Abstract
Natural killer (NK) cells have emerged as a potent alternative immunotherapeutic approach to T cell therapy for cancer. Despite promising results from preclinical and clinical studies, numerous challenges have limited the application of NK cell-based therapy, including poor expansion of NK cells in vitro, their short in vivo life span, time-intensiveness, treatment complexities, and the cost burden of the treatment. Recent advancements in the development of immune cell-delivering nanosystems have led to promising strategies to overcome these limitations and enhance NK cell toxicity towards cancer cells. This review first summarizes the biological roles of NK cells and their tumoricidal mechanisms. NK cells, in the context of the immune system and the tumor microenvironment, have reportedly provided novel insights into specific therapeutic targets. Eventually, various strategies targeting NK cells using nanoplatforms to modulate the NK cell responses for effective cancer immunotherapy are described herein. Altogether, this review discusses the potential of nanotechnology in advancements in NK cell-based onco-immunotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Various microorganisms occupy niches within the human body, some of which are beneficial, and some are hazardous, considering their asymptomatic existence. The immune system responds to foreign pathogens through innate and adaptive immune responses; the former responding rapidly to pathogens to eliminate them, the latter requiring the recognition and formation of immunological memory before initiating an immune response (Flajnik and Du Pasquier 2004). As the first line of defense, natural killer (NK) cells, a prototypical innate lymphoid cell lineage, defend the host against microbial infections and tumors through potent cytolytic effects (Hamerman et al. 2005).
NK cells are considered the most cytotoxic cells against tumors in vitro (Bassani et al. 2019). NK cells reported serving as a promising candidate for adoptive cell therapy for in vivo cancer treatment (Ogbomo et al. 2011; Kim et al. 2019). In this field of adoptive cell therapy, chimeric antigen receptor (CAR)-NK cell therapy offers numerous advantages over CAR-T cell therapy. First, CAR-NK cells recognize the cancer cells through their receptors, thus targeting multiple antigens and mitigating tumor immune evasion by downregulating CAR-targeted antigens (Souza-Fonseca-Guimaraes et al. 2019). Second, unlike CAR-T cells, CAR-NK cells do not undergo clonal expansion in vivo, which can cause the “cytokine storm” resulting in severe side effects (Shimabukuro-Vornhagen et al. 2018; Lee and Kim 2019). Lastly, stringent HLA matching is not required for the cytotoxic function of NK cells, thus ameliorating the risk of graft-versus-host disease, which has been observed in numerous cases of CAR-T cell therapies (Bollino and Webb 2017). Moreover, apart from their cytolytic function, NK cells reportedly promote both innate and adaptive immune responses by secreting cytokines including interferon gamma (IFN)-γ and tumor necrosis factor (TNF)-α (Reefman et al. 2010; Oth et al. 2018). However, numerous issues limited its application in cancer treatment, including the sources of NK cells (Hu et al. 2019), time-intensiveness, methodological complexities for ex vivo expansion (Tanaka et al. 2019), the limited in vivo life span (Nayar et al. 2015), poor infiltration to solid tumors (Phung et al. 2019b), and the immunosuppressive tumor microenvironment (TME) (Tormoen et al. 2018).
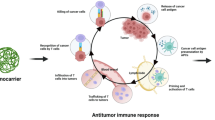
This review summarizes various approaches using nanosystems to overcome the limitations of NK cell-based therapy and enhance the toxicity of NK cells towards cancer cells (Fig. 1). Prior to these approaches, the biological properties of NK cells are discussed, including tumor cell recognition, the interplay between NK cells and other immune cells, and immunosuppressive factors within the TME. Finally, the prospects for improving cancer treatment based on NK cell activity are discussed herein.
The intervention of nanoparticles for improved NK cell activity against cancers. Nanoparticles can promote the cytotoxic function of NK cells directly via targeting their activating or inhibitory receptors and block immune checkpoint components. Distinctly, nanoparticles can also enhance NK cell activation indirectly via targeting dendritic cells and T cells, which result in the elevated NK cell stimulatory cytokines or inhibiting the immunosuppressive cells, such as regulatory T cells (Tregs), and myeloid-derived suppressor cells (MDSCs) as well as their secreted cytokines, such as transforming growth factor-beta (TGF-β), IL-10, adenosine. MICA/B major histocompatibility complex (MHC) class I chain-related protein A/B, HLA human leukocyte antigen, DNAM-1 DNAX Accessory Molecule-1, NKG2A natural killer group 2, member A, NKG2D natural killer group 2, member D, KIR killer cell immunoglobulin-like receptor, PD-1 programmed cell death protein 1, PD-L1 programmed death-ligand 1, CTLA-4 cytotoxic T-lymphocyte-associated protein 4, A2AR adenosine A2A receptor
“Show me your identity”: NK cells detect cell surface abnormalities before exerting cytotoxicity
NK cells recognize stress cells and tumor cells in a major histocompatibility complex (MHC)-independent manner (Paul and Lal 2017), and thus spontaneously eradicating various cancers without requiring prior sensitization and HLA or MHC matching. Through immune receptors, NK cells detect abnormal cells either on the basis of the lack of identifying molecules such as MHC class I, which bind to inhibitory receptors, or the upregulation of ligands for activating NK cell receptors (Morvan and Lanier 2016). They also directly eliminate cancer cells by triggering antibody-dependent cellular cytotoxicity (ADCC) (Wang et al. 2015).
Moreover, the interaction between mature NK cells and target cells induces the release of perforin (a membrane-disrupting protein) and granzymes (a family of proteolytic enzymes) from NK cells, followed by target cell lysis (Voskoboinik et al. 2015). In addition, NK cell activation upregulates TNF family members on the cell surface to mediate tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)- or Fas ligand (FasL)-induced apoptosis pathways (Kumar 2018).
In the past few decades, the identification of various activating signaling pathways that regulate NK cell activation has yielded promising targets to enhance NK cell activity towards cancer cells (Table 1). Furthermore, several cytokines, including type I IFN, IL-2, IL-12, IL-15, IL-18, and IL-21 are crucial for NK activation and proliferation (Freeman et al. 2015). However, IL-2 plays a contrasting role in immunomodulation. First, it stimulates effector immune cells that impede tumor progression (Mortara et al. 2018; Coyne and Narayanan 2019). However, it also functions as an immunosuppressor by maintaining the inhibitory function of Foxp3+ regulatory T cells (Tregs) and negatively regulating activated effector T cells and NK cells via activation-induced cell death (AICD), resulting in immune tolerance (Marks-Konczalik et al. 2000; Nguyen et al. 2019a; Ou et al. 2019). IL-15 distinctly inhibits AICD and does not activate Tregs (Waldmann et al. 2001), while inducing the activation and proliferation of cytotoxic T cells (CTLs) and NK cells (Zhang et al. 2018). IL-21, a member of 4-helical cytokines closest to IL-15, reportedly elevate NK cell progenitor maturation and activate the tumor lytic effects of NK cells through interaction with natural killer group 2, member D (NKG2D) (Takaki et al. 2005). Surface molecules, NKG2D and CD16 activate NK cells, thus facilitating onco-immunotherapy (Morvan and Lanier 2016). CD16 is reportedly one of the most important receptors mediating NK cell effector function by enabling them to detect antibody-coated target cells and exert ADCC (Naeim 2008). However, CD16 has been considered low-affinity Fc receptor, which limits the efficacy of the therapeutic anti-CD16 antibody, e.g., Rituximab (Snyder et al. 2018). NK Group 2D (NKG2D) binds MHC class I polypeptide-related sequence A, B (MICA, MICB) which are abundantly expressed in human (Dhar and Wu 2018).
The expression of CARs helps NK cells effectively eliminate solid tumors (Yong et al. 2018). The single-chain variable fragment (scFv) is recruited to develop NKG2D-based CARs with co-stimulation by Dap10, 4-1BB, or CD28, being clinically promising (Barrow and Colonna 2019). In another approach, bi- and tri-specific killer engagers (BiKEs and TriKEs) are encoded by a scFv against CD16 and tumor antigens. Owing to their small size (50–75 kDa), BiKEs and TriKEs have an enhanced biodistribution and further enhance NK cell-mediated tumor rejection (Davis et al. 2017).
“Dance with me”: NK cells in the context of the immune system and tumor microenvironment
The cross-talk of dendritic cells (DCs) with various immune effector cells, including NK cells has been previously reported. DCs, upon maturation, directly enhance NK cell activation by interacting with several NK cell-activating receptors including NKG2D, NKp30, and NKp46, and/or indirectly via DC-produced IL-2, IL-12, IL-15, and IL-18 (Van Elssen et al. 2014; Ashraf et al. 2019). NK cells in turn recruit conventional type 1 DCs by expressing DC chemoattractants such as chemokine (C–C motif) ligand 5 (CCL5) and chemokine (C motif) ligand (XCL1) to enhance the anti-cancer efficacy (Bottcher et al. 2018). Regarding the association between NK cells and T cells, NK cells potentially regulate T cell immunity via cytokine secretion or direct cytolytic activity of NK cells. Moreover, NK cells potentially induce IFN-γ signaling on CD4+ T cells, which results in their differentiation into TH1 helper cells. However, NK cells can reduce CD8+ T cell activation or even eliminate CD4+ and CD8+ T cells (Crouse et al. 2015).
A major factor contributing to the failure of cancer immunotherapy is the abundance of immune suppressors in the TME (Sau et al. 2018; Cho 2019; Park and Youn 2019; Phung et al. 2019a). The tumor elicits several mechanisms to alter the responses of effector immune cells, such as recruitment of immunosuppressive cells, upregulation of immune checkpoint molecules, and increased production of immunosuppressive cytokines including transforming growth factor (TGF)-β (Choi and Moon 2018; Mahjub et al. 2018). TGF-β potently suppresses both innate and adaptive immune responses, playing an important role in tumor progression via effector T cell and NK cell exclusion while increasing the Treg population in the TME (Heo et al. 2019). TGF-β suppresses NK cell effector function by downregulating transcription factors and activating receptors including CD16, NKG2D, and NKp30, decreasing cytokine production, and preventing NK activation via IL-12, IL-15, and IL-18 (Foltz et al. 2018). Besides activating receptors, NK cells express various inhibitory markers that maintain NK cells in the inactive state and limit their cytotoxic function (Table 1). By inhibiting these pathways, the anti-tumor activity of NK cells is induced.
“BiKE or CAR? Doesn’t matter, I need a road”: the integration of nanotechnology in boosting NK cell-based immunotherapies
Enhancement of NK cell activity by targeting activating receptors
NK cells are activated and expanded upon induction of cytokines or stimulating molecules. Nanocarriers have been used to incorporate various such molecules including membrane-bound IL-15 (Oyer et al. 2015), membrane-bound IL-21 (Foltz et al. 2018), IL-15 plasmids (Liu et al. 2018), and anti-CD16 antibody (Loftus et al. 2018). Oyer et al. proposed a particle-based strategy using plasma membrane (PM) particles derived from genetically engineered leukemia K562 cells co-expressing membrane-bound IL-15 and 4-1BB ligands (K562-mbIL15-41BBL) to selectively expand NK cells from peripheral blood mononuclear cells (PBMCs) (Oyer et al. 2015). PM particles led to markedly greater NK cell expansion than those stimulated with free soluble IL-15 and 1-4BB ligands. Furthermore, these expanded NK cells exhibited high cytotoxicity against several leukemia cell lines. However, long-term expansion of NK cells stimulated by feeder cells expressing membrane-bound IL-15 was reportedly limited via senescence potentially resulting from telomere shortening. Moreover, NK cells activated by IL-15 decreased the level of effector receptor CD16 via proteolysis of metalloprotease-17 (Romee et al. 2013). To overcome this limitation, Denman et al. reported that genetically modified artificial antigen-presenting cells (aAPCs) expressing membrane-bound IL-21 (mbIL21) prolong NK cell expansion up to 6 weeks without senescence and produce more cytokines than mbIL15-expressing aAPCs (Denman et al. 2012). Accordingly, Oyer et al. generated particles isolated from K562-mb21-41BBL cells (PM21) to effectively expand NK cells from the PBMCs ex vivo and in vivo (Oyer et al. 2016). In a murine model, administration of PM21 particles increased the population of peripheral blood NK cells; this study further investigated the effect of K562-mb21-41BBL cell-derived exosomes (EX21) on NK cell expansion in vivo and found that EX21 efficiently expanded NK cells and in vivo administration of NK cells expanded via EX21 displayed equivalent antitumor efficacy to those stimulated with PM21 (Oyer et al. 2017). Furthermore, Liu et al. encapsulated IL15-harboring plasmids into DOTAP and MPEG-PLA (DMA) nanoparticles. The DMA-pIL15 complex significantly inhibited tumor growth by inhibiting angiogenesis, promoting apoptosis, and reducing proliferation by activating host immunity (Liu et al. 2018). Moreover, Loftus et al. used the nanoscale graphene oxide (NGO) as a template to mimic the signaling receptor nanoclusters to activate NK cells by targeting the CD16 receptor. In particular, NGO was functionalized with the 8-arm star, amine-terminated poly(ethylene glycol) (PEG) via an EDC coupling reaction. Thereafter, biotin was conjugated with the free amine groups of the PEGylated NGO, followed by the streptavidin coating. Finally, streptavidin-coated PEGylated NGO-biotin was linked to the anti-CD16 antibody to achieve the antibody-functionalized nanoscale NGO clusters (NGO-α-hCD16). Their study reported that NGO-α-hCD16 specifically binds to human NK cells through the CD16 receptor. Moreover, the biomimetic nanocluster successfully promoted the cytolytic function of NK cells via the production of IFN-γ and CD107a upregulation (Loftus et al. 2018).
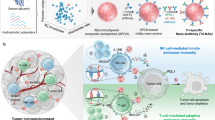
Mediation of NK cell toxicity via APC regulation
Several nano-vaccines targeting DCs not only augment cytotoxic T cells but also promote NK cell activation and proliferation. DC-targeted Poly(g-glutamic acid)-based vaccines co-delivering antigenic OVA and poly(I:C), a toll-like receptor-3 (TLR) stimulator, markedly increased the NK cell population and their activation in vivo and promoted NK cell homing to the tumor tissue (Kim et al. 2017). Furthermore, Kang et al. proposed necroptic tumor cell-mimetic vaccines (αHSP70p-CM-CaP) constructed by a phospholipid bilayer and a phosphate calcium (CaP) core to target both DCs and NK cells at lymph nodes for melanoma treatment (Kang et al. 2018b) (Fig. 2). The exterior surface of the bilayer of the vaccines was reconstituted with membrane protein B16OVA and peptide functionalized-αHSP70 (αHSP70), and the targeting ligand activating DCs and NK cells upon interaction with the CD49 receptor (Specht et al. 2015), CpG, and TLR-9 agonist were encapsulated in the CaP core. Vaccines with artificial PMs were efficiently delivered to the lymph node and induced the expansion of IFN-γ-producing CD8+ T cells and NKG2D+ NK cells. Furthermore, a combination of the αHSP70p-CM-CaP vaccine with anti-PD-1 therapy resulted in significant tumor regression in B16OVA tumor-bearing mice.
In another approach, extracellular vesicles, especially exosomes, have emerged as cancer therapy platforms owing to their biocompatibility, long circulation property, the reflection of their relevant parent cell identities, and easy modification to harbor therapeutic agents (Bach et al. 2017; Manandhar et al. 2018). In both preclinical and clinical settings, exosomes derived from matured DCs directly activate NK cells, and the binding of TNF molecules on the exosome membrane to their corresponding receptors results in elevated IFN-γ production in NK cells (Reiners et al. 2014). In this context, several studies have attempted to engineer DC-derived exosomes (DEX) to promote in vivo NK cell activation. Viaud et al. reported that DEX-expressing membrane-bound NKG2D and IL-15Rα ligand directly mediate NK cell activation and proliferation in humans and mice. A phase I clinical study reported that DEX immunization significantly increased the population of circulating NK cells and restored their NKG2D-dependent function (Viaud et al. 2009). In another study, DEX generated from poly(I:C) and tumor antigens-pulsed DCs robustly stimulated and recruited antigen-specific CTLs and NK cells to the tumor, significantly inhibiting tumor growth in a mouse model of melanoma (Damo et al. 2015).
Co-delivery of NK cells and nanoparticles via direct conjugation
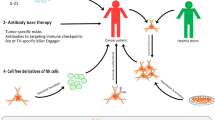
Chandrasekaran et al. adorned liposomes with TRAIL and anti-NK1.1 proteins via maleimide-thiol chemistry, mediating the conjugation of liposomes with natural killer cells. Targeting of NK cells with TRAIL liposomes prolonged their presence within the tumor-draining lymph nodes, further inhibiting the spread of cancer cells from the primary site (Fig. 3) (Chandrasekaran et al. 2016). Siegler et al. further induced the expression of chimeric antigen receptors to redirect their antitumor specificity. Furthermore, paclitaxel-loaded cross-linked multilamellar liposomal vesicles were conjugated with the NK cell surface. This combination of immunotherapy and chemotherapeutic boosted the antitumor efficacy in Her2- and CD19-overexpressing cancer models (Siegler et al. 2017).

(Reprinted with permission from Chandrasekaran et al. 2016. Copyright © 2016 Elsevier Ltd.)
Schematic representation of liposome formulations used herein. Liposomes were adorned with TRAIL and anti-NK1.1 antibodies via maleimide-thiol chemistry. Liposomal anti-NK1.1 mediated the conjugation of liposomes to NK1.1-expressing natural killer cells to form “super” natural killer cells (Chandrasekaran et al. 2016).
Nanoparticles to direct NK cells to tumor tissue
The ideal strategy for enhanced tumor infiltration of NK cells is to upregulate the NK cell-activating ligands and trigger the production of NK cell stimulatory cytokines in the TME. Tan et al. reported an approach to enhance the migration of activated T cells and NK cells to tumors through delivery of a DNA fragment encoding NKG2D ligand and IL-21 (dsNKG2D–IL-21) to the TME. The fused dsNKG2D–IL-21 gene was effectively delivered to the tumor through chitosan-based nanoparticles, resulting in enhanced secretion of the NKG2D ligand and IL-21 by tumor cells, thereby promoting T and NK cell activation and accumulation in the tumor tissue. The authors observed that administration of dsNKG2D–IL-21-chitosan nanoparticles considerably delayed tumor growth and prolonged the life span of treated mice (Tan et al. 2017). Furthermore, Meraz et al. developed cationic liposomes delivering tumor suppressor candidate 2 (TUSC2) plasmid DNA to the tumor tissue to investigate its potential to activate and increase the NK cell population in the TME (Meraz et al. 2018b). TUSC2 is a potent tumor suppressor gene in lung cancer (Rimkus et al. 2017). However, TUCS2 mRNA is reportedly downregulated or suppressed in approximately 80% of tumors, which is associated with low overall survival (Rimkus et al. 2017). TUCS2 majorly contributes to cancer therapy, e.g., in inducing tumor cell apoptosis, inhibiting signaling for drug resistance, regulating crucial cytokines to maintain homeostasis, and stimulating innate and adaptive immunity (e.g., IL-2, IL-15). NK cell-mediated cancer eradication is also an important arm of TUCS2 gene therapy. A pervious study generated a cationic liposome comprising DOTAP and cholesterol to form a complex with the TUCS2 and reported that intravenous administration of the lipoplexes increased the number of NK cells in the TME of tumor-bearing mice, probably owing to the TUCS-2 upregulation-mediated release of proinflammatory cytokines and IL-15 while decreasing the population of Tregs and myeloid-derived suppressor cells (MDSCs) (Fig. 4). In the same context of nanoparticle-induced cytokine production by NK cells in the TME facilitating NK cell delivery to tumors, a PEGylated liposome co-encapsulated cdGMP, a STING agonist, and MPLA, a TLR-4 agonist, potently induced type I interferons in the TME, thus increasing the delivery of NK cells to the tumor (Atukorale et al. 2019). NK cells could also be directed to the tumor by sensing chemokine attraction. NK cells could be directed to tumors via an external simulator, actively migrating through chemokine signaling. Park et al. developed immunomodulatory Degradex® poly(lactic-co-glycolic acid) microspheres containing recombinant IFN-γ. Their delivery induced the release of chemokines in hepatocellular carcinoma, thus increasing NK cell infiltration (Park et al. 2017).

(Reprinted with permission from Meraz et al. 2018a, b. Copyright© 2018, American Association for Cancer Research)
Combinatorial delivery of liposomes harboring tumor suppressor candidate 2 (TUSC2) with anti-PD-1 therapy increased the number of tumor-infiltrating NK and CD8 T cells while decreasing the number of myeloid-derived suppressor cells (MDSCs) and regulatory T cells (Tregs) (Meraz et al. 2018a).
Using a different approach, several studies have reported the potential of an external magnetic field to guide NK cells to the tumor. NK cells were engineered with magnetic nanoparticles ex vivo, infused in the body, and traced via external magnetic exposure (Jang et al. 2012; Oh et al. 2012; Wu et al. 2018; Burga et al. 2019). Jang et al. loaded silica-decorated superparamagnetic iron oxide (Fe3O4/SiO2) conjugated with fluorophore (Cyanine 5.5) into NK cells (Jang et al. 2012). Thereafter, the magnetic nanoparticle-modified NK cells were intravenously injected into B cell lymphoma-bearing mice and exposed to a magnetic field (Fig. 5). Consequently, NK cell infiltration in tumors was increased by 17-fold when applying the magnetic field, and nanoparticle labeling did not affect NK cell function. However, brief intratumor retention of NK cells was observed upon removal of the magnetic field. To overcome this limitation, Wu et al. implanted a magnetic plate to the hypodermic tumor, followed by injection of NK cells loaded with magnetic nanoparticles comprising an Fe3O4 core and polydopamine layer (Wu et al. 2018). This strategy showed improved accumulation and retention of NK in tumors, resulting in significantly enhanced therapeutic efficacy.
Direction of nanoparticles to tumor sites using NK cell materials
Owing to the natural immunosurveillance properties of diseased/stress cells, NK cell membrane-associated components are recruited to form the NKsome. NK-NPs selectively accumulate in the tumor and eliminate primary tumor growth (Deng et al. 2018; Pitchaimani et al. 2018, 2019; Zhu et al. 2018). Pitchaimani et al. isolated the receptor proteins from activated NK-92 cells and infused them into liposomes to form NKsomes. Doxorubicin loaded NKsomes inhibited the tumor growth up to 78.5% after marked accumulation of vesicles at the tumor area (Fig. 6) (Pitchaimani et al. 2018).
Release NK cell activity by targeting immunosuppressive pathways
Park et al. reported a strategy to overcome TGF-β-mediated immune inhibition in the TME to reverse the anti-tumor activity of CTLs and NK cells. Using liposomal polymeric gels (nLGs), they co-delivered TGF-β inhibitor and IL-2 to the tumor site, reporting marked tumor inhibition and enhanced survival of tumor-bearing mice (Park et al. 2012). In particular, nLGs were generated by extruding the complex of TGF-β inhibitor and acrylated cyclodextrin, IL-2, PLA-PEG crosslinker, IL-2, DSPE-PEG-NH2, phosphatidylcholine, and cholesterol, followed by ultraviolet irradiation, resulting in polymerization of the crosslinker and cyclodextrin complex, subsequently encapsulating the TGF-β inhibitor and IL-2 within the hydrogel core. After intravenous administration, nLGs effectively accumulated at the tumor site owning to the enhanced permeation and retention (EPR) effect. The proportion of activated CD8+ T cells and NK cells in the tumor tissue was markedly increased, while the number of Tregs was reduced.
Immunosuppressive cells are critical for inhibiting NK cell function and inducing tumorigenesis. Tumor-associated dendritic cells (TADCs) have recently been considered potential targets for cancer immunotherapy. Although DCs are essential for initiation of anti-tumor immune responses, they are typically inactivated and dysfunctional under cancerous conditions; this is associated with effector T cell deletion and an increase in Tregs, thereby leading to immune tolerance (Truong et al. 2019). Thus, the redirection of dysfunctional TADCs to fully functional DCs is important for promoting immunotherapeutic effects against cancer. TLR agonists including poly(I:C), imiquimod, and CpG are commonly used to activate DCs, subsequently inducing CTL responses towards cancer (Bastola and Lee 2019; Tran et al. 2019; Wang et al. 2019). However, accumulating evidence indicates that TADCs respond poorly to TLR stimulators, thus deterring the development of effective cancer immunotherapy (Idoyaga et al. 2007). These studies have identified the crucial role of microRNAs (miRs) in regulating TLR simulation. Inhibition of miR-148a efficiently restores the sensitivity of TADCs to TLR-3/4 agonists. These findings suggest a rational approach to combine TLR stimulation with miR-148a inhibitor (miR-148ai) to synergistically reprogram TADCs to robustly facilitate anticancer immune responses. Recently, Liu et al. co-entrapped miR-148ai with poly(I:C), a the TLR-3 agonist, and OVA antigen into polypeptide micelles to formulate the polypeptide micelle/poly (I:C)/OVA/148ai (PMP/OVA/148ai) nanovaccines and reported that vaccination with the PMP/OVA/148ai led to increased mature DCs in the spleen and tumor, reduced Tregs and MDSCs in the tumor. Furthermore, upon administration of PMP/OVA/148ai, the cell population in the TME of effector immune cells was significantly elevated (2- to 3-times for CD4+ and CD8+ T cells, and 3- to 4-times for NK cells), thus markedly reducing the tumor burden (Liu et al. 2016).
“Where do we go from here? Start from the end”: conclusions and future directions
Advancements in materials science have placed nanotechnology at the frontier of onco-immunotherapy with several properties including: (1) the incorporation of multiple therapeutic agents in the same platform, thus facilitating effective combination therapies (Nguyen et al. 2017; Hwang et al. 2018; Le et al. 2018; Phung et al. 2019c), (2) chemical or biological modification of nanoparticles to specifically deliver payload agents to NK cells through surface functionalization with targeting ligands (Choi and Han 2018; Al-azzawi and Masheta 2019), (3) nanoparticle-mediated protection of bio-macromolecular components including peptides, nucleic acids, and proteins from in vivo degradation, resulting in increased therapeutic efficacy (Ghosh et al. 2018; Nguyen and Jeong 2018; Park et al. 2018; Jang et al. 2019; Lee 2019). NK cell-based therapy has attracted increasing attention in studies on cancer treatment. However, despite the marked potential of NK cells to eliminate tumor cells, the clinical translation of NK cell therapy remains challenging, probably owing to difficulties in the mass production of NK cells, their poor persistence and delivery at the tumor site, and a reduction in their activity upon administration to patients, together with numerous immunosuppressive factors in the TME. In this context, mobilization of host NK cells by nanoparticles might be a potential alternative to NK cell therapy. In several preclinical and clinical studies, engineered nanoparticles delivering therapeutic agents including antibodies, stimulatory cytokines, genes, or adjuvants have increased NK cell activity and proliferation and promoted NK cell migration to tumor sites, thus markedly inhibiting tumor progression.
However, few studies have thus far evaluated the potential of nanoparticles in modulating the anti-tumor activity of NK cells and the degree of therapeutic efficacy of NK cells, probably owing to their close proximity with the other lymphocytes and thus deterring treatment via specifically targeting NK cells (Nutt and Huntington 2019). However, simultaneous expression of several markers in NK cells and the other effector immune cells including T lymphocytes might provide an interesting therapeutic approach to synergistically augment the activity of both cell types to treat cancers. Similar to T cells, NK cells express numerous immune checkpoint molecules on the surface (e.g., CTLA-4, TIM-3, and PD-1) (Stojanovic et al. 2014; Huang et al. 2015; Xu et al. 2015), along with immunosuppressive intracellular signaling components (e.g., Cbl-b, CDk8) (Guillerey et al. 2016). Thus, therapies targeting these shared components not only elevate T cell activity but also improve NK cell toxicity towards the tumor. These findings suggest a promising strategy to design combinatorial treatment-based nanoplatforms to stimulate both T cell and NK cell responses to cancers.
Until recently, NK cell therapy has exhibited higher efficacy against hematological cancers rather than solid tumors, probably owing to their low tumor-infiltrating potential (Habif et al. 2019). The generation of nanoparticles targeting the TME to upregulate NK cell-activating ligands and stimulatory cytokines might be a potential approach to improve the delivery of NK cells to solid tumors. The discovery of the immunomodulatory effects of numerous approved chemotherapeutic drugs has been paradigm-shifting in cancer therapy. Indeed, chemotherapies upregulate NK cell-activating factors, thus improving NK cell-mediated recognition and eradication of tumor cells (Carotta 2016). Furthermore, nanoparticles reportedly increased the therapeutic efficacy and safety of chemotherapy (Lee et al. 2018; Nguyen et al. 2018a, b; Nguyen et al. 2019b; Pham et al. 2019; Soe et al. 2019). Therefore, regarding the elevation in ligand-mediated NK cell activation in tumor tissues, nanoparticles delivering chemotherapeutic agents to the tumor could be an effective strategy, especially in combination with different therapeutic agents to enhance anticancer activity.
Finally, numerous studies on NK cell biology and the efficacy of cancer treatment in both preclinical and clinical settings provide insights into the anti-tumor effects of NK cells, along with the interplay between the immune system and tumors. Furthermore, advancements in high-throughput technologies and big data sciences might further elucidate NK cell biomarkers crucial for the development of therapeutic agents and nanosystems specifically targeting NK cells for effective cancer immunotherapy.
References
Al-Azzawi S, Masheta D (2019) Designing a drug delivery system for improved tumor treatment and targeting by functionalization of a cell-penetrating peptide. J Pharm Investig 49:643–654. https://doi.org/10.1007/s40005-018-00424-w
Ashraf MU, Jeong Y, Roh S-E, Bae Y-S (2019) Transendothelial migration (TEM) of in vitro generated dendritic cell vaccine in cancer immunotherapy. Arch Pharmacal Res 42:582–590. https://doi.org/10.1007/s12272-019-01145-w
Atukorale PU, Raghunathan SP, Raguveer V, Moon TJ, Zheng C, Bielecki PA, Wiese ML, Goldberg AL, Covarrubias G, Hoimes CJ, Karathanasis E (2019) Nanoparticle encapsulation of synergistic immune agonists enables systemic codelivery to tumor sites and IFNbeta-driven antitumor immunity. Cancer Res 79:5394–5406. https://doi.org/10.1158/0008-5472.Can-19-0381
Bach DH, Hong JY, Park HJ, Lee SK (2017) The role of exosomes and miRNAs in drug-resistance of cancer cells. Int J Cancer 141:220–230. https://doi.org/10.1002/ijc.30669
Barrow AD, Colonna M (2019) Exploiting NK cell surveillance pathways for cancer therapy. Cancers 11:55. https://doi.org/10.3390/cancers11010055
Bassani B, Baci D, Gallazzi M, Poggi A, Bruno A, Mortara L (2019) Natural killer cells as key players of tumor progression and angiogenesis: old and novel tools to divert their pro-tumor activities into potent anti-tumor effects. Cancers (Basel). https://doi.org/10.3390/cancers11040461
Bastola R, Lee S (2019) Physicochemical properties of particulate vaccine adjuvants: their pivotal role in modulating immune responses. J Pharm Investig 49:279–285. https://doi.org/10.1007/s40005-018-0406-4
Bollino D, Webb TJ (2017) Chimeric antigen receptor-engineered natural killer and natural killer T cells for cancer immunotherapy. Transl Res 187:32–43. https://doi.org/10.1016/j.trsl.2017.06.003
Bottcher JP, Bonavita E, Chakravarty P, Blees H, Cabeza-Cabrerizo M, Sammicheli S, Rogers NC, Sahai E, Zelenay S, Reis E, Sousa C (2018) NK cells stimulate recruitment of cdc1 into the tumor microenvironment promoting cancer immune control. Cell 172:1022–1037.e14. https://doi.org/10.1016/j.cell.2018.01.004
Burga RA, Khan DH, Agrawal N, Bollard CM, Fernandes R (2019) Designing magnetically responsive biohybrids composed of cord blood-derived natural killer cells and iron oxide nanoparticles. Bioconjug Chem 30:552–560. https://doi.org/10.1021/acs.bioconjchem.9b00048
Carotta S (2016) Targeting NK cells for anticancer immunotherapy: clinical and preclinical approaches. Front Immunol 7:152–152. https://doi.org/10.3389/fimmu.2016.00152
Chandrasekaran S, Chan MF, Li J, King MR (2016) Super natural killer cells that target metastases in the tumor draining lymph nodes. Biomaterials 77:66–76. https://doi.org/10.1016/j.biomaterials.2015.11.001
Cho H-J (2019) Recent progresses in the development of hyaluronic acid-based nanosystems for tumor-targeted drug delivery and cancer imaging. J Pharma Investig. https://doi.org/10.1007/s40005-019-00448-w
Choi YH, Han HK (2018) Nanomedicines: current status and future perspectives in aspect of drug delivery and pharmacokinetics. J Pharm Investig 48:43–60. https://doi.org/10.1007/s40005-017-0370-4
Choi H, Moon A (2018) Crosstalk between cancer cells and endothelial cells: implications for tumor progression and intervention. Arch Pharmacal Res 41:711–724. https://doi.org/10.1007/s12272-018-1051-1
Coyne CP, Narayanan L (2019) Anti-neoplastic cytotoxicity by complementary simultaneous selective “targeted” delivery for pulmonary adenocarcinoma: fludarabine-(5′-phosphoramidate)-[anti-IGF-1R] in dual-combination with dexamethasone-(C21-phosphoramidate)-[anti-EGFR]. J Pharm Investig 49:173–193. https://doi.org/10.1007/s40005-018-0401-9
Crouse J, Xu HC, Lang PA, Oxenius A (2015) NK cells regulating T cell responses: mechanisms and outcome. Trends Immunol 36:49–58. https://doi.org/10.1016/j.it.2014.11.001
Damo M, Wilson DS, Simeoni E, Hubbell JA (2015) TLR-3 stimulation improves anti-tumor immunity elicited by dendritic cell exosome-based vaccines in a murine model of melanoma. Sci Rep 5:17622. https://doi.org/10.1038/srep17622
Davis ZB, Vallera DA, Miller JS, Felices M (2017) Natural killer cells unleashed: checkpoint receptor blockade and BiKE/TriKE utilization in NK-mediated anti-tumor immunotherapy. Semin Immunol 31:64–75. https://doi.org/10.1016/j.smim.2017.07.011
Deng G, Sun Z, Li S, Peng X, Li W, Zhou L, Ma Y, Gong P, Cai L (2018) Cell-membrane immunotherapy based on natural killer cell membrane coated nanoparticles for the effective inhibition of primary and abscopal tumor growth. ACS Nano 12:12096–12108. https://doi.org/10.1021/acsnano.8b05292
Denman CJ, Senyukov VV, Somanchi SS, Phatarpekar PV, Kopp LM, Johnson JL, Singh H, Hurton L, Maiti SN, Huls MH, Champlin RE, Cooper LJ, Lee DA (2012) Membrane-bound IL-21 promotes sustained ex vivo proliferation of human natural killer cells. PLoS ONE 7:e30264. https://doi.org/10.1371/journal.pone.0030264
Dhar P, Wu JD (2018) NKG2D and its ligands in cancer. Curr Opin Immunol 51:55–61. https://doi.org/10.1016/j.coi.2018.02.004
Di Santo JP (2006) Natural killer cell developmental pathways: a question of balance. Annu Rev Immunol 24:257–286
Flajnik MF, Du Pasquier L (2004) Evolution of innate and adaptive immunity: can we draw a line? Trends Immunol 25:640–644. https://doi.org/10.1016/j.it.2004.10.001
Foltz JA, Moseman JE, Thakkar A, Chakravarti N, Lee DA (2018) TGFbeta imprinting during activation promotes natural killer cell cytokine hypersecretion. Cancers (Basel). https://doi.org/10.3390/cancers10110423
Freeman BE, Raue HP, Hill AB, Slifka MK (2015) Cytokine-mediated activation of NK cells during viral infection. J Virol 89:7922–7931. https://doi.org/10.1128/jvi.00199-15
Ghosh D, Peng X, Leal J, Mohanty R (2018) Peptides as drug delivery vehicles across biological barriers. J Pharm Investig 48:89–111. https://doi.org/10.1007/s40005-017-0374-0
Guillerey C, Huntington ND, Smyth MJ (2016) Targeting natural killer cells in cancer immunotherapy. Nat Immunol 17:1025–1036. https://doi.org/10.1038/ni.3518
Habif G, Crinier A, Andre P, Vivier E, Narni-Mancinelli E (2019) Targeting natural killer cells in solid tumors. Cell Mol Immunol 16:415–422. https://doi.org/10.1038/s41423-019-0224-2
Hamerman JA, Ogasawara K, Lanier LL (2005) NK cells in innate immunity. Curr Opin Immunol 17:29–35. https://doi.org/10.1016/j.coi.2004.11.001
Heo MJ, Yun J, Kim SG (2019) Role of non-coding RNAs in liver disease progression to hepatocellular carcinoma. Arch Pharmacal Res 42:48–62. https://doi.org/10.1007/s12272-018-01104-x
Hu W, Wang G, Huang D, Sui M, Xu Y (2019) Cancer Immunotherapy based on natural killer cells: current progress and new opportunities. Front Immunol 10:1205–1205. https://doi.org/10.3389/fimmu.2019.01205
Huang BY, Zhan YP, Zong WJ, Yu CJ, Li JF, Qu YM, Han S (2015) The PD-1/B7-H1 pathway modulates the natural killer cells versus mouse glioma stem cells. PLoS ONE 10:e0134715. https://doi.org/10.1371/journal.pone.0134715
Hwang HS, Shin H, Han J, Na K (2018) Combination of photodynamic therapy (PDT) and anti-tumor immunity in cancer therapy. J Pharma Investig 48:143–151. https://doi.org/10.1007/s40005-017-0377-x
Idoyaga J, Moreno J, Bonifaz L (2007) Tumor cells prevent mouse dendritic cell maturation induced by TLR ligands. Cancer Immunol Immunother 56:1237–1250. https://doi.org/10.1007/s00262-006-0275-y
Jang E-S, Shin J-H, Ren G, Park M-J, Cheng K, Chen X, Wu JC, Sunwoo JB, Cheng Z (2012) The manipulation of natural killer cells to target tumor sites using magnetic nanoparticles. Biomaterials 33:5584–5592. https://doi.org/10.1016/j.biomaterials.2012.04.041
Jang MH, Kim CH, Yoon HY, Sung SW, Goh MS, Lee ES, Shin DJ, Choi YW (2019) Steric stabilization of RIPL peptide-conjugated liposomes and in vitro assessment. J Pharm Investig 49:115–125. https://doi.org/10.1007/s40005-018-0392-6
Kang T, Huang Y, Zhu Q, Cheng H, Pei Y, Feng J, Xu M, Jiang G, Song Q, Jiang T (2018a) Necroptotic cancer cells-mimicry nanovaccine boosts anti-tumor immunity with tailored immune-stimulatory modality. Biomaterials 164:80–97
Kang T, Huang Y, Zhu Q, Cheng H, Pei Y, Feng J, Xu M, Jiang G, Song Q, Jiang T, Chen H, Gao X, Chen J (2018b) Necroptotic cancer cells-mimicry nanovaccine boosts anti-tumor immunity with tailored immune-stimulatory modality. Biomaterials 164:80–97. https://doi.org/10.1016/j.biomaterials.2018.02.033
Kim SY, Noh YW, Kang TH, Kim JE, Kim S, Um SH, Oh DB, Park YM, Lim YT (2017) Synthetic vaccine nanoparticles target to lymph node triggering enhanced innate and adaptive antitumor immunity. Biomaterials 130:56–66. https://doi.org/10.1016/j.biomaterials.2017.03.034
Kim N, Lee HH, Lee HJ, Choi WS, Lee J, Kim HS (2019) Natural killer cells as a promising therapeutic target for cancer immunotherapy. Arch Pharm Res 42:591–606. https://doi.org/10.1007/s12272-019-01143-y
Kumar S (2018) Natural killer cell cytotoxicity and its regulation by inhibitory receptors. Immunology 154:383–393
Le Q-V, Choi J, Oh Y-K (2018) Nano delivery systems and cancer immunotherapy. J Pharm Investig 48:527–539. https://doi.org/10.1007/s40005-018-0399-z
Lee M-K (2019) Clinical usefulness of liposomal formulations in cancer therapy: lessons from the experiences of doxorubicin. J Pharm Investig 49:203–214. https://doi.org/10.1007/s40005-018-0398-0
Lee YH, Kim CH (2019) Evolution of chimeric antigen receptor (CAR) T cell therapy: current status and future perspectives. Arch Pharm Res 42:607–616. https://doi.org/10.1007/s12272-019-01136-x
Lee H, Nguyen TT, Kim M, Jeong JH, Park JB (2018) The effects of biodegradable poly(lactic-co-glycolic acid)-based microspheres loaded with quercetin on stemness, viability and osteogenic differentiation potential of stem cell spheroids. J Periodontal Res 53:801–815. https://doi.org/10.1111/jre.12569
Liu L, Yi H, Wang C, He H, Li P, Pan H, Sheng N, Ji M, Cai L, Ma Y (2016) Integrated nanovaccine with microRNA-148a inhibition reprograms tumor-associated dendritic cells by modulating miR-148a/DNMT1/SOCS1 Axis. J Immunol 197:1231–1241. https://doi.org/10.4049/jimmunol.1600182
Liu X, Li Y, Sun X, Muftuoglu Y, Wang B, Yu T, Hu Y, Ma L, Xiang M, Guo G, You C, Gao X, Wei Y (2018) Powerful anti-colon cancer effect of modified nanoparticle-mediated IL-15 immunogene therapy through activation of the host immune system. Theranostics 8:3490–3503. https://doi.org/10.7150/thno.24157
Loftus C, Saeed M, Davis DM, Dunlop IE (2018) Activation of human natural killer cells by graphene oxide-templated antibody nanoclusters. Nano Lett 18:3282–3289. https://doi.org/10.1021/acs.nanolett.8b01089
Mahjub R, Jatana S, Lee SE, Qin Z, Pauli G, Soleimani M, Madadi S, Li SD (2018) Recent advances in applying nanotechnologies for cancer immunotherapy. J Control Release 288:239–263. https://doi.org/10.1016/j.jconrel.2018.09.010
Manandhar S, Kothandan VK, Oh J, Yoo SH, Hwang J, Hwang SR (2018) A pharmaceutical investigation into exosomes. J Pharm Investig 48:617–626. https://doi.org/10.1007/s40005-018-0391-7
Marks-Konczalik J, Dubois S, Losi JM, Sabzevari H, Yamada N, Feigenbaum L, Waldmann TA, Tagaya Y (2000) IL-2-induced activation-induced cell death is inhibited in IL-15 transgenic mice. Proc Natl Acad Sci USA 97:11445–11450. https://doi.org/10.1073/pnas.200363097
Meraz IM, Majidi M, Cao X, Lin H, Li L, Wang J, Baladandayuthapani V, Rice D, Sepesi B, Ji L (2018a) TUSC2 immunogene therapy synergizes with anti–PD-1 through enhanced proliferation and infiltration of natural killer cells in syngeneic Kras-mutant mouse lung cancer models. Cancer Immunol Res 6:163–177
Meraz IM, Majidi M, Cao X, Lin H, Li L, Wang J, Baladandayuthapani V, Rice D, Sepesi B, Ji L, Roth JA (2018b) TUSC2 Immunogene therapy synergizes with Anti-PD-1 through enhanced proliferation and infiltration of natural killer cells in syngeneic kras-mutant mouse lung cancer models. Cancer Immunol Res 6:163–177. https://doi.org/10.1158/2326-6066.Cir-17-0273
Mortara L, Balza E, Bruno A, Poggi A, Orecchia P, Carnemolla B (2018) Anti-cancer therapies employing IL-2 cytokine tumor targeting: contribution of innate, adaptive and immunosuppressive cells in the anti-tumor efficacy. Front Immunol 9:2905. https://doi.org/10.3389/fimmu.2018.02905
Morvan MG, Lanier LL (2016) NK cells and cancer: you can teach innate cells new tricks. Nat Rev Cancer 16:7–19. https://doi.org/10.1038/nrc.2015.5
Naeim F (2008) Chapter 2—Principles of immunophenotyping. In: Naeim F, Rao PN, Grody WW (eds) Hematopathology. Academic Press, Oxford, pp 27–55
Nayar S, Dasgupta P, Galustian C (2015) Extending the lifespan and efficacies of immune cells used in adoptive transfer for cancer immunotherapies: a review. Oncoimmunology 4:e1002720–e1002720. https://doi.org/10.1080/2162402X.2014.1002720
Nguyen TT, Jeong JH (2018) Development of a single-jet electrospray method for producing quercetin-loaded poly(lactic-co-glycolic acid) microspheres with prolonged-release patterns. J Drug Deliv Sci Technol 47:268–274. https://doi.org/10.1016/j.jddst.2018.07.005
Nguyen HT, Tran TH, Thapa RK, Phung CD, Shin BS, Jeong J-H, Choi H-G, Yong CS, Kim JO (2017) Targeted co-delivery of polypyrrole and rapamycin by trastuzumab-conjugated liposomes for combined chemo-photothermal therapy. Int J Pharm 527:61–71. https://doi.org/10.1016/j.ijpharm.2017.05.034
Nguyen HT, Phung CD, Thapa RK, Pham TT, Tran TH, Jeong JH, Ku SK, Choi HG, Yong CS, Kim JO (2018) Multifunctional nanoparticles as somatostatin receptor-targeting delivery system of polyaniline and methotrexate for combined chemo-photothermal therapy. Acta Biomater 68:154–167. https://doi.org/10.1016/j.actbio.2017.12.033
Nguyen HT, Byeon JH, Phung CD, Pham LM, Ku SK, Yong CS, Kim JO (2019a) Method for the instant in-flight manufacture of black phosphorus to assemble core@shell nanocomposites for targeted photoimmunotherapy. ACS Appl Mater Interfaces 11:24959–24970. https://doi.org/10.1021/acsami.9b04632
Nguyen HT, Soe ZC, Yang KY, Phung CD, Nguyen LT-T, Jeong J-H, Jin SG, Choi H-G, Ku SK, Yong CS, Kim JO (2019b) Transferrin-conjugated pH-sensitive platform for effective delivery of porous palladium nanoparticles and paclitaxel in cancer treatment. Colloids Surf B: Biointerfaces 176:265–275. https://doi.org/10.1016/j.colsurfb.2019.01.010
Nutt SL, Huntington ND (2019) 17—Cytotoxic T lymphocytes and natural killer cells. In: Rich RR, Fleisher TA, Shearer WT, Schroeder HW, Frew AJ, Weyand CM (eds) Clinical immunology, 5th edn. Content Repository Only!, London, pp 247–259.e1
Ogbomo H, Cinatl J, Mody CH, Forsyth PA (2011) Immunotherapy in gliomas: limitations and potential of natural killer (NK) cell therapy. Trends Mol Med 17:433–441. https://doi.org/10.1016/j.molmed.2011.03.004
Oh CH, Shin J-H, Sunwoo J, Cheng Z, Jang E-S (2012) Magnetic nanoparticles induced natural killer (NK) cell control to target tumor site. J Nucl Med 53:1574
Oth T, Habets THPM, Germeraad WTV, Zonneveld MI, Bos GMJ, Vanderlocht J (2018) Pathogen recognition by NK cells amplifies the pro-inflammatory cytokine production of monocyte-derived DC via IFN-γ. BMC Immunol 19:8–8. https://doi.org/10.1186/s12865-018-0247-y
Ou W, Jiang L, Gu Y, Soe ZC, Kim BK, Gautam M, Poudel K, Pham LM, Phung CD, Chang J-H, Kim JR, Ku SK, Yong CS, Kim JO (2019) Regulatory T cells tailored with pH-responsive liposomes shape an immuno-antitumor milieu against tumors. ACS Appl Mater Interfaces 11:36333–36346. https://doi.org/10.1021/acsami.9b11371
Oyer JL, Igarashi RY, Kulikowski AR, Colosimo DA, Solh MM, Zakari A, Khaled YA, Altomare DA, Copik AJ (2015) Generation of highly cytotoxic natural killer cells for treatment of acute myelogenous leukemia using a feeder-free, particle-based approach. Biol Blood Marrow Transplant 21:632–639. https://doi.org/10.1016/j.bbmt.2014.12.037
Oyer JL, Pandey V, Igarashi RY, Somanchi SS, Zakari A, Solh M, Lee DA, Altomare DA, Copik AJ (2016) Natural killer cells stimulated with PM21 particles expand and biodistribute in vivo: clinical implications for cancer treatment. Cytotherapy 18:653–663. https://doi.org/10.1016/j.jcyt.2016.02.006
Oyer J, Gitto SB, Khederzadeh S, Shaver K, Lee DA, Altomare D, Copik A (2017) Assessment of antitumor function of NK cells expanded with exosomes from K562.mb21 cells. J Clin Oncol 35:132–132. https://doi.org/10.1200/JCO.2017.35.7_suppl.132
Park SM, Youn JI (2019) Role of myeloid-derived suppressor cells in immune checkpoint inhibitor therapy in cancer. Arch Pharm Res 42:560–566. https://doi.org/10.1007/s12272-019-01165-6
Park J, Wrzesinski SH, Stern E, Look M, Criscione J, Ragheb R, Jay SM, Demento SL, Agawu A, Licona Limon P, Ferrandino AF, Gonzalez D, Habermann A, Flavell RA, Fahmy TM (2012) Combination delivery of TGF-beta inhibitor and IL-2 by nanoscale liposomal polymeric gels enhances tumour immunotherapy. Nat Mater 11:895–905. https://doi.org/10.1038/nmat3355
Park W, Gordon AC, Cho S, Huang X, Harris KR, Larson AC, Kim D-H (2017) Immunomodulatory magnetic microspheres for augmenting tumor-specific infiltration of natural killer (NK) cells. ACS Appl Mater Interfaces 9:13819–13824. https://doi.org/10.1021/acsami.7b02258
Park J, Kim S, Kim K (2018) Bone morphogenetic protein-2 associated multiple growth factor delivery for bone tissue regeneration. J Pharm Investig 48:187–197. https://doi.org/10.1007/s40005-017-0382-0
Paul S, Lal G (2017) The molecular mechanism of natural killer cells function and its importance in cancer immunotherapy. Front Immunol 8:1124. https://doi.org/10.3389/fimmu.2017.01124
Pham TT, Nguyen HT, Phung CD, Pathak S, Regmi S, Ha D-H, Kim JO, Yong CS, Kim SK, Choi J-E, Yook S, Park J-B, Jeong J-H (2019) Targeted delivery of doxorubicin for the treatment of bone metastasis from breast cancer using alendronate-functionalized graphene oxide nanosheets. J Ind Eng Chem 76:310–317. https://doi.org/10.1016/j.jiec.2019.03.055
Phung CD, Nguyen HT, Choi JY, Pham TT, Acharya S, Timilshina M, Chang J-H, Kim J-H, Jeong J-H, Ku SK, Choi H-G, Yong CS, Kim JO (2019a) Reprogramming the T cell response to cancer by simultaneous, nanoparticle-mediated PD-L1 inhibition and immunogenic cell death. J Controlled Release 315:126–138. https://doi.org/10.1016/j.jconrel.2019.10.047
Phung CD, Nguyen HT, Tran TH, Choi HG, Yong CS, Kim JO (2019b) Rational combination immunotherapeutic approaches for effective cancer treatment. J Control Release 294:114–130. https://doi.org/10.1016/j.jconrel.2018.12.020
Phung DC, Nguyen HT, Phuong Tran TT, Jin SG, Yong CS, Truong DH, Tran TH, Kim JO (2019c) Combined hyperthermia and chemotherapy as a synergistic anticancer treatment. J Pharm Investig 49:519–526. https://doi.org/10.1007/s40005-019-00431-5
Pitchaimani A, Nguyen TDT, Aryal S (2018) Natural killer cell membrane infused biomimetic liposomes for targeted tumor therapy. Biomaterials 160:124–137. https://doi.org/10.1016/j.biomaterials.2018.01.018
Pitchaimani A, Nguyen TDT, Marasini R, Eliyapura A, Azizi T, Jaberi-Douraki M, Aryal S (2019) Biomimetic natural killer membrane camouflaged polymeric nanoparticle for targeted bioimaging. Adv Funct Mater 29:1806817. https://doi.org/10.1002/adfm.201806817
Reefman E, Kay JG, Wood SM, Offenhauser C, Brown DL, Roy S, Stanley AC, Low PC, Manderson AP, Stow JL (2010) Cytokine secretion is distinct from secretion of cytotoxic granules in NK cells. J Immunol 184:4852–4862. https://doi.org/10.4049/jimmunol.0803954
Reiners KS, Dassler J, Coch C, Pogge Von Strandmann E (2014) Role of exosomes released by dendritic cells and/or by tumor targets: regulation of NK cell plasticity. Front Immunol 5:91. https://doi.org/10.3389/fimmu.2014.00091
Rimkus T, Sirkisoon S, Harrison A, Lo HW (2017) Tumor suppressor candidate 2 (TUSC2, FUS-1) and human cancers. Discov Med 23:325–330
Romee R, Foley B, Lenvik T, Wang Y, Zhang B, Ankarlo D, Luo X, Cooley S, Verneris M, Walcheck B, Miller J (2013) NK cell CD16 surface expression and function is regulated by a disintegrin and metalloprotease-17 (ADAM17). Blood 121:3599–3608. https://doi.org/10.1182/blood-2012-04-425397
Sau S, Alsaab HO, Bhise K, Alzhrani R, Nabil G, Iyer AK (2018) Multifunctional nanoparticles for cancer immunotherapy: a groundbreaking approach for reprogramming malfunctioned tumor environment. J Control Release 274:24–34. https://doi.org/10.1016/j.jconrel.2018.01.028
Shimabukuro-Vornhagen A, Gödel P, Subklewe M, Stemmler HJ, Schlößer HA, Schlaak M, Kochanek M, Böll B, Von Bergwelt-Baildon MS (2018) Cytokine release syndrome. J ImmunoTher. Cancer 6:56. https://doi.org/10.1186/s40425-018-0343-9
Siegler EL, Kim YJ, Chen X, Siriwon N, Mac J, Rohrs JA, Bryson PD, Wang P (2017) Combination cancer therapy using chimeric antigen receptor-engineered natural killer cells as drug carriers. Mol Ther 25:2607–2619. https://doi.org/10.1016/j.ymthe.2017.08.010
Snyder KM, Hullsiek R, Mishra HK, Mendez DC, Li Y, Rogich A, Kaufman DS, Wu J, Walcheck B (2018) Expression of a recombinant high affinity IgG Fc receptor by engineered NK cells as a docking platform for therapeutic mAbs to target cancer cells. Front Immunol 9:2873–2873. https://doi.org/10.3389/fimmu.2018.02873
Soe ZC, Ou W, Gautam M, Poudel K, Kim BK, Pham LM, Phung CD, Jeong J-H, Jin SG, Choi H-G, Ku SK, Yong CS, Kim JO (2019) Development of folate-functionalized PEGylated zein nanoparticles for ligand-directed delivery of paclitaxel. Pharmaceutics 11:562. https://doi.org/10.3390/pharmaceutics11110562
Souza-Fonseca-Guimaraes F, Cursons J, Huntington ND (2019) The emergence of natural killer cells as a major target in cancer immunotherapy. Trends Immunol 40:142–158. https://doi.org/10.1016/j.it.2018.12.003
Specht HM, Ahrens N, Blankenstein C, Duell T, Fietkau R, Gaipl US, Gunther C, Gunther S, Habl G, Hautmann H, Hautmann M, Huber RM, Molls M, Offner R, Rodel C, Rodel F, Schutz M, Combs SE, Multhoff G (2015) Heat shock protein 70 (Hsp70) peptide activated natural killer (NK) cells for the treatment of patients with non-small cell lung cancer (NSCLC) after radiochemotherapy (RCTx) - from preclinical studies to a clinical phase II trial. Front Immunol 6:162. https://doi.org/10.3389/fimmu.2015.00162
Stojanovic A, Fiegler N, Brunner-Weinzierl M, Cerwenka A (2014) CTLA-4 is expressed by activated mouse NK cells and inhibits NK Cell IFN-gamma production in response to mature dendritic cells. J Immunol 192:4184–4191. https://doi.org/10.4049/jimmunol.1302091
Takaki R, Hayakawa Y, Nelson A, Sivakumar PV, Hughes S, Smyth MJ, Lanier LL (2005) IL-21 enhances tumor rejection through a NKG2D-dependent mechanism. J Immunol 175:2167–2173. https://doi.org/10.4049/jimmunol.175.4.2167
Tan L, Han S, Ding S, Xiao W, Ding Y, Qian L, Wang C, Gong W (2017) Chitosan nanoparticle-based delivery of fused NKG2D-IL-21 gene suppresses colon cancer growth in mice. Int J Nanomed 12:3095–3107. https://doi.org/10.2147/ijn.S128032
Tanaka Y, Nakazawa T, Nakamura M, Nishimura F, Matsuda R, Omoto K, Shida Y, Murakami T, Nakagawa I, Motoyama Y, Morita H, Tsujimura T, Nakase H (2019) Ex vivo-expanded highly purified natural killer cells in combination with temozolomide induce antitumor effects in human glioblastoma cells in vitro. PLoS ONE 14:e0212455. https://doi.org/10.1371/journal.pone.0212455
Tormoen GW, Crittenden MR, Gough MJ (2018) Role of the immunosuppressive microenvironment in immunotherapy. Adv Radiat Oncol 3:520–526. https://doi.org/10.1016/j.adro.2018.08.018
Tran TH, Tran TTP, Truong DH, Nguyen HT, Pham TT, Yong CS, Kim JO (2019) Toll-like receptor-targeted particles: a paradigm to manipulate the tumor microenvironment for cancer immunotherapy. Acta Biomater 94:82–96. https://doi.org/10.1016/j.actbio.2019.05.043
Truong DH, Tran TTP, Nguyen HT, Phung CD, Pham TT, Yong CS, Kim JO, Tran TH (2019) Modulating T-cell-based cancer immunotherapy via particulate systems. J Drug Target 27:145–163. https://doi.org/10.1080/1061186X.2018.1474360
Van Elssen CH, Oth T, Germeraad WT, Bos GM, Vanderlocht J (2014) Natural killer cells: the secret weapon in dendritic cell vaccination strategies. Clin Cancer Res 20:1095–1103. https://doi.org/10.1158/1078-0432.Ccr-13-2302
Viaud S, Terme M, Flament C, Taieb J, André F, Novault S, Escudier B, Robert C, Caillat-Zucman S, Tursz T, Zitvogel L, Chaput N (2009) Dendritic cell-derived exosomes promote natural killer cell activation and proliferation: a role for NKG2D ligands and IL-15Ralpha. PLoS ONE 4:e4942–e4942. https://doi.org/10.1371/journal.pone.0004942
Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S (2008) Functions of natural killer cells. Nat Immunol 9:503
Voskoboinik I, Whisstock JC, Trapani JA (2015) Perforin and granzymes: function, dysfunction and human pathology. Nat Rev Immunol 15:388–400. https://doi.org/10.1038/nri3839
Waldmann TA, Dubois S, Tagaya Y (2001) Contrasting roles of IL-2 and IL-15 in the life and death of lymphocytes: implications for immunotherapy. Immunity 14:105–110
Wang W, Erbe AK, Hank JA, Morris ZS, Sondel PM (2015) NK cell-mediated antibody-dependent cellular cytotoxicity in cancer immunotherapy. Front Immunol 6:368. https://doi.org/10.3389/fimmu.2015.00368
Wang N, Geng C, Sun H, Wang X, Li F, Liu X (2019) Hesperetin ameliorates lipopolysaccharide-induced acute lung injury in mice through regulating the TLR4-MyD88-NF-kappaB signaling pathway. Arch Pharm Res 42:1063–1070. https://doi.org/10.1007/s12272-019-01200-6
Wu L, Zhang F, Wei Z, Li X, Zhao H, Lv H, Ge R, Ma H, Zhang H, Yang B, Li J, Jiang J (2018) Magnetic delivery of Fe3O4@polydopamine nanoparticle-loaded natural killer cells suggest a promising anticancer treatment. Biomater Sci 6:2714–2725. https://doi.org/10.1039/c8bm00588e
Xu L, Huang Y, Tan L, Yu W, Chen D, Lu C, He J, Wu G, Liu X, Zhang Y (2015) Increased Tim-3 expression in peripheral NK cells predicts a poorer prognosis and Tim-3 blockade improves NK cell-mediated cytotoxicity in human lung adenocarcinoma. Int Immunopharmacol 29:635–641. https://doi.org/10.1016/j.intimp.2015.09.017
Yong S-B, Chung JY, Song Y, Kim Y-H (2018) Recent challenges and advances in genetically-engineered cell therapy. J Pharm Investig 48:199–208. https://doi.org/10.1007/s40005-017-0381-1
Zhang M, Wen B, Anton OM, Yao Z, Dubois S, Ju W, Sato N, Dilillo DJ, Bamford RN, Ravetch JV, Waldmann TA (2018) IL-15 enhanced antibody-dependent cellular cytotoxicity mediated by NK cells and macrophages. Proc Natl Acad Sci USA 115:E10915–e10924. https://doi.org/10.1073/pnas.1811615115
Zhu L, Gangadaran P, Kalimuthu S, Oh JM, Baek SH, Jeong SY, Lee SW, Lee J, Ahn BC (2018) Novel alternatives to extracellular vesicle-based immunotherapy—exosome mimetics derived from natural killer cells. Artif Cells Nanomed Biotechnol 46:S166–s179. https://doi.org/10.1080/21691401.2018.1489824
Acknowledgements
This research study was supported by the Yeungnam University in 2019.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Phung, C.D., Tran, T.H. & Kim, J.O. Engineered nanoparticles to enhance natural killer cell activity towards onco-immunotherapy: a review. Arch. Pharm. Res. 43, 32–45 (2020). https://doi.org/10.1007/s12272-020-01218-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-020-01218-1