Abstract
Cancer immunotherapy has emerged as an effective therapeutic strategy to treat cancer. Among diverse immune populations, invariant natural killer T (iNKT) cells have shown potent antitumor activity by linking innate and adaptive immune systems. Upon activation by lipid antigens on CD1d molecules, iNKT cells rapidly produce various cytokines and trigger antitumor immunity directly or indirectly by activating other antitumor immune cells. Administration of a representative iNKT cell ligand alpha-galactosylceramide (α-GalCer) or α-GalCer-pulsed APCs effectively stimulates iNKT cells and thereby induces antitumor effects. In this review, we will introduce the biology and importance of NKT cells in antitumor immunity. Previous studies have demonstrated that iNKT cells not only activate various immune cells but also reinvigorate exhausted immune cells in the tumor microenvironment. Furthermore, we will summarize the major clinical trials utilizing iNKT-based immunotherapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recent clinical trials using cancer immunotherapy have shown promising outcomes, and results are still evolving through continuous studies (Pardoll 2012; Mahoney et al. 2015; Sharma and Allison 2015). It is now generally thought that immune cells such as T cells and NK cells recognize and eliminate cancerous cells. However, many studies have also shown that these killer cells have limited potency at the advanced stage of tumors because their activity is braked by immune suppressive environmental factors, including PD-1, Tim-3 and LAG-3, and these cells became hyporesponsive exhausted cells via continuous antigenic stimulation (Wherry 2011; Cho 2017). Thus, overcoming this dysfunctional state of immune cells in tumor microenvironments is challenging. Immune checkpoint blockades such as anti-PD-1 and anti-CTLA4 antibodies rescue the dysfunction of immune systems to fight against tumors, but the clinical efficacy is only experienced in a portion of patients (Wolchok et al. 2013; Pauken and Wherry 2015). Although NKT cells comprise a small portion of immune cells, they can eradicate tumors, and we have recently found that iNKT cell activation reinvigorates exhausted CD8 T cells and NK cells (Seo et al. 2017; Bae et al. 2018). Here, we review the biology and function of NKT cells in the antitumor immunity and propose a strategy to overcome immune exhaustion via iNKT cell activation.
NKT cell biology
It is known that NKT cells are a distinct subset of immune cells co-expressing T cell receptor (TCR) and NK lineage markers (Kawano et al. 1997; Benlagha et al. 2002). The NKT cell receptor generally consists of an invariant TCRα and semi-variant TCRβ chain and recognizes lipid antigens presented by the non-classical MHC class I molecule, CD1d, which is expressed on various immune cells including monocytes, macrophages, dendritic cells (DCs), and B cells (Kawano et al. 1997; Exley et al. 2000; Benlagha et al. 2002; Godfrey and Berzins 2007).
There are 3 types of NKT cells: type I NKT, type II NKT, and NKT-like cells. Type I NKT cells are the most prevalent type of NKT cells and have a restricted TCR recombinant, the so-called invariant NKT (iNKT) cells (Godfrey et al. 2004). In mice, iNKT cells express semi-invariant TCRs with a limited Vβ repertoire, including Vβ2, Vβ7, and Vβ8.2 (Gapin et al. 2001). In humans, iNKT cells are characterized by the expression of the invariant Vα24-Jα18 TCRα chain paired with the Vβ11 TCRβ chain (Zhou et al. 2004). The representative iNKT cell ligand is α-GalCer (also known as KRN 7000) (Fig. 1), a synthetic glycosphingolipid originally isolated from a marine sponge Agelas mauritianus (Kobayashi et al. 1995).
Mode of iNKT cell activation by α-GalCer. α-GalCer loaded on CD1d molecules in antigen presenting cells (APCs) can be recognized by invariant T cell receptors (TCRs) on iNKT cells. Activated iNKT cells can initiate the production of cytokines such as IL-4 and IFNγ. Upregulated CD40L on activated iNKT cells induces activation signals on APCs via CD40-CD40L
In contrast to iNKT cells, type II NKT cells express variant TCRs and are called non-classical NKT cells (Godfrey et al. 2004; Terabe et al. 2005). Despite the diverse recombination of TCRs, type II NKT cells are also found to recognize antigens in a CD1d-dependent manner (Terabe et al. 2005). Among the 3 types of NKT cells, iNKT cells are known to have superior antitumor activity than any other type of NKT cells (Cui et al. 1997; Smyth and Godfrey 2000). Thus, we will hereafter focus on iNKT cells and their roles in cancer immunotherapy.
Antitumor mechanisms of iNKT cells
iNKT cells are known to exert antitumor activity in direct or indirect manners (Cui et al. 1997; Smyth and Godfrey 2000). iNKT cells can rapidly produce various and large quantities of cytokines in response to stimuli, which link innate and adaptive immunity (Smyth and Godfrey 2000). Tumors expressing CD1d molecules can be directly killed by these iNKT cells through the Fas–FasL interaction, perforin, granzyme B, and tumor necrosis factor α-related apoptosis-inducing ligand (TRAIL) (Kawano et al. 1998).
As mentioned above, iNKT cells secrete various cytokines upon activation and indirectly activate antitumor mechanisms (Coquet et al. 2007, 2008). iNKT cells produce IFNγ, IL-2, -4, -5, -6, -10, -13, -17, -21, -22, TNF-α, TGF-β, and GM-CSF, which affect a broad spectrum of immune cells, including dendritic cells, macrophages, neutrophils, NK cells, and T and B cells (Coquet et al. 2007, 2008). In addition, activated iNKT cells express CD40L and induce the maturation of DCs through CD40–CD40L interaction (Kitamura et al. 1999). Mature DCs then express higher costimulatory molecules such as CD40, CD80 and CD86 and produce larger quantities of IL-12, which in turn activate IFNγ secretion from iNKT cells, NK cells, and CD8 T cells, and accelerate this positive feedback loop (Cui et al. 1997; Kitamura et al. 1999; Taraban et al. 2008). Moreover, DCs upregulate the expression of NKG2D ligands and CD70, a ligand for CD27, and contribute to killer cell activation (Cui et al. 1997; Kawano et al. 1997; Taraban et al. 2008). Consequently, this action of iNKT cells turns on antitumor mechanisms to eliminate tumor cells. Furthermore, CD8α+ DCs cross-primed by iNKT cells contribute to antitumor immunity via secretion of the chemokine CCL17, which attracts and primes CD8 T cells through CCL17-CCR4 (Semmling et al. 2010). B cells are also known to play a role in iNKT-induced antitumor immunity via upregulated IgG production for antibody-dependent cellular cytotoxicity (ADCC) (Moreno et al. 2008).
Immune exhaustion in tumor microenvironments and reinvigoration via activation of iNKT cells
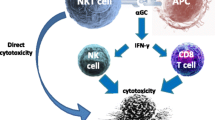
In the tumor environment, T and NK cells are frequently exhausted, which is characterized by high expression of co-inhibitory receptors such as PD-1, Tim-3, LAG3, and TIGIT (Wherry 2011; Mognol et al. 2017). These exhausted T and NK cells are defective in proliferation and effector functions. However, our group has recently found that iNKT cell activation by α-GalCer efficiently reverses the function of exhausted tumor NK cells via IL-21 (Seo et al. 2017, 2018) (Fig. 2). Further studies have shown that activation of iNKT cells by α-GalCer reinvigorates not only NK cells but also exhausted CD8 T cells in tumors via IL-2 and IL-12 (Bae et al. 2018). These results revealed that iNKT cell activation plays a crucial role in overcoming immune exhaustion in the tumor microenvironment, suggesting that a combination of iNKT cell agonists is an excellent strategy for current cancer immunotherapy.
The effect of activated iNKT cells on anti-tumor immunity. Upon activation of iNKT cells by α-GalCer, iNKT cells secrete IFNγ rapidly. Matured APCs, by costimulatory signals, including CD40-CD40L, start to produce IL-12. IL-12 triggers IFNγ secretion by NKT cells as well as NK cells. IFNγ stimulates the antitumor activities of both CD8 T cells and NK cells. The exhaustion of NK cells in advanced tumors could be reversed by IL-21 secreted by activated NKT cells. Furthermore, IL-2 and IL-12 could reinvigorate exhausted CD8 T cells in the tumor microenvironment. Not only iNKT cells but also activated CD8 T and NK cells participate in antitumor immunity
Despite the potent antitumor activity of α-GalCer, we need to optimize the dose and schedule of this therapy because repetitive injection of α-GalCer was shown to induce NKT cell anergy (Parekh et al. 2005; Sullivan and Kronenberg 2005). We and other groups have found that the administration of α-GalCer-loaded APCs showed prolonged and enhanced NKT cell responses compared to the injection of free α-GalCer (Chang et al. 2005; Chung et al. 2006; Kim et al. 2008, 2014a, b) and IL-2 has enough potency to break anergic NKT states (Parekh et al. 2005). In addition, PD-1 is highly induced by α-GalCer stimulation, supporting the idea that the combination of anti-PD-1 with α-GalCer can be an efficient way to treat advanced tumors (Chang et al. 2008; Parekh et al. 2009; Bae et al. 2018).
Clinical trials harnessing iNKT cells
Numerous clinical attempts utilizing NKT cells have been made and are on-going in cancer patients. A representative iNKT cell ligand, α-Galcer, was used to activate the antitumor effects of iNKT cells in cancer patients. A phase 1 clinical study was conducted by slow i.v. administration of various doses of α-GalCer in patients with solid tumors and 7 out of 24 patients showed stable disease progression without serious adverse effects (Table 1) (Giaccone et al. 2002). Nieda et al. conducted a phase 1 clinical study using α-GalCer-pulsed, monocyte derived immature DCs in patients with metastatic malignant tumors and demonstrated that α-GalCer-pulsed DCs increased the activation of T cells, the number and cytotoxicity of NK cells, and the levels of IFNγ and IL-12 in sera (Nieda et al. 2004). Chang et al. used α-GalCer-loaded mature DCs in the clinical trials of advanced cancer patients. They clearly showed more than 100-fold expansion of iNKT cells after injecting patients with α-GalCer-loaded mature DCs compared to unloaded DCs. Interleukin-12 p40 and IFNγ inducible protein-10 (IP-10) in sera were also increased in α-GalCer-loaded mature DC-treated groups (Chang et al. 2005). Motohashi et al. performed phase 1 and -2 clinical trials in patients with advanced non-small cell lung cancer using this α-GalCer-pulsed APCs, from total PBMCs with GM-CSF and IL-2 for 7 days. This trial also observed higher numbers of NK and NKT cells and increased levels of IFNγ in the peripheral blood (Motohashi et al. 2009). Nagato et al. also conducted a clinical study using α-GalCer-loaded APCs and showed a significant increase in iNKT cell numbers among tumor-infiltrating lymphocytes (TILs) and increased IFNγ production in TILs (Nagato et al. 2012).
Autologous B cells and monocytes from PBMCs can be alternatively chosen for the APC compartment. Choi et al. showed that B cells and monocytes (B/Mo) pulsed with α-GalCer and transfected with recombinant HPV E6/E7 gene by a 1-day process efficiently activated NK and NKT cells as well as HPV E6/E7 specific CD4 and CD8 T cells in HPV type 16 or 18 positive recurrent cervical carcinoma patients (Choi et al. 2018).
The number of iNKT cells is significantly reduced in cancer patients (Giaccone et al. 2002). To supplement NKT cells in cancer patients, clinical trials using in vitro-expanded NKT cells were conducted in patients with advanced or non-small cell lung cancer. Exley et al. used expanded autologous NKT cells from PBMCs with IL-2 and CD3 mAb and observed significant type 1 responses in iNKT cell recipients with grade 1–2 toxicity (Exley et al. 2017). Yamasaki et al. performed a phase 2 clinical study using ex vivo expanded NKT cells with IL-2 and α-GalCer and APCs pulsed with α-GalCer in patients with head and neck squamous cell carcinoma. Fifty percent of the patients showed stable disease progression (Yamasaki et al. 2011).
Conclusion
Cancer immunotherapy is one of the most powerful tools for the treatment of tumors. However, the current immunotherapy shows a limited response rate in cancer patients (Ribas and Wolchok 2018). Activation of iNKT cells has been shown to quickly and persistently potentiate antitumor responses by not only priming various immune cells but also reinvigorating exhausted CD8 T and NK cells. To date, clinical trials using this method have shown that they induce promising results in diverse cancer patients without severe adverse effects. Thus, the combination of iNKT cell-based immunotherapy is a good option for cancer patients who are resistant to current immunotherapies.
References
Bae E-A, Seo H, Kim B-S, Choi J, Jeon I, Shin K-S, Koh C-H, Song B, Kim I-K, Min BS, Han YD, Shin SJ, Kang C-Y (2018) Activation of NKT cells in an anti-PD-1–resistant tumor model enhances antitumor immunity by reinvigorating exhausted CD8 T cells. Cancer Res 78:5315–5326
Benlagha K, Kyin T, Beavis A, Teyton L, Bendelac A (2002) A thymic precursor to the NK T cell lineage. Science 296:553–555
Chang DH, Osman K, Connolly J, Kukreja A, Krasovsky J, Pack M, Hutchinson A, Geller M, Liu N, Annable R (2005) Sustained expansion of NKT cells and antigen-specific T cells after injection of α-galactosyl-ceramide loaded mature dendritic cells in cancer patients. J Exp Med 201:1503–1517
Chang W-S, Kim J-Y, Kim Y-J, Kim Y-S, Lee J-M, Azuma M, Yagita H, Kang C-Y (2008) Cutting edge: programmed death-1/programmed death ligand 1 interaction regulates the induction and maintenance of invariant NKT cell anergy. J Immunol 181:6707–6710
Cho JH (2017) Immunotherapy for non-small-cell lung cancer: current status and future obstacles. Immune Netw 17:378–391
Choi C, Choi H, Lee J, Kang E, Cho D, Kim Y, Kim D, Seo H, Park M, Kim W, Oh T, Kang C-Y, Kim B-G (2018) 960P Phase I study of BVAC-C in HPV type 16 or 18 positive recurrent cervical carcinoma: safety, clinical activity and immunologic correlates. Ann Oncol 29(mdy285):168
Chung Y, Kim B-S, Kim Y-J, Ko H-J, Ko S-Y, Kim D-H, Kang C-Y (2006) CD1d-restricted T cells license B cells to generate long-lasting cytotoxic antitumor immunity in vivo. Cancer Res 66:6843–6850
Coquet JM, Kyparissoudis K, Pellicci DG, Besra G, Berzins SP, Smyth MJ, Godfrey DI (2007) IL-21 is produced by NKT cells and modulates NKT cell activation and cytokine production. J Immunol 178:2827–2834
Coquet JM, Chakravarti S, Kyparissoudis K, Mcnab FW, Pitt LA, Mckenzie BS, Berzins SP, Smyth MJ, Godfrey DI (2008) Diverse cytokine production by NKT cell subsets and identification of an IL-17–producing CD4 − NK1. 1 − NKT cell population. Proc Natl Acad Sci USA 105:11287–11292
Cui J, Shin T, Kawano T, Sato H, Kondo E, Toura I, Kaneko Y, Koseki H, Kanno M, Taniguchi M (1997) Requirement for Vα14 NKT cells in IL-12-mediated rejection of tumors. Science 278:1623–1626
Exley M, Garcia J, Wilson SB, Spada F, Gerdes D, Tahir SM, Patton KT, Blumberg RS, Porcelli S, Chott A, Balk SP (2000) CD1d structure and regulation on human thymocytes, peripheral blood T cells, B cells and monocytes. Immunology 100:37–47
Exley MA, Friedlander P, Alatrakchi N, Vriend L, Yue S, Sasada T, Zeng W, Mizukami Y, Clark J, Nemer D (2017) Adoptive transfer of invariant NKT cells as immunotherapy for advanced melanoma: a phase I clinical trial. Clin Cancer Res 23:3510–3519
Gapin L, Matsuda JL, Surh CD, Kronenberg M (2001) NKT cells derive from double-positive thymocytes that are positively selected by CD1d. Nat Immunol 2:971
Giaccone G, Punt CJ, Ando Y, Ruijter R, Nishi N, Peters M, Von Blomberg BME, Scheper RJ, Van Der Vliet HJ, Van Den Eertwegh AJ (2002) A phase I study of the natural killer T-cell ligand α-galactosylceramide (KRN7000) in patients with solid tumors. Clin Cancer Res 8:3702–3709
Godfrey DI, Berzins SP (2007) Control points in NKT-cell development. Nat Rev Immunol 7:505
Godfrey DI, Macdonald HR, Kronenberg M, Smyth MJ, Van Kaer L (2004) NKT cells: what’s in a name? Nat Rev Immunol 4:231
Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E (1997) CD1d-restricted and TCR-mediated activation of Vα14 NKT cells by glycosylceramides. Science 278:1626–1629
Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Sato H, Kondo E, Harada M, Koseki H, Nakayama T (1998) Natural killer-like nonspecific tumor cell lysis mediated by specific ligand-activated Vα14 NKT cells. Proc Natl Acad Sci USA 95:5690–5693
Kim YJ, Ko HJ, Kim YS, Kim DH, Kang S, Kim JM, Chung Y, Kang CY (2008) α-Galactosylceramide-loaded, antigen-expressing B cells prime a wide spectrum of antitumor immunity. Int J Cancer 122:2774–2783
Kim E-K, Jeon I, Seo H, Park Y-J, Song B, Lee K-A, Jang Y, Chung Y, Kang C-Y (2014a) Tumor-derived osteopontin suppresses antitumor immunity by promoting extramedullary myelopoiesis. Cancer Res 74:6705–6716
Kim E, Seo H, Chae M, Jeon I, Song B, Park Y, Ahn H, Yun C, Kang CY (2014b) Enhanced antitumor immunotherapeutic effect of B-cell-based vaccine transduced with modified adenoviral vector containing type 35 fiber structures. Gene Ther 21:106
Kitamura H, Iwakabe K, Yahata T, Nishimura S-I, Ohta A, Ohmi Y, Sato M, Takeda K, Okumura K, Van Kaer L (1999) The natural killer T (NKT) cell ligand α-galactosylceramide demonstrates its immunopotentiating effect by inducing interleukin (IL)-12 production by dendritic cells and IL-12 receptor expression on NKT cells. J Exp Med 189:1121–1128
Kobayashi E, Motoki K, Uchida T, Fukushima H, Koezuka Y (1995) KRN7000, a novel immunomodulator, and its antitumor activities. Oncol Res 7:529–534
Mahoney KM, Rennert PD, Freeman GJ (2015) Combination cancer immunotherapy and new immunomodulatory targets. Nat Rev Drug Discov 14:561
Mognol GP, Spreafico R, Wong V, Scott-Browne JP, Togher S, Hoffmann A, Hogan PG, Rao A, Trifari S (2017) Exhaustion-associated regulatory regions in CD8+ tumor-infiltrating T cells. Proc Natl Acad Sci USA 114:E2776–E2785
Moreno M, Mol BM, Von Mensdorff-Pouilly S, Verheijen RH, Von Blomberg BME, Van Den Eertwegh AJ, Scheper RJ, Bontkes HJ (2008) Toll-like receptor agonists and invariant natural killer T-cells enhance antibody-dependent cell-mediated cytotoxicity (ADCC). Cancer Lett 272:70–76
Motohashi S, Nagato K, Kunii N, Yamamoto H, Yamasaki K, Okita K, Hanaoka H, Shimizu N, Suzuki M, Yoshino I (2009) A phase I–II study of α-galactosylceramide-pulsed IL-2/GM-CSF-cultured peripheral blood mononuclear cells in patients with advanced and recurrent non-small cell lung cancer. J Immunol 182:2492–2501
Nagato K, Motohashi S, Ishibashi F, Okita K, Yamasaki K, Moriya Y, Hoshino H, Yoshida S, Hanaoka H, Fujii S-I (2012) Accumulation of activated invariant natural killer T cells in the tumor microenvironment after α-galactosylceramide-pulsed antigen presenting cells. J Clin Immunol 32:1071–1081
Nieda M, Okai M, Tazbirkova A, Lin H, Yamaura A, Ide K, Abraham R, Juji T, Macfarlane DJ, Nicol AJ (2004) Therapeutic activation of Vα24+ Vβ11+ NKT cells in human subjects results in highly coordinated secondary activation of acquired and innate immunity. Blood 103:383–389
Pardoll DM (2012) The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12:252
Parekh VV, Wilson MT, Olivares-Villagómez D, Singh AK, Wu L, Wang C-R, Joyce S, Van Kaer L (2005) Glycolipid antigen induces long-term natural killer T cell anergy in mice. J Clin Invest 115:2572–2583
Parekh VV, Lalani S, Kim S, Halder R, Azuma M, Yagita H, Kumar V, Wu L, Van Kaer L (2009) PD-1/PD-L blockade prevents anergy induction and enhances the anti-tumor activities of glycolipid-activated invariant NKT cells. J Immunol 182:2816–2826
Pauken KE, Wherry EJ (2015) Overcoming T cell exhaustion in infection and cancer. Trends Immunol 36:265–276
Ribas A, Wolchok JD (2018) Cancer immunotherapy using checkpoint blockade. Science 359:1350–1355
Semmling V, Lukacs-Kornek V, Thaiss CA, Quast T, Hochheiser K, Panzer U, Rossjohn J, Perlmutter P, Cao J, Godfrey DI (2010) Alternative cross-priming through CCL17-CCR4-mediated attraction of CTLs toward NKT cell-licensed DCs. Nat Immunol 11:313
Seo H, Jeon I, Kim B-S, Park M, Bae E-A, Song B, Koh C-H, Shin K-S, Kim I-K, Choi K, Oh T, Min J, Min BS, Han YD, Kang S-J, Shin SJ, Chung Y, Kang C-Y (2017) IL-21-mediated reversal of NK cell exhaustion facilitates anti-tumour immunity in MHC class I-deficient tumours. Nat Commun 8:15776
Seo H, Kim B-S, Bae E-A, Min BS, Han YD, Shin SJ, Kang C-Y (2018) IL21 therapy combined with PD-1 and Tim-3 blockade provides enhanced NK cell antitumor activity against MHC class I-deficient tumors. Cancer Immunol Res 6:685–695
Sharma P, Allison JP (2015) Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell 161:205–214
Smyth MJ, Godfrey DI (2000) NKT cells and tumor immunity—a double-edged sword. Nat Immunol 1:459
Sullivan BA, Kronenberg M (2005) Activation or anergy: NKT cells are stunned by α-galactosylceramide. J Clin Invest 115:2328–2329
Taraban VY, Martin S, Attfield KE, Glennie MJ, Elliott T, Elewaut D, Van Calenbergh S, Linclau B, Al-Shamkhani A (2008) Invariant NKT cells promote CD8+ cytotoxic T cell responses by inducing CD70 expression on dendritic cells. J Immunol 180:4615–4620
Terabe M, Swann J, Ambrosino E, Sinha P, Takaku S, Hayakawa Y, Godfrey DI, Ostrand-Rosenberg S, Smyth MJ, Berzofsky JA (2005) A nonclassical non-Vα14 Jα18 CD1d-restricted (type II) NKT cell is sufficient for down-regulation of tumor immunosurveillance. J Exp Med 202:1627–1633
Wherry EJ (2011) T cell exhaustion. Nat Immunol 12:492
Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon R-A, Reed K (2013) Nivolumab plus ipilimumab in advanced melanoma. New Engl J Med 369:122–133
Yamasaki K, Horiguchi S, Kurosaki M, Kunii N, Nagato K, Hanaoka H, Shimizu N, Ueno N, Yamamoto S, Taniguchi M (2011) Induction of NKT cell-specific immune responses in cancer tissues after NKT cell-targeted adoptive immunotherapy. Clin Immunol 138:255–265
Zhou D, Mattner J, Cantu C, Schrantz N, Yin N, Gao Y, Sagiv Y, Hudspeth K, Wu Y-P, Yamashita T (2004) Lysosomal glycosphingolipid recognition by NKT cells. Science 306:1786–1789
Acknowledgements
This work was supported by grants from the Basic Science Research Program (NRF-2015R1A2A1A10055844) and the Bio & Medical Technology Development Program (NRF-2016M3A9B5941426).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no potential conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bae, EA., Seo, H., Kim, IK. et al. Roles of NKT cells in cancer immunotherapy. Arch. Pharm. Res. 42, 543–548 (2019). https://doi.org/10.1007/s12272-019-01139-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-019-01139-8