Abstract
Regulated cell death (RCD) pathways, such as pyroptosis, apoptosis, and necroptosis, are essential for maintaining the body’s balance, defending against pathogens, and eliminating abnormal cells that could lead to diseases like cancer. Although these pathways operate through distinct mechanisms, recent genetic and pharmacological studies have shown that they can interact and influence each other. The concept of “PANoptosis” has emerged, highlighting the interplay between pyroptosis, apoptosis, and necroptosis, especially during cellular responses to infections. This article provides a concise overview of PANoptosis and its molecular mechanisms, exploring its implications in various diseases. The review focuses on the extensive interactions among different RCD pathways, emphasizing the role of PANoptosis in infections, cytokine storms, inflammatory diseases, and cancer. Understanding PANoptosis is crucial for developing novel treatments for conditions involving infections, sterile inflammations, and cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cell death, an evolutionarily conserved and fundamental process for every aspect of organismal survival including development and homeostasis, is also considered as a part of innate immune responses. It regulates various physiological processes during diverse challenges, ranging from physical perturbations and microbial invasions. Cell death can be classified into regulated or non-regulated types, depending on the nature of the triggering signals and the array of molecules involved (Peng et al. 2022).
Regulated cell death (RCD) is a form of cell death that is intensively regulated by intracellular signaling pathways, whereas non-RCD occurs accidentally as a consequence of unexpected injury to cells (Tang et al. 2019). Furthermore, RCD can be categorized into lytic and non-lytic cell death based on morphological features and molecular mechanisms (Lee et al. 2023; Tweedell et al. 2023). Apoptosis is classified as non-lytic cell death as the cell retains its membrane integrity while undergoing well-defined cellular changes including chromatin condensation, nuclear fragmentation, shrinkage of cytoplasmic contents, and membrane blebbing (Elmore 2007). Conversely, pyroptosis and necroptosis represent conventional lytic cell death that features the elimination of cell remnants and the release of potent inflammatory mediators (Shi et al. 2015; Peng et al. 2022). Consequently, apoptosis is typically regarded as “immunologically silent,” whereas pyroptosis and necroptosis are considered as more “aggressive” types of cell death (Shi et al. 2015). The orchestration of these regulated cellular demises is underpinned by an intricate ensemble of molecules. Previously, apoptosis, necroptosis, and pyroptosis were considered as discrete phenomena in the landscape of cellular fate. However, as our understanding of RCD continues to evolve, revealing an expanding spectrum of sterile and non-sterile triggers, a crescendo of evidence has emerged, suggesting intricate interplays among these pathways, which conceptualized a unified mode of cell death: PANopsosis (Wang and Kanneganti 2021). PANoptosis has been associated with a range of diseases such as pathogen infections, autoinflammatory disorders, cytokine storms, and cancer (Karki and Kanneganti 2021). In this review, we aim to provide an overview mainly focusing on the mechanism of PANoptosis. We then elucidate the fundamental components of PANoptosis and explore the latest discoveries regarding the molecular and functional interplay between different RCD pathways that have contributed to conceiving the concept of PANoptosis. Finally, we discuss the pathological contexts related to these RCDs and discuss potential therapeutic approaches to adjust them.
Mechanism of pyroptosis, apoptosis, and necroptosis in RCD
Pyroptosis
Pyroptosis is induced by the formation of pores on the plasma membrane, mediated by members of the gasdermin family such as gasdermin D (GSDMD) or gasdermin E (GSDME) (Wang et al. 2017). This process is facilitated by inflammatory caspases, mainly CASP1, which trigger the activation of GSDMD and the release of pro-inflammatory cytokines such as mature interleukin (IL)-1β and IL-18 (Wang and Kanneganti 2021). This process is initiated by the recognition of damage-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs) by cytosolic pattern recognition receptors (PRRs). Notable PRRs include nucleotide-binding oligomerization domain-like receptor (NLR)-family, pyrin domain (PYD)-containing 1 (NLRP1), NLR-family, PYD-containing 3 (NLRP3), NLR-family apoptosis inhibitory protein–NLR-family, caspase activation and recruitment domain (CARD)-containing 4 (NAIP–NLRC4), absent in melanoma 2 (AIM2), and pyrin. Activation of these sensors leads to their oligomerization, often followed by the recruitment of apoptosis-associated speck-like protein containing a CARD (ASC) through homotypic PYD-PYD or CARD-CARD interactions, resulting in the formation of an inflammasome complex. CASP1 is subsequently recruited to the inflammasome complex through homotypic CARD-CARD interactions. In certain inflammasome sensors, like NLRP1 and NAIP/NLRC4, the presence of CARD domains allows for direct interaction between the multimerized inflammasome sensor and CASP1. Once integrated into the inflammasome complex, CASP1 undergoes auto-proteolysis and becomes activated (Christgen and Kanneganti 2020). Following activation, CASP1 acts on its other substrates and processes the immature forms of IL-1β and IL-18 into their mature forms.
Additionally, active CASP1 cleaves the executor of pyroptotic cell death, GSDMD. Cleavage of GSDMD results in the formation of membrane pores via the N-terminus of the protein, enabling the release of mature IL-1β and IL-18. Recent discoveries have unveiled that fine-tuned, reversible palmitoylation regulates GSDMD cleavage and pore formation, suggesting additional regulatory events in inflammasome-mediated pyroptosis (Du et al. 2024; Zhang et al. 2024). CASP11 in mice and CASP 4/5 in humans can also cleave GSDMD (He et al. 2015; Kayagaki et al. 2015; Sborgi et al. 2016). However, these caspases are not known to process inflammatory cytokines to their mature forms. In a non-canonical model of inflammasome activation, bacterial lipopolysaccharide (LPS) binding to CASP11 and CASP 4/5 triggers caspase activation and subsequent GSDMD cleavage (Fig. 1).
Inflammasome activation and pyroptosis. Specific sensors detect pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs). These sensors then assemble to form an inflammasome such as NLRP1, NLRP3, NAIP-NLRC4, AIM2, and pyrin. Active caspase (CASP) 1 plays a crucial role by cleaving pro-IL-18 and pro-IL-1β into their mature forms. Additionally, active CASP1, along with CASP11 in mice or CASP4/5 in humans and CASP-8, cleaves gasdermin D (GSDMD), releasing its N-terminal region. This liberated N-terminal region undergoes oligomerization, ultimately leading to the formation of pores in the plasma membrane. Moreover, active CASP8, through the activation of CASP3, also cleaves gasdermin E (GSDME). The formation of these pores in the plasma membrane results in cell lysis, leading to the release of intracellular contents, as well as the inflammatory cytokines IL-18 and IL-1β
Apoptosis
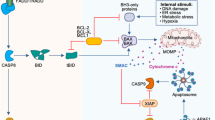
Apoptosis is executed through the sequential activation of initiating caspases of extrinsic or intrinsic apoptosis. Extrinsic apoptosis is regulated by cell-surface death receptor signaling, the formation of the death-inducing signaling complex (DISC), and the activation of CASP8 or CASP10. Activation of death receptors such as Fas (CD95) and TNF receptor (TNFR) occurs through ligand binding. This activation leads to the recruitment of cytoplasmic adapter protein, Fas-associated death domain (FADD), followed by the recruitment of CASP8/10. Active CASP8/10 then cleave and activate their substrates, including executioner CASP3 and CASP7 (Christgen et al. 2022). In intrinsic apoptosis, internal stimuli, such as DNA damage agents and stress, trigger the activation of pro-apoptotic Bcl-2 family proteins, specifically BAX, BAK, and BID. This activation leads to a process called mitochondrial outer membrane permeabilization (MOMP), causing the release of cytochrome c (Cyt c) into the cytosol (Chipuk et al. 2006). Subsequently, Cyt c binds to apoptotic peptidase activating factor 1 (APAF1) monomers, prompting the formation of the apoptosome through the oligomerization of APAF1. The binding of Cyt c to APAF1 results in a structural change that facilitates the exchange of ADP for ATP, a crucial step for the complete assembly of the apoptosome (Cecconi 1999). These combined actions of Cyt c binding and nucleotide exchange lead to the creation of a death platform, consisting of a central hub formed by seven components and an auxiliary, asymmetric wheel with CARDs extending outward (Taylor et al. 2008). The intrinsic initiator caspase, caspase-9, becomes activated as it is recruited to this extended surface through homotypic interactions between CARDs. CASP9 then cleaves and activates executioners such as CASP3 and CASP7 (Elmore 2007; Li et al. 2017) (Fig. 2).
Molecular mechanisms of extrinsic and intrinsic apoptosis. Extrinsic apoptosis: Binding of a ligand to one of several death receptors, such as Fas or TNF receptor (TNFR), initiates the recruitment of Fas-associated death domain (FADD), followed by the activation of CASP8 or CASP10. Intrinsic apoptosis: DNA damaging agents and stress disrupt the mitochondrial outer membrane and increase mitochondrial outer membrane permeabilization (MOMP). The cytochrome C released from the mitochondria interacts with apoptotic peptidase activating factor 1 (APAF1) to form apoptosome, which activates initiator CASP9. The two pathways converge at activation of the effector caspases (CASP3 and CASP7)
Necroptosis
Necroptosis is elicited by the development of pores with mixed lineage kinase domain-like pseudokinase (MLKL), resulting from the phosphorylation of MLKL controlled by the receptor-interacting protein kinase 1 (RIPK1) and RIPK3 signaling pathway (Murphy et al. 2013). Necroptosis is often described as a ‘back-up’ form of cell death, as many instances of necroptosis stem from the initiation of apoptosis but its failure to proceed. Consequently, pathogens that inhibit apoptosis execution such as CASP8 can trigger necroptosis, leading to the lytic nature of this programmed cell death pathway. Given that this cell death pathway primarily activates in pathological contexts, there is a growing interest in the development of therapeutics targeting necroptosis.
The absence or inhibition of CASP8 results in the activation of RIPK1 and the formation of the necrosome (Zhao et al. 2012). Active RIPK1 then initiates the phosphorylation and activation of RIPK3, which subsequently phosphorylates the pseudokinase MLKL. Once phosphorylated, MLKL translocates to the cellular membrane and forms pores by assembling its helical N-terminal domains. This process leads to a form of cell death characterized by cellular membrane disruption known as necroptosis (Sun et al. 2012; Cai et al. 2014). The result of necroptosis is the release of intracellular contents, including damage-associated molecular patterns (DAMPs), which, in turn, trigger inflammatory responses.
Necroptosis can occur through two mechanisms: a RIPK1-dependent pathway and a RIPK1-independent pathway. In the latter case, it involves interactions between RIP homotypic interaction motif (RHIM) domains of RIPK3 and other proteins that also contain RHIM domains, such as TIR domain-containing adapter-inducing interferon-β (TRIF) (Kaiser et al. 2013), and Z-DNA binding protein 1 (ZBP1) (Kesavardhana et al. 2017), also known as DAI (Fig. 3). Some chemicals and chemotherapeutic drugs such as arsenite or cisplatin induce stress granule, where ZBP1 is localized and interacts with RIPK3 to activate MLKLs leading to necroptosis (Szczerba et al. 2023; Yang et al. 2023). Moreover, it has been reported that extracellular osmotic stress could directly activate RIPK3 to undergo necroptosis upon change of cytosolic pH regulated by NA+/H+ exchanger protein solute carrier family 9 member A1 (SLC9A1), independent of interaction through RHIM domain (Zhang et al. 2022). These studies expand our understanding of necroptosis from canonical RCD as summarized to RIPK1-RIPK3-MLKL axis under CASP inhibition, to the more comprehensive and physiologically relevant form of cell death.
Molecular mechanisms of necroptosis. CASP8 inhibited, RIPK1 and RIPK3 form necrosomes through homotypic interactions with RHIM, resulting in phosphorylation of MLKL. Phosphorylated MLKL undergoes oligomerization and induces membrane rupture in an RIPK1-dependent manner. Without RIPK1, the RHIM domains found in RIPK3 and other proteins containing RHIM domains like TRIF and ZBP1 interact, leading to necroptosis. The formation of pores in the plasma membrane results in cell lysis, leading to the release of intracellular contents, as well as damage-associated molecular patterns (DAMPs)
Interconnectedness of multiple cell death pathways
The interconnectedness of various cell death pathways, including pyroptosis, apoptosis, and necroptosis, has become increasingly evident in recent studies. Initially thought to be distinct, these pathways are now recognized to intersect and influence each other in intricate ways.
Crosstalk between pyroptosis and apoptosis
Pyroptosis, triggered by inflammatory caspases like CASP1 and CASP11, was originally associated with CASP1-dependent apoptosis-like RCD. However, new insights have shown that when macrophages lack GSDMD, a key executioner in pyroptosis and downstream molecules of CASP1 and CASP11, inflammasome activation can lead to CASP3-mediated RCD (Wang et al. 2017). With no GSDMD, CASP1 activation leads to RCD via the BH3 Interacting Domain Death Agonist (Bid)-CASP9-CASP3 pathway. It suggests that apoptosis triggered by inflammasome may be significant for neurons and mast cells because they have minimal or no GSDMD expression (Tsuchiya et al. 2019). Additionally, the activation of NLRP1b and NLRC4 enhances CASP8 activation in the absence of downstream inflammasome components. When WT macrophages are infected with Salmonella, CASP1, and CASP8 co-localize with ASC particles although CASP8 is not necessary to cause Salmonella-induced pyroptosis (Man et al. 2014). However, ASC co-localizes with FADD, an adaptor molecule for CASP8, to drive CASP8-dependent apoptosis in Casp1–/– cells upon NLRC4 inflammasome activation (Van Opdenbosch et al. 2017). Furthermore, AIM2 inflammasome triggers, Francisella infection or DNA electroporation, recruit and activate CASP8 through ASC, leading to CASP3 activation in Casp1–/– cells (Pierini et al. 2012; Sagulenko et al. 2013). Besides its traditional role in extrinsic apoptosis, CASP8 has been shown to affect inflammasome activation and pyroptosis. CASP8 enhances NLRP3 priming and post-translational modification, activating NLRP3 inflammasome (Gurung et al. 2014). In response to NLRP3 inflammasome activating triggers, CASP8 is recruited into the inflammasome complex along with its function in upregulating Nlrp3 and Il1b (Gurung et al. 2016). Furthermore, with TAK1 inhibition, CASP8 induces GSDMD cleavage and NLRP3 inflammasome activation (Orning et al. 2018). Consistent with the functions of CASP8, FADD also controls inflammasome activation and pyroptosis in macrophages treated with NLRP3 inflammasome triggers (Gurung et al. 2014). The crosstalk between pyroptosis and apoptosis is not limited to inflammasome triggers. During chemotherapy, CASP3 can induce pyroptosis by cleaving GSDME and may also cleave N-terminal GSDMD, reducing GSDMD-mediated pyroptosis (Taabazuing et al. 2017; Wang et al. 2017) (Fig. 1). Bile acid-driven APAF1 apoptosomes containing CASP11 promote CASP3 to be cleaved, which drives GSDME-dependent pyroptosis (Xu et al. 2021). These findings underscore the reciprocal regulation between pyroptosis and apoptosis.
Crosstalk between necroptosis and apoptosis
Relatively to other crosstalk mechanisms, the relationships between necroptosis and apoptosis, which regulate homeostasis, are more definitive than those with pyroptosis. Apoptotic CASP8 can prevent necroptosis during embryonic development as CASP8 deletion does not cause embryonic mortality without RIPK3 or MLKL (Kaiser et al. 2011; Dillon et al. 2012). RIPK1 inhibits CASP8-mediated apoptosis by regulating the transcription of pro-survival cellular FLICE-like inhibitory protein (cFLIP) (Dillon et al. 2012; Mifflin et al. 2020). Since RIPK1 has a role in both necroptosis and apoptosis, Ripk1–/– mice can only be rescued by deleting both CASP8 and RIPK3 during embryonic development (Dillon et al. 2014). When CASP8 is intact, RIPK1 deficiency leads to CASP8-mediated apoptosis, while co-deletion of RIPK1 and CASP8 drives susceptibility to RIPK1-independent necroptosis mediated by TRIF or IFN-driven RIPK3 activation (Dillon et al. 2014). These recent studies highlight the interconnectedness of apoptosis and necroptosis. Recently, the loss of enzymatic activity of CASP8 induces necroptosis, wherein further inhibition of necroptosis causes pyroptosis driven by NLRP3 inflammasome activation, implying that CASP8 serves as a regulator of three RCD pathways (Fritsch et al. 2019).
Crosstalk between pyroptosis and necroptosis
Studies are relatively limited to suggest a connection between pyroptosis and necroptosis. Pyroptosis generally occurs as a consequence of inflammasome activation. Various triggers, including NLRP3 sensing cellular perturbations, CASP11 recognizing LPS, AIM2 detecting dsDNA, and NLRC4 responding to flagellin and toxins from Salmonella Typhimurium, initiate pyroptosis. Similarly, cellular alterations during infection with Bacillus anthracis and Clostridium difficile activate NLRP1 and pyrin inflammasomes, respectively (Zheng et al. 2020a). Moreover, necroptosis, which has mostly been studied using tumor necrosis factor (TNF)-α in combination with zVAD-fmk (zVAD, a pan-caspase inhibitor), is initiated when TNF-α triggers necroptosis upon inhibition of CASP8 by zVAD (Wu et al. 2011). Certain viruses, such as human cytomegalovirus (CMV), murine CMV, and vaccinia virus encode viral inhibitors that counteract caspase activation, leading to necroptosis (Nailwal and Chan 2019).
Activation of NLRP3 inflammasome in cells in response to necroptosis triggers suggests a connection between pyroptosis and necroptosis. Upon caspase inhibition, toll-like receptor 3 (TLR3) signaling activates the NLRP3 inflammasome during necroptosis involving RIPK3 (Kang et al. 2015). NLRP3 inflammasome can be activated in a potassium efflux-dependent and independent manner (Munoz-Planillo et al. 2013; Gross et al. 2016). Necroptotic MLKL pores induce K+ efflux as well as the release of other DAMPs, which could cause NLRP3 inflammasome activation (Frank and Vince 2019). These studies shed light on the interactions between pyroptosis and necroptosis pathways.
PANoptosis
Cells get exposed to multiple ligands and inhibitors during infection with pathogens, resulting in diverse signaling pathways that culminate in various types of cell death. For instance, infection of macrophages with viruses such as influenza A virus (IAV), herpes simplex virus 1 (HSV1), and corona, bacteria such as Francisella, Yersinia, and Salmonella and fungi such as Candida and Aspergillus, induce pyroptosis, apoptosis, and necroptosis, accompanied by inflammatory cytokine release (Banoth et al. 2020; Malireddi et al. 2020a; Lee et al. 2021; Han et al. 2023). Furthermore, engaging multiple receptors through combinatorial triggers in macrophages can induce various forms of cell death, underscoring the complexity of physiological signaling pathways. This intricate interplay has been termed the PANoptosome complex, giving rise to the concept of PANoptosis as a multifaceted mode of cell death (Christgen et al. 2020a, b; Wang et al. 2022). An overview of the characteristics of RCD, including pyroptosis, apoptosis, necroptosis, and PANoptosis, is presented in Table 1. Recent studies have revealed that PANoptosis is implicated in a wide range of human diseases encompassing infections, cytokine release syndrome, sterile inflammatory diseases, and cancer, which are discussed in the following sections. Therefore, regulation of PANoptosis provides a significant therapeutic strategy to prevent or ameliorate those pathological conditions.
PANoptosis in infection
Invading pathogens and the diseases they cause are closely associated with PANoptosis, which can play either a detrimental or beneficial role. For instance, PANoptosis induced by SARS-CoV-2 infection has a deleterious effect concerning disease severity. During SARS-CoV-2 infection, elevated levels of inflammatory cytokines, particularly TNF-α and interferon-gamma (IFN-γ), synergistically trigger PANoptosis via the Janus kinase (JAK)-signal transducer and activator of transcription (STAT1)-interferon regulatory factor 1 (IRF1) axis (Karki et al. 2021a). This TNF-α and IFN-γ mediated PANoptosis is relevant to accelerating cytokine shock syndromes, resulting in extensive damage to internal organs and subsequent poor prognosis (Karki and Kanneganti 2021; Karki et al. 2021a).
Despite the general anti-viral properties of interferons, IFN therapy has demonstrated detrimental effects in patients with COVID-19 and animals challenged with SARS-CoV-2. Among IFN-stimulated genes (ISGs) upregulated by IFNs, the Z-DNA binding protein 1 (ZBP1) signaling pathway triggers PANoptosis in macrophages infected with β-coronaviruses. Notably, exogenous IFN treatment increased mortality in wild-type (WT) mice infected with β-coronaviruses including mouse hepatitis virus (MHV) and SARS-CoV-2, but not in Zbp1–/– mice. This suggests that ZBP1-mediated PANoptosis hampers the therapeutic efficacy of IFN in patients with COVID-19 (Karki et al. 2022).
PANoptosis can also exert beneficial effects in several pathogenic contexts. Infection of macrophages with pathogens such as IAV induces PANoptosis in macrophages in a ZBP1-dependent manner. When infected with IAV, CASP6 binds to RIPK3 and facilitates the interaction between ZBP1 and RIPK3, ultimately leading to ZBP1-dependent PANoptosis and contributing to host defense (Zheng et al. 2020b) (Fig. 4A). Similarly, HSV1 and Francisella novicida induces AIM2-dependent PANoptosis. Deletion of AIM2 has shown a significant increase in lethality compared to WT mice upon infection with HSV1 or Francisella novicida, underscoring the crucial role of AIM2-mediated PANoptosis in host defense. Additionally, pyrin and ZBP1 collectively mediate AIM2 responses, inducing inflammatory cell death and thus enhancing host defense against these pathogens (Lee et al. 2021) (Fig. 4B). Furthermore, infections of macrophages with bacteria such as Salmonella Typhimurium and Yersinia and fungi such as Candida albicans and Aspergillus fumigatus, induced PANoptosis with the release of inflammatory cytokines (Banoth et al. 2020; Christgen et al. 2020b, a; Malireddi et al. 2020a). Deleting key molecules involved in PANoptosis, including CASP1, CASP11, RIPK3, and CASP8, protects macrophages from cell death during S. Typhimurium infection. Interestingly, individual deletion of these cell death components does not fully prevent cell death, underscoring the critical role of PANoptosis in response to S. Typhimurium infection (Christgen et al. 2020b, a).
PANoptosome and PANoptosis. A Certain pathogens such as IAV, and other agents such as IFN plus KPT-330 induce ZBP1 PANoptosome assembly. ZBP1 senses IAV or endogenous Z-nucleic acids (Z-NA) to assemble ZBP1 PANoptosomes consisting of ZBP1 and other cell death molecules. CASP6 potentiates the interaction between RIPK3 and ZBP1. B AIM2 senses double-stranded DNA (dsDNA) during infections caused by HSV1 or Francisella, leading to the assembly of the AIM2 PANoptosome. C NLRP12 plays a crucial role in inducing lytic cell death in response to the combination of heme and PAMPs such as Pam3CSK4 and LPS. NLRP12 also forms a PANoptosome, serving as an integral component in this cellular process
Altogether, the impact of PANoptosis on the host can be either beneficial or detrimental, contingent upon the specific infection context, as summarized in Table 2. Further investigations are warranted to comprehensively understand this ambivalent nature and elucidate the underlying mechanisms, paving the way for novel therapeutic approaches.
PANoptosis in cytokine release syndrome
Cytokine release syndrome (CRS) is a severe systemic inflammatory condition characterized by the excessive release of cytokines and the overactivation of immune cells in response to various stimuli, including PAMPs or DAMPs (Karki and Kanneganti 2021). While cytokines and chemokines are essential for maintaining cellular homeostasis and combating infections (Tse and Takeuchi 2023), an uncontrolled and exaggerated release of these molecules can lead to cytokine storms, which can be life-threatening. Among pro-inflammatory cytokines, the synergism of TNF-α and IFN-γ induces PANoptosis in macrophages (Karki et al. 2021a). Notably, intraperitoneal injection of TNF-α and IFN-γ in mice results in death, reflecting the major symptoms of CRS observed in patients with conditions like COVID-19. TNF-α and IFN-γ stimulate the production of STAT1 and IRF1-dependent nitric oxide (NO), triggering CASP8 activation and leading to PANoptosis in murine macrophages (Karki et al. 2021a).
Additionally, while fever can confer beneficial effects during infection, heatstroke is linked to worse outcomes due to cytokine storms (Walter et al. 2016). The pathological responses to heatstroke are exacerbated by prior infections, potentially accelerating cell death (Han et al. 2024). Indeed, cells pre-infected with E. coli or C. rodentium or stimulated with LPS exhibit robust activation of the NLRP3 inflammasome and PANoptosis, mediated by CASP1, CASP8, RIPK3, and CASP11. Moreover, the absence of nerve injury-induced protein 1 (Ninjurin 1, NINJ1) reduces inflammatory cell death and lethality induced by heatstroke, highlighting the role of NINJ1 in PANoptosis, cytokine storm and heatstroke. Furthermore, NINJ1 expression is upregulated by both heatstroke and LPS, and its oligomerization depends on CASP8 and RIPK3, suggesting NINJ1 acts downstream of these molecules (Han et al. 2024). However, regarding the role of ZBP1 and MLKL in heat stress-induced cell death, there are conflicting findings (Yuan et al. 2022; Han et al. 2024).
The activation of these cell death pathways results in additional cytokines and alarmins, which act as warning signals to neighboring immune and stromal cells, intensifying cytokine production and contributing to the cytokine storm phenomenon (Karki and Kanneganti 2021). Understanding the mechanisms underlying abnormal cytokine release and the interplay between cell death pathways is crucial for developing innovative treatments for cytokine storm-related conditions.
Several mammalian proteins with RHIM domains, such as RIPK1, RIPK3, ZBP1, and TRIF, are involved in PANoptosis in response to cytokines, PAMPS, DAMPs, and inflammation, leading to CRS (Karki and Kanneganti 2021, 2023b). The functions of these proteins related to CRS have been studied in mice and humans. For instance, RIPK1 deficiency is highly lethal characterized by CRS with systemic inflammation, severe anemia, and neutrophilia. This fatal phenotype can be prevented by co-deleting CASP8 and RIPK3, indicating that inhibition of CASP8 and RIPK3-mediated inflammatory cell death is critical in controlling CRS and fatality (Karki and Kanneganti 2021).
RHIM proteins also play a pivotal role in triggering cell death pathways during microbial infections, just as they are implicated in sterile systemic inflammation, leading to CRS. Certain microbes, like Staphylococcus aureus (S. aureus) and Yersinia pestis, induce inflammatory cell death by releasing pathogen-associated molecular patterns (PAMPs) or toxins into host cells (Kitur et al. 2015; Malireddi et al. 2020b). For instance, S. aureus produces α-hemolysin and α-toxin (Hla), which prompt inflammatory cell death through the RIPK3 and NLRP3 signaling pathways. This leads to the release of numerous inflammatory cytokines and results in severe lung disease in mice (Kitur et al. 2015). Although most infections trigger similar cytokine responses, the precise reason why only certain bacteria or viruses can result in a cytokine storm remains elusive.
PANoptosis in inflammatory diseases
Multiple preclinical studies have indicated that PANoptosis is involved in sterile inflammatory disorders. For instance, Pstpip2cmo mice have a mutation causing severe inflammation in bones as well as in skin and foot, resembling the human condition of chronic recurrent multifocal osteomyelitis (Chitu et al. 2009). The osteomyelitis in Pstpip2cmo mice critically relies on IL-1β (Lukens et al. 2014). Combined deletion of CASP1, CASP8, and RIPK3–essential components for PANoptosis–prevents disease progression in these mice. Footpads of Pstpip2cmoCasp1–/–Casp8–/–Ripk3–/– mice showed reduced IL-1β processing compared to those in Pstpip2cmoCasp1–/– or Pstpip2cmoCasp8–/–Ripk3–/– mice, suggesting the compensatory roles of CASP1 and CASP8 in IL-1β processing (Lukens et al. 2014; Gurung et al. 2016). PANoptosis induced by ZBP1 might have a significant pathological role in inflammatory diseases caused by hyperproduction of interferons. Individuals afflicted by uncommon autoimmune disorders such as Aicardi-Goutières syndrome (AGS) have exhibited adenosine deaminase acting on RNA 1 (ADAR1) loss of function or melanoma differentiation-associated gene 5 (MDA5) gain of function mutations (Karki and Kanneganti 2023a). Moreover, loss of ADAR1p150 expression or mutations in the ADAR1 Zα domain contributes to interferonopathy, conditions observed in patients with AGS and bilateral striatal necrosis (Karki and Kanneganti 2023a). However, these conditions can be recovered by either the ZBP1 or the ZBP1 Zα domain removal, suggesting that ZBP1–mediated PANoptosis drives pathology in AGS caused by ADAR1 loss (Hubbard et al. 2022; Jiao et al. 2022). Indeed, the Zα domain of ADAR1 interacts with the counterpart domain of ZBP1 to prevent PANoptosis. In addition, ZBP1 deletion also mitigates severe bowel inflammation in intestinal epithelial cells lacking SET domain bifurcated histone lysine methyltransferase 1 (SETDB1) in mice (Wang et al. 2020). Furthermore, it has been reported that PANoptosis is associated with some pathologies of hemolytic diseases. NLRP12-triggered PANoptosis has been suggested to drive acute kidney injury and lethality in a hemolytic model. NLRP12 triggers PANoptosis in response to heme plus PAMPs. Importantly, NLRP12 expression is upregulated in diseases associated with hemolysis, as well as hemolytic diseases, such as β-thalassemia or sickle cell disease (SCD) (Sundaram et al. 2023) (Fig. 4C). PANoptosis has been observed in bone marrow of patients with myelodysplastic syndromes, which are thought to be caused by an inflammatory bone marrow microenvironment and cell death of hematopoietic stem/progenitor cells (Liu et al. 2024). The genes related to type I IFN and PANoptosis contribute to Sjogren’s syndrome, a systemic autoimmune disease (Yang et al. 2024). All the evidence suggests the potential of PANoptosis as a possible therapeutic target in inflammatory diseases.
PANoptosis and cancer
Cancer, which stands as one of the foremost global public health concerns, possesses two biological hallmarks; (1) unregulated proliferation and (2) resistance to cell death (Hanahan and Weinberg 2011). Previously, chemotherapeutics and radiotherapy were actively deployed to treat cancer, yet they yielded only minimal success within clinical settings. These approaches usually induce apoptosis to clear the cancerous cells. Furthermore, there are several approaches to treating cancer by targeting transcription factors (Park et al. 2023), histone deacetylase (Park et al. 2021b), tumor-derived metabolite (Byun 2023), developing antibody–drug conjugate (Maiti et al. 2023), NK cell-based cancer immunotherapy (Park et al. 2022), exosomes (Kim 2022), and natural product-based combination therapy (Park et al. 2021a). However, cancer cells tend to develop resistance to cell death induced by therapeutic agents in various ways (Malireddi et al. 2021). For instance, cancer cells exploit intrinsic anti-apoptotic signaling machinery to circumvent apoptotic cell death (Mohammad et al. 2015). Therefore, this limitation in curing highly resistant cancer could be surmounted by utilizing different modes of cell death other than apoptosis or, by even engaging all types of cell death simultaneously to effectively eradicate malignant cells. In this regard, PANoptosis may have great potential as an alternative detour for its very specific anticancer properties compared to pyroptosis and necroptosis, which are still highly context-dependent.
IRF1 and ZBP1-mediated cell death signaling pathways have provided a glimpse of the therapeutic potential of PANoptosis for cancer treatment. In a mouse model of melanoma, TNF-α and IFN-γ demonstrated a tumor-suppressive effect (Malireddi et al. 2021). TNF-α and IFN-γ induced PANoptosis relies on STAT1/IRF1 signaling axis, implying the role of IRF1 in tumor suppression (Malireddi et al. 2021). Indeed, Irf1–/– mice exhibited an increased tumor burden in the colon compared to WT counterparts, which could be attributed to defective PANoptosis in the absence of IRF1 (Karki et al. 2020). The immune regulator IRF1 has been reported to be involved in PANoptosis triggered by various agents, indicating that modulating the activation of molecules in the IRF1 pathway could aid in the treatment of cancer (Sharma et al. 2023). ZBP1-mediated PANoptosis is regulated by ADAR1 and is also associated with tumorigenesis, thereby giving insight into a novel anticancer drug strategy. The abrogation of ADAR1 elevates PANoptosis and suppresses melanoma and colon cancer growth in vivo, while ZBP1 deficiency rescues both tumor growth. ADAR1, which contains a Zα domain like ZBP1 does, can interact with ZBP1 via the Zα domain upon IFN treatment, leading to suppression of PANoptosis (Karki et al. 2021b). Overall, PANoptosis could be a prospective alternative to bypass tumor resistance against cell death.
Future perspective for therapeutic aspects
Blocking individual pathways for pyroptosis, apoptosis, or necroptosis may not be sufficient to inhibit cell death during infection. However, simultaneous inhibition of upstream molecules like CASP8, RIPK3, and CASP1 effectively prevents cell death across different triggers. For instance, while the absence of individual CASP1, CASP3, CASP7, or RIPK3 fails to suppress cell death, simultaneous deletion of CASP1, CASP8, and RIPK3 provides extensive protection against NLRP12-dependent cell death triggered by heme plus PAMPs, implying that NLRP12 drives PANoptosis (Sundaram et al. 2023). Similarly, individual loss of cell death components such as CASP1, CASP3, CASP7, CASP11, RIPK3, and MLKL does not abrogate cell death induced by heat stress plus LPS, whereas combined loss of CASP1, CASP11, CASP8, and RIPK3 provides significant protection (Han et al. 2024). Thus, targeting PANoptosis holds promise as a crucial therapeutic strategy for the treatment of numerous diseases. To block PANoptosis, the neutralization of TNF-α and IFN-γ, which are PANoptosis triggers during SARS-CoV-2 infection, could provide therapeutic potential against COVID-19 and CRS, as it can prevent inflammatory cell death and its ensuing harmful consequences. Indeed, inhibition of TNF-α and IFN-γ by neutralizing antibodies ameliorated the mortality rate of mice infected with SARS-CoV-2 (Karki and Kanneganti 2021; Karki et al. 2021a). Moreover, targeting specific PANoptosis components, such as GSDMD, caspases, or RIPK3, provides the potential for mitigating the pathological consequences of excessive cell death during infection. For cancer therapy, when ADAR1 is sequestered in the nucleus, ZBP1 can interact with RIPK3 via the RHIM domain to promote potent cell death signaling cascades (Karki et al. 2021b; Karki and Kanneganti 2023a). This possibly explains the failure of early clinical efforts to employ IFNs as an anticancer therapeutic approach (Einhorn and Grander 1996). IFN-γ upregulates both ZBP1 and ADAR1 expressions, leaving ADAR1 to impede antitumor cell death by ZBP1. Thus, the sequestration of ADAR1 in the nucleus using nuclear export inhibitors (NEIs) such as KPT-330 along with IFN could markedly augment the effect of IFN therapies. In the mouse melanoma model, the combination of IFN-γ and KPT-330 has shown a dramatic tumor regression by ZBP1-dependent PANoptosis, suggesting a practicable approach to overcome the barriers of the current strategy (Karki et al. 2021b) (Fig. 4A). Continued research promises to deepen our understanding of detrimental or beneficial effects of PANoptosis and further establish novel ways to harness it for potential therapeutic strategy.
Conclusion
Our review highlights the intricate landscape of RCD, emphasizing the interconnectedness and cross-regulation among various cell death pathways, including pyroptosis, apoptosis, and necroptosis. Through the lens of evolving research, we have explored the emerging concept of PANoptosis, a unified mode of cell death orchestrated by a complex interplay of molecular signaling pathways. PANoptosis represents a multifaceted response to diverse challenges, ranging from pathogen infections to sterile inflammatory conditions. In infectious contexts, pathogens such as viruses, bacteria, and fungi can induce PANoptosis in host cells, contributing either to host defense or pathogenesis, depending on the specific infection scenario. Furthermore, PANoptosis plays a pivotal role in inflammatory diseases, including cytokine release syndrome and autoimmune disorders, where excessive or dysregulated cell death exacerbates tissue damage and systemic inflammation. Moreover, the therapeutic implications of PANoptosis extend to cancer, offering a promising avenue for novel treatment strategies. By targeting key molecules involved in PANoptotic signaling pathways, such as IRF1 and ZBP1, it may be possible to exploit PANoptosis as a means to overcome cancer cell resistance and enhance tumor suppression.
While significant progress has been made in understanding the mechanisms and implications of RCD, including the recently proposed PANoptosis, several limitations persist in current research efforts. Addressing these limitations and charting new directions for future research is crucial for advancing our knowledge and potentially harnessing these processes for therapeutic benefit. (1) Our understanding of the intricate molecular mechanisms governing RCD pathways remains incomplete. There is a need to delve deeper into the signaling cascades, protein interactions, and regulatory networks involved in apoptosis, pyroptosis, necroptosis, and PANoptosis. (2) One of the challenges in studying RCD pathways is their context dependency and specificity. The outcomes of these processes can vary depending on cell type, physiological conditions, and the nature of the stimulus. Future research should aim to unravel the context-dependent factors that influence the activation and regulation of RCD pathways, thereby providing insights into their diverse roles in health and disease. (3) While RCD pathways hold promise as therapeutic targets for various diseases, identifying specific molecules or targets within these pathways remains a challenge. Moreover, the development of therapeutic interventions targeting RCD must consider the potential side effects and unintended consequences of modulating these pathways. Future research should focus on the identification, validation, and preclinical testing of novel therapeutic targets and strategies aimed at modulating RCD for therapeutic benefit. (4) Translating findings from basic research on RCD pathways into clinically applicable therapies requires robust biomarkers and diagnostic tools.
Overall, our comprehensive analysis underscores the importance of understanding PANoptosis in various pathological contexts and its potential as a therapeutic target. Further research into the molecular mechanisms and regulatory networks governing PANoptosis is essential for unlocking its full therapeutic potential and advancing precision medicine approaches for a wide range of diseases.
Data availability
This paper is a review paper and does not contain any data.
References
Banoth B, Tuladhar S, Karki R, Sharma BR, Briard B, Kesavardhana S, Burton A, Kanneganti TD (2020) ZBP1 promotes fungi-induced inflammasome activation and pyroptosis, apoptosis, and necroptosis (PANoptosis). J Biol Chem 295:18276–18283. https://doi.org/10.1074/jbc.RA120.015924
Byun JK (2023) Tumor lactic acid: a potential target for cancer therapy. Arch Pharm Res 46:90–110. https://doi.org/10.1007/s12272-023-01431-8
Cai Z, Jitkaew S, Zhao J, Chiang HC, Choksi S, Liu J, Ward Y, Wu LG, Liu ZG (2014) Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat Cell Biol 16:55–65. https://doi.org/10.1038/ncb2883
Cecconi F (1999) Apaf1 and the apoptotic machinery. Cell Death Differ 6:1087–1098. https://doi.org/10.1038/sj.cdd.4400602
Chipuk JE, Bouchier-Hayes L, Green DR (2006) Mitochondrial outer membrane permeabilization during apoptosis: the innocent bystander scenario. Cell Death Differ 13:1396–1402. https://doi.org/10.1038/sj.cdd.4401963
Chitu V, Ferguson PJ, De Bruijn R, Schlueter AJ, Ochoa LA, Waldschmidt TJ, Yeung YG, Stanley ER (2009) Primed innate immunity leads to autoinflammatory disease in PSTPIP2-deficient cmo mice. Blood 114:2497–2505. https://doi.org/10.1182/blood-2009-02-204925
Christgen S, Kanneganti TD (2020) Inflammasomes and the fine line between defense and disease. Curr Opin Immunol 62:39–44. https://doi.org/10.1016/j.coi.2019.11.007
Christgen S, Zheng M, Kesavardhana S, Karki R, Malireddi RKS, Banoth B, Place DE, Briard B, Sharma BR, Tuladhar S, Samir P, Burton A, Kanneganti TD (2020a) Identification of the PANoptosome: a molecular platform triggering pyroptosis, apoptosis, and necroptosis (PANoptosis). Front Cell Infect Microbiol 10:237. https://doi.org/10.3389/fcimb.2020.00237
Christgen S, Zheng M, Kesavardhana S, Karki R, Malireddi RKS, Banoth B, Place DE, Briard B, Sharma BR, Tuladhar S, Samir P, Burton A, Kanneganti TD (2020b) Identification of the PANoptosome: a molecular platform triggering pyroptosis, apoptosis, and necroptosis (PANoptosis). Front Cell Infect Microbiol. https://doi.org/10.3389/fcimb.2020.00237
Christgen S, Tweedell RE, Kanneganti TD (2022) Programming inflammatory cell death for therapy. Pharmacol Ther 232:108010. https://doi.org/10.1016/j.pharmthera.2021.108010
Dillon CP, Oberst A, Weinlich R, Janke LJ, Kang TB, Ben-Moshe T, Mak TW, Wallach D, Green DR (2012) Survival function of the FADD-CASPASE-8-cFLIP(L) complex. Cell Rep 1:401–407. https://doi.org/10.1016/j.celrep.2012.03.010
Dillon CP, Weinlich R, Rodriguez DA, Cripps JG, Quarato G, Gurung P, Verbist KC, Brewer TL, Llambi F, Gong YN, Janke LJ, Kelliher MA, Kanneganti TD, Green DR (2014) RIPK1 blocks early postnatal lethality mediated by caspase-8 and RIPK3. Cell 157:1189–1202. https://doi.org/10.1016/j.cell.2014.04.018
Du G, Healy LB, David L, Walker C, El-Baba TJ, Lutomski CA, Goh B, Gu B, Pi X, Devant P, Fontana P, Dong Y, Ma X, Miao R, Balasubramanian A, Puthenveetil R, Banerjee A, Luo HR, Kagan JC, Oh SF, Robinson CV, Lieberman J, Wu H (2024) ROS-dependent S-palmitoylation activates cleaved and intact gasdermin D. Nature 630:437–446. https://doi.org/10.1038/s41586-024-07373-5
Einhorn S, Grander D (1996) Why do so many cancer patients fail to respond to interferon therapy? J Interferon Cytokine Res 16:275–281. https://doi.org/10.1089/jir.1996.16.275
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35:495–516. https://doi.org/10.1080/01926230701320337
Frank D, Vince JE (2019) Pyroptosis versus necroptosis: similarities, differences, and crosstalk. Cell Death Differ 26:99–114. https://doi.org/10.1038/s41418-018-0212-6
Fritsch M, Gunther SD, Schwarzer R, Albert MC, Schorn F, Werthenbach JP, Schiffmann LM, Stair N, Stocks H, Seeger JM, Lamkanfi M, Kronke M, Pasparakis M, Kashkar H (2019) Caspase-8 is the molecular switch for apoptosis, necroptosis and pyroptosis. Nature 575:683–687. https://doi.org/10.1038/s41586-019-1770-6
Gross CJ, Mishra R, Schneider KS, Medard G, Wettmarshausen J, Dittlein DC, Shi H, Gorka O, Koenig PA, Fromm S, Magnani G, Cikovic T, Hartjes L, Smollich J, AaB R, Cooper MA, Schmidt-Supprian M, Schuster M, Schroder K, Broz P, Traidl-Hoffmann C, Beutler B, Kuster B, Ruland J, Schneider S, Perocchi F, Gross O (2016) K(+) efflux-independent NLRP3 inflammasome activation by small molecules targeting mitochondria. Immunity 45:761–773. https://doi.org/10.1016/j.immuni.2016.08.010
Gurung P, Anand PK, Malireddi RK, Vande Walle L, Van Opdenbosch N, Dillon CP, Weinlich R, Green DR, Lamkanfi M, Kanneganti TD (2014) FADD and caspase-8 mediate priming and activation of the canonical and noncanonical Nlrp3 inflammasomes. J Immunol 192:1835–1846. https://doi.org/10.4049/jimmunol.1302839
Gurung P, Burton A, Kanneganti TD (2016) NLRP3 inflammasome plays a redundant role with caspase 8 to promote IL-1beta-mediated osteomyelitis. Proc Natl Acad Sci U S A 113:4452–4457. https://doi.org/10.1073/pnas.1601636113
Han JH, Tweedell RE, Kanneganti TD (2023) Evaluation of caspase activation to assess innate immune cell death. J vis Exp. https://doi.org/10.3791/64308
Han JH, Karki R, Malireddi RKS, Mall R, Sarkar R, Sharma BR, Klein J, Berns H, Pisharath H, Pruett-Miller SM, Bae SJ, Kanneganti TD (2024) NINJ1 mediates inflammatory cell death, PANoptosis, and lethality during infection conditions and heat stress. Nat Commun 15:1739. https://doi.org/10.1038/s41467-024-45466-x
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674. https://doi.org/10.1016/j.cell.2011.02.013
He WT, Wan H, Hu L, Chen P, Wang X, Huang Z, Yang ZH, Zhong CQ, Han J (2015) Gasdermin D is an executor of pyroptosis and required for interleukin-1beta secretion. Cell Res 25:1285–1298. https://doi.org/10.1038/cr.2015.139
Hubbard NW, Ames JM, Maurano M, Chu LH, Somfleth KY, Gokhale NS, Werner M, Snyder JM, Lichauco K, Savan R, Stetson DB, Oberst A (2022) ADAR1 mutation causes ZBP1-dependent immunopathology. Nature 607:769–775. https://doi.org/10.1038/s41586-022-04896-7
Jiao H, Wachsmuth L, Wolf S, Lohmann J, Nagata M, Kaya GG, Oikonomou N, Kondylis V, Rogg M, Diebold M, Troder SE, Zevnik B, Prinz M, Schell C, Young GR, Kassiotis G, Pasparakis M (2022) ADAR1 averts fatal type I interferon induction by ZBP1. Nature 607:776–783. https://doi.org/10.1038/s41586-022-04878-9
Kaiser WJ, Upton JW, Long AB, Livingston-Rosanoff D, Daley-Bauer LP, Hakem R, Caspary T, Mocarski ES (2011) RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature 471:368–372. https://doi.org/10.1038/nature09857
Kaiser WJ, Sridharan H, Huang C, Mandal P, Upton JW, Gough PJ, Sehon CA, Marquis RW, Bertin J, Mocarski ES (2013) Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J Biol Chem 288:31268–31279. https://doi.org/10.1074/jbc.M113.462341
Kang S, Fernandes-Alnemri T, Rogers C, Mayes L, Wang Y, Dillon C, Roback L, Kaiser W, Oberst A, Sagara J, Fitzgerald KA, Green DR, Zhang J, Mocarski ES, Alnemri ES (2015) Caspase-8 scaffolding function and MLKL regulate NLRP3 inflammasome activation downstream of TLR3. Nat Commun 6:7515. https://doi.org/10.1038/ncomms8515
Karki R, Kanneganti TD (2021) The ‘cytokine storm’: molecular mechanisms and therapeutic prospects. Trends Immunol 42:681–705. https://doi.org/10.1016/j.it.2021.06.001
Karki R, Kanneganti TD (2023a) ADAR1 and ZBP1 in innate immunity, cell death, and disease. Trends Immunol 44:201–216. https://doi.org/10.1016/j.it.2023.01.001
Karki R, Kanneganti TD (2023b) PANoptosome signaling and therapeutic implications in infection: central role for ZBP1 to activate the inflammasome and PANoptosis. Curr Opin Immunol 83:102348. https://doi.org/10.1016/j.coi.2023.102348
Karki R, Sharma BR, Lee E, Banoth B, Malireddi RKS, Samir P, Tuladhar S, Mummareddy H, Burton AR, Vogel P, Kanneganti TD (2020) Interferon regulatory factor 1 regulates PANoptosis to prevent colorectal cancer. JCI Insight. https://doi.org/10.1172/jci.insight.136720
Karki R, Sharma BR, Tuladhar S, Williams EP, Zalduondo L, Samir P, Zheng M, Sundaram B, Banoth B, Malireddi RKS, Schreiner P, Neale G, Vogel P, Webby R, Jonsson CB, Kanneganti TD (2021a) Synergism of TNF-alpha and IFN-gamma triggers inflammatory cell death, tissue damage, and mortality in SARS-CoV-2 infection and cytokine shock syndromes. Cell 184(149–168):e117. https://doi.org/10.1016/j.cell.2020.11.025
Karki R, Sundaram B, Sharma BR, Lee S, Malireddi RKS, Nguyen LN, Christgen S, Zheng M, Wang Y, Samir P, Neale G, Vogel P, Kanneganti TD (2021b) ADAR1 restricts ZBP1-mediated immune response and PANoptosis to promote tumorigenesis. Cell Rep 37:109858. https://doi.org/10.1016/j.celrep.2021.109858
Karki R, Lee S, Mall R, Pandian N, Wang Y, Sharma BR, Malireddi RS, Yang D, Trifkovic S, Steele JA, Connelly JP, Vishwanath G, Sasikala M, Reddy DN, Vogel P, Pruett-Miller SM, Webby R, Jonsson CB, Kanneganti TD (2022) ZBP1-dependent inflammatory cell death, PANoptosis, and cytokine storm disrupt IFN therapeutic efficacy during coronavirus infection. Sci Immunol 7:6294. https://doi.org/10.1126/sciimmunol.abo6294
Kayagaki N, Stowe IB, Lee BL, O’rourke K, Anderson K, Warming S, Cuellar T, Haley B, Roose-Girma M, Phung QT, Liu PS, Lill JR, Li H, Wu J, Kummerfeld S, Zhang J, Lee WP, Snipas SJ, Salvesen GS, Morris LX, Fitzgerald L, Zhang Y, Bertram EM, Goodnow CC, Dixit VM (2015) Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526:666–671. https://doi.org/10.1038/nature15541
Kesavardhana S, Kuriakose T, Guy CS, Samir P, Malireddi RKS, Mishra A, Kanneganti TD (2017) ZBP1/DAI ubiquitination and sensing of influenza vRNPs activate programmed cell death. J Exp Med 214:2217–2229. https://doi.org/10.1084/jem.20170550
Kim SB (2022) Function and therapeutic development of exosomes for cancer therapy. Arch Pharm Res 45:295–308. https://doi.org/10.1007/s12272-022-01387-1
Kitur K, Parker D, Nieto P, Ahn DS, Cohen TS, Chung S, Wachtel S, Bueno S, Prince A (2015) Toxin-induced necroptosis is a major mechanism of Staphylococcus aureus lung damage. PLoS Pathog 11:e1004820. https://doi.org/10.1371/journal.ppat.1004820
Lee S, Karki R, Wang Y, Nguyen LN, Kalathur RC, Kanneganti TD (2021) AIM2 forms a complex with pyrin and ZBP1 to drive PANoptosis and host defence. Nature 597:415–419. https://doi.org/10.1038/s41586-021-03875-8
Lee E, Song CH, Bae SJ, Ha KT, Karki R (2023) Regulated cell death pathways and their roles in homeostasis, infection, inflammation, and tumorigenesis. Exp Mol Med 55:1632–1643. https://doi.org/10.1038/s12276-023-01069-y
Li P, Zhou L, Zhao T, Liu X, Zhang P, Liu Y, Zheng X, Li Q (2017) Caspase-9: structure, mechanisms and clinical application. Oncotarget 8:23996–24008. https://doi.org/10.18632/oncotarget.15098
Liu S, Joshi K, Zhang L, Li W, Mack R, Runde A, Hagen PA, Barton K, Breslin P, Ji HL, Kini AR, Wang Z, Zhang J (2024) Caspase 8 deletion causes infection/inflammation-induced bone marrow failure and MDS-like disease in mice. Cell Death Dis 15:278. https://doi.org/10.1038/s41419-024-06660-3
Lukens JR, Gurung P, Vogel P, Johnson GR, Carter RA, Mcgoldrick DJ, Bandi SR, Calabrese CR, Vande Walle L, Lamkanfi M, Kanneganti TD (2014) Dietary modulation of the microbiome affects autoinflammatory disease. Nature 516:246–249. https://doi.org/10.1038/nature13788
Maiti R, Patel B, Patel N, Patel M, Patel A, Dhanesha N (2023) Antibody drug conjugates as targeted cancer therapy: past development, present challenges and future opportunities. Arch Pharm Res 46:361–388. https://doi.org/10.1007/s12272-023-01447-0
Malireddi RKS, Gurung P, Kesavardhana S, Samir P, Burton A, Mummareddy H, Vogel P, Pelletier S, Burgula S, Kanneganti TD (2020ba) Innate immune priming in the absence of TAK1 drives RIPK1 kinase activity–independent pyroptosis, apoptosis, necroptosis, and inflammatory disease. J Exp Med. https://doi.org/10.1084/jem.20191644
Malireddi RKS, Gurung P, Kesavardhana S, Samir P, Burton A, Mummareddy H, Vogel P, Pelletier S, Burgula S, Kanneganti TD (2020b) Innate immune priming in the absence of TAK1 drives RIPK1 kinase activity-independent pyroptosis, apoptosis, necroptosis, and inflammatory disease. J Exp Med. https://doi.org/10.1084/jem.20191644
Malireddi RKS, Karki R, Sundaram B, Kancharana B, Lee S, Samir P, Kanneganti TD (2021) Inflammatory cell death, PANoptosis, mediated by cytokines in diverse cancer lineages inhibits tumor growth. Immunohorizons 5:568–580. https://doi.org/10.4049/immunohorizons.2100059
Man SM, Hopkins LJ, Nugent E, Cox S, Gluck IM, Tourlomousis P, Wright JA, Cicuta P, Monie TP, Bryant CE (2014) Inflammasome activation causes dual recruitment of NLRC4 and NLRP3 to the same macromolecular complex. Proc Natl Acad Sci U S A 111:7403–7408. https://doi.org/10.1073/pnas.1402911111
Mifflin L, Ofengeim D, Yuan J (2020) Receptor-interacting protein kinase 1 (RIPK1) as a therapeutic target. Nat Rev Drug Discov 19:553–571. https://doi.org/10.1038/s41573-020-0071-y
Mohammad RM, Muqbil I, Lowe L, Yedjou C, Hsu HY, Lin LT, Siegelin MD, Fimognari C, Kumar NB, Dou QP, Yang H, Samadi AK, Russo GL, Spagnuolo C, Ray SK, Chakrabarti M, Morre JD, Coley HM, Honoki K, Fujii H, Georgakilas AG, Amedei A, Niccolai E, Amin A, Ashraf SS, Helferich WG, Yang X, Boosani CS, Guha G, Bhakta D, Ciriolo MR, Aquilano K, Chen S, Mohammed SI, Keith WN, Bilsland A, Halicka D, Nowsheen S, Azmi AS (2015) Broad targeting of resistance to apoptosis in cancer. Semin Cancer Biol 35(Suppl):S78–S103. https://doi.org/10.1016/j.semcancer.2015.03.001
Munoz-Planillo R, Kuffa P, Martinez-Colon G, Smith BL, Rajendiran TM, Nunez G (2013) K(+) efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity 38:1142–1153. https://doi.org/10.1016/j.immuni.2013.05.016
Murphy JM, Czabotar PE, Hildebrand JM, Lucet IS, Zhang JG, Alvarez-Diaz S, Lewis R, Lalaoui N, Metcalf D, Webb AI, Young SN, Varghese LN, Tannahill GM, Hatchell EC, Majewski IJ, Okamoto T, Dobson RC, Hilton DJ, Babon JJ, Nicola NA, Strasser A, Silke J, Alexander WS (2013) The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism. Immunity 39:443–453. https://doi.org/10.1016/j.immuni.2013.06.018
Nailwal H, Chan FK (2019) Necroptosis in anti-viral inflammation. Cell Death Differ 26:4–13. https://doi.org/10.1038/s41418-018-0172-x
Orning P, Weng D, Starheim K, Ratner D, Best Z, Lee B, Brooks A, Xia S, Wu H, Kelliher MA, Berger SB, Gough PJ, Bertin J, Proulx MM, Goguen JD, Kayagaki N, Fitzgerald KA, Lien E (2018) Pathogen blockade of TAK1 triggers caspase-8–dependent cleavage of gasdermin D and cell death. Science 362:1064–1069. https://doi.org/10.1126/science.aau2818
Park J, Lee GE, An HJ, Lee CJ, Cho ES, Kang HC, Lee JY, Lee HS, Choi JS, Kim DJ, Choi JS, Cho YY (2021a) Kaempferol sensitizes cell proliferation inhibition in oxaliplatin-resistant colon cancer cells. Arch Pharm Res 44:1091–1108. https://doi.org/10.1007/s12272-021-01358-y
Park SJ, Joo SH, Lee N, Jang WJ, Seo JH, Jeong CH (2021b) ACY-241, an HDAC6 inhibitor, overcomes erlotinib resistance in human pancreatic cancer cells by inducing autophagy. Arch Pharm Res 44:1062–1075. https://doi.org/10.1007/s12272-021-01359-x
Park EJ, Jun HW, Na IH, Lee HK, Yun J, Kim HS, Kim Y, Hong JT, Han SB (2022) CD48-expressing non-small-cell lung cancer cells are susceptible to natural killer cell-mediated cytotoxicity. Arch Pharm Res 45:1–10. https://doi.org/10.1007/s12272-021-01365-z
Park M, Kang KW, Kim JW (2023) The role and application of transcriptional repressors in cancer treatment. Arch Pharm Res 46:1–17. https://doi.org/10.1007/s12272-023-01427-4
Peng F, Liao M, Qin R, Zhu S, Peng C, Fu L, Chen Y, Han B (2022) Regulated cell death (RCD) in cancer: key pathways and targeted therapies. Signal Transduct Target Ther 7:286. https://doi.org/10.1038/s41392-022-01110-y
Pierini R, Juruj C, Perret M, Jones CL, Mangeot P, Weiss DS, Henry T (2012) AIM2/ASC triggers caspase-8-dependent apoptosis in Francisella-infected caspase-1-deficient macrophages. Cell Death Differ 19:1709–1721. https://doi.org/10.1038/cdd.2012.51
Sagulenko V, Thygesen SJ, Sester DP, Idris A, Cridland JA, Vajjhala PR, Roberts TL, Schroder K, Vince JE, Hill JM, Silke J, Stacey KJ (2013) AIM2 and NLRP3 inflammasomes activate both apoptotic and pyroptotic death pathways via ASC. Cell Death Differ 20:1149–1160. https://doi.org/10.1038/cdd.2013.37
Sborgi L, Ruhl S, Mulvihill E, Pipercevic J, Heilig R, Stahlberg H, Farady CJ, Muller DJ, Broz P, Hiller S (2016) GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J 35:1766–1778. https://doi.org/10.15252/embj.201694696
Sharma BR, Karki R, Rajesh Y, Kanneganti TD (2023) Immune regulator IRF1 contributes to ZBP1-, AIM2-, RIPK1-, and NLRP12-PANoptosome activation and inflammatory cell death (PANoptosis). J Biol Chem 299:105141. https://doi.org/10.1016/j.jbc.2023.105141
Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, Shao F (2015) Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526:660–665. https://doi.org/10.1038/nature15514
Sun L, Wang H, Wang Z, He S, Chen S, Liao D, Wang L, Yan J, Liu W, Lei X, Wang X (2012) Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 148:213–227. https://doi.org/10.1016/j.cell.2011.11.031
Sundaram B, Pandian N, Mall R, Wang Y, Sarkar R, Kim HJ, Malireddi RKS, Karki R, Janke LJ, Vogel P, Kanneganti TD (2023) NLRP12-PANoptosome activates PANoptosis and pathology in response to heme and PAMPs. Cell 186(2783–2801):e2720. https://doi.org/10.1016/j.cell.2023.05.005
Szczerba M, Johnson B, Acciai F, Gogerty C, Mccaughan M, Williams J, Kibler KV, Jacobs BL (2023) Canonical cellular stress granules are required for arsenite-induced necroptosis mediated by Z-DNA-binding protein 1. Sci Signal 16:0837. https://doi.org/10.1126/scisignal.abq0837
Taabazuing CY, Okondo MC, Bachovchin DA (2017) Pyroptosis and apoptosis pathways engage in bidirectional crosstalk in monocytes and macrophages. Cell Chem Biol 24(507–514):e504. https://doi.org/10.1016/j.chembiol.2017.03.009
Tang D, Kang R, Berghe TV, Vandenabeele P, Kroemer G (2019) The molecular machinery of regulated cell death. Cell Res 29:347–364. https://doi.org/10.1038/s41422-019-0164-5
Taylor RC, Cullen SP, Martin SJ (2008) Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol 9:231–241. https://doi.org/10.1038/nrm2312
Tse KM, Takeuchi O (2023) Innate immune sensing of pathogens and its post-transcriptional regulations by RNA-binding proteins. Arch Pharm Res 46:65–77. https://doi.org/10.1007/s12272-023-01429-2
Tsuchiya K, Nakajima S, Hosojima S, Thi Nguyen D, Hattori T, Le Manh T, Hori O, Mahib MR, Yamaguchi Y, Miura M, Kinoshita T, Kushiyama H, Sakurai M, Shiroishi T, Suda T (2019) Caspase-1 initiates apoptosis in the absence of gasdermin D. Nat Commun 10:2091. https://doi.org/10.1038/s41467-019-09753-2
Tweedell RE, Kumar SP, Kanneganti TD (2023) Innate sensing pathways: Defining new innate immune and inflammatory cell death pathways has shaped translational applications. PLoS Biol 21:e3002022. https://doi.org/10.1371/journal.pbio.3002022
Van Opdenbosch N, Van Gorp H, Verdonckt M, Saavedra PHV, De Vasconcelos NM, Goncalves A, Vande Walle L, Demon D, Matusiak M, Van Hauwermeiren F, D’hont J, Hochepied T, Krautwald S, Kanneganti TD, Lamkanfi M (2017) Caspase-1 engagement and TLR-induced c-FLIP expression suppress ASC/caspase-8-dependent apoptosis by inflammasome sensors NLRP1b and NLRC4. Cell Rep 21:3427–3444. https://doi.org/10.1016/j.celrep.2017.11.088
Walter EJ, Hanna-Jumma S, Carraretto M, Forni L (2016) The pathophysiological basis and consequences of fever. Crit Care 20:200. https://doi.org/10.1186/s13054-016-1375-5
Wang Y, Kanneganti TD (2021) From pyroptosis, apoptosis and necroptosis to PANoptosis: a mechanistic compendium of programmed cell death pathways. Comput Struct Biotechnol J 19:4641–4657. https://doi.org/10.1016/j.csbj.2021.07.038
Wang Y, Gao W, Shi X, Ding J, Liu W, He H, Wang K, Shao F (2017) Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature 547:99–103. https://doi.org/10.1038/nature22393
Wang R, Li H, Wu J, Cai ZY, Li B, Ni H, Qiu X, Chen H, Liu W, Yang ZH, Liu M, Hu J, Liang Y, Lan P, Han J, Mo W (2020) Gut stem cell necroptosis by genome instability triggers bowel inflammation. Nature 580:386–390. https://doi.org/10.1038/s41586-020-2127-x
Wang Y, Pandian N, Han JH, Sundaram B, Lee S, Karki R, Guy CS, Kanneganti TD (2022) Single cell analysis of PANoptosome cell death complexes through an expansion microscopy method. Cell Mol Life Sci 79:531. https://doi.org/10.1007/s00018-022-04564-z
Wu YT, Tan HL, Huang Q, Sun XJ, Zhu X, Shen HM (2011) zVAD-induced necroptosis in L929 cells depends on autocrine production of TNFalpha mediated by the PKC-MAPKs-AP-1 pathway. Cell Death Differ 18:26–37. https://doi.org/10.1038/cdd.2010.72
Xu W, Che Y, Zhang Q, Huang H, Ding C, Wang Y, Wang G, Cao L, Hao H (2021) Apaf-1 pyroptosome senses mitochondrial permeability transition. Cell Metab 33(424–436):e410. https://doi.org/10.1016/j.cmet.2020.11.018
Yang T, Wang G, Zhang M, Hu X, Li Q, Yun F, Xing Y, Song X, Zhang H, Hu G, Qian Y (2023) Triggering endogenous Z-RNA sensing for anti-tumor therapy through ZBP1-dependent necroptosis. Cell Rep 42:113377. https://doi.org/10.1016/j.celrep.2023.113377
Yang Y, Zhang H, Xiao X, Guo M (2024) PANoptosis features, a humanized NSG murine model of Sjogren’s syndrome. DNA Cell Biol. https://doi.org/10.1089/dna.2023.0374
Yuan F, Cai J, Wu J, Tang Y, Zhao K, Liang F, Li F, Yang X, He Z, Billiar TR, Wang H, Su L, Lu B (2022) Z-DNA binding protein 1 promotes heatstroke-induced cell death. Science 376:609–615. https://doi.org/10.1126/science.abg5251
Zhang W, Fan W, Guo J, Wang X (2022) Osmotic stress activates RIPK3/MLKL-mediated necroptosis by increasing cytosolic pH through a plasma membrane Na(+)/H(+) exchanger. Sci Signal 15:5881. https://doi.org/10.1126/scisignal.abn5881
Zhang N, Zhang J, Yang Y, Shan H, Hou S, Fang H, Ma M, Chen Z, Tan L, Xu D (2024) A palmitoylation-depalmitoylation relay spatiotemporally controls GSDMD activation in pyroptosis. Nat Cell Biol 26:757–769. https://doi.org/10.1038/s41556-024-01397-9
Zhao J, Jitkaew S, Cai Z, Choksi S, Li Q, Luo J, Liu ZG (2012) Mixed lineage kinase domain-like is a key receptor interacting protein 3 downstream component of TNF-induced necrosis. Proc Natl Acad Sci U S A 109:5322–5327. https://doi.org/10.1073/pnas.1200012109
Zheng D, Liwinski T, Elinav E (2020a) Inflammasome activation and regulation: toward a better understanding of complex mechanisms. Cell Discov 6:36. https://doi.org/10.1038/s41421-020-0167-x
Zheng M, Karki R, Vogel P, Kanneganti TD (2020b) Caspase-6 is a key regulator of innate immunity, inflammasome activation, and host defense. Cell 181(674–687):e613. https://doi.org/10.1016/j.cell.2020.03.040
Acknowledgements
This work was supported by new faculty startup funds from Seoul National University (2023-0024 to R.K), and by a grant (RS-2023-00251395) from the National Research Foundation of Korea (NRF). This work was also supported by a NRF grant funded by the Korean government (MSIT) (RS-2024-00355169 to J.H.H), and by Woosuk University (to J.H.H).
Funding
Funding was provided by New faculty startup funds from Seoul National University (Grant No. 2023-0024), National Research Foundation of Korea (Grant No. RS-2023-00251395), Woosuk University, National Research Foundation of Korea (Grant No. RS-2024-00355169).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declared no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bae, H., Jang, Y., Karki, R. et al. Implications of inflammatory cell death-PANoptosis in health and disease. Arch. Pharm. Res. 47, 617–631 (2024). https://doi.org/10.1007/s12272-024-01506-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-024-01506-0