Abstract
With the in-depth investigation of various diseases, angiogenesis has gained increasing attention. Among the contributing factors to angiogenesis research, endothelial epigenetics has emerged as an influential player. Endothelial epigenetic therapy exerts its regulatory effects on endothelial cells by controlling gene expression, RNA, and histone modification within these cells, which subsequently promotes or inhibits angiogenesis. As a result, this therapeutic approach offers potential strategies for disease treatment. The purpose of this review is to outline the pertinent mechanisms of endothelial cell epigenetics, encompassing glycolysis, lactation, amino acid metabolism, non-coding RNA, DNA methylation, histone modification, and their connections to specific diseases and clinical applications. We firmly believe that endothelial cell epigenetics has the potential to become an integral component of precision medicine therapy, unveiling novel therapeutic targets and providing new directions and opportunities for disease treatment.
Graphical Abstract
In recent years, with the deepening of people’s understanding of diseases, angiogenesis has been paid more and more attention. Endothelial cells, as the key cells in angiogenesis, play an important role in the field of angiogenesis research. Glycolysis, lactation, pentose phosphate pathway, amino acid metabolism, non-coding RNA, DNA methylation, histone modification, etc., all have an impact on endothelial cells and thus affect angiogenesis. Endothelial cell epigenetics is expected to become part of precision medicine treatments. Individual treatment plans can be implemented for patients, and precision medicine treatment strategies can be realized. Through epigenetic studies of endothelial cells, new drugs or therapeutic regimens can be developed for clinical application to reduce pain in patients and delay disease progression. Combined with other therapeutic strategies, it can control and guide the formation and reconstruction of blood vessels, and play different roles in different diseases such as diabetes, cardiovascular diseases, and tumors.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endothelial cells are key components of the inner lining of blood vessels, known as the intima. They have essential functions in maintaining the integrity and functionality of blood vessels, such as regulating blood flow, participating in immune responses, and facilitating vessel formation and remodeling [1]. Epigenetics refers to changes in gene expression and chromatin structure and modification that do not involve alterations in the DNA sequence [2]. In the context of endothelial cells, epigenetic regulation plays a crucial role in various cellular processes related to angiogenesis, the formation of new blood vessels. This includes controlling endothelial cell proliferation, migration, and the formation of lumens within new vessels [3, 4]. Epigenetic mechanisms involved in endothelial cell regulation primarily include DNA methylation, histone modifications, and the activity of non-coding RNA molecules. These mechanisms can influence gene expression by modifying how genes are packaged and accessed in the chromatin structure.
Endothelial Cell
Endothelial cells are slender, flat, polygonal cells laid on the inner membrane of the blood vessel wall. These cells are tightly connected to each other, creating a barrier that prevents blood from penetrating into the vessel wall. In addition to their role as a physical barrier, endothelial cells also play a critical role in regulating various aspects of vascular function. For example, endothelial cells release nitric oxide (NO) [5], an important vasodilator that relaxes vascular smooth muscle and increases the diameter of the vessel cavity, thereby reducing vascular resistance [6, 7]. In the regulation of inflammatory response, endothelial cells can secrete cell adhesion molecules, cytokines, chemokines, and so on, which can control the adhesion and migration of white blood cells, thereby regulating the progress of inflammatory response [8, 9].
Angiogenesis
Angiogenesis is the process by which new blood vessels are formed from the existing vascular system in order to support various physiological processes like growth, tissue repair, and remodeling [10, 11]. This process involves the proliferation and migration of endothelial cells, the degradation of the basement membrane, and the action of various cytokines [12, 13]. Angiogenesis takes on different roles in different diseases. For example, in wound healing and myocardial infarction, new blood vessels can promote the recovery of damaged tissue and function and help the healing of the disease [14, 15]. However, in tumors, new blood vessels contribute to the growth and metastasis of tumors. Therefore, we can develop new therapeutic strategies by understanding the molecular mechanisms and regulatory factors of angiogenesis [11, 16].
Factors that affect angiogenesis include pro-angiogenic factors such as hypoxia-inducible factor (HIF), macrophages, vascular endothelial growth factor (VEGF), HGF, and non-coding RNA and anti-angiogenic factors such as thromboprotein-1 and interleukin (IL)-12 [17, 18].
Epigenetics
Over the past few decades, advancements in the field of genetics have primarily focused on unraveling the sequence of DNA to understand the impact of genes on biological traits and genetic diseases. Traditional genetics holds that genes determine an individual’s characteristics. However, in recent years, an emerging discipline, epigenetics, has attracted wide attention and exploration. It includes DNA methylation, histone modification, non-coding RNA, etc., which can alter the accessibility and activity of genes, thereby regulating their expression levels [19, 20].
DNA methylation is a critical mechanism in epigenetics and has been extensively studied. It involves the addition of methyl groups to DNA molecules, which can block the binding of transcription factors to gene regions, leading to a reduction in gene expression levels. DNA methylation plays a key role not only in regulating gene expression during normal development but also in the onset and progression of disease [21]. Histones are proteins that are tightly bound to DNA and regulate gene availability and activity through site-specific chemical modifications, such as methylation, acylation, and phosphorylation. Histone modifications can affect the structure of chromatin and thus gene transcription and expression. Different types of tissues and cells differ in terms of histone modification, which also leads to differences in their expression and function. Non-coding RNA is another essential mechanism in epigenetics. Non-coding RNA refers to RNA molecules produced during transcription that interact with DNA and proteins to influence gene expression and regulation. They participate in the regulatory network of gene expression through regulation at the post-transcriptional level [22]. Beyond this, many other mechanisms reveal the complexity of the interaction between genes and the environment, providing new perspectives for the risk assessment of individual differences and diseases (Fig. 1).
The process of angiogenesis. Vascular endothelial cells are affected by both pro-angiogenic factors and anti-angiogenic factors, among which pro-angiogenic factors include HIF, macrophages, monocytes, VEGF family, non-coding RNAs, HGF, angiotensin II, asTF, classical Wnt pathway, and so on, and anti-angiogenic factors include TSP-1, IL-12, non-coding RNAs, etc. The balance between pro-angiogenic and anti-angiogenic factors determines the outcome of neovascularization. Depending on the degree of action of these factors, endothelial cells can proliferate, migrate, and form new blood vessels in a controlled manner
Metabolic Mechanism
Glycolysis
Glycolysis is a cellular metabolic pathway that converts glucose into pyruvate or lactic acid, generating energy and metabolites. It is an important process that provides energy to the body [23]. Endothelial cells use glycolysis to produce ATP, rather than oxidative phosphorylation, to maintain their normal function and promote germination, proliferation, and migration [24], a process that requires active remodeling of the cytoskeleton, and when endothelial cells (ECs) migrate to form new blood vessels, they extend dynamically and rapidly, moving filamentous and lamellar and pulling the cells forward, a process that is energy-demanding [25]. Pyruvate and lactic acid, produced during glycolysis, regulate the expression of angiogenic factors, such as VEGF and hypoxia-inducible factor-1α (HIF-α) and so on, which play an important stimulating role in the angiogenesis process [26]. However, HIF-1α increases the expression of a good deal of glycolytic enzymes, and HIF-1α is a known inducer of pyruvate dehydrogenase kinase (PDK), which inactivates pyruvate dehydrogenase (PDH) through a phosphorylation mechanism, thereby inhibiting the entry of pyruvate into the tricarboxylic acid (TCA) cycle and its conversion to acetyl-CoA [27]. Furthermore, increased glycolytic metabolism promotes angiogenesis at the leading edge of the vessel through a molecule called PFKFB3, which is involved in regulating glycolysis. When PFKFB3 is silenced or inhibited, it impairs vascular sprouting and thus angiogenesis [23].
Recent research suggests that phosphoglycerate dehydrogenase (GAPDH), a key enzyme in the glycolysis pathway, can impact the proliferation and migration of vascular endothelial cells, thereby promoting angiogenesis [28]. GAPDH also facilitates the production of vital NADH cofactors and activates specific signaling pathways, including PI3K/Akt and ERK1/2, which further influence the angiogenesis process [29]. Another critical enzyme in glycolysis, pyruvate kinase (PKM2), plays a significant role in angiogenesis, particularly in tumors, by promoting endothelial cell proliferation, migration, and adhesion to the extracellular matrix. Under hypoxic conditions, interactions between HIF-1α and the NF-κB subunit p65/RelA regulate the activation of the PKM2 promoter, leading to its nuclear translocation and regulating the expression of VEGF, further promoting angiogenesis [30, 31].
Glycolytic Side Branches: Pentose Phosphate Pathway
Pentose phosphate pathway (PPP) is a vital pathway in cell metabolism that operates in conjunction with glycolysis. It plays a different role in glucose metabolism from glycolysis and also has a profound impact on the process of angiogenesis. The pentose phosphate pathway is through the production of sugar alcohol phosphate and NADPH [32], among which NADPH plays an important role in the process of angiogenesis and is a cofactor in many reducing reactions in cells, including the reduction of the antioxidant glutathione and the activation of nitric reductase. These responses help maintain the function and homeostasis of vascular endothelial cells and promote angiogenesis. One key function of PPP is to provide biosynthetic precursors needed for cell growth and division, such as ribose 5-phosphate, an essential nucleotide building block, and NADPH, which is used for the biosynthesis of fatty acids, cholesterol, proline, tetrahydrofolate, and deoxyribonucleotides [33]. In the process of angiogenesis, cells need nucleic acid synthesis and cell proliferation to maintain cell growth and division and promote angiogenesis. Finally, the pentose phosphate pathway works by providing adequate NADPH, a key intracellular reducing agent that is necessary for the glutathione system and other ROS scavengers to maintain redox homeostasis. High levels of ROS can damage DNA, proteins, and lipids, leading to genomic instability. NADPH can maintain the antioxidant capacity of cells; reduce oxidative stress damage to cells, thereby promoting cell survival and proliferation; reduce cell apoptosis; and thus promote angiogenesis [34].
Overall, the PPP significantly impacts angiogenesis by supplying reducing reaction NADPH to cells, positively influencing the growth and function of vascular endothelial cells, and aiding in the regulation of the angiogenesis process.
Lactation
In a hypoxic environment, cells are unable to produce enough energy through the oxidative phosphorylation process of mitochondria to provide the cells with the required ATP through this rapid energy-producing metabolic pathway. Lactic acid can directly act on vascular endothelial cells and promote the production and release of VEGF [35, 36]. VEGF is one of the main regulatory factors of angiogenesis, which can promote endothelial cell proliferation and migration and promote the angiogenesis process. In addition, lactic acid can further induce vascular endothelial cell migration and lumen formation by regulating enzymes [37, 38] and growth factor activity in the extracellular matrix. Lactic acid can also activate HIF signaling pathways, thereby promoting angiogenesis. HIF is an important cellular adaptive factor that is activated in hypoxic environments and regulates the expression of many genes, including VEGF and angiogenic receptors. Lactic acid indirectly promotes the activation of HIF signaling pathways by inhibiting the degradation of HIF-1α protein and enhancing its transcriptional activity, thereby enhancing the ability of cells to engage in angiogenesis and promote the formation of new blood vessels in hypoxic environments [39, 40]. Finally, the binding of lactic acid to GPR81 can activate a variety of signaling pathways, including downstream cAMP and adenylate cyclase signaling pathways, thereby promoting angiogenesis. The binding of lactic acid to MCT1 can affect extracellular acid–base balance and mediate signal transduction related to angiogenesis [41, 42].
Recent studies have shown that pyruvate kinase (PKM2), a key enzyme in the lactate metabolic pathway, regulates endothelial cell proliferation and migration, ultimately affecting angiogenesis. Furthermore, attention has been paid to the role of lactate dehydrogenase A (LDHA) in angiogenesis. LDHA is one of the key enzymes in the glycolysis pathway, which can convert pyruvate produced by glycolysis into lactic acid. Lactic acid accumulates in inflammation and tumor microenvironment and is secreted in large quantities to ensure the energy needs of rapidly growing cancer cells, while excessive lactic acid will produce extracellular acidosis, thus promoting the proliferation, migration, and lumen formation of vascular endothelial cells, thus promoting the occurrence of angiogenesis [43]. In addition, some studies have found that the process of angiogenesis can be affected by regulating the lactate/pyruvate ratio. When pyruvate is reduced to lactic acid, NADH is oxidized to NAD, which, in turn, promotes the further reduction of pyruvate to lactic acid and the production of H+. The decrease in pH in the extracellular environment ultimately promotes angiogenesis. Therefore, by inhibiting LDHA activity or increasing pyruvate concentration, the lactic acid/pyruvate ratio can be reduced. Thus, the proliferation and migration of vascular endothelial cells are inhibited, and the formation of angiogenesis is reduced [44] (Fig. 2).
Metabolic mechanism of glycolysis, pentose phosphate pathway, and lactation. Glycolysis, lactation, and pentose phosphate pathways interact and influence each other. In the hypoxic environment, the body cannot meet the normal energy metabolism, thus producing a large amount of lactic acid, which can directly act on the HIF pathway and VEGF signaling pathway to promote angiogenesis. However, G6P produced by the glycolytic pathway can also enter the pentose phosphate pathway to produce NADPH, which leads to endothelial cell proliferation, migration, and eventually, angiogenesis
Amino Acid Metabolism
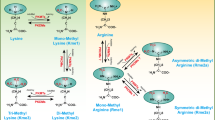
Amino acid metabolism refers to a series of chemical reaction processes related to the synthesis, degradation, and transformation of amino acids in living organisms. Amino acids serve as the building blocks of proteins and the basis of important metabolic pathways in many organisms. Amino acid metabolism plays an important role in maintaining the normal function, growth and development, energy supply, and stress response of organisms. Glutamine is the most consumed amino acid by ECs and plays a significant role in tip and stem cell localization during blood vessel formation [45, 46]. In the process of angiogenesis, amino acids can provide the basic nitrogen source for the synthesis of some important biological molecules, such as amino acids, nucleic acids, and proteins. It can also produce energy through protein breakdown, providing the energy needed for the vascular endothelial cells. Glutathione produced by EC via glutamine is used for redox homeostasis, and depletion of glutamine makes EC vulnerable to ROS-induced damage [47]. Glutamine catabolism produces glutamic acid, which can be converted into ornithine. Ornithine then leads to the production of pro-angiogenic factors such as NO, promoting angiogenesis [48, 49]. Amino acid metabolism can also promote the expression of pro-angiogenic factors, thus promoting the proliferation and migration of endothelial cells, thus promoting angiogenesis [50].
Another amino acid that has attracted attention is arginine. Arginine is an important amino acid that can participate in several metabolic pathways through enzymes such as arginine decarboxylase (ADC) and dimethylarginine dimethylaminohydrolase (DDAH). Recent studies have found that arginine and its metabolite NO have important regulatory effects on vascular function and angiogenesis. Increased production of nitric oxide triggers activation of the induced form of nitric oxide synthase (iNOS), which prompts l-arginine to produce more NO, a gas signaling molecule that plays a key role in vascular endothelial cells, inducing both nitrifying and oxidizing DNA damages. It leads to chronic inflammation and promotes endothelial cell function and angiogenesis [51].
In addition, arginine and its metabolites may also regulate angiogenesis by influencing metabolic pathways and amino acid transport in vascular endothelial cells. For instance, arginine can inhibit a protein complex called mammalian target of rapamycin complex 1 (mTORC1), thereby regulating the metabolism and angiogenesis of endothelial cells. Impaired mTORC1 activity leads to inhibition of cell proliferation and growth [52]. Additionally, the activation of PI3K/protein kinase B (Akt)/mTOR network can control a variety of cellular activities, such as mRNA translation, cell cycle progression, gene transcription, inhibition of apoptosis, autophagy, and metabolism [53]. Furthermore, inhibited VEGF expression can lead to growth inhibition and decreased angiogenesis signaling [54].
DNA Methylation
DNA methylation is a common form of epigenetic modification that involves chemical modifications to the DNA molecule, the addition of methyl groups to the genome. It helps maintain genome stability, regulates gene expression, and is involved in cell differentiation and development. DNA methylation occurs as modification of cytosine nucleotides placed within CpG dinucleotides. Abnormal methylation usually occurs in many pathological diseases in the form of hypermethylation of CpGs within the gene promoter region, which induces inhibition of gene expression [55, 56]. It has been found that DNA hypermethylation is associated with inhibiting gene silencing, while promoter hypomethylation leads to gene overexpression and global genomic instability [57], among which abnormal DNA methylation is the most studied epigenetic mechanism in ECs [58, 59]. In addition, DNA methylation is also involved in the regulation of endothelial cell inflammation. Endothelial cells can participate in the regulation of inflammatory response by releasing cell adhesion molecules, cytokines, and chemokines [60]. Abnormal DNA methylation can affect the expression and function of these regulatory factors, and thus affects the inflammatory response of endothelial cells, and then affects the process of angiogenesis. It can also regulate the expression of angiogenesis-related transcription factors and cytokines such as VEGF, fibroblast growth factor 2 (FGF-2), HIF-1α, and so on [61], thereby affecting angiogenesis. In some disease states, abnormal DNA methylation modification may also lead to angiogenesis disorders, for example in diseases such as atherosclerosis, hypertension, and diabetes, and abnormal DNA methylation leads to endothelial cell function disorders, thus affecting the health of blood vessels.
At present, some studies use gene editing technology to edit the expression levels and activities of key enzymes such as DNA methylase and demethylase, thereby changing gene expression and cell growth state, resulting in the expression imbalance of pro-angiogenic factors and anti-angiogenic factors, and subsequently resulting in angiogenesis disorders [62]. VEGF, its receptor, and eNOS (an essential gene essential for the promotion of angiogenesis) are also controlled by the methylation status of their gene promoters, mainly manifested as the expression of VEGF, and eNOS is downregulated by methyl-CPG-binding domain protein 2 (MBD2) binding. Methylated protein readers regulate endothelial function in physiological and disease states, thereby affecting angiogenesis [55, 63].
Non-coding RNA
The categories and functions of non-coding RNA are shown in Table 1.
Histone Modification
Histone modification is a process of chemical modification of histones on chromosomes. Histone modification changes chromatin structure and function by adding or removing different chemical markers on histone molecules, which, in turn, affects gene expression. Common histone modifications include acetylation, methylation, phosphorylation, ubiquitination, SUMOylation, serine/threonine dephosphorylation, etc. These modifications can interact individually or in complex ways to form histone codes that regulate gene expression at the chromatin level in response to changes in cell development, growth, signal transduction, disease, and more.
Acetylation Modification
Acetylation can relax the chromatin structure, make the gene promoter region more prone to transcription factor binding and gene transcriptional activity, promote the binding of VEGF and its receptor (VEGFR), enhance the activity of VEGF/VEGFR signaling pathway, stimulate HIF-1α [83], and promote the proliferation and migration of endothelial cells, thereby promoting angiogenesis.
Recent studies have shown that acetylation modification can directly affect the formation of angiogenesis by regulating the acetylation levels of some key transcription factors, affecting the proliferation and migration ability of endothelial cells. Acetylation modification can also affect the structure and function of chromatin by regulating the acetylation state of histones, and then regulate the expression of genes related to angiogenesis. Examples include histone deacetylases (HDACs) and histone acetyltransferases (HATs). HDACs remove acetyl groups from histones, thereby inhibiting gene transcription. Specifically, HDAC1 is recruited to the promoters of pro-angiogenic cytokines IL-8 and VEGF, partially inhibiting angiogenesis. Interestingly, HDAC1 has also been found to be involved in the pro-angiogenic mechanism in the cytosol. The interstitial flow between endothelial cells rapidly alters HDAC1 activity, shifting VEGF-induced angiogenesis from an HDAC1-independent to an HDAC1-dependent mechanism [84]. This suggests that HDAC1 can be deacetylated to promote neovascularization. HDACs play diverse roles in regulating cell proliferation, migration, angiogenesis, immune evasion, and therapeutic resistance, making them attractive targets for clinical treatments [85, 86]. HATs can catalyze the acetylation of histones and enhance gene transcription, thus affecting the proliferation, migration, and angiogenesis of endothelial cells. In addition, acetylation modification can also regulate gene expression by interacting with other epigenetic modifications such as DNA methylation and methylation.
Phosphorylation Modification
Phosphorylation can activate multiple signaling pathways in angiogenesis. For example, VEGF signaling pathway, ERK/MAPK [87] and PI3K/Akt [88], and others, which regulate the activity of transcription factors or cotranscription factors, affect the transcriptional activity of gene promoter region and further promote angiogenesis. Moreover, phosphorylation can also regulate extracellular matrix attachment and the formation of vascular intima, and influence the morphology and function of endothelial cells by regulating the interaction between extracellular matrix molecules and cell adhesion molecules. For example, the increased expression of POSTN, an ECM protein, will enhance the expression of VEGF and promote Erk phosphorylation, thus enhancing angiogenesis [89].
Ubiquitination Modification and SUMOylation Modification
Although ubiquitination and ubiquitination have very similar enzyme cascades, they play different roles. In addition to activating related signaling pathways, such as the VEGF pathway, HIF pathway, and NOTCH signaling pathway, SUMO protein can promote cell proliferation, migration, and resistance to apoptosis, and improve the ability of endothelial cells to form tubes and branches [90, 91]. By regulating protein degradation pathways, such as the ubiquitin–proteasome system and autophagy, ubiquitin modification enables old blood vessels to subside, while maintaining and regulating the stability and function of new blood vessel tissues, which promotes angiogenesis to a certain extent (Fig. 3).
Metabolic mechanism of DNA methylation and histone modification. DNA methylation refers to the addition of methyl groups to DNA molecules, thereby regulating the HIF pathway and VEGF pathway, releasing inflammatory factors, participating in the regulation of inflammatory response, and affecting the inflammatory response of endothelial cells, thereby affecting the proliferation, migration, formation of the cavity, and angiogenesis of endothelial cells. Histone modification can affect the VEGF signaling pathway, PI3K/Akt, ERK/MAPK, and other signaling pathways, and also play a certain role in promoting the process of angiogenesis
Therapeutic Strategies
Diabetes
High glucose status can lead to impaired function of various cells such as endothelial cells and pathological changes of blood vessels. In diabetes, abnormal DNA methylation can contribute to the loss of endothelial cell function. Drugs that affect DNA methylation and histone modification already exist, such as DNMT inhibitors (e.g., Aza) and histone deacetylase inhibitors (HDACi, e.g., VPA and TSA), which promote β-cell development, proliferation, and differentiation. It also regulates its function by preventing inflammatory cell damage, improving insulin resistance, and preventing microvascular complications in the later stages of diabetes [92]. Furthermore, these epigenetic drugs have been tested and used in the treatment of various clinical diseases. For example, Aza is the first-line drug for the treatment of high-risk myelodysplastic syndromes, and there is a growing literature showing that histone deacetylase can regulate glucose homeostasis and islet function, so we expect that in diabetes, application of DNA methyltransferase inhibitors such as 5-azocytosine (5-AZA) or histone deacetylase inhibitors can restore endothelial cell function and promote angiogenesis [93]. In a recent study, Ou et al. [92] targeted demethylation of the cell cycle regulator CDKN1C site to inhibit p57 expression and induce β-cell replication. Together, these studies support future epigenetic editing attempts in diabetes therapy [94]. Moreover, miRNAs are also involved in key pathways of insulin secretion, insulin signaling, and inflammation in the development of diabetes. By regulating the expression level of miRNA, it is possible to affect the expression of related genes and the activity of signaling pathways.
Cardiovascular Diseases
Cardiovascular diseases are often accompanied by endothelial cell dysfunction and vascular intimal inflammation. Epigenetic therapy targeting endothelial cells can help repair the damage to endothelial cell function by regulating DNA methylation, histone modification, and non-coding RNA modification. This therapy aims to restore the integrity and function of endothelial cells, and produce a variety of angiogenic factors such as VEGF and basic fibroblast growth factor (bFGF). It regulates the signaling pathways related to angiogenesis, such as PI3K/Akt and ERK1/2; reduces inflammatory response; inhibits the activation of immune cells and the release of inflammatory mediators; regulates the adhesion of endothelial cells; promotes the interaction between peripheral immune cells and endothelial cells; maintains the stability and integrity of blood vessels; and ultimately promotes angiogenesis.
At present, the study of DNA methylation for cardiovascular disease is still in the development stage. Since the changes in DNA methylation are reversible, this offers encouraging prospects for the treatment of the disease. Studies have found that high methylation levels of ABCA1 are associated with coronary heart disease and aging. Acetylsalicylic acid (ASA) treatment can reduce the DNA methylation level of ABCA1, thus reducing the occurrence of atherosclerosis and coronary heart disease, and acetylsalicylic acid is currently in the third stage of clinical trials [95]. Arunachalam et al. [96] proved that resveratrol, a histone acetylation inhibitor, can improve metabolic disorders, atherosclerosis, and coronary heart disease by upregulating SIRT1 in endothelial cells. Currently, resveratrol is also in the second stage of clinical trials for the treatment of coronary heart disease [96]. Inclisiran (ALN-PCSSC) is a long-acting RNA interference (RNAi) therapeutic agent that inhibits the synthesis of the proprotein convertase subtilisin proteinase-kexin type 9 (PCSK9). Inclisiran was observed in phase 1–3 clinical trials to have a low rate of adverse events and significantly lower LDL cholesterol levels. Inclisiran may offer a new approach for lowering low-density lipoprotein cholesterol (LDL-C) and a more successful RNA drug for cardiovascular disease [97]. Although some epigenetic drugs have not been widely used in clinical practice in cardiovascular disease, it is believed that in the future, through continuous exploration and large-scale clinical studies, more emerging epigenetic drugs for the treatment of cardiovascular diseases will be created to better improve the symptoms and prognosis of patients with cardiovascular diseases.
Tumor
During tumor development, tumor cells release angiogenic factors that stimulate endothelial cells in surrounding tissues to form new blood vessels to supply nutrients and oxygen needed by the tumor. Therefore, in the treatment of tumor patients, we need to inhibit the formation of new blood vessels in the tumor. By inhibiting the expression of VEGF and its receptors, it can reduce the angiogenesis capacity of endothelial cells and limit the growth and spread of tumors. Inhibition of tumor necrosis factor (TNF)-α, IL, etc., reduces inflammatory response, thereby inhibiting tumor-related angiogenesis. It can also promote the expression of angiogenesis inhibitors, such as regulating the production of angiostatin and endostatin. These factors can inhibit endothelial cell proliferation and angiogenesis, thereby limiting tumor growth and expansion.
EZH2 is a crucial component epigenetic regulatory mechanism that mediates histone methylation. In recent years, many small molecule inhibitors of EZH2 have been developed, some of which have entered various stages of clinical trials. For example, tazemetostat (EPZ-6438, trade name Tazverik) showed excellent efficacy and tolerability in clinical trials, and the FDA introduced it for the treatment of epithelioid sarcoma and follicular lymphoma in 2020. In addition, five different small-molecule EZH2 inhibitors and a dual EZH1/2 inhibitor, valemetostat (DS-3201b), are being evaluated in a series of clinical trials [98]. Bromine domain and off-terminal (BET) family member proteins play a key role in the epigenetic inheritance of histone Kac modifications, and abnormal activation of BET proteins is strongly associated with various human diseases, including cancer. For this reason, inhibition of BET bromine domain proteins (BBIs) is considered a promising therapy for BET-related diseases, and over the last few decades, 70 clinical trials have been conducted. However, drug resistance and adverse events pose significant challenges to the development of BBIs. Nevertheless, optimism remains high for the future of BET inhibitor drug development [99]. Other HDAC inhibitors, such as vorinostat, romidepsin, panobinostat, and belinostat, have also been approved for the treatment of some tumors [100,101,102,103].
Conclusions and Future Prospects
We can foresee that endothelial cell epigenetics will hold immense potential and promising prospects in the field of angiogenesis research. Individualized treatment plans can be implemented on patients to achieve precision medicine treatment strategies. Through epigenetic research on endothelial cells, new drugs or treatment programs can be developed for clinical application to reduce patients’ pain and delay disease progression. Combined with other treatment strategies, it can control and guide the generation and reconstruction of blood vessels and play different roles in different diseases.
References
Sturtzel C. Endothelial cells. Adv Exp Med Biol. 2017;1003:71–91. https://doi.org/10.1007/978-3-319-57613-8_4.
Wegner M, Pioruńska-Stolzmann M, Jagodziński PP. The impact of chromatin modification on the development of chronic complications in patients with diabetes. Postepy Hig Med Dosw. 2015;69:964–8. https://doi.org/10.5604/17322693.1165198.
Katoh M. Therapeutics targeting angiogenesis: genetics and epigenetics, extracellular miRNAs and signaling networks (Review). Int J Mol Med. 2013;32(4):763–7. https://doi.org/10.3892/ijmm.2013.1444.
Cheng C, Wang Y, Xue Q, Huang Y, Wang X, Liao F, et al. CircRnas in atherosclerosis, with special emphasis on the spongy effect of circRnas on miRnas. Cell Cycle. 2023;22(5):527–41. https://doi.org/10.1080/15384101.2022.2133365.
Mesquita A, Matsuoka M, Lopes SA, Pernambuco FP, Cruz AS, Nader HB, et al. Nitric oxide regulates adhesiveness, invasiveness, and migration of anoikis-resistant endothelial cells. Braz J Med Biol Res. 2022;55:11612. https://doi.org/10.1590/1414-431X2021e11612.
Bahadoran Z, Mirmiran P, Kashfi K, Ghasemi A. Vascular nitric oxide resistance in type 2 diabetes. Cell Death Dis. 2023;14(7):410. https://doi.org/10.1038/s41419-023-05935-5.
Shimokawa H. Reactive oxygen species in cardiovascular health and disease: special references to nitric oxide, hydrogen peroxide, and Rho-kinase. J Clin Biochem Nutri. 2020;66(2):83–91. https://doi.org/10.3164/jcbn.19-119.
Dalal PJ, Muller WA, Sullivan DP. Endothelial cell calcium signaling during barrier function and inflammation. Am J Pathol. 2020;190(3):535–42. https://doi.org/10.1016/j.ajpath.2019.11.004.
Wautier JL, Wautier MP. Vascular permeability in diseases. Int J Mol Sci. 2022;23(7):3645. https://doi.org/10.3390/ijms23073645.
Dudley AC, Griffioen AW. Pathological angiogenesis: mechanisms and therapeutic strategies. Angiogenesis. 2023;26(3):313–47. https://doi.org/10.1007/s10456-023-09876-7.
Parmar D, Apte M. Angiopoietin inhibitors: a review on targeting tumor angiogenesis. Eur J Pharmacol. 2021;899:174021. https://doi.org/10.1016/j.ejphar.2021.174021.
Akwii RG, Sajib MS, Zahra FT, Mikelis CM. Role of angiopoietin-2 in vascular physiology and pathophysiology. Cells. 2019;8(5):471. https://doi.org/10.3390/cells8050471.
Dalton AC, Shlamkovitch T, Papo N, Barton WA. Constitutive association of Tie1 and Tie2 with endothelial integrins is functionally modulated by angiopoietin-1 and fibronectin. PLoS ONE. 2016;11(10):e0163732. https://doi.org/10.1371/journal.pone.0163732.
Han C, Barakat M, DiPietro LA. Angiogenesis in wound repair: too much of a good thing. Cold Spring Harb Perspect Biol. 2022;14(10):a041225. https://doi.org/10.1101/cshperspect.a041225.
Wu X, Reboll MR, Korf-Klingebiel M, Wollert KC. Angiogenesis after acute myocardial infarction. Cardiovasc Res. 2021;117(5):1257–73. https://doi.org/10.1093/cvr/cvaa287.
Lugano R, Ramachandran M, Dimberg A. Tumor angiogenesis: causes, consequences, challenges and opportunities. Cell Mol Life Sci: CMLS. 2020;77(9):1745–70. https://doi.org/10.1007/s00018-019-03351-7.
Vimalraj S. A concise review of VEGF, PDGF, FGF, Notch, angiopoietin, and HGF signalling in tumor angiogenesis with a focus on alternative approaches and future directions. Int J Biol Macromol. 2022;221:1428–38. https://doi.org/10.1016/j.ijbiomac.2022.09.129.
Kaštelan S, Orešković I, Bišćan F, Kaštelan H, Gverović AA. Inflammatory and angiogenic biomarkers in diabetic retinopathy. Biochemia Medica. 2020;30(3):030502. https://doi.org/10.11613/BM.2020.030502.
Li Y. Modern epigenetics methods in biological research. Methods. 2021;187:104–13. https://doi.org/10.1016/j.ymeth.2020.06.022.
Peixoto P, Cartron PF, Serandour AA, Hervouet E. From 1957 to nowadays: a brief history of epigenetics. Int J Mol Sci. 2020;21(20):7571. https://doi.org/10.3390/ijms21207571.
Greenberg M, Bourc’his D. The diverse roles of DNA methylation in mammalian development and disease. Nat Rev Mol Cell Biol. 2019;20(10):590–607. https://doi.org/10.1038/s41580-019-0159-6.
Bure IV, Nemtsova MV, Kuznetsova EB. Histone modifications and non-coding RNAs: mutual epigenetic regulation and role in pathogenesis. Int J Mol Sci. 2022;23(10):5801. https://doi.org/10.3390/ijms23105801.
Li L, Wang M, Ma Q, Ye J, Sun G. Role of glycolysis in the development of atherosclerosis. Am J Physiol Cell Physiol. 2022;323(2):C617–29. https://doi.org/10.1152/ajpcell.00218.2022.
He X, Zeng H, Chen JX. Emerging role of SIRT3 in endothelial metabolism, angiogenesis, and cardiovascular disease. J Cell Physiol. 2019;234(3):2252–65. https://doi.org/10.1002/jcp.27200.
Li X, Sun X, Carmeliet P. Hallmarks of endothelial cell metabolism in health and disease. Cell Metab. 2019;30(3):414–33. https://doi.org/10.1016/j.cmet.2019.08.011.
Kierans SJ, Taylor CT. Regulation of glycolysis by the hypoxia-inducible factor (HIF): implications for cellular physiology. J Physiol. 2021;599(1):23–37. https://doi.org/10.1113/JP280572.
Tirpe AA, Gulei D, Ciortea SM, Crivii C, Berindan-Neagoe I. Hypoxia: overview on hypoxia-mediated mechanisms with a focus on the role of HIF genes. Int J Mol Sci. 2019;20(24):6140. https://doi.org/10.3390/ijms20246140.
Sirover MA. Pleiotropic effects of moonlighting glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in cancer progression, invasiveness, and metastases. Cancer Metastasis Rev. 2018;37(4):665–76. https://doi.org/10.1007/s10555-018-9764-7.
Chiche J, Ricci JE, Pouysségur J. Tumor hypoxia and metabolism – towards novel anticancer approaches. Ann Endocrinol. 2013;74(2):111–4. https://doi.org/10.1016/j.ando.2013.02.004.
Zahra K, Dey T, Ashish MSP, Pandey U. Pyruvate kinase M2 and cancer: the role of PKM2 in promoting tumorigenesis. Front Oncol. 2020;10:159. https://doi.org/10.3389/fonc.2020.00159.
İlhan M. Non-metabolic functions of pyruvate kinase M2: PKM2 in tumorigenesis and therapy resistance. Neoplasma. 2022;69(4):747–54. https://doi.org/10.4149/neo_2022_220119N77.
Movahed ZG, Yarani R, Mohammadi P, Mansouri K. Sustained oxidative stress instigates differentiation of cancer stem cells into tumor endothelial cells: pentose phosphate pathway, reactive oxygen species and autophagy crosstalk. Biomed Pharmacother. 2021;139:111643. https://doi.org/10.1016/j.biopha.2021.111643.
TeSlaa T, Ralser M, Fan J, Rabinowitz JD. The pentose phosphate pathway in health and disease. Nat Metab. 2023;5(8):1275–89. https://doi.org/10.1038/s42255-023-00863-2.
Ge T, Yang J, Zhou S, Wang Y, Li Y, Tong X. The role of the pentose phosphate pathway in diabetes and cancer. Front Endocrinol. 2020;11:365. https://doi.org/10.3389/fendo.2020.00365.
Gao Y, Zhou H, Liu G, Wu J, Yuan Y, Shang A. Tumor microenvironment: lactic acid promotes tumor development. J Immunol Res. 2022;2022:3119375. https://doi.org/10.1155/2022/3119375.
Kumar VB, Viji RI, Kiran MS, Sudhakaran PR. Endothelial cell response to lactate: implication of PAR modification of VEGF. J Cell Physiol. 2007;211(2):477–85. https://doi.org/10.1002/jcp.20955.
Thakur A, Qiu G, Xu C, Han X, Yang T, Ng SP, et al. Label-free sensing of exosomal MCT1 and CD147 for tracking metabolic reprogramming and malignant progression in glioma. Sci Adv. 2020;6(26):eaaz6119. https://doi.org/10.1126/sciadv.aaz6119.
Logozzi M, Spugnini E, Mizzoni D, Di Raimo R, Fais S. Extracellular acidity and increased exosome release as key phenotypes of malignant tumors. Cancer Metastasis Rev. 2019;38(1–2):93–101. https://doi.org/10.1007/s10555-019-09783-8.
Nagao A, Kobayashi M, Koyasu S, Chow C, Harada H. HIF-1-dependent reprogramming of glucose metabolic pathway of cancer cells and its therapeutic significance. Int J Mol Sci. 2019;20(2):238. https://doi.org/10.3390/ijms20020238.
Singh L, Aldosary S, Saeedan AS, Ansari MN, Kaithwas G. Prolyl hydroxylase 2: a promising target to inhibit hypoxia-induced cellular metabolism in cancer cells. Drug Discovery Today. 2018;23(11):1873–82. https://doi.org/10.1016/j.drudis.2018.05.016.
Brown TP, Ganapathy V. Lactate/GPR81 signaling and proton motive force in cancer: role in angiogenesis, immune escape, nutrition, and Warburg phenomenon. Pharmacol Ther. 2020;206:107451. https://doi.org/10.1016/j.pharmthera.2019.107451.
Baltazar F, Afonso J, Costa M, Granja S. Lactate beyond a waste metabolite: metabolic affairs and signaling in malignancy. Front Oncol. 2020;10:231. https://doi.org/10.3389/fonc.2020.00231.
Micaily I, Roche M, Ibrahim MY, Martinez-Outschoorn U, Mallick AB. Metabolic pathways and targets in chondrosarcoma. Front Oncol. 2021;11:772263. https://doi.org/10.3389/fonc.2021.772263.
Dunaway LS, Pollock JS. HDAC1: an environmental sensor regulating endothelial function. Cardiovasc Res. 2022;118(8):1885–903. https://doi.org/10.1093/cvr/cvab198.
Huang H, Vandekeere S, Kalucka J, Bierhansl L, Zecchin A, Brüning U, et al. Role of glutamine and interlinked asparagine metabolism in vessel formation. EMBO J. 2017;36(16):2334–52. https://doi.org/10.15252/embj.201695518.
Payen VL, Mina E, Van Hée VF, Porporato PE, Sonveaux P. Monocarboxylate transporters in cancer. Molecular. Metabolism. 2020;33:48–66. https://doi.org/10.1016/j.molmet.2019.07.006.
DeBerardinis RJ, Cheng T. Q’s next: the diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene. 2010;29(3):313–24. https://doi.org/10.1038/onc.2009.358.
Kucharzewska P, Welch JE, Svensson KJ, Belting M. Ornithine decarboxylase and extracellular polyamines regulate microvascular sprouting and actin cytoskeleton dynamics in endothelial cells. Exp Cell Res. 2010;316(16):2683–91. https://doi.org/10.1016/j.yexcr.2010.05.033.
Matsunaga T, Weihrauch DW, Moniz MC, Tessmer J, Warltier DC, Chilian WM. Angiostatin inhibits coronary angiogenesis during impaired production of nitric oxide. Circulation. 2002;105(18):2185–91. https://doi.org/10.1161/01.cir.0000015856.84385.e9.
Li X, Kumar A, Carmeliet P. Metabolic pathways fueling the endothelial cell drive. Annu Rev Physiol. 2019;81:483–503. https://doi.org/10.1146/annurev-physiol-020518-114731.
Rana T. Influence and implications of the molecular paradigm of nitric oxide underlying inflammatory reactions of the gastrointestinal tract of dog: a major hallmark of inflammatory bowel disease. Inflamm Bowel Dis. 2022;28(8):1280–8. https://doi.org/10.1093/ibd/izac017.
Özdemir BH, Özdemir AA. How exercise affects the development and progression of hepatocellular carcinoma by changing the biomolecular status of the tumor microenvironment. Exp Clin Transplant: Off J Middle East Soc Organ Transplant. 2022. https://doi.org/10.6002/ect.2021.0456.
Laukkanen S, Veloso A, Yan C, Oksa L, Alpert EJ, Do D, et al. Therapeutic targeting of LCK tyrosine kinase and mTOR signaling in T-cell acute lymphoblastic leukemia. Blood. 2022;140(17):1891–906. https://doi.org/10.1182/blood.2021015106.
Huang JJ, Hsieh JJ. The therapeutic landscape of renal cell carcinoma: from the Dark Age to the Golden Age. Semin Nephrol. 2020;40(1):28–41. https://doi.org/10.1016/j.semnephrol.2019.12.004.
Aspriţoiu VM, Stoica I, Bleotu C, Diaconu CC. Epigenetic regulation of angiogenesis in development and tumors progression: potential implications for cancer treatment. Front Cell Dev Biol. 2021;9:689962. https://doi.org/10.3389/fcell.2021.689962.
Xie Z, Zhou Z, Yang S, Zhang S, Shao B. Epigenetic regulation and therapeutic targets in the tumor microenvironment. Mol Biomed. 2023;4(1):17. https://doi.org/10.1186/s43556-023-00126-2.
Kulis M, Esteller M. DNA methylation and cancer. Adv Genet. 2010;70:27–56. https://doi.org/10.1016/B978-0-12-380866-0.60002-2.
Gaur P, Hunt CR, Pandita TK. Emerging therapeutic targets in esophageal adenocarcinoma. Oncotarget. 2016;7(30):48644–55. https://doi.org/10.18632/oncotarget.8777.
Kaz AM, Grady WM. Epigenetic biomarkers in esophageal cancer. Cancer Lett. 2014;342(2):193–9. https://doi.org/10.1016/j.canlet.2012.02.036.
Das D, Karthik N, Taneja R. Crosstalk between inflammatory signaling and methylation in cancer. Front Cell Dev Biol. 2021;9:756458. https://doi.org/10.3389/fcell.2021.756458.
Mohammed FH, Cemic F, Hemberger J, Giri S. Biological skin regeneration using epigenetic targets. Drug Discovery Today. 2023;28(4):103495. https://doi.org/10.1016/j.drudis.2023.103495.
Bhat SM, Prasad PR, Joshi MB. Novel insights into DNA methylation-based epigenetic regulation of breast tumor angiogenesis. Int Rev Cell Mol Biol. 2023;380:63–96. https://doi.org/10.1016/bs.ircmb.2023.04.002.
Dubey R, Prabhakar PK, Gupta J. Epigenetics: key to improve delayed wound healing in type 2 diabetes. Mol Cell Biochem. 2022;477(2):371–83. https://doi.org/10.1007/s11010-021-04285-0.
Su W, Huo Q, Wu H, Wang L, Ding X, Liang L, et al. The function of LncRNA-H19 in cardiac hypertrophy. Cell Biosci. 2021;11(1):153. https://doi.org/10.1186/s13578-021-00668-4.
Voellenkle C, Garcia-Manteiga JM, Pedrotti S, Perfetti A, De Toma I, Da Silva D, et al. Implication of Long noncoding RNAs in the endothelial cell response to hypoxia revealed by RNA-sequencing. Sci Rep. 2016;6:24141. https://doi.org/10.1038/srep24141.
Zhu A, Chu L, Ma Q, Li Y. Long non-coding RNA H19 down-regulates miR-181a to facilitate endothelial angiogenic function. Artif Cells, Nanomed Biotechnol. 2019;47(1):2698–705. https://doi.org/10.1080/21691401.2019.1634577.
Shi X, Wei YT, Li H, Jiang T, Zheng XL, Yin K, et al. Long non-coding RNA H19 in atherosclerosis: what role. Mol Med. 2020;26(1):72. https://doi.org/10.1186/s10020-020-00196-w.
Zhang Y, Yang M, Yang S, Hong F. Role of noncoding RNAs and untranslated regions in cancer: a review. Medicine. 2022;101(33):e30045. https://doi.org/10.1097/MD.0000000000030045.
Li ZX, Zhu QN, Zhang HB, Hu Y, Wang G, Zhu YS. MALAT1: a potential biomarker in cancer. Cancer Manag Res. 2018;10:6757–68. https://doi.org/10.2147/CMAR.S169406.
Braga EA, Fridman MV, Burdennyy AM, Filippova EA, Loginov VI, Pronina IV, et al. Regulation of the key epithelial cancer suppressor miR-124 function by competing endogenous RNAs. Int J Mol Sci. 2022;23(21):13620. https://doi.org/10.3390/ijms232113620.
He B, Peng F, Li W, Jiang Y. Interaction of lncRNA-MALAT1 and miR-124 regulates HBx-induced cancer stem cell properties in HepG2 through PI3K/Akt signaling. J Cell Biochem. 2019;120(3):2908–18. https://doi.org/10.1002/jcb.26823.
Du M, Chen W, Zhang W, Tian XK, Wang T, Wu J, et al. TGF-β regulates the ERK/MAPK pathway independent of the SMAD pathway by repressing miRNA-124 to increase MALAT1 expression in nasopharyngeal carcinoma. Biomed Pharmacother. 2018;99:688–96. https://doi.org/10.1016/j.biopha.2018.01.120.
Al-Rugeebah A, Alanazi M, Parine NR. MEG3: an oncogenic long non-coding RNA in different cancers. Pathol Oncol Res: POR. 2019;25(3):859–74. https://doi.org/10.1007/s12253-019-00614-3.
Zhang L, Zhao F, Li W, Song G, Kasim V, Wu S. The biological roles and molecular mechanisms of long non-coding RNA MEG3 in the hallmarks of cancer. Cancers. 2022;14(24):6032. https://doi.org/10.3390/cancers14246032.
Zhao Y, Liu Y, Zhang Q, Liu H, Xu J. The mechanism underlying the regulation of long non-coding RNA MEG3 in cerebral ischemic stroke. Cell Mol Neurobiol. 2023;43(1):69–78. https://doi.org/10.1007/s10571-021-01176-2.
Yang QY, Yu Q, Zeng WY, Zeng M, Zhang XL, Zhang YL, et al. Killing two birds with one stone: miR-126 involvement in both cancer and atherosclerosis. Eur Rev Med Pharmacol Sci. 2022;26(17):6145–68. https://doi.org/10.26355/eurrev_202209_29632.
Soofiyani SR, Hosseini K, Ebrahimi T, Forouhandeh H, Sadeghi M, Beirami SM, et al. Prognostic value and biological role of miR-126 in breast cancer. MicroRNA (Shariqah, United Arab Emirates). 2022;11(2):95–103. https://doi.org/10.2174/1876402914666220428123203.
Tirpe A, Gulei D, Tirpe GR, Nutu A, Irimie A, Campomenosi P, et al. Beyond conventional: the new horizon of anti-angiogenic microRNAs in non-small cell lung cancer therapy. Int J Mol Sci. 2020;21(21):8002. https://doi.org/10.3390/ijms21218002.
Xie J, Wu W, Zheng L, Lin X, Tai Y, Wang Y, et al. Roles of microRNA-21 in skin wound healing: a comprehensive review. Front Pharmacol. 2022;13:828627. https://doi.org/10.3389/fphar.2022.828627.
Maryam M, Naemi M, Hasani SS. A comprehensive review on oncogenic miRNAs in breast cancer. J Gen. 2021;100:15.
Amini S, Abak A, Sakhinia E, Abhari A. MicroRNA-221 and microRNA-222 in common human cancers: expression, function, and triggering of tumor progression as a key modulator. Lab Med. 2019;50(4):333–47. https://doi.org/10.1093/labmed/lmz002.
Di Martino MT, Arbitrio M, Caracciolo D, Cordua A, Cuomo O, Grillone K, et al. miR-221/222 as biomarkers and targets for therapeutic intervention on cancer and other diseases: a systematic review. Mol Ther Nucleic Acids. 2022;27:1191–224. https://doi.org/10.1016/j.omtn.2022.02.005.
Li G, Tian Y, Zhu WG. The roles of histone deacetylases and their inhibitors in cancer therapy. Front Cell Dev Biol. 2020;8:576946. https://doi.org/10.3389/fcell.2020.576946.
Hai R, Yang D, Zheng F, Wang W, Han X, Bode AM, et al. The emerging roles of HDACs and their therapeutic implications in cancer. Eur J Pharmacol. 2022;931:175216. https://doi.org/10.1016/j.ejphar.2022.175216.
Qiu X, Zhu L, Wang H, Tan Y, Yang Z, Yang L, et al. From natural products to HDAC inhibitors: an overview of drug discovery and design strategy. Bioorg Med Chem. 2021;52:116510. https://doi.org/10.1016/j.bmc.2021.116510.
Wasik A, Ratajczak-Wielgomas K, Badzinski A, Dziegiel P, Podhorska-Okolow M. The role of periostin in angiogenesis and lymphangiogenesis in tumors. Cancers. 2022;14(17):4225. https://doi.org/10.3390/cancers14174225.
Guo YJ, Pan WW, Liu SB, Shen ZF, Xu Y, Hu LL. ERK/MAPK signalling pathway and tumorigenesis. Exp Ther Med. 2020;19(3):1997–2007. https://doi.org/10.3892/etm.2020.8454.
Revathidevi S, Munirajan AK. Akt in cancer: mediator and more. Semin Cancer Biol. 2019;59:80–91. https://doi.org/10.1016/j.semcancer.2019.06.002.
Makkar R, Behl T, Arora S. Role of HDAC inhibitors in diabetes mellitus. Curr Res Transl Med. 2020;68(2):45–50. https://doi.org/10.1016/j.retram.2019.08.001.
Rabellino A, Andreani C, Scaglioni PP. Roles of ubiquitination and SUMOylation in the regulation of angiogenesis. Curr Issues Mol Biol. 2020;35:109–26. https://doi.org/10.21775/cimb.035.109.
Wang M, Jiang X. The significance of SUMOylation of angiogenic factors in cancer progression. Cancer Biol Ther. 2019;20(2):130–7. https://doi.org/10.1080/15384047.2018.1523854.
Ou K, Yu M, Moss NG, Wang YJ, Wang AW, Nguyen SC, et al. Targeted demethylation at the CDKN1C/p57 locus induces human β cell replication. J Clin Investig. 2019;129(1):209–14. https://doi.org/10.1172/JCI99170.
Ling C, Rönn T. Epigenetics in human obesity and type 2 diabetes. Cell Metab. 2019;29(5):1028–44. https://doi.org/10.1016/j.cmet.2019.03.009.
Crescenti A, Solà R, Valls RM, Caimari A, Del Bas JM, Anguera A, et al. Cocoa consumption alters the global DNA methylation of peripheral leukocytes in humans with cardiovascular disease risk factors: a randomized controlled trial. PLoS ONE. 2013;8(6):e65744. https://doi.org/10.1371/journal.pone.0065744.
Guay SP, Légaré C, Houde AA, Mathieu P, Bossé Y, Bouchard L. Acetylsalicylic acid, aging and coronary artery disease are associated with ABCA1 DNA methylation in men. Clin Epigenetics. 2014;6(1):14. https://doi.org/10.1186/1868-7083-6-14.
Arunachalam G, Yao H, Sundar IK, Caito S, Rahman I. SIRT1 regulates oxidant- and cigarette smoke-induced eNOS acetylation in endothelial cells: role of resveratrol. Biochem Biophys Res Commun. 2010;393(1):66–72. https://doi.org/10.1016/j.bbrc.2010.01.080.
Fitzgerald K, White S, Borodovsky A, Bettencourt BR, Strahs A, Clausen V, et al. A highly durable RNAi therapeutic inhibitor of PCSK9. N Engl J Med. 2017;376(1):41–51. https://doi.org/10.1056/NEJMoa1609243.
Zeng J, Zhang J, Sun Y, Wang J, Ren C, Banerjee S, et al. Targeting EZH2 for cancer therapy: from current progress to novel strategies. Eur J Med Chem. 2022;238:114419. https://doi.org/10.1016/j.ejmech.2022.114419.
Guo J, Zheng Q, Peng Y. BET proteins: biological functions and therapeutic interventions. Pharmacol Ther. 2023;243:108354. https://doi.org/10.1016/j.pharmthera.2023.108354.
Dokmanovic M, Clarke C, Marks PA. Histone deacetylase inhibitors: overview and perspectives. Mol Cancer Res: MCR. 2007;5(10):981–9. https://doi.org/10.1158/1541-7786.MCR-07-0324.
Quintás-Cardama A, Santos FP, Garcia-Manero G. Histone deacetylase inhibitors for the treatment of myelodysplastic syndrome and acute myeloid leukemia. Leukemia. 2011;25(2):226–35. https://doi.org/10.1038/leu.2010.276.
Eleutherakis-Papaiakovou E, Kanellias N, Kastritis E, Gavriatopoulou M, Terpos E, Dimopoulos MA. Efficacy of panobinostat for the treatment of multiple myeloma. J Oncol. 2020;2020:7131802. https://doi.org/10.1155/2020/7131802.
Sawas A, Radeski D, O’Connor OA. Belinostat in patients with refractory or relapsed peripheral T-cell lymphoma: a perspective review. Ther Adv Hematol. 2015;6(4):202–8. https://doi.org/10.1177/2040620715592567.
Acknowledgements
All figures were created in BioRender.com.
Funding
This study was supported by the National Natural Science Foundation of China (82070455, 82370457) and the Key Research and Development Project of Jiangsu Province (BE2022780).
Author information
Authors and Affiliations
Contributions
Yue Cai designed, collected, and interpreted the data and drafted, revised, and approved the manuscript; Lihua Li, Chen Shao, Yiliu Chen, and Zhongqun Wang interpreted the data and drafted, revised, and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Associate Editor Judith C. Sluimer oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cai, Y., Li, L., Shao, C. et al. Therapeutic Strategies for Angiogenesis Based on Endothelial Cell Epigenetics. J. of Cardiovasc. Trans. Res. 17, 816–827 (2024). https://doi.org/10.1007/s12265-024-10485-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-024-10485-y