Abstract
The susceptibility of cancer cells to natural killer (NK) cell–mediated cytotoxicity depends on the balance of activating and inhibitory ligands expressed on their surface. Although many types of cancer cells are killed by NK cells, non-small-cell lung cancer (NSCLC) cells are relatively resistant to NK cell–mediated cytotoxicity. In this study, we showed that several NSCLC cell lines have differential sensitivity to NK cell–mediated cytotoxicity: NCI-H522 cells were highly sensitive, but A549, NCI-H23, NCI-H1915, and NCI-H1299 were resistant. Among activating ligands such as CD48, HLA-A/B/G, ICAM-1, MICA/B, and ULBPs, only CD48 rendered NCI-H522 cells susceptible to NK cell–mediated cytotoxicity, which was proved by using CD48 siRNA and neutralizing antibody. CD48-positive NCI-H522 cells established a more stable contact with NK cells than did CD48-negative A549 and CD48 siRNA cell–transfected NCI-H522 cells. Taken together, these data demonstrate that CD48-positive NSCLC cells might be susceptible to NK cell–mediated cytotoxicity, which provide information on how to stratify NSCLC patients potentially responsive to NK-cell therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Non-small-cell lung cancer (NSCLC) accounts for about 85% of all lung cancers and remains the leading cause of cancer death worldwide, despite the advances in surgery, chemotherapy, and radiotherapy (Pockley et al. 2020; Tripathi et al. 2020). Recently, immune checkpoint inhibitors targeting PD-1 or PD-L1, either as single agents or in combination with chemotherapy, have emerged as an important treatment option, but only approximately 15% of patients with NSCLC respond to this combination therapy (Poznanski et al. 2021; Lin et al. 2020; Yang et al. 2020). Natural killer (NK) cell therapy might help to overcome the limitation of this combination therapy for NSCLC (Hamilton and Plangger 2021).
NK cell cytotoxicity against cancer cells is regulated by signals from activating (e.g., CD226, 2B4, NKG2D) and inhibitory receptors (e.g., NKG2A, TIGIT, KLRG-1), which are concomitantly expressed on individual NK cells at different densities (Claus et al. 2016; Kim et al. 2019c; Bae et al. 2019; Phung et al. 2020). After engagement of activating receptors, NK cells may induce the death of cancer cells by several mechanisms: releasing perforin and granzymes, direct binding to death receptors including Fas and TNFR on cancer cells, and induction of complement- or antibody-dependent cell–mediated cytotoxicity (Bradley et al. 1998; Talanian et al. 1997; Abel et al. 2018). Although several clinical trials of human NK cells in combination with other therapies have shown a prolonged survival of patients with NSCLC, the overall survival of patients with advanced NSCLC remains less than 2 years (Pockley et al. 2020; Multhoff et al. 2020; Xie et al. 2019; Shevtsov et al. 2019).
Tonn et al. (2013) established an allogeneic NK cell therapy, which was based on the NK-92 cell line, originally derived from a malignant non-Hodgkin’s lymphoma. Unlike primary NK cells, whose expansion capability varies among donors, NK-92 cells can be continuously expanded with phenotypical and functional characteristics similar to those of primary NK cells (Yang et al. 2020). Importantly, the lack of most of the inhibitory receptors enables high cytotoxicity of NK-92 cells against cancer cells (Yang et al. 2020). Safety and antitumor activity of NK-92 cells to various types of cancers have been reported in preclinical and clinical trials, but a substantial proportion of patients with NSCLC do not profit from NK-92 cell therapies (Klingemann et al. 2016). This resistance of NSCLC to NK cell therapy might be due to the genetic alterations in activating/inhibitory ligands on NSCLC cells (Lee 2020; Campbell and Hasegawa 2013).
CD48 is the member of the signaling lymphocyte activation marker family and identified as an important molecule in immune cell functions, with roles in cancer, autoimmunity, and allergy (McArdel et al. 2016). CD48 exists in both membrane-bound and soluble form, although how the soluble CD48 is generated has been unknown (Low and Saltiel 1988). CD48 is downregulated in acute myeloid leukemia and multiple myeloma cells, although the expression level of CD48 in lung cancer cells has not been reported (Sanchez-Correa et al. 2011). Anti-CD48 antibody kills multiple myeloma cells in vitro and inhibits their growth in vivo (Hosen et al. 2012). CD48 ligands 2B4 (CD244), which is expressed on NK cells, T cells, B cells, dendritic cells, and monocytes (Sun et al. 2021).
In this study, we examined the susceptibility of NSCLC cells to NK cell–mediated cytotoxicity by analyzing the expression of activating and inhibitory ligands on their surface. Our data demonstrate that CD48-positive NSCLC cells were susceptible to NK-92 cell–mediated cytotoxicity and suggest that the mechanism of this susceptibility might rely on an increase in contact stability between NK-92 and NSCLC by CD48.
Materials and methods
Cell lines and culture
NK-92 cells, an IL-2-dependent NK cell line, were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA) and maintained in α-minimum essential medium without ribonucleosides or deoxyribonucleosides, supplemented with 12.5% fetal bovine serum and 12.5% horse serum, 2 mM l-glutamine, 0.2 mM inositol, 0.02 mM folic acid, 0.1 mM 2-mercaptoethanol, 100 U/ml penicillin, 100 μg/ml streptomycin (Sigma-Aldrich, Burlington, MA, USA), and 200 U/ml human recombinant IL-2 (Bayer HealthCare Pharmaceuticals, Emeryville, CA, USA). NSCLC cell lines (A549, NCI-H23, NCI-H522, NCI-H1299, and NCI-H1915), and human myeloid leukemia K562 cells were purchased from ATCC and maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin (Sigma-Aldrich).
Flow cytometry
Anti-CD56-PE, anti-2B4-PE, anti-LFA-1-FITC, anti-CD16-FITC, anti-CD3-FITC, anti-ICAM-1-APC, anti-NKG2D-APC, anti-2B4 antibodies, anti-IgG1-PE, anti-IgG2a-PE, anti-IgG1-FITC, anti-IgG2a-FITC, anti-IgG2a-APC and anti-IgG1-APC were purchased from BD Biosciences (San Jose, CA, USA). Anti-ILT-2-PE, anti-KIR2DL4-APC, anti-IgG2b-APC and anti-IgG1-APC antibodies were purchased from Biolegend (San Diego, CA, USA). Cells were analyzed using a FACSCalibur flow cytometer (BD Biosciences). Data were processed using Cell Quest Pro software (BD Biosciences) (Kim et al. 2018).
Degranulation assay
To analyze exocytosis, NK-92 cells (1 × 105 cells) were mixed with target cells (1 × 105 cells), centrifuged at 50×g for 1 min, and incubated for 2 h at 37 °C in the presence of anti-CD107a-PE antibody (BD Biosciences). Cells were harvested, washed with medium, stained with anti-CD56-APC antibody, and analyzed using a flow cytometer (Kim et al. 2019b).
Cytotoxicity assay
The cytotoxicity of NK-92 cells to target cells was assessed using a lactate dehydrogenase (LDH) release assay (Takara, Shiga, Japan). Target cells were plated at a density of 1 × 105 cells/well in a 96-well plate and co-cultured with NK-92 cells at various effector-to-target cell ratios for 4 h at 37 °C (Kim et al. 2017). Cytotoxicity was calculated using the following equation: [(experimental release − effector spontaneous release − target spontaneous release) / (target maximum release − target spontaneous release)] × 100%.
RT-PCR and western blotting
Total RNA was extracted from cells using TRIZOL Reagent (Invitrogen, Waltham, MD, USA). For RT-PCR, cDNA was synthesized from 300 ng total RNA using an RT kit (Bioneer, Daejeon, Korea) and mRNA levels of activating and inhibitory ligands were assessed by PCR (Roche Applied Science, Penzberg, Upper Bavaria, Germany). PCR products were fractionated on 1% agarose gels and stained with 5 μg/ml ethidium bromide (Lee et al. 2020b).
Whole cell lysates were prepared using total lysis buffer (Lonza, Basel, Switzerland). Protein concentration was measured by Bradford assay. Equal amounts of total protein were fractionated by 10% SDS-PAGE and transferred to pure nitrocellulose membranes. Membranes were blocked with 5% nonfat dry milk in TBS containing 0.1% Tween 20 (TTBS) for 1 h and then incubated overnight at 4 °C with an appropriate dilution of primary antibody in 5% BSA (in TTBS) for 2 h. Antibodies against human granzyme A, granzyme B, and perforin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and against human HLA-G and CD48 from Abcam (Cambridge, UK). Blots were incubated with biotinylated secondary antibody for 1 h and then with HRP-conjugated streptavidin for 1 h (Kim et al. 2019a; Ryu et al. 2020). Signals were detected by enhanced chemiluminescence (Amersham Pharmacia Biotech, Piscataway, NJ, USA) using a Chemi Doc™ XRS + machine (Bio-Rad, Hercules, CA, USA).
Small interfering RNA (siRNA) and overexpression of CD48
siRNAs targeting human CD48 and HLA-G was purchased from Bioneer. The following sequences were used: CD48 (GenBank accession number NM 001778.2), 5′-CCU UCC CAA AGG AGC UCC AdTdT-3′, 5′-GUU GAA AAA GAC UGG GAA UdTdT-3′, 5′-CCA AGC CUG UCA UCA AAA UdTdT-3′; HLA-G (GenBank accession number NM 002127.3), 5′-CAG GUG CUG UUU UUG UUC UdTdT-3′, 5′-GAG AGA UUG AUG GGU UAA UdTdT-3′, 5′-UGA ACA GUA UGC CUA CGA UdTdT-3′; negative control, 5′-CCU ACG CCA CCA AUU UCG UdTdT-3′. Target cells were transfected with 100 nM siRNAs using Lipofectamine RNAiMAX reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) and incubated at 37 °C in a CO2 incubator for 48 h (Lee et al. 2020a).
Human TrueORF™ CD48 plasmid was purchased from OriGene Technologies (Rockville, MD, USA). The CD48 coding sequence was cloned into the expression vector pCMV6-Entry to generate a construct with a C-terminal Myc-DDK tag. A549 cells were transfected with 1 μg/ml of the pCMV6-CD48 plasmid using Lipofectamine 2000 reagent (Invitrogen Life Technologies). Cells were incubated at 37 °C in a CO2 incubator for 48 h.
Time-lapse imaging
Culture dishes (35 mm; Corning, Corning, NY, USA) were pre-coated with 1% Matrigel® Basement Membrane Matrix (BD Biosciences, Bedford, MA, USA) in serum-free medium for 24 h at 4 °C. NK-92 cells were labeled with 5 μM 5-chloromethylfluorescein diacetate (CMFDA) (Invitrogen, San Diego, CA, USA) in serum-free medium for 10 min at 37 °C and washed twice with medium. Target cells (1 × 105 cells/ml) and NK-92 cells (2.5 × 105 cells/ml) were added onto the Matrigel-coated dishes, and propidium iodide (Life Technologies, Carlsbad, CA, USA) was added at 2 μg/ml. Cells were pre-incubated for 30 min under a Biostation IM-Q microscope equipped with a ×20 magnification objective (numeric aperture 0.5). Images were acquired in three channels (phase contrast; CMFDA, green filter; and propidium iodine, red filter) every 2 min for 8 h and analyzed by using Imaris software version 7.2 (Bitplane, Zurich, Switzerland). Propidium iodide–stained target cells were considered dead. Cells were automatically tracked by using spot analysis with the autoregressive motion algorithm, and broken tracks were manually reconnected after removal of background spots. The number of contacts that lasted for > 6 min was counted (Kim et al. 2017).
Statistics
Data represent the mean ± SEM of at least 3 independent experiments, each performed in triplicate; p values were calculated using one-way ANOVA in GraphPad Software (San Diego, CA, USA). Mann–Whitney test was used in Fig. 5.
Results
NSCLC cells have different susceptibility to NK-92 cell–mediated cytotoxicity
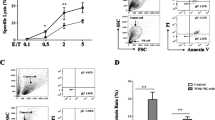
First, we characterized effector NK-92 cells. Our NK-92 cells were CD3−CD16−CD56+ (Fig. 1A) and expressed the inhibitory receptors KIR2DL4 and ILT-2 (Fig. 1B), the activating receptors NKG2D and 2B4, and the adhesion molecule LFA-1 (Fig. 1C). NK-92 cells also expressed the cytotoxic molecules granzyme A, granzyme B, and perforin (Fig. 1D). Then, we examined the cytotoxic potential of NK-92 cells against NSCLC cells. NK-92 cells weakly lysed A549, NCI-H23, NCI-H1915, and NCI-H1299 cells, but they strongly killed NCI-H522 cells; the latter effect was similar to the their killing potency against reference target K562 cells (Fig. 1E). These data suggest that various NSCLC cells might have different susceptibility to NK cell–mediated cytotoxicity.
Differential susceptibility of NSCLC cells to NK-92 cell–mediated cytotoxicity. A NK-92 cells were stained with PE-conjugated CD56, FITC-conjugated CD3, and FITC-conjugated CD16 antibodies, and their phenotypes were analyzed by flow cytometry. B, C Expression levels of the inhibitory receptors KIR2DL4 and ILT-2 (B), activating receptors NKG2D and 2B4, and adhesion molecule LFA-1 (C) on NK-92 cells was analyzed by flow cytometry. Filled histograms represent isotype control. D Detection of granzyme A, granzyme B, and perforin in NK-92 cells by western blotting. All images were manually arranged according to the size. E NK-92 cells were co-cultured with the NSCLC cell lines A549, NCI-H23, NCI-H522, NCI-H1299, and NCI-H1915 for 4 h at the indicated effector-to-target ratios. K-562 cells were used as positive control. Viability of target cells was analyzed by the LDH assay (n = 3)
Sensitive NCI-H522 cells highly express CD48
Next, we examined why NCI-H522 cells were highly susceptible to NK-92 cell–mediated cytotoxicity. Considering the importance of activating and inhibitory ligands in NK cell–mediated cytotoxicity (Claus et al. 2016; Kim et al. 2019c), we analyzed the expression levels of several ligands on NSCLC cells. Among several activating ligands, CD48 was highly expressed in NCI-H522 cells but not in other NSCLC cells (Fig. 2A–D). Among several inhibitory ligands, HLA-G was highly expressed in A549 cells, but not in NCI-H522 cells (Fig. 2E). These data prompted us to hypothesize that CD48 might render NCI-H522 cells highly sensitive to or HLA-G might render A549 cells resistant to NK-92 cell–mediated cytotoxicity. As an activating ligand, ICAM-1 on cancer cells can binds LFA-1 on NK cells, which increases the killing of cancer cells by NK cells (Minetto et al. 2019). Our data showed that ICAM-1 expression was decreased in NCI-H522 cells, which were susceptible to NK cell-mediated cytotoxicity (Fig. 2A). Thus, we presumed that ICAM-1 might not be involved in determination of the susceptibility of NCI-H522 cells to NK cell-mediated cytotoxicity and excluded ICAM-1 in our next experiments.
Expression of the activating and inhibitory ligands in NSCLC cells. A The mRNA level of the activating ligands CD48, ICAM-1, MICA, MICB, and ULBPs, in A549 and NCI-H522 cell lines was assessed by RT-PCR. B Expression levels of CD48 in six NSCLC cell lines were assessed by RT-PCR. C The protein levels of CD48 in A549 and NCI-H522 cell lines were assessed by western blotting. D The surface expression levels of CD48 in A549 and NCI-H522 cells were determined by flow cytometry. E The mRNA levels of the inhibitory ligands HLA-A, -B, and -G in A549 and NCI-H522 cell lines were assessed by RT-PCR
To check our hypothesis, we used siRNAs and blocking antibody. NCI-H522 cells transfected with CD48 siRNA became more resistant to NK-92 cell–mediated cytotoxicity (Fig. 3A) and induced less degranulation in NK-92 cells than control NCI-H522 cells did (Fig. 3B). A549 cells were resistant to NK-92 cell–mediated cytotoxicity regardless of the transfection with HLA-G siRNA (Fig. 3C). We also treated NK-92 cells with blocking antibody against 2B4, the receptor of CD48, which reduced their cytotoxicity to NCI-H522 cells (Fig. 3D). When we overexpressed CD48 in A549 cells (Fig. 3E and F), they became sensitive to NK-92 cell–mediated cytotoxicity (Fig. 3G) and induced higher degranulation in NK-92 cells than control A549 cells (Fig. 3H). Overall, these data suggest that CD48 is a key molecule that makes NCI-H522 cells susceptible to NK-92 cell–mediated cytotoxicity.
Role of CD48 in the susceptibility of NSCLC cells to NK-92 cell-mediated cytotoxicity at the population level. A, B NCI-H522 cells were transfected with CD48 siRNAs and then co-cultured with NK-92 cells for 4 h. The expression level of CD48 in NCI-H522 was assessed by western blotting, and the viability of target cells was determined by the LDH assay (A). The expression level of CD107a in NK-92 cells, which indicates their degranulation, was examined using a flow cytometer (B). C A549 cells were transfected with HLA-G siRNAs and then co-cultured with NK-92 cells for 4 h. The expression level of HLA-G in A549 was assessed by western blotting, and the viability of target cells was determined by the LDH assay. D NK-92 cells were treated with isotype or anti-2B4 antibodies (0.1–10 μg/ml) for 2 h. After washing, NK-92 cells were mixed with NCI-H522 cells for 4 h. Viability of NCI-H522 cells was determined by the LDH assay (n = 3). E, H A549 cells were transfected with the pCMV6-CD48 plasmid for 48 h and then co-cultured with NK-92 cells. The expression level of CD48 on A549 cells was assessed by western blotting (E). A549 cells were stained with FITC-conjugated CD48 antibodies, and their phenotypes were analyzed by flow cytometry (F). A549 cells were cocultured with NK-92 cells for 4 h and their viability was determined by the LDH assay (G). The expression level of CD107a in NK-92 cells was examined by flow cytometry (H). *p < 0.01 vs control
CD48-expressing NCI-H522 cells establish stable contacts with NK-92 cells
Next, we examined the role of CD48 in the lysis of NCI-H522 cells by NK-92 cells. CD48 is known as an inducer of degranulation in NK cells as well as an adhesion molecule (Hoffmann et al. 2011; Roda-Navarro et al. 2004). Using time-lapse imaging, we examined the effect of CD48 on the adhesive interaction between individual NSCLC and NK-92 cells. Over 8 h, NK-92 cells killed 44% of NCI-H522 cells but only 2% of A549 cells (Fig. 4A and B; Movies S1–S4). CD48 siRNA transfection of NCI-H522 cells or the presence of anti-2B4 blocking antibody reduced NK-92 cell–mediated killing of NCI-H522 cells (Fig. 4A and B). All cytotoxicity data at the single-cell level were similar to those at the population level.
Role of CD48 in the susceptibility of NSCLC cells to NK-92 cell-mediated cytotoxicity at the single-cell level. CMFDA-labeled NK-92 cells were co-cultured for 8 h with A549, NCI-H522, or CD48 siRNA-transfected NCI-H522 cells. CMFDA-labeled NK-92 cells were pre-treated with anti-2B4 Ab for 2 h and co-cultured with NCI-H522 cells for 8 h. Propidium iodide (1 μg/ml) was added to the medium from the beginning of co-culture to detect dead cells. Interactions between cells were filmed by Biostation IM-Q at 2-min intervals for 8 h (9 movies from three independent experiments for group) and were analyzed by Imaris software. A Representative photos are shown (magnification, ×100; scale bar, 10 μm). Merged images of bright and fluorescence images are in left and fluorescence images in right. B Ratios of dead NSCLC cells were calculated every 1 h. *p < 0.01 vs NK-94 + NCI-H522 group
We next analyzed the contact modes of NK-92 and A549 or NCI-H522 cells. Some NK-92 cells moved without contact, some contacted target cells without killing them, and some contacted and killed target cells (Fig. 5A; Movies S5 and S6). The ratios of NK-92 cells contacting target cells per total NK-92 cells show in the movies were similar in all experimental groups, suggesting that CD48 did not affect the frequency of contacts between NK cells and target cells (Fig. 5B). Each contact continued on average for 120 min regardless of CD48 expression. However, the outcomes of the contacts depended on the types of NSCLC cells: 73% of contacts led to the lysis of NCI-H522 cells, but only 7% led to the lysis of A549 cells (Fig. 5C). CD48 siRNA and anti-2B4 blocking antibody reduced the ratios of killing contacts between NK-92 cells and NCI-H522 cells (Fig. 5C).
Role of CD48 in contact stability between NSCLC and NK-92 cells. NSCLC cells and NK-92 cells were imaged and analyzed as shown in Fig. 4. A Representative photos of non-killing and killing contact (original magnification, 100 × ; electronically zoomed; scale bar, 10 μm). B Ratios of NK cells contacting NSCLC cells (n = 9 movies). C Ratios of killing contacts to total contacts (n = 113, 81, 73 and 63 from the left). D Representative images of stable and unstable contacts (original magnification, ×100; electronically zoomed; scale bar, 10 μm). E Representative profile of NK cell moving speed. F Ratios of stable contacts to total contacts (n = 192, 134, 103, and 96 from the left). *p < 0.01
Finally, we analyzed contact stability. Some NK-92 cells moved on the surface of target cells at a mean speed of < 2 μm/min (called stable contact), while others occasionally moved at > 2 μm/min (called unstable contact) (Fig. 5D and 5E; Movies S7 and S8). NK-92 cells showed more stable contacts with NCI-H522 cells than with A549 cells (Fig. 5F). CD48 siRNA and anti-2B4 blocking antibody decreased the ratios of stable contacts between NK-92 cells and NCI-H522 cells (Fig. 5F). Overall, these data suggest that NK-92 cells establish stable contacts with efficiently kill CD48-positive NCI-H522 cells, but not CD48-negative A549 cells.
Discussion
Quantifying the dying process of individual cancer cells in defined conditions is useful for understanding the diversity in the susceptibility of target cells to NK cell–mediated cytotoxicity. To this end, we employed time-lapse imaging to compare the susceptibility of NSCLC cell lines to NK-92 cell–mediated cytotoxicity. We found that CD48-positive NSCLC cells were sensitive to NK cell–mediated cytotoxicity, but CD48-negative cells were resistant. As a potential mechanism, we demonstrated that CD48 increased the stability of contacts between NSCLC cells and NK cells.
CD48, a member of the signaling lymphocytic activation molecule family, participates in adhesion and activation of immune cells (McArdel et al. 2016). How does CD48 on NSCLC cells increase their susceptibility to NK cell–mediated cytotoxicity? Binding of CD48 to 2B4 on NK cells induces the phosphorylation of immunoreceptor tyrosine-based switch motifs in the latter, the recruitment of the adaptor protein SAP, and subsequent activation of downstream signaling to increase cytotoxicity and cytokine production by NK cells (Tangye et al. 1999). In addition, 2B4 is likely important for integrin activation and the induction of a high-affinity state of LFA-1 on NK cells, followed by the enhanced adhesion to and killing of cancer cells by NK cells (Hoffmann et al. 2011). Consistently, our data showed that NK-92 cells established stable contacts with and efficiently killed CD48-positive NSCLC cells.
Our live-cell imaging data allowed us to explain the contact dynamics of NK-92 cells around cancer cells at a single-cell level. In an 8-h imaging experiment, non-contacting NK-92 cells migrated randomly at a mean speed of 2.3 μm/min (data not shown), lower than 5 μm/min reported for IL-2-activated NK cells, which were isolated from mouse spleen cells (Kim et al. 2017, 2018). Almost all of the NK-92 cells contacted NSCLC cells regardless of CD48 expression on target cells. Each NK-92 cells contacted multiple targets and contacted the same target more than once (data not shown). An interesting observation in our study was that NK-92 cells showed similar contact duration (120 min on average) with CD48-positive and -negative NSCLC cells, but the outcomes were different: we observed strong killing of CD48-positive NSCLC cells and weak killing of CD48-negative cells. During the contact, NK-92 cells stayed at the same position on the surface of CD48-positive NSCLC cells (stable contact), but moved around on CD48-negative cells (unstable contact). Our analysis of NK-92 cell behavior on CD48-positive NSCLC cells allowed us to predict the cytotoxic mechanisms of NK-92 cells from the point of view of contact dynamics. We tentatively conclude that contact stability is more important than contact duration for delivering a lethal hit to cancer cells.
Further studies will be required to understand the activation mechanisms of NK cells by CD48. First, on NK cells, 2B4 has been described as both an activating and an inhibitory receptor, either promoting or inhibiting target cell lysis (Garni-Wagner et al. 1993; Schatzle et al. 1999; Lee et al. 2004; Vaidya et al. 2005; Chlewicki et al. 2008; Sun et al. 2021). Although SAP–Fyn signaling promotes and EAT-2 signaling inhibits NK cell function, the molecular mechanisms are not fully elucidated (Veillette 2010). Further work is needed to understand how 2B4 regulates NK cells positively and negatively. Second, CD48 has another binding partner, CD2, on NK cells. Although CD48 binds CD2 weakly (Kd ~ 90 μM) and 2B4 strongly (Kd 8–16 μM), further study is needed to examine the compensatory and redundant effects of these interactions in NK cells (Brown et al. 1998; Arulanandam et al. 1993). Third, since both CD2 and 2B4 are also expressed on T cells (Heng et al. 2008; Sun et al. 2021), it will be interesting to study the susceptibility of CD48-positive NSCLC cells to T cell–mediated cytotoxicity.
Although the prognostic significance of CD48 in lung cancer patients has not been reported, several reports imply the clinical meaning of CD48 in patients with acute myeloid leukemia (AML) (Sanchez-Correa et al. 2011; Wang et al. 2020, 2021). The expression of 2B4 was decreased on NK cells from AML patients and AML from the majority of patients did not express CD48 (Sanchez-Correa et al. 2012). Over-expression of CD48 in AML activated NK cell functions in vivo (Wang et al. 2020; Veillette 2010). These results suggest that AML can escape immunosurveillance of NK cells by decreasing CD48 expression (Sanchez-Correa et al. 2011; Wang et al. 2020).
Significant progress in the NK cell therapy of NSCLC is critically dependent on better understanding of the nature of effector NK cells and target NSCLC cells. The effectiveness of NK cells can be tempered by cancer-associated changes in the expression of activating and inhibitory ligands. Our data demonstrate that CD48-positive NSCLC cells are susceptible to NK cell–mediated cytotoxicity and suggest that CD48 might be used as a good biomarker to stratify NSCLC patients responsive to NK cell therapy.
References
Abel AM, Yang C, Thakar MS, Malarkannan S (2018) Natural killer cells: development, maturation, and clinical utilization. Front Immunol 9:1869. https://doi.org/10.3389/fimmu.2018.01869
Arulanandam AR, Moingeon P, Concino MF, Recny MA, Kato K, Yagita H, Koyasu S, Reinherz EL (1993) A soluble multimeric recombinant CD2 protein identifies CD48 as a low affinity ligand for human CD2: divergence of CD2 ligands during the evolution of humans and mice. J Exp Med 177:1439–1450. https://doi.org/10.1084/jem.177.5.1439
Bae EA, Seo H, Kim IK, Jeon I, Kang CY (2019) Roles of NKT cells in cancer immunotherapy. Arch Pharm Res 42:543–548.
Bradley M, Zeytun A, Rafi-Janajreh A, Nagarkatti PS, Nagarkatti M (1998) Role of spontaneous and interleukin-2-induced natural killer cell activity in the cytotoxicity and rejection of Fas+ and Fas- tumor cells. Blood 92:4248–4255.
Brown MH, Boles K, Van Der Merwe PA, Kumar V, Mathew PA, Barclay AN (1998) 2B4, the natural killer and T cell immunoglobulin superfamily surface protein, is a ligand for CD48. J Exp Med 188:2083–2090. https://doi.org/10.1084/jem.188.11.2083
Campbell KS, Hasegawa J (2013) Natural killer cell biology: an update and future directions. J Allergy Clin Immunol 132:536–544. https://doi.org/10.1016/j.jaci.2013.07.006
Chlewicki LK, Velikovsky CA, Balakrishnan V, Mariuzza RA, Kumar V (2008) Molecular basis of the dual functions of 2B4 (CD244). J Immunol 180:8159–8167. https://doi.org/10.4049/jimmunol.180.12.8159
Claus M, Wingert S, Watzl C (2016) Modulation of natural killer cell functions by interactions between 2B4 and CD48 in cis and in trans. Open Biol. https://doi.org/10.1098/rsob.160010
Garni-Wagner BA, Purohit A, Mathew PA, Bennett M, Kumar V (1993) A novel function-associated molecule related to non-MHC-restricted cytotoxicity mediated by activated natural killer cells and T cells. J Immunol 151:60–70. PMID: 8326140
Hamilton G, Plangger A (2021) The impact of NK cell-based therapeutics for the treatment of lung cancer for biologics: targets and therapy. Biologics 15:265–277. https://doi.org/10.2147/BTT.S290305
Heng TS, Painter MW, Immunological Genome Project C (2008) The Immunological Genome Project: networks of gene expression in immune cells. Nat Immunol 9:1091–1094. https://doi.org/10.1038/ni1008-1091
Hoffmann SC, Cohnen A, Ludwig T, Watzl C (2011) 2B4 engagement mediates rapid LFA-1 and actin-dependent NK cell adhesion to tumor cells as measured by single cell force spectroscopy. J Immunol 186:2757–2764. https://doi.org/10.4049/jimmunol.1002867
Hosen N, Ichihara H, Mugitani A, Aoyama Y, Fukuda Y, Kishida S, Matsuoka Y, Nakajima H, Kawakami M, Yamagami T, Fuji S, Tamaki H, Nakao T, Nishida S, Tsuboi A, Iida S, Hino M, Oka Y, Oji Y, Sugiyama H (2012) CD48 as a novel molecular target for antibody therapy in multiple myeloma. Br J Haematol 156:213–224. https://doi.org/10.1111/j.1365-2141.2011.08941.x
Kim JS, Shin BR, Lee HK, Lee JH, Kim KH, Choi JE, Ji AY, Hong JT, Kim Y, Han SB (2017) Cd226(-/-) natural killer cells fail to establish stable contacts with cancer cells and show impaired control of tumor metastasis in vivo. Oncoimmunology 6:e1338994. https://doi.org/10.1080/2162402X.2017.1338994
Kim J, Kim JS, Lee HK, Kim HS, Park EJ, Choi JE, Choi YJ, Shin BR, Kim EY, Hong JT, Kim Y, Han SB (2018) CXCR3-deficient natural killer cells fail to migrate to B16F10 melanoma cells. Int Immunopharmacol 63:66–73. https://doi.org/10.1016/j.intimp.2018.07.026
Kim HS, Lee JS, Lee HK, Park EJ, Jeon HW, Kang YJ, Lee TY, Kim KS, Bae SC, Park JH, Han SB (2019a) Mesenchymal stem cells ameliorate renal inflammation in adriamycin-induced nephropathy. Immune Netw 19:e36. https://doi.org/10.4110/in.2019.19.e36
Kim JS, Kim B, Lee HK, Kim HS, Park EJ, Choi YJ, Ahn GB, Yun J, Hong JT, Kim Y, Han SB (2019b) Characterization of morphological changes of B16 melanoma cells under natural killer cell attack. Int Immunopharmacol 67:366–371. https://doi.org/10.1016/j.intimp.2018.12.037
Kim N, Lee HH, Lee HJ, Choi WS, Lee J, Kim HS (2019c) Natural killer cells as a promising therapeutic target for cancer immunotherapy. Arch Pharm Res 42:591–606. https://doi.org/10.1007/s12272-019-01143-y
Klingemann H, Boissel L, Toneguzzo F (2016) Natural killer cells for immunotherapy—advantages of the NK-92 cell line over blood NK cells. Front Immunol 7:91. https://doi.org/10.3389/fimmu.2016.00091
Lee JK (2020) Sesamolin promotes cytolysis and migration activity of natural killer cells via dendritic cells. Arch Pharm Res 43:462–474. https://doi.org/10.1007/s12272-020-01229-y
Lee KM, Mcnerney ME, Stepp SE, Mathew PA, Schatzle JD, Bennett M, Kumar V (2004) 2B4 acts as a non-major histocompatibility complex binding inhibitory receptor on mouse natural killer cells. J Exp Med 199:1245–1254. https://doi.org/10.1084/jem.20031989
Lee HK, Kim EY, Kim HS, Park EJ, Lee HJ, Lee TY, Kim KS, Bae SC, Hong JT, Kim Y, Han SB (2020a) Effect of human mesenchymal stem cells on xenogeneic T and B cells isolated from lupus-prone MRL.Fas (lpr) mice. Stem Cells Int 2020:5617192. https://doi.org/10.1155/2020/5617192
Lee HK, Kim HS, Pyo M, Park EJ, Jang S, Jun HW, Lee TY, Kim KS, Bae SC, Kim Y, Hong JT, Yun J, Han SB (2020b) Phorbol ester activates human mesenchymal stem cells to inhibit B cells and ameliorate lupus symptoms in MRL.Fas (lpr) mice. Theranostics 10:10186–10199. https://doi.org/10.7150/thno.46835
Lin M, Luo H, Liang S, Chen J, Liu A, Niu L, Jiang Y (2020) Pembrolizumab plus allogeneic NK cells in advanced non-small cell lung cancer patients. J Clin Investig 130:2560–2569. https://doi.org/10.1172/JCI132712
Low MG, Saltiel AR (1988) Structural and functional roles of glycosyl-phosphatidylinositol in membranes. Science 239:268–275. https://doi.org/10.1126/science.3276003
Mcardel SL, Terhorst C, Sharpe AH (2016) Roles of CD48 in regulating immunity and tolerance. Clin Immunol 164:10–20. https://doi.org/10.1016/j.clim.2016.01.008
Minetto P, Guolo F, Pesce S, Greppi M, Obino V, Ferretti E, Sivori S, Genova C, Lemoli RM, Marcenaro E (2019) Harnessing NK cells for cancer treatment. Front Immunol 10:2836. https://doi.org/10.3389/fimmu.2019.02836
Multhoff G, Seier S, Stangl S, Sievert W, Shevtsov M, Werner C, Pockley AG, Blankenstein C, Hildebrandt M, Offner R, Ahrens N, Kokowski K, Hautmann M, Rodel C, Fietkau R, Lubgan D, Huber R, Hautmann H, Duell T, Molls M, Specht H, Haller B, Devecka M, Sauter A, Combs SE (2020) Targeted natural killer cell-based adoptive immunotherapy for the treatment of patients with NSCLC after radiochemotherapy: a randomized Phase II Clinical Trial. Clin Cancer Res 26:5368–5379. https://doi.org/10.1158/1078-0432.CCR-20-1141
Phung CD, Tran TH, Kim JO (2020) Engineered nanoparticles to enhance natural killer cell activity towards onco-immunotherapy: a review. Arch Pharm Res 43:32–45. https://doi.org/10.1007/s12272-020-01218-1
Pockley AG, Vaupel P, Multhoff G (2020) NK cell-based therapeutics for lung cancer. Expert Opin Biol Ther 20:23–33. https://doi.org/10.1080/14712598.2020.1688298
Poznanski SM, Ritchie TM, Fan IY, El-Sayes A, Portillo AL, Ben-Avi R, Rojas EA, Chew MV, Shargall Y, Ashkar AA (2021) Expanded human NK cells from lung cancer patients sensitize patients’ PDL1-negative tumors to PD1-blockade therapy. J Immunother Cancer. https://doi.org/10.1136/jitc-2020-001933
Roda-Navarro P, Mittelbrunn M, Ortega M, Howie D, Terhorst C, Sanchez-Madrid F, Fernandez-Ruiz E (2004) Dynamic redistribution of the activating 2B4/SAP complex at the cytotoxic NK cell immune synapse. J Immunol 173:3640–3646. https://doi.org/10.4049/jimmunol.173.6.3640
Ryu D, Lee JH, Kwak MK (2020) NRF2 level is negatively correlated with TGF-beta1-induced lung cancer motility and migration via NOX4-ROS signaling. Arch Pharm Res 43:1297–1310. https://doi.org/10.1007/s12272-020-01298-z
Sanchez-Correa B, Morgado S, Gayoso I, Bergua JM, Casado JG, Arcos MJ, Bengochea ML, Duran E, Solana R, Tarazona R (2011) Human NK cells in acute myeloid leukaemia patients: analysis of NK cell-activating receptors and their ligands. Cancer Immunol Immunother 60:1195–1205. https://doi.org/10.1007/s00262-011-1050-2
Sanchez-Correa B, Gayoso I, Bergua JM, Casado JG, Morgado S, Solana R, Tarazona R (2012) Decreased expression of DNAM-1 on NK cells from acute myeloid leukemia patients. Immunol Cell Biol 90:109–115. https://doi.org/10.1038/icb.2011.15
Schatzle JD, Sheu S, Stepp SE, Mathew PA, Bennett M, Kumar V (1999) Characterization of inhibitory and stimulatory forms of the murine natural killer cell receptor 2B4. Proc Natl Acad Sci USA 96:3870–3875. https://doi.org/10.1073/pnas.96.7.3870
Shevtsov M, Pitkin E, Ischenko A, Stangl S, Khachatryan W, Galibin O, Edmond S, Lobinger D, Multhoff G (2019) Ex vivo Hsp70-activated NK cells in combination with PD-1 inhibition significantly increase overall survival in preclinical models of glioblastoma and lung cancer. Front Immunol 10:454. https://doi.org/10.3389/fimmu.2019.00454
Sun L, Gang X, Li Z, Zhao X, Zhou T, Zhang S, Wang G (2021) Advances in understanding the roles of CD244 (SLAMF4) in immune regulation and associated diseases. Front Immunol 12:648182. https://doi.org/10.3389/fimmu.2021.648182
Talanian RV, Yang X, Turbov J, Seth P, Ghayur T, Casiano CA, Orth K, Froelich CJ (1997) Granule-mediated killing: pathways for granzyme B-initiated apoptosis. J Exp Med 186:1323–1331. https://doi.org/10.1084/jem.186.8.1323
Tangye SG, Lazetic S, Woollatt E, Sutherland GR, Lanier LL, Phillips JH (1999) Cutting edge: human 2B4, an activating NK cell receptor, recruits the protein tyrosine phosphatase SHP-2 and the adaptor signaling protein SAP. J Immunol 162:6981–6985. PMID: 10358138
Tonn T, Schwabe D, Klingemann HG, Becker S, Esser R, Koehl U, Suttorp M, Seifried E, Ottmann OG, Bug G (2020) Treatment of patients with advanced cancer with the natural killer cell line NK-92. Cytotherapy 15(12):1563–1570. https://doi.org/10.1016/j.jcyt.2013.06.017
Tripathi SK, Rengasamy KRR, Biswal BK (2020) Plumbagin engenders apoptosis in lung cancer cells via caspase-9 activation and targeting mitochondrial-mediated ROS induction. Arch Pharm Res 43:242–256. https://doi.org/10.1007/s12272-020-01221-6
Vaidya SV, Stepp SE, Mcnerney ME, Lee JK, Bennett M, Lee KM, Stewart CL, Kumar V, Mathew PA (2005) Targeted disruption of the 2B4 gene in mice reveals an in vivo role of 2B4 (CD244) in the rejection of B16 melanoma cells. J Immunol 174:800–807. https://doi.org/10.4049/jimmunol.174.2.800
Veillette A (2010) SLAM-family receptors: immune regulators with or without SAP-family adaptors. Cold Spring Harb Perspect Biol 2:a002469. https://doi.org/10.1101/cshperspect.a002469
Wang Z, Xiao Y, Guan W, Wang M, Chen J, Zhang L, Li Y, Xiong Q, Wang H, Wang M, Li Y, Lv N, Li Y, Wang L, Yu L (2020) Acute myeloid leukemia immune escape by epigenetic CD48 silencing. Clin Sci 134:261–271. https://doi.org/10.1042/CS20191170
Wang Z, Guan W, Wang M, Chen J, Zhang L, Xiao Y, Wang L, Li Y, Yu L (2021) AML1-ETO inhibits acute myeloid leukemia immune escape by CD48. Leuk Lymphoma 62:937–943. https://doi.org/10.1080/10428194.2020.1849680
Xie S, Wu Z, Niu L, Chen J, Ma Y, Zhang M (2019) Preparation of highly activated natural killer cells for advanced lung cancer therapy. Onco Targets Ther 12:5077–5086. https://doi.org/10.2147/OTT.S201924
Yang S, Cao B, Zhou G, Zhu L, Wang L, Zhang L, Kwok HF, Zhang Z, Zhao Q (2020) Targeting B7–H3 immune checkpoint with chimeric antigen receptor-engineered natural killer cells exhibits potent cytotoxicity against non-small cell lung cancer. Front Pharmacol 11:1089. https://doi.org/10.3389/fphar.2020.01089
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grants funded by the Korea government (MSIT) (2017R1A5A2015541 and 2020R1A2C2004200).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (MP4 4825 kb) Movie S1. NK-92 cell cytotoxicity against A549 cells. Cells were imaged every 2 min for 8 h (magnification, 100×; accelerated 1,200× over real time).
Supplementary file2 (MP4 15564 kb) Movie S2. NK-92 cell cytotoxicity against NCI-H522 cells. Cells were imaged every 2 min for 8 h (magnification, 100×; accelerated 1,200× over real time).
Supplementary file3 (MP4 6249 kb) Movie S3. NK-92 cell cytotoxicity against NCI-H522 cells transfected with CD48 siRNA. Cells were imaged every 2 min for 8 h (magnification, 100×; accelerated 1,200× over real time).
Supplementary file4 (MP4 4596 kb) Movie S4. NK-92 cell cytotoxicity against NCI-H522 cells in the presence of anti-2B4 blocking antibodies. Cells were imaged every 2 min for 8 h (magnification, 100×; accelerated 1,200× over real time).
Supplementary file5 (MP4 2188 kb) Movie S5. Non-killing contacts between NK-92 cells and NCI-H522 cells. Cells were imaged every 2 min for 8 h (magnification, 100×; electronically zoomed; accelerated 1,200× over real time).
Supplementary file6 (MP4 1155 kb) Movie S6. Killing contacts between NK-92 cells and NCI-H522 cells. NK-92 cells and NCI-H522 cells were imaged every 2 min for 8 h (magnification, 100 ×; electronically zoomed; accelerated 1,200x over real-time).
Supplementary file7 (MP4 2946 kb) Movie S7. Stable contacts between NK-92 cells and NCI-H522 cells. NK-92 cells and NCI-H522 cells were imaged every 2 min for 8 h (magnification, 100 ×; electronically zoomed; accelerated 1,200x over real-time).
Supplementary file8 (MP4 2183 kb) Movie S8. Unstable contacts between NK-92 cells and NCI-H522 cells. NK-92 cells and NCI-H522 cells were imaged every 2 min for 8 h (magnification, 100 ×; electronically zoomed; accelerated 1,200x over real-time).
Rights and permissions
About this article
Cite this article
Park, E.J., Jun, H.W., Na, I.H. et al. CD48-expressing non-small-cell lung cancer cells are susceptible to natural killer cell–mediated cytotoxicity. Arch. Pharm. Res. 45, 1–10 (2022). https://doi.org/10.1007/s12272-021-01365-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-021-01365-z