 2021;12: 787116.
Yan M, Zheng M, Niu R, Yang X, Tian S, Fan L, Li Y, Zhang S. Roles of tumor-associated neutrophils in tumor metastasis and its clinical applications. Front Cell Dev Biol. 2022;10: 938289.
Rocamora-Reverte L, Melzer FL, Würzner R, Weinberger B. The Complex Role of Regulatory T Cells in Immunity and Aging. Front Immunol. 2021;11: 616949.
Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors in cancer therapy: a focus on T-regulatory cells. Immunol Cell Biol. 2018;96(1):21–33.
Huang Y, Wang H, Yue X, Li X. Bone serves as a transfer station for secondary dissemination of breast cancer. Bone Res. 2023;11(1):21.
Omokehinde T, Johnson RW. GP130 Cytokines in Breast Cancer and Bone. Cancers. 2020;12(2):326.
Sole C, Arnaiz E, Manterola L, Otaegui D, Lawrie CH. The circulating transcriptome as a source of cancer liquid biopsy biomarkers. Semin Cancer Biol. 2019;58:100–8.
Klinge CM. Non-Coding RNAs in Breast Cancer: Intracellular and Intercellular Communication. Non-coding RNA. 2018;4(4):40.
Zografos E, Zagouri F, Kalapanida D, Zakopoulou R, Kyriazoglou A, Apostolidou K, Gazouli M, Dimopoulos MA. Prognostic role of microRNAs in breast cancer: A systematic review. Oncotarget. 2019;10(67):7156–78.
Ghafouri-Fard S, KhanbabapourSasi A, Abak A, Shoorei H, Khoshkar A, Taheri M. Contribution of miRNAs in the Pathogenesis of Breast Cancer. Front Oncol. 2021;11: 768949.
Adinew GM, Taka E, Mendonca P, Messeha SS, Soliman KFA. The Anticancer Effects of Flavonoids through miRNAs Modulations in Triple-Negative Breast Cancer. Nutrients. 2021;13(4):1212.
Wieder R. Awakening of Dormant Breast Cancer Cells in the Bone Marrow. Cancers. 2023;15(11):3021.
Peng Y, Wang X, Guo Y, Peng F, Zheng N, He B, Ge H, Tao L, Wang Q. Pattern of cell-to-cell transfer of microRNA by gap junction and its effect on the proliferation of glioma cells. Cancer Sci. 2019;110(6):1947–58.
Zarzynska, J. M. (2017) The Role of Stem Cells in Breast Cancer. Breast Cancer - From Biology to Medicine. In Tech
Corcoran KE, Malhotra A, Molina CA, Rameshwar P. Stromal-derived factor-1alpha induces a non-canonical pathway to activate the endocrine-linked Tac1 gene in non-tumorigenic breast cells. J Mol Endocrinol. 2008;40(3):113–23.
Reddy BY, Greco SJ, Patel PS, Trzaska KA, Rameshwar P. RE-1-silencing transcription factor shows tumor-suppressor functions and negatively regulates the oncogenic TAC1 in breast cancer cells. Proc Natl Acad Sci USA. 2009;106(11):4408–13.
Lim PK, Bliss SA, Patel SA, Taborga M, Dave MA, Gregory LA, Greco SJ, Bryan M, Patel PS, Rameshwar P. Gap junction-mediated import of microRNA from bone marrow stromal cells can elicit cell cycle quiescence in breast cancer cells. Cancer Res. 2011;71(5):1550–60.
Bliss SA, Sinha G, Sandiford OA, Williams LM, Engelberth DJ, Guiro K, Isenalumhe LL, Greco SJ, Ayer S, Bryan M, Kumar R, Ponzio NM, Rameshwar P. Mesenchymal Stem Cell-Derived Exosomes Stimulate Cycling Quiescence and Early Breast Cancer Dormancy in Bone Marrow. Cancer Res. 2016;76(19):5832–44.
Kogure A, Kosaka N, Ochiya T. Cross-talk between cancer cells and their neighbors via miRNA in extracellular vesicles: an emerging player in cancer metastasis. J Biomed Sci. 2019;26(1):7.
Liu Q, Peng F, Chen J. The Role of Exosomal MicroRNAs in the Tumor Microenvironment of Breast Cancer. Int J Mol Sci. 2019;20(16):3884.
Asgarpour K, Shojaei Z, Amiri F, Ai J, Mahjoubin-Tehran M, Ghasemi F, ArefNezhad R, Hamblin MR, Mirzaei H. Exosomal microRNAs derived from mesenchymal stem cells: cell-to-cell messages. Cell Commun Signal. 2020;18(1):149. https://doi.org/10.1186/s12964-020-00650-6.
Shu L, Wang Z, Wang Q, Wang Y, Zhang X. Signature miRNAs in peripheral blood monocytes of patients with gastric or breast cancers. Open Biol. 2018;8(10):180051.
Tavakoli F, Sartakhti JS, Manshaei MH, Basanta D. Cancer immunoediting: A game theoretical approach. In Silico Biol. 2021;14(1–2):1–12.
Kim SK, Cho SW. The evasion mechanisms of cancer immunity and drug intervention in the tumor microenvironment. Front Pharmacol. 2022;13: 868695.
Xing Y, Wang Z, Lu Z, Xia J, Xie Z, Jiao M, Liu R, Chu Y. MicroRNAs: immune modulators in cancer immunotherapy. Immunother Adv. 2021;1(1):ltab006.
He Z, Zhang S. Tumor-Associated Macrophages and Their Functional Transformation in the Hypoxic Tumor Microenvironment. Front Immunol. 2021;12: 741305.
Gao J, Liang Y, Wang L. Shaping Polarization Of Tumor-Associated Macrophages In Cancer Immunotherapy. Front Immunol. 2022;13: 888713.
Petty AJ, Yang Y. Tumor-associated macrophages: implications in cancer immunotherapy. Immunotherapy. 2017;9(3):289–302.
Chatterjee B, Saha P, Bose S, Shukla D, Chatterjee N, Kumar S, Tripathi PP, Srivastava AK. MicroRNAs: As Critical Regulators of Tumor- Associated Macrophages. Int J Mol Sci. 2020;21(19):7117.
Boutilier AJ, Elsawa SF. Macrophage Polarization States in the Tumor Microenvironment. Int J Mol Sci. 2021;22(13):6995.
Yang J, Zhang Z, Chen C, Liu Y, Si Q, Chuang TH, Li N, Gomez-Cabrero A, Reisfeld RA, Xiang R, Luo Y. MicroRNA-19a-3p inhibits breast cancer progression and metastasis by inducing macrophage polarization through downregulated expression of Fra-1 proto-oncogene. Oncogene. 2014;33(23):3014–23.
Hong BS, Ryu HS, Kim N, Kim J, Lee E, Moon H, Kim KH, Jin MS, Kwon NH, Kim S, Kim D, Chung DH, Jeong K, Kim K, Kim KY, Lee HB, Han W, Yun J, Kim JI, Noh DY, Moon HG. Tumor Suppressor miRNA-204-5p Regulates Growth, Metastasis, and Immune Microenvironment Remodeling in Breast Cancer. Cancer Res. 2019;79(7):1520–34.
Ma S, Liu M, Xu Z, Li Y, Guo H, Ge Y, Liu Y, Zheng D, Shi J. A double feedback loop mediated by microRNA-23a/27a/24–2 regulates M1 versus M2 macrophage polarization and thus regulates cancer progression. Oncotarget. 2016;7(12):13502–19.
Zonari E, Pucci F, Saini M, Mazzieri R, Politi LS, Gentner B, Naldini L. A role for miR-155 in enabling tumor-infiltrating innate immune cells to mount effective antitumor responses in mice. Blood. 2013;122(2):243–52.
Kwon Y, Kim M, Kim Y, Jung HS, Jeoung D. Exosomal MicroRNAs as Mediators of Cellular Interactions Between Cancer Cells and Macrophages. Front Immunol. 2020;11:1167.
Chen X, Song E. Turning foes to friends: targeting cancer-associated fibroblasts. Nat Rev Drug Discov. 2019;18(2):99–115.
Ziani L, Chouaib S, Thiery J. Alteration of the Antitumor Immune Response by Cancer-Associated Fibroblasts. Front Immunol. 2018;9:414.
Shah SH, Miller P, Garcia-Contreras M, Ao Z, Machlin L, Issa E, El-Ashry D. Hierarchical paracrine interaction of breast cancer associated fibroblasts with cancer cells via hMAPK-microRNAs to drive ER-negative breast cancer phenotype. Cancer Biol Ther. 2015;16(11):1671–81.
Chatterjee A, Jana S, Chatterjee S, Wastall LM, Mandal G, Nargis N, Roy H, Hughes TA, Bhattacharyya A. MicroRNA-222 reprogrammed cancer-associated fibroblasts enhance growth and metastasis of breast cancer. Br J Cancer. 2019;121(8):679–89.
Donnarumma E, Fiore D, Nappa M, Roscigno G, Adamo A, Iaboni M, Russo V, Affinito A, Puoti I, Quintavalle C, Rienzo A, Piscuoglio S, Thomas R, Condorelli G. Cancer-associated fibroblasts release exosomal microRNAs that dictate an aggressive phenotype in breast cancer. Oncotarget. 2017;8(12):19592–608.
Al-Ansari MM, Aboussekhra A. miR-146b-5p mediates p16-dependent repression of IL-6 and suppresses paracrine procarcinogenic effects of breast stromal fibroblasts. Oncotarget. 2015;6(30):30006–16.
Tang X, Hou Y, Yang G, Wang X, Tang S, Du YE, Yang L, Yu T, Zhang H, Zhou M, Wen S, Xu L, Liu M. Stromal miR-200s contribute to breast cancer cell invasion through CAF activation and ECM remodeling. Cell Death Differ. 2016;23(1):132–45.
Dysthe M, Parihar R. Myeloid-Derived Suppressor Cells in the Tumor Microenvironment. Adv Exp Med Biol. 2020;1224:117–40.
Deng Z, Rong Y, Teng Y, Zhuang X, Samykutty A, Mu J, Zhang L, Cao P, Yan J, Miller D, Zhang HG. Exosomes miR-126a released from MDSC induced by DOX treatment promotes lung metastasis. Oncogene. 2017;36(5):639–51.
Song L, Dong G, Guo L, Graves DT. The function of dendritic cells in modulating the host response. Mol Oral Microbiol. 2018;33(1):13–21.
Del Prete A, Salvi V, Soriani A, Laffranchi M, Sozio F, Bosisio D, Sozzani S. Dendritic cell subsets in cancer immunity and tumor antigen sensing. Cell Mol Immunol. 2023;20(5):432–47.
Lee SS, Cheah YK. The Interplay between MicroRNAs and Cellular Components of Tumour Microenvironment (TME) on Non-Small-Cell Lung Cancer (NSCLC) Progression. J Immunol Res. 2019;2019:3046379.
Scalavino V, Liso M, Serino G. Role of microRNAs in the Regulation of Dendritic Cell Generation and Function. Int J Mol Sci. 2020;21(4):1319.
Liang X, Liu Y, Mei S, Zhang M, Xin J, Zhang Y, Yang R. MicroRNA-22 impairs anti-tumor ability of dendritic cells by targeting p38. PLoS ONE. 2015;10(3): e0121510.
Zhang M, Shi Y, Zhang Y, Wang Y, Alotaibi F, Qiu L, Wang H, Peng S, Liu Y, Li Q, Gao D, Wang Z, Yuan K, Dou FF, Koropatnick J, Xiong J, Min W. miRNA-5119 regulates immune checkpoints in dendritic cells to enhance breast cancer immunotherapy. Cancer Immunol, Immunother: CII. 2020;69(6):951–67.
De Sanctis F, Ugel S, Facciponte J, Facciabene A. The dark side of tumor-associated endothelial cells. Semin Immunol. 2018;35:35–47.
Maishi N, Annan DA, Kikuchi H, Hida Y, Hida K. Tumor Endothelial Heterogeneity in Cancer Progression. Cancers. 2019;11(10):1511.
Soheilifar MH, Masoudi-Khoram N, Madadi S, Nobari S, Maadi H, Keshmiri Neghab H, Amini R, Pishnamazi M. Angioregulatory microRNAs in breast cancer: Molecular mechanistic basis and implications for therapeutic strategies. J Adv Res. 2021;37:235–53.
Zhu N, Zhang D, Xie H, Zhou Z, Chen H, Hu T, Bai Y, Shen Y, Yuan W, Jing Q, Qin Y. Endothelial-specific intron-derived miR-126 is down-regulated in human breast cancer and targets both VEGFA and PIK3R2. Mol Cell Biochem. 2011;351(1–2):157–64.
Okeke EB, Uzonna JE. The Pivotal Role of Regulatory T Cells in the Regulation of Innate Immune Cells. Front Immunol. 2019;10:680.
Shan F, Somasundaram A, Bruno TC, Workman CJ, Vignali DAA. Therapeutic targeting of regulatory T cells in cancer. Trends Cancer. 2022;8(11):944–61.
Kos K, de Visser KE. The Multifaceted Role of Regulatory T Cells in Breast Cancer. Annu Rev Cancer Biol. 2021;5:291–310.
Soheilifar MH, Vaseghi H, Seif F, Ariana M, Ghorbanifar S, Habibi N, Papari Barjasteh F, Pornour M. Concomitant overexpression of mir-182-5p and mir-182-3p raises the possibility of IL-17-producing Treg formation in breast cancer by targeting CD3d, ITK, FOXO1, and NFATs: A meta-analysis and experimental study. Cancer Sci. 2021;112(2):589–603.
Pei X, Wang X, Li H. LncRNA SNHG1 regulates the differentiation of Treg cells and affects the immune escape of breast cancer via regulating miR-448/IDO. Int J Biol Macromol. 2018;118(Pt A):24–30.
Rodríguez-Galán A, Fernández-Messina L, Sánchez-Madrid F. Control of Immunoregulatory Molecules by miRNAs in T Cell Activation. Front Immunol. 2018;9:2148.
Hippen KL, Loschi M, Nicholls J, MacDonald KPA, Blazar BR. Effects of MicroRNA on Regulatory T Cells and Implications for Adoptive Cellular Therapy to Ameliorate Graft-versus-Host Disease. Front Immunol. 2018;9:57.
Hu Y, Wang C, Li Y, Zhao J, Chen C, Zhou Y, Tao Y, Guo M, Qin N, Ren T, Wen Z, Xu L. MiR-21 controls in situ expansion of CCR6+ regulatory T cells through PTEN/AKT pathway in breast cancer. Immunol Cell Biol. 2015;93(8):753–64.
Xuan X, Tian C, Zhao M, Sun Y, Huang C. Mesenchymal stem cells in cancer progression and anticancer therapeutic resistance. Cancer Cell Int. 2021;21(1):595.
Li X, Fan Q, Peng X, Yang S, Wei S, Liu J, Yang L, Li H. Mesenchymal/stromal stem cells: necessary factors in tumour progression. Cell Death Discov. 2022;8(1):333.
Wang N, Pei B, Yuan X, Yi C, Wiredu Ocansey DK, Qian H, Mao F. Emerging roles of mesenchymal stem cell-derived exosomes in gastrointestinal cancers. Front Bioeng Biotechnol. 2022;10:1019459.
Pakravan K, Babashah S, Sadeghizadeh M, Mowla SJ, Mossahebi-Mohammadi M, Ataei F, Dana N, Javan M. MicroRNA-100 shuttled by mesenchymal stem cell-derived exosomes suppresses in vitro angiogenesis through modulating the mTOR/HIF-1α/VEGF signaling axis in breast cancer cells. Cell Oncol (Dordr). 2017;40(5):457–70.
Hughes AM, Kolb AD, Shupp AB, Shine KM, Bussard KM. Printing the Pathway Forward in Bone Metastatic Cancer Research: Applications of 3D Engineered Models and Bioprinted Scaffolds to Recapitulate the Bone-Tumor Niche. Cancers. 2021;13(3):507.
Back J, Nguyen MN, Li L, Lee S, Lee I, Chen F, Gillinov L, Chung YH, Alder KD, Kwon HK, Yu KE, Dussik CM, Hao Z, Flores MJ, Kim Y, Ibe IK, Munger AM, Seo SW, Lee FY. Inflammatory conversion of quiescent osteoblasts by metastatic breast cancer cells through pERK1/2 aggravates cancer-induced bone destruction. Bone Res. 2021;9(1):43.
Haider MT, Smit DJ, Taipaleenmäki H. MicroRNAs: Emerging Regulators of Metastatic Bone Disease in Breast Cancer. Cancers. 2022;14(3):729.
Liu X, Cao M, Palomares M, Wu X, Li A, Yan W, Fong MY, Chan WC, Wang SE. Metastatic breast cancer cells overexpress and secrete miR-218 to regulate type I collagen deposition by osteoblasts. Breast Cancer Res: BCR. 2018;20(1):127.
Marini, F., Chifman, J., Tooze, J., Gomez-Manzano, C., & Bussard, K. M. Osteoblasts are educated into a tumor-associated stromal cell by disseminated breast cancer cells and mediate breast cancer cell proliferation in the bone microenvironment [abstract]. In: Proceedings of the American Association for Cancer Research Annual Meeting 2017; 2017 Apr 1–5; Washington, DC. Philadelphia (PA): AACR; Cancer Res 2017; 77(13 Suppl): Abstract nr 5890.
Puppo M, Taipaleenmäki H, Hesse E, Clézardin P. Non-coding RNAs in bone remodelling and bone metastasis: Mechanisms of action and translational relevance. Br J Pharmacol. 2021;178(9):1936–54.
Liu J, Dang L, Wu X, Li D, Ren Q, Lu A, Zhang G. microRNA-Mediated Regulation of Bone Remodeling: A Brief Review. JBMR plus. 2019;3(9): e10213.
Li D, Liu J, Guo B, Liang C, Dang L, Lu C, He X, Cheung HY, Xu L, Lu C, He B, Liu B, Shaikh AB, Li F, Wang L, Yang Z, Au DW, Peng S, Zhang Z, Zhang BT, Pan X, Qian A, Shang P, Xiao L, Wong CK, Xu J, Bian Z, Liang Z, Guo DA, Zhu H, Tan W, Lu A, Zhang G. Osteoclast-derived exosomal miR-214-3p inhibits osteoblastic bone formation. Nat Commun. 2016;7:10872.
Orso F, Quirico L, Virga F, Penna E, Dettori D, Cimino D, Coppo R, Grassi E, Elia AR, Brusa D, Deaglio S, Brizzi MF, Stadler MB, Provero P, Caselle M, Taverna D. miR-214 and miR-148b Targeting Inhibits Dissemination of Melanoma and Breast Cancer. Can Res. 2016;76(17):5151–62.
Wang W, Liu Y, Guo J, He H, Mi X, Chen C, Xie J, Wang S, Wu P, Cao F, Bai L, Si Q, Xiang R, Luo Y. miR-100 maintains phenotype of tumor-associated macrophages by targeting mTOR to promote tumor metastasis via Stat5a/IL-1ra pathway in mouse breast cancer. Oncogenesis. 2018;7(12):97.
Zhong Y, Yi C. MicroRNA-720 suppresses M2 macrophage polarization by targeting GATA3. Biosci Rep. 2016;36(4): e00363.
Li Y, Zhao L, Shi B, Ma S, Xu Z, Ge Y, Liu Y, Zheng D, Shi J. Functions of miR-146a and miR-222 in Tumor-associated Macrophages in Breast Cancer. Sci Rep. 2015;5:18648.
Acknowledgements
This work was supported by the Indian Council of Medical Research, India [No.5/13/05/2019/NCD-III to N.S.] and the Department of Science &Technology [DST/INSPIRE Fellowships: 2019/IF190170 to R. L. A. and 2021/IF210073 to I. S.].
Funding
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this study.
Author information
Authors and Affiliations
Contributions
RLA wrote the manuscript. IS helped in preparing the figures. NS designed and reviewed the content and secured funding for this research.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Akshaya, R.L., Saranya, I. & Selvamurugan, N. MicroRNAs mediated interaction of tumor microenvironment cells with breast cancer cells during bone metastasis. Breast Cancer 30, 910–925 (2023). https://doi.org/10.1007/s12282-023-01491-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-023-01491-0