Abstract
The sodium-glucose cotransporter 2 (SGLT2) is responsible for most glucose reabsorption in the kidney and has been proposed as a novel therapeutic target for the treatment of type 2 diabetes. In the present study, the combinatory effects of SGLT2 selective inhibitor ipragliflozin and various antidiabetic drugs in high-fat diet and streptozotocin-nicotinamide-induced type 2 diabetic mice were investigated. Ipragliflozin dose-dependently increased urinary glucose excretion and improved glucose tolerance. In addition, each antidiabetic drug (mitiglinide, glibenclamide, sitagliptin, insulin, metformin, voglibose, or rosiglitazone) also significantly improved glucose tolerance without affecting urinary glucose excretion. Combination treatment of ipragliflozin with each antidiabetic drug additively improved glucose tolerance. In these experiments, ipragliflozin-induced increases in urinary glucose excretion were not influenced by combination treatment with antidiabetic drugs. Further, ipragliflozin did not affect antidiabetic drug-induced insulinotropic action (mitiglinide and glibenclamide), increases in plasma glucagon-like peptide-1 and insulin levels via inhibition of dipeptidyl peptidase 4 activity (sitagliptin), increases in plasma insulin level (insulin), decreases in hepatic phosphoenolpyruvate carboxykinase activity (metformin), inhibition of small intestinal disaccharidase activity (voglibose), or improvement of impaired insulin secretion (rosiglitazone). These results suggest that combination treatment of ipragliflozin with various antidiabetic drugs additively enhances the improvement in glucose tolerance without affecting each drug’s unique pharmacological effects. Ipragliflozin may therefore be expected to be effective when administered as part of a combination regimen in the treatment of type 2 diabetes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The number of patients with diabetes is dramatically increasing due to increasing prevalence of obesity and physical inactivity and is expected to rise to 366 million in 2030 (Wild et al. 2004). Approximately 90 % of all patients with diabetes have type 2 diabetes, a progressive metabolic disease characterized by chronic hyperglycemia due to insulin resistance and impaired insulin secretion. However, while many antidiabetic drugs have been developed and used for treatment, most type 2 diabetic patients’ therapeutic goals are still not achieved (Nathan et al. 2008), highlighting the need for efficient new therapeutic strategies, including combination therapy, for treating type 2 diabetes.
In recent years, inhibitors of sodium-glucose cotransporter (SGLT) 2, which stimulate glucose excretion in the urine, have been proposed as novel drugs for treating type 2 diabetes (Chao 2014), with several shown to improve hyperglycemia in this patient population (Abdul-Ghani et al. 2012). In our research, we identified the SGLT2 selective inhibitor ipragliflozin, which possesses potent and selective SGLT2 inhibitory activity and reduces hyperglycemia through inducing urinary glucose excretion in type 2 diabetic animal models as well as human patients (Tahara et al. 2012; Kashiwagi et al. 2014). Because SGLT2 inhibitors exert their antihyperglycemic effects via urinary glucose excretion, differing from the pharmacological mechanisms of existing antidiabetic drugs (insulin secretion, inhibition of gluconeogenesis, improvement of insulin resistance, inhibition of carbohydrate digestion), an additive effect may be expected when administered in combination. Indeed, coadministration of SGLT2 inhibitors and some antidiabetic drugs has been reported to additively improve hyperglycemia and diabetic complications (Bailey et al. 2013; Bays 2013). However, few studies have evaluated the coadministration effects of SGLT2 inhibitors with many variations of antidiabetic drugs commonly used in clinical settings or assessed the effects of coadministration on the pharmacological mechanism of those drugs.
Here, we investigated the effects of combination treatment of the SGLT2 selective inhibitor ipragliflozin with various antidiabetic drugs mitiglinide (short-acting insulin secretagogue: stimulates insulin release by closing the ATP-sensitive potassium (KATP) channel in pancreatic β-cells), glibenclamide (sulfonylurea: stimulates insulin release by closing the KATP channels on pancreatic β-cells), insulin, sitagliptin (dipeptidyl peptidase 4 [DPP-4] inhibitor: stimulates glucose-dependent insulin release via glucagon-like peptide 1 [GLP-1] by inhibiting its degradation by DPP-4), metformin (biguanide: reduces gluconeogenesis by increasing AMP-activated protein kinase signaling, resulting in inhibition of gluconeogenetic genes such as phosphoenolpyruvate carboxykinase [PEPCK] in the liver), voglibose (α-glucosidase inhibitor: reduces glucose absorption by inhibiting intestinal disaccharidases, sucrase and maltase), and rosiglitazone (thiazolidinedione: improves insulin resistance by activating peroxisome proliferator-activated receptor-γ [PPAR-γ] in fat and muscle) (Tran et al. 2015) on antidiabetic and mechanism-based pharmacological effects in high-fat diet and streptozotocin-nicotinamide-induced type 2 diabetic mice, which exhibit moderate glucose intolerance with impairment of insulin secretion and insulin resistance.
Materials and methods
Materials
Ipragliflozin and sitagliptin were synthesized at Astellas Pharma Inc. (Ibaraki, Japan). Streptozotocin, metformin, and glibenclamide were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA), and insulin (Novolin®R 100) was purchased from Novo Nordisk Pharma, Ltd. (Tokyo, Japan). Mitiglinide (Glufast®), rosiglitazone maleate (Avandia®), and voglibose (BASEN®) were purchased from Kissei Pharmaceutical Co., Ltd. (Nagano, Japan), GlaxoSmithKline (Philadelphia, PA, USA), and Takeda Pharmaceutical Company, Ltd. (Osaka, Japan), respectively, and purified at Astellas Pharma Inc. Insulin was diluted with physiological saline and administered intraperitoneally. All other drugs were dissolved or suspended in 0.5 % methylcellulose solution and administered orally via stomach tube. The vehicle treatment group received a 0.5 % methylcellulose solution orally. Doses of all drugs were expressed as the free base form.
Animal models
Male ICR mice were purchased from Japan SLC, Inc. (Shizuoka, Japan) at age 6 weeks and fed either a normal chow diet consisting (as a percentage of total calories [kcal]) of 10 % fat, 70 % carbohydrate, and 20 % protein (total caloric energy value = 3.85 kcal/g; D12450B; Research Diets, Inc., New Brunswick, NJ, USA) or a high-fat diet consisting of 45 % fat, 35 % carbohydrate, and 20 % protein (total caloric energy value = 4.73 kcal/g; D12451; Research Diet, Inc.). High-fat diet and streptozotocin-nicotinamide-induced type 2 diabetic mice created by intraperitoneally administering nicotinamide (1000 mg/kg) and streptozotocin (150 mg/kg), as previously described (Tahara et al. 2013), were grouped to attain uniform mean blood glucose levels among groups. All mice were housed under conventional conditions with controlled temperature, humidity, and light (12-h light–dark cycle) and were provided diet and water ad libitum. Animals were handled and cared for in accordance with the Guide for the Care and Use of Laboratory Animals, and all procedures were approved by the Animal Ethical Committee of Astellas Pharma Inc.
Effects of ipragliflozin and antidiabetic drugs on glucose tolerance
Examination 1: Single treatment at various doses (0.01–1 mg/kg) of ipragliflozin was conducted in diabetic mice fasted overnight. Liquid meal (ENSURE® H containing carbohydrates at 206 mg/mL, fat at 53 mg/mL, and protein at 53 mg/mL; Abbott, Osaka, Japan) was orally administered via stomach tube at a volume of 20 mL/kg at 30 min after drug dosing, after which blood samples for measurement of blood glucose levels were taken at each point for 2 h.
Examination 2: Single treatment of ipragliflozin (0.1 mg/kg), mitiglinide (1 mg/kg), glibenclamide (3 mg/kg), sitagliptin (1 mg/kg), insulin (0.5 IU/kg), metformin (300 mg/kg), or voglibose (0.3 mg/kg), or combination treatment of ipragliflozin and each antidiabetic drug was conducted in diabetic mice fasted overnight. In all groups except the mitiglinide-treated group, liquid meal was orally administered 30 min after drug dosing, after which blood samples were taken at each point for 2 h. In the mitiglinide-treated groups, ipragliflozin was administered 30 min before liquid meal loading, but mitiglinide was administered just before the loading, meaning that combination administration times for these two drugs were staggered. To investigate additional parameters, the experimental protocol described above was repeated, except blood samples for measurement of plasma insulin, GLP-1, and DPP-4 activity were collected 10 min after liquid meal loading, and liver samples for measurement of PEPCK activity and upper small intestine for measurement of disaccharidase (maltase and sucrase) activities were collected under isoflurane anesthesia 1 h after liquid meal loading.
Examination 3: Diabetic mice were treated with vehicle or rosiglitazone (3 mg/kg, q.d.) for 7 days prior to fasting. At 12 h after their last meal, mice were treated with vehicle or ipragliflozin (0.1 mg/kg), and after 30 min, liquid meal was administered, and blood samples for measurement of blood glucose levels were collected. To investigate additional parameters, the experimental protocol described above was repeated, except blood samples for measurement of plasma insulin level were collected 10 min after liquid meal loading as described above.
Effects of ipragliflozin and antidiabetic drugs on urinary glucose excretion
Examination 1: Single treatment at various doses (0.01–1 mg/kg) of ipragliflozin was conducted in diabetic mice, and spontaneously voided urine was collected for 24 h while the animals were kept in metabolic cages. After urine volume had been measured, the glucose concentration in the urine was measured.
Examination 2: Treatment of ipragliflozin (0.1 mg/kg, q.d.), mitiglinide (1 mg/kg, b.i.d.), glibenclamide (3 mg/kg, b.i.d.), sitagliptin (1 mg/kg, q.d.), insulin (0.5 IU/kg, b.i.d.), metformin (300 mg/kg, b.i.d.), or voglibose (0.3 mg/kg, b.i.d.), or combination treatment of ipragliflozin and each antidiabetic drug was conducted in diabetic mice, and spontaneously voided urine was collected.
Examination 3: Diabetic mice were treated with vehicle or rosiglitazone (3 mg/kg, q.d.) for 7 days, after which animals were treated with vehicle or ipragliflozin (0.1 mg/kg, q.d.), and spontaneously voided urine was collected.
Biochemical determination
Blood and urinary glucose levels were measured using Glucose CII-Test reagent (Wako Pure Chemicals, Osaka, Japan). Plasma insulin and active GLP-1 levels were determined using an insulin EIA test kit (Morinaga Institute of Biological Sciences, Inc., Kanagawa, Japan) and an active GLP-1 ELISA kit (Linco Research, Inc., St. Charles, MO, USA), respectively. Activities of plasma DPP-4, liver PEPCK, and upper small intestinal disaccharidases (maltase and sucrase) were measured as described previously, with some modification (Tahara et al. 2009; Hansen et al. 1976; Dahlqvist 1984).
Statistical analysis
The experimental results are expressed as the mean ± S.E.M. The significance of differences between two groups was determined using Student’s t test. The significance of differences between multiple groups was assessed using Dunnett’s or Tukey’s multiple comparison test. A value of P < 0.05 was taken as significant. Statistical and data analyses were conducted using GraphPad Prism 5 (GraphPad Software, La Jolla, CA, USA).
Results
Effects of single or combination treatment with ipragliflozin, mitiglinide, glibenclamide, sitagliptin, and insulin on glucose tolerance
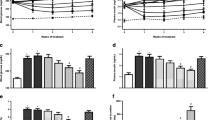
Compared to normal mice, significant increases in nonfasting blood glucose (normal: 145 ± 4 mg/dL, diabetic: 257 ± 7 mg/dL; P < 0.05) and plasma insulin (normal: 1.28 ± 0.04 ng/mL, diabetic: 2.86 ± 0.03 ng/mL; P < 0.05) levels were noted among diabetic mice. During the liquid meal tolerance test, glucose tolerance in diabetic mice declined due to loss of insulin secretion. Single treatment of ipragliflozin (0.01–1 mg/kg) dose-dependently improved glucose tolerance without significantly affecting plasma insulin levels (tended to decrease slightly), an effect which was significant at doses of 0.03 mg/kg or higher (Fig. 1). We selected the subminimum effective dose (0.1 mg/kg) for investigating the pharmacological effects of ipragliflozin. Single treatment with mitiglinide (1 mg/kg), glibenclamide (3 mg/kg), sitagliptin (1 mg/kg), or insulin (0.5 IU/kg) also significantly improved glucose tolerance with increases in plasma insulin levels (Fig. 2). Combination treatment of ipragliflozin with each of these antidiabetic drugs significantly and additively improved glucose tolerance without change in antidiabetic drug-induced increases in plasma insulin levels. While sitagliptin also significantly increased plasma GLP-1 level and decreased plasma DPP-4 activity in addition to effects on plasma insulin levels, combination treatment with ipragliflozin did not affect these parameters (Fig. 3).
Effects of ipragliflozin on blood glucose and plasma insulin levels during the liquid meal tolerance test in type 2 diabetic mice. a Time course of changes in the blood glucose level and b the area under the blood glucose concentration–time curve (AUC) during the liquid meal tolerance test. c Plasma insulin level at 10 min into the liquid meal tolerance test. The values are the mean ± SEM for four animals in each group. *P < 0.05 versus normal group, #P < 0.05 versus diabetic vehicle group
Effects of single or combination treatment with ipragliflozin and mitiglinide, glibenclamide, sitagliptin, or insulin on blood glucose and plasma insulin levels during the liquid meal tolerance test in type 2 diabetic mice. a Time course of changes in the blood glucose level and b the area under the blood glucose concentration–time curve (AUC) during the liquid meal tolerance test. c Plasma insulin level at 10 min into the liquid meal tolerance test. The values are the mean ± SEM for four animals in each group. *P < 0.05 versus normal group, #P < 0.05 versus diabetic vehicle group, $P < 0.05 versus each antidiabetic drug group
Effects of single or combination treatment with ipragliflozin (0.1 mg/kg) and sitagliptin (1 mg/kg) on plasma GLP-1 level and DPP-4 activity during the liquid meal tolerance test in type 2 diabetic mice. Plasma a GLP-1 level and (B) DPP-4 activity at 10 min into the liquid meal tolerance test. The values are the mean ± SEM for four animals in each group. #P < 0.05 versus diabetic vehicle group
Effects of single or combination treatment with ipragliflozin, metformin, and voglibose on glucose tolerance
Single treatment with metformin or voglibose significantly improved glucose tolerance without affecting plasma insulin levels (Figs. 4a–c and 5a–c). Combination treatment with ipragliflozin and these antidiabetic drugs also significantly and additively improved glucose tolerance without change plasma insulin levels. Metformin significantly reduced hepatic PEPCK activities (Fig. 4d), and voglibose significantly decreased activities of small intestinal disaccharidases, maltase (Fig. 5d), and sucrase (data not shown). Combination treatment with ipragliflozin did not affect these parameters.
Effects of single or combination treatment with ipragliflozin and metformin on blood glucose, plasma insulin and hepatic PEPCK activity during the liquid meal tolerance test in type 2 diabetic mice. a Time course of changes in the blood glucose level and b the area under the blood glucose concentration–time curve (AUC) during the liquid meal tolerance test. c Plasma insulin level at 10 min and (D) hepatic PEPCK activity at 1 h into the liquid meal tolerance test. The values are the mean ± SEM for four animals in each group. *P < 0.05 versus normal group, #P < 0.05 versus diabetic vehicle group, $P < 0.05 versus metformin group
Effects of single or combination treatment with ipragliflozin and voglibose on blood glucose, plasma insulin and intestinal maltase activity during the liquid meal tolerance test in type 2 diabetic mice. a Time course of changes in the blood glucose level and b the area under the blood glucose concentration–time curve (AUC) during the liquid meal tolerance test. c Plasma insulin level at 10 min and d intestinal maltase activity at 1 h into the liquid meal tolerance test. The values are the mean ± SEM for four animals in each group. *P < 0.05 versus normal group, #P < 0.05 versus diabetic vehicle group, $P < 0.05 versus voglibose group
Effects of single or combination treatment with ipragliflozin and rosiglitazone on glucose tolerance
Compared to normal mice, significant increases in the fasting blood glucose (normal: 109 ± 5 mg/dL, diabetic: 135 ± 4 mg/dL) and plasma insulin (normal: 0.74 ± 0.13 ng/mL, diabetic: 1.25 ± 0.13 ng/mL) levels in type 2 diabetic mice were observed (Fig. 6); diabetic mice exhibited mild insulin resistance. One-week repeated treatment with rosiglitazone (3 mg/kg) significantly reduced fasting blood glucose and plasma insulin levels and improved glucose tolerance and impaired insulin secretion during the liquid meal tolerance test. Single treatment of ipragliflozin or combination treatment with rosiglitazone significantly and additively improved glucose tolerance without affecting rosiglitazone-induced improvement of impaired insulin secretion.
Effects of single or combination treatment of ipragliflozin and rosiglitazone on blood glucose and plasma insulin levels in type 2 diabetic mice. Rosiglitazone (3 mg/kg) was repeatedly administered for 7 days, and ipragliflozin (0.1 mg/kg) was singly administered 30 min before liquid meal loading. a Blood glucose and plasma insulin levels under fasting conditions. b Time course of changes in the blood glucose level and the area under the blood glucose concentration–time curve (AUC) during the liquid meal tolerance test. c Plasma insulin level at 10 min into the liquid meal tolerance test. The values are the mean ± SEM for four animals in each group. *P < 0.05 versus normal group, #P < 0.05 versus diabetic vehicle group, $P < 0.05 versus rosiglitazone group
Effects of single or combination treatment with ipragliflozin and antidiabetic drugs on urinary glucose excretion
Compared to normal mice, slight but significant glucosuria (normal: 2.0 ± 0.4 mg/day, diabetic: 6.2 ± 1.6 mg/day) was observed in diabetic mice. Ipragliflozin (0.01–1 mg/kg) dose-dependently increased urinary glucose excretion, and this effect was significant at doses of 0.03 mg/kg or higher (Fig. 7). None of the antidiabetic drugs evaluated in this study significantly affected urinary glucose excretion (Fig. 8). Combination treatment of ipragliflozin and each antidiabetic drug significantly increased urinary glucose excretion, and these effects were not remarkably changed compared with that observed on single treatment of ipragliflozin. In addition, ipragliflozin slightly increased urine volume concomitant with urinary glucose excretion, and combination treatment with each antidiabetic drug did not affect the ipragliflozin-induced increase in urine volume.
Effects of single or combination treatment of ipragliflozin (0.1 mg/kg, q.d.) and a mitiglinide (1 mg/kg, b.i.d.), b glibenclamide (3 mg/kg, b.i.d.), c sitagliptin (1 mg/kg, q.d.), d insulin (0.5 IU/kg, b.i.d.), e metformin (300 mg/kg, b.i.d.), f voglibose (0.3 mg/kg, b.i.d.), or g rosiglitazone (3 mg/kg, q.d.) on urinary glucose excretion in type 2 diabetic mice. The values are the mean ± SEM for four animals in each group. *P < 0.05 versus normal group, #P < 0.05 versus diabetic vehicle group
Discussion
When type 2 diabetic patients are treated with a single antidiabetic drug, long-term glycemic control is often not realized or maintained because of safety and tolerability issues, loss of efficacy over time, and inability to affect declining pancreatic β-cell function; therefore, many patients require combination therapy (Turner et al. 1999). Metformin and sulfonylurea, either alone or in combination, are commonly used as an initial oral antidiabetic treatment for type 2 diabetic patients. However, glycemic control has been unable to be achieved or maintained in many diabetic patients (Turner et al. 1999; U.K. Prospective Diabetes Study Group 1998), and no significant effects on cardiovascular disease mortality have been noted (Rao et al. 2008). Novel, efficacious, and well-tolerated therapies that can be utilized along with existing antidiabetic drugs are therefore in high demand. Owing to the polygenic nature of type 2 diabetes and the side effects caused by existing antidiabetic drugs, combination therapy with existing and upcoming antidiabetic drugs with different mechanisms is expected to improve hyperglycemia and minimize risk factors effectively and safely. Given that SGLT2 selective inhibitors possess a mechanism different from those of existing antidiabetic drugs, these inhibitors are expected to be promising new options for treating type 2 diabetic patients. Here, we investigated the effects of combination treatment with the SGLT2 selective inhibitor ipragliflozin and various antidiabetic drugs—namely mitiglinide, glibenclamide, sitagliptin, insulin, metformin, voglibose, and rosiglitazone—on antidiabetic and mechanism-based pharmacological effects in high-fat diet and streptozotocin-nicotinamide-induced type 2 diabetic mice.
Compared with normal mice, diabetic mice exhibited a mild decline in glucose tolerance due to loss of early-phase insulin secretion during the liquid meal tolerance test. Ipragliflozin significantly improved glucose tolerance, without changes in plasma insulin level, by increasing urinary glucose excretion. A single treatment with mitiglinide, glibenclamide, sitagliptin, or insulin also significantly improved glucose tolerance, with an accompanying increase in plasma insulin levels. Combination treatment with ipragliflozin and these insulinotropic drugs and insulin additively improved glucose tolerance. We previously found that ipragliflozin improved glucose tolerance with a concomitant slight decrease in plasma insulin level in type 2 diabetic mice (Tahara et al. 2013). This previous finding therefore makes the effect of combination treatment of ipragliflozin with insulinotropic drugs and insulin on plasma insulin levels an interesting point to investigate. In the present study, we were able to confirm that ipragliflozin did not affect the increase in plasma insulin levels induced by these insulinotropic drugs and insulin. Similarly, the DPP-4 inhibitor sitagliptin induces glucose-dependent insulinotropic action by increasing plasma GLP-1 levels, and ipragliflozin also did not affect sitagliptin-induced inhibition in DPP-4 activity or increases in GLP-1 level. Here, we investigated effects of the subminimum effective dose (0.1 mg/kg) of ipragliflozin. We confirmed that effects of increase in plasma insulin levels induced by insulin and insulinotropic drugs were not remarkably reduced by coadministration of a high dose (3 mg/kg) of ipragliflozin. These results suggest that the efficacies of insulinotropic drugs and insulin are maintained even when coadministered with ipragliflozin. We previously confirmed that effective doses of ipragliflozin did not reduce fasting blood glucose levels in normal mice; does not increase risk of hypoglycemia (Tahara et al. 2012). Under the same experimental conditions, insulin and the examined insulinotropic drugs other than sitagliptin have been shown to reduce fasting blood glucose levels significantly at effective doses; given that reduction of fasting blood glucose levels is believed to be related to the risk of hypoglycemia, and combination treatment of ipragliflozin did not aggravate drug-induced reductions in fasting blood glucose levels (unpublished data). These results suggest that combination treatment of effective doses of ipragliflozin and insulinotropic drugs or insulin will not increase risk of hypoglycemia and may therefore be a safe, effective option for treating type 2 diabetes, however, further studies in clinical settings are necessary to clarify the safety of ipragliflozin.
Single treatment with metformin significantly improved glucose tolerance without affecting plasma insulin level. Biguanides, including metformin, effectively improve glucose tolerance, hyperglycemia, and insulin resistance by reducing hepatic glucose production. Some of these pharmacologic effects have been shown to be mediated by a reduction in PEPCK activity in liver (Kim et al. 2008). In the present study, diabetic mice exhibited an increase in PEPCK activity in liver, which is correlated with subsequent increases in hepatic gluconeogenesis and secretion of glucose; metformin potently decreased this PEPCK activity concomitant with improvement in glucose tolerance. In addition, metformin-induced decrease in hepatic PEPCK activity was not influenced by coadministration of ipragliflozin. These results suggest that ipragliflozin does not affect the metformin-induced decrease in hepatic gluconeogenesis and secretion of glucose via PEPCK. In contrast, SGLT2 inhibitors, dapagliflozin and empagliflozin, have been reported to increase endogenous glucose production with a concomitant increase in plasma glucagon levels in type 2 diabetic patients (Merovci et al. 2014; Ferrannini et al. 2014). In the present study, however, we did not investigate the effects of ipragliflozin on glucose production. As such, further research is needed to clarify these effects and the mechanisms by which SGLT2 inhibitors including ipragliflozin may cause them in type 2 diabetic models. At present, metformin is widely used to treat type 2 diabetes and seems useful in demonstrating the additive effect with SGLT2 inhibitors for controlling blood glucose level. However, a rare but potentially serious side effect of metformin administration is lactic acidosis, underscoring the importance of confirming that coadministration with ipragliflozin does not exacerbate lactic acidosis. In our preliminary experiments, metformin significantly reduced blood glucose levels while increasing plasma lactic acid levels during the glucose tolerance test in KK/Ay type 2 diabetic mice, and coadministration of ipragliflozin did not exacerbate metformin-induced increase in lactic acid level. Because lactic acid level may also be influenced by mild dehydration resulting from SGLT2 inhibitors-induced osmotic diuresis, additional and detailed examinations should be undertaken to confirm the effect on lactic acidosis. However, our present findings suggest that combination treatment of ipragliflozin and biguanides may represent a promising alternative option for treating type 2 diabetes.
α-Glucosidase inhibitors, including voglibose, slow intestinal absorption of dietary carbohydrates by inhibiting digestion of disaccharides via maltase and sucrase. Slowing the absorption of carbohydrate from the small intestine in this manner subsequently reduces postprandial hyperglycemia (Matsuo et al. 1992). In the present study, treatment with voglibose alone significantly improved glucose tolerance by inhibiting small intestinal glucosidase activities. Combination treatment with ipragliflozin and voglibose additively improved glucose tolerance, and voglibose-induced inhibition of small intestinal glucosidase activities was not influenced by coadministration with ipragliflozin. Major side effects associated with α-glucosidase inhibitors are gastrointestinal symptoms, such as flatulence, diarrhea, and abdominal discomfort, due to osmotic water retention by and fermentation of non-absorbed disaccharides in the intestine (Lebovitz 1997). We previously confirmed that voglibose induced mild diarrhea, mainly due to osmotic water retention caused by an increase in non-absorbed disaccharides content in the intestines of normal mice (Tahara et al. 2012 and unpublished data). In contrast, ipragliflozin did not increase intestinal contents of carbohydrate including disaccharides, and combination treatment with voglibose did not exacerbate intestinal water retention or diarrhea compared with that observed on single treatment of voglibose (unpublished data). Voglibose has been reported to improve not only postprandial hyperglycemia but also obesity, as demonstrated by metabolic profiles including body weight, fat mass, and plasma lipid parameters in high-fat diet-induced obese mice, by inhibiting dietary carbohydrate digestion in small intestine (Do et al. 2014). Ipragliflozin exerted similar action, improving not only hyperglycemia but also obesity in type 2 diabetic mice (Tahara et al. 2013) and high-fat diet-induced obese rats (Yokono et al. 2014) by increasing glucose excretion into urine. These findings suggest that coadministration of ipragliflozin and α-glucosidase inhibitors might improve not only hyperglycemia but also obesity, highlighting these drugs’ potential therapeutic use in patients with type 2 diabetes.
Risk of diabetic micro- and macrovascular complications is known to be related to hyperglycemia and insulin resistance (Laakso 2010). Rosiglitazone, a member of the thiazolidinedione class of antidiabetic agents, counteracts insulin resistance by binding to the transcription factor PPAR-γ which then promotes the synthesis of glucose transporters and activates adipocyte differentiation (Yki-Järvinen 2004). These PPAR-γ agonists, including rosiglitazone, exerted no significant pharmacological effects such as improvement of insulin resistance in a single administration; we therefore performed one-week repeated administration of rosiglitazone in the present study. Repeated administration of rosiglitazone significantly improved glucose tolerance and impaired insulin secretion, which may be attributable to improvement in insulin resistance. Combination treatment of ipragliflozin (single administration) and rosiglitazone (one-week repeated administration) additively improved glucose tolerance without affecting improvement in impaired insulin secretion. The major side effect of PPAR-γ agonists is weight gain with concomitant increase in adipose mass (Kahn et al. 2006; Stienstra et al. 2008). In the present study, one-week repeated administration of rosiglitazone significantly increased epididymal adipose tissue mass and body weight. However, repeated administration of ipragliflozin slightly but significantly reduced these parameters, and when administered concomitantly, the significant increases in fat and body weights were mitigated (unpublished data). In addition, we previously confirmed that repeated administration of ipragliflozin improved glucose tolerance and impaired insulin secretion, possibly by way of attenuation of glucose toxicity and insulin resistance in type 2 diabetic mice (Tahara et al. 2013). In the present study, the repeated administration period of rosiglitazone was relatively short, at 1 week, and combination with ipragliflozin was conducted as a single dosing. Investigating in detail the efficacy, safety, and effects of coadministration on adverse reactions in response to rosiglitazone, such as weight gain, will require examining these effects over long-term combinatory treatment of a PPAR-γ agonist and ipragliflozin in type 2 diabetic animals. However, these results suggest that combination treatment of ipragliflozin and PPAR-γ agonists represent a promising treatment option for improving insulin resistance among type 2 diabetes patients.
Ipragliflozin increases urinary glucose excretion via the inhibition of renal SGLT2. In the present study, ipragliflozin dose-dependently and significantly increased urinary glucose excretion in type 2 diabetic mice. None of the antidiabetic drugs evaluated in our study significantly affected ipragliflozin-induced increase in urinary glucose excretion, although a slight decreasing trend (5–20 %) was noted. These slight decreases in urinary glucose excretion may be dependent with antidiabetic drug-induced improvement in hyperglycemia. In addition, while ipragliflozin did slightly increase urine volume concomitant with urinary glucose excretion, combination treatment with each antidiabetic drug did not affect ipragliflozin-induced increase in urine volume. These results suggest that ipragliflozin-induced increase in urinary glucose excretion are not influenced by coadministration with various antidiabetic drugs and that ipragliflozin exerts its antidiabetic effects under a variety of combination therapy regimens in type 2 diabetic patients. To our knowledge, no information is available concerning the antihyperglycemic and mechanism-based pharmacological effects (e.g. glucose tolerance, insulin secretion, increase in GLP-1 level, and urinary glucose excretion) induced by coadministration of SGLT2 inhibitor and various antidiabetic drugs under identical experimental conditions in a single animal model. As such, we believe the present findings will prove useful in defining the combinatory effects of SGLT2 inhibitors and antidiabetic drugs. However, in the present study, we investigated some main pharmacological effects of antidiabetic drugs in single or short-term administration; detailed long-term treatment examinations measuring additional pharmacological effects should be undertaken to confirm the combinatory effects of ipragliflozin and various antidiabetic drugs.
In summary, we demonstrated that combination treatment with ipragliflozin and various antidiabetic drugs additively improved glucose tolerance without affecting each drug’s unique pharmacological effects in type 2 diabetic mice. These results suggest that combination of the SGLT2 selective inhibitor ipragliflozin and these antidiabetic drugs may prove useful in clinical practice for improving symptoms of type 2 diabetes, such as hyperglycemia and insulin resistance.
References
Abdul-Ghani, M.A., L. Norton, and R.A. DeFronzo. 2012. Efficacy and safety of SGLT2 inhibitors in the treatment of type 2 diabetes mellitus. Current Diabetes Reports 12: 230–238.
Bailey, C.J., J.L. Gross, D. Hennicken, N. Iqbal, T.A. Mansfield, and J.F. List. 2013. Dapagliflozin add-on to metformin in type 2 diabetes inadequately controlled with metformin: a randomized, double-blind, placebo-controlled 102-week trial. BMC Medicine. doi:10.1186/1741-7015-11-43.
Bays, H. 2013. Sodium glucose co-transporter type 2 (SGLT2) inhibitors: targeting the kidney to improve glycemic control in diabetes mellitus. Diabetes Therapy 4: 195–220.
Chao, E.C. 2014. SGLT-2 inhibitors: a new mechanism for glycemic control. Clinical Diabetes 32: 4–11.
Dahlqvist, A. 1984. Assay of intestinal disaccharidases. Scandinavian Journal of Clinical and Laboratory Investigation 44: 169–172.
Do, H.J., T. Jin, J.H. Chung, J.W. Hwang, and M.J. Shin. 2014. Voglibose administration regulates body weight and energy intake in high fat-induced obese mice. Biochemical and Biophysical Research Communications 443: 1110–1117.
Ferrannini, E., E. Muscelli, S. Frascerra, S. Baldi, A. Mari, T. Heise, U.C. Broedl, and H.J. Woerle. 2014. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. The Journal of Clinical Investigation 124: 499–508.
Hansen, R.J., H. Hinze, and H. Holzer. 1976. Assay of phosphoenolpyruvate carboxykinase in crude yeast extracts. Analytical Biochemistry 74: 576–584.
Kahn, S.E., S.M. Haffner, M.A. Heise, W.H. Herman, R.R. Holman, N.P. Jones, et al. 2006. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. New England Journal of Medicine 355: 2427–2443.
Kashiwagi, A., K. Kazuta, Y. Takinami, S. Yoshida, A. Utsuno, and I. Nagase. 2014. Ipragliflozin improves glycemic control in Japanese patients with type 2 diabetes mellitus: the BRIGHTEN study. Diabetol International. doi:10.1007/s13340-014-0164-0.
Kim, Y.D., K.G. Park, Y.S. Lee, Y.Y. Park, D.K. Kim, B. Nedumaran, W.G. Jang, W.J. Cho, J. Ha, I.K. Lee, C.H. Lee, and H.S. Choi. 2008. Metformin inhibits hepatic gluconeogenesis through AMP-activated protein kinase-dependent regulation of the orphan nuclear receptor SHP. Diabetes 57: 306–314.
Laakso, M. 2010. Cardiovascular disease in type 2 diabetes from population to man to mechanisms. Diabetes Care 33: 442–449.
Lebovitz, H.E. 1997. Alpha-Glucosidase inhibitors. Endocrinology and Metabolism Clinics of North America 26: 539–551.
Matsuo, T., H. Odaka, and H. Ikeda. 1992. Effect of an intestinal disaccharidase inhibitor (AO-128) on obesity and diabetes. The American Journal of Clinical Nutrition 55: S314–S317.
Merovci, A., C. Solis-Herrera, G. Daniele, R. Eldor, T.V. Fiorentino, D. Tripathy, J. Xiong, Z. Perez, L. Norton, M.A. Abdul-Ghani, and R.A. DeFronzo. 2014. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. The Journal of Clinical Investigation 124: 509–514.
Nathan, D.M., J.B. Buse, M.B. Davidson, E. Ferrannini, R.R. Holman, R. Sherwin, and B. Zinman. 2008. Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: update regarding thiazolidinediones: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 31: 173–175.
Rao, A.D., N. Kuhadiya, K. Reynolds, and V.A. Fonseca. 2008. Is the combination of sulfonylureas and metformin associated with an increased risk of cardiovascular disease or all-cause mortality?: a meta-analysis of observational studies. Diabetes Care 31: 1672–1678.
Stienstra, R., C. Duval, S. Keshtkar, J. van der Laak, S. Kersten, and M. Müller. 2008. Peroxisome proliferator-activated receptor gamma activation promotes infiltration of alternatively activated macrophages into adipose tissue. Journal of Biological Chemistry 283: 22620–22627.
Tahara, A., A. Matsuyama-Yokono, R. Nakano, Y. Someya, M. Hayakawa, and M. Shibasaki. 2009. Evaluation of the antidiabetic effects of dipeptidyl peptidase-IV inhibitor ASP8497 in streptozotocin-nicotinamide-induced mildly diabetic mice. Pharmacology 83: 177–187.
Tahara, A., E. Kurosaki, M. Yokono, D. Yamajuku, R. Kihara, Y. Hayashizaki, T. Takasu, M. Imamura, L. Qun, H. Tomiyama, Y. Kobayashi, A. Noda, M. Sasamata, and M. Shibasaki. 2012. Pharmacological profile of ipragliflozin (ASP1941), a novel selective SGLT2 inhibitor, in vitro and in vivo. Naunyn-Schmiedeberg’s Archives of Pharmacology 385: 423–436.
Tahara, A., E. Kurosaki, M. Yokono, D. Yamajuku, R. Kihara, Y. Hayashizaki, T. Takasu, M. Imamura, Q. Li, H. Tomiyama, Y. Kobayashi, A. Noda, M. Sasamata, and M. Shibasaki. 2013. Effects of SGLT2 selective inhibitor ipragliflozin on hyperglycemia, hyperlipidemia, hepatic steatosis, oxidative stress, inflammation, and obesity in type 2 diabetic mice. European Journal of Pharmacology 715: 246–255.
Tran, L., A. Zielinski, A.H. Roach, J.A. Jende, A.M. Householder, E.E. Cole, S.A. Atway, M. Amornyard, M.L. Accursi, S.W. Shieh, and E.E. Thompson. 2015. The pharmacologic treatment of type 2 diabetes: oral medications. Annals of Pharmacotherapy. doi:10.1177/1060028015573010.
Turner, R.C., C.A. Cull, V. Frighi, and R.R. Holman. 1999. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. JAMA 281: 2005–2012.
U.K. Prospective Diabetes Study Group. 1998. UKPDS 28: a randomized trial of efficacy of early addition of metformin in sulfonylurea-treated type 2 diabetes. U.K. Prospective Diabetes Study Group. Diabetes Care 21: 87–92.
Wild, S., G. Roglic, A. Green, R. Sicree, and H. King. 2004. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 27: 1047–1053.
Yki-Järvinen, H. 2004. Thiazolidinediones. New England Journal of Medicine 351: 1106–1118.
Yokono, M., T. Takasu, Y. Hayashizaki, K. Mitsuoka, R. Kihara, Y. Muramatsu, S. Miyoshi, A. Tahara, E. Kurosaki, Q. Li, H. Tomiyama, M. Sasamata, M. Shibasaki, and Y. Uchiyama. 2014. SGLT2 selective inhibitor ipragliflozin reduces body fat mass by increasing fatty acid oxidation in high-fat diet-induced obese rats. European Journal of Pharmacology 727: 66–74.
Acknowledgments
The authors thank Drs. Yuichi Tomura, Hideaki Minoura, Yuka Hayashizaki, Shoji Takakura, Seiji Kaku, and Wataru Uchida (Astellas Pharma Inc.) for their valuable comments and continuing encouragement.
Conflict of interest
The authors declare that no conflict of interest exists in the present study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tahara, A., Takasu, T., Yokono, M. et al. Effects of the combination of SGLT2 selective inhibitor ipragliflozin and various antidiabetic drugs in type 2 diabetic mice. Arch. Pharm. Res. 39, 259–270 (2016). https://doi.org/10.1007/s12272-015-0621-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-015-0621-8