Abstract
The hypomethylating agents (HAs), azacitidine and decitabine, have emerged as an alternative to initial and salvage therapy in patients with acute myeloid leukemia (AML). Little is known about how AML responds to hypomethylating agents after standard therapy, and the activity of these agents in a real-world setting is not well studied. We retrospectively examined data for 75 consecutive AML patients at Wake Forest from 2002 to 2011 treated with HAs either as first-line (n = 34), salvage (n = 28), or consolidation (n = 13) therapy. We collected data on age, gender, race, Charlson comorbidity index (CCI), cytogenetics, type of treatment, complete remission (CR), complete remission with incomplete count recovery (CRi), and survival. Statistical analysis was performed using Kaplan–Meier estimates and Cox proportional hazards models. Frontline response rate (CR + CRi) was 26.5 %, and median overall survival (OS) was 3.4 months (95 % CI 1.3–7.4), with 18 % alive at 1 year. In the salvage cohort, the response rate was significantly lower compared to frontline (3.6 versus 26.5 %, p = 0.017). Despite the reduced response, OS from time of HA treatment was longer than frontline at 8.2 months (CI 4.8–10.3). In the consolidation cohort, OS was 13.8 months (CI 8.0–21.6) with one patient in remission more than 30 months from diagnosis. These data suggest that prior cytotoxic therapy decreases marrow response rates to HAs but not survival. Furthermore, use of hypomethylating agents for consolidation resulted in a median overall survival over 1 year in a cohort of older patients. This suggests that hypomethylating agents have activity in all phases of AML treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute myeloid leukemia (AML) is an aggressive, genetically heterogenous malignancy of the bone marrow. There are an estimated 13,000 new cases and 10,000 deaths annually in the USA [1]. Following diagnosis, patients with AML are generally treated with induction chemotherapy. This therapy typically consists of the combination of cytarabine and an anthracycline, most commonly daunorubicin [2]. Once remission is achieved, patients are treated with consolidation therapy in the form of high or standard doses of cytarabine or stem cell transplantation [2]. With induction chemotherapy, 60–70 % of patients achieve a complete remission, but the 5-year estimated survival rate is only 20–30 % [3]. Despite advances in therapy, 30–40 % of AML patients do not achieve a remission [2, 4]. In cases of induction failure or relapsed disease, no standard regimen exists, and median estimated survival ranges from 3 to 12 months [5].
The prognosis is even worse for older patients, often defined as 60 years of age or older. In this age group, only 50 % of patients will achieve a remission with standard induction therapy, and long-term survival is less than 10 % [6–8]. Elderly patients do not tolerate traditional chemotherapy regimens as well as younger patients. Treatment-related mortality from these regimens may be as high as 25 % in the elderly, compared to 5–10 % in younger patients [9]. Poor outcomes continue in older patients beyond the initial hospitalization, with the median survival reported to be 2–12 months [10]. Reasons for these poor outcomes include both differences in tumor biology and common conditions of older patients. The elderly have an increased incidence of preexisting myelodysplastic syndrome, poor-risk cytogenetics, and multidrug-resistant leukemia, all of which independently carry a poor prognosis [7]. In addition to the negative prognostic factors related to the leukemia itself, elderly patients have decreased functional status and increased comorbidities. This combination results in a high prevalence of aggressive, drug-resistant leukemia in patients least equipped to tolerate the disease or the intensive treatment options. There is currently no standard treatment regimen for elderly patients who are unsuitable for conventional chemotherapy at diagnosis, consolidation, or relapse. Defining the optimal treatment of elderly AML patients is of increasing importance, as it is predicted that by 2031, AML incidence will rise by 38 % in the elderly [11].
In recent years, the hypomethylating agents, azacitidine and decitabine, have emerged as alternatives to initial and salvage therapy in patients with AML. These agents are used in patients unable to tolerate traditional induction chemotherapy. The hypomethylating agents are cytidine analogs that are incorporated into DNA during replication and inhibit DNA methyltransferases, leading to reduced DNA methylation. This alteration in DNA methylation is thought to result in increased expression of tumor suppressor genes, leading to leukemic cell apoptosis. There is also some evidence that these agents can directly damage DNA and that azacitidine alters RNA stability [12]. Some studies have suggested that hypomethylating agents can induce higher response rates than standard chemotherapy in high-risk cytogenetic groups [13]. Little is known about how AML responds to salvage therapy with hypomethylating agents after standard induction chemotherapy, and the use of these agents in the treatment of AML is currently evolving.
The purpose of this study was to better understand the response rates of patients who received hypomethylating agents as first-line therapy, in consolidation after standard induction chemotherapy, or as salvage therapy.
Methods
Study design
We retrospectively reviewed data for 75 consecutive AML patients treated with either azacitidine or decitabine and seen at Wake Forest University from 2002 to 2011. Patients received one of these agents as either first-line, salvage, or consolidation therapy. Patients who received no previous therapy were termed first line. Patients who responded to cytotoxic induction chemotherapy and then received azacitidine or decitabine were termed consolidation. Patients who received azacitidine or decitabine after relapse or lack of response to cytotoxic induction therapy were termed salvage. All assignments were based on collaborative review by two physicians. The flow chart for inclusion and exclusion is shown in Fig. 1.
Data collection
Information was primarily obtained from our institution’s electronic medical records system using structured data extraction, chart review, and ICD-9 codes. We collected data on age, gender, race, cytogenetic characteristics, type of treatment, complete remission (CR), complete remission with incomplete count recovery (CRi), partial response (PR), and death or date of last contact. Comorbidities were obtained from the date of diagnosis or prior using ICD-9 codes. Regimens of hypomethylating agents were grouped into either 10-day decitabine treatment, 5-day decitabine treatment, or azacitidine treatment. A single cycle of 10-day decitabine was sufficient to assign a patient to this treatment group.
We collected data on overall survival from date of diagnosis, survival from date of induction regimen administration, survival from date of hypomethylating agent administration, cytogenetic risk stratification score based on Southwest Oncology Group guidelines [14], number of relapses, and the Charlson comorbidity index (CCI) [15].
Statistics
Descriptive measures presented include percentages, means, and Kaplan–Meier estimates of median survival. Fisher’s exact test was used to compare group differences in categorical variables, specifically in marrow response rates and comorbidity scores. We used a Cox proportional hazards model to adjust for age, race, gender, and treatment group and to estimate the effect of comorbidity burden on survival outcomes.
Results
Patient data
Patient demographics are listed in Table 1. Patients in the frontline, salvage, and consolidation cohorts of this study were predominantly elderly (median ages of 74.5, 65.5, and 73) with only 8 of 75 patients younger than 60. The frontline cohort had a significantly higher comorbidity burden with a mean CCI score of 2.35 compared to 1.32 for the salvage cohort and 1.15 for the consolidation cohort (p values of 0.0355 and 0.0077, respectively). This is consistent with frontline patients not being considered candidates for traditional induction chemotherapy. After adjusting for baseline covariates, the CCI score trended with survival though did not reach significance (p = 0.2651). In addition to advanced age, patients in this study were also poor risk on the basis of tumor biology with 32 % of patients having poor-risk cytogenetics and 33 % having either secondary or therapy-related AML. Overall survival from time of hypomethylating agent (HA) treatment for each cohort is shown in Fig. 2.
Hypomethylating agents have activity in first-line treatment of AML
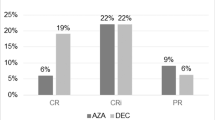
Decitabine and azacitidine have activity in AML, as shown in several clinical trials. However, reports in real-world settings have cast some doubt on their utility [16]. The FDA recently declined to give decitabine approval for an indication in AML because a pivotal phase III trial failed to show a survival benefit except in an unplanned post hoc analysis [17]. To assess the activity of these agents at our institution, we examined the outcomes of 34 patients treated with hypomethylating agents as their first line of therapy. They represent a poor-risk cohort with advanced age (median age of 74.5), 16 of 34 were either secondary or therapy-related AML, 13 of 34 had adverse cytogenetics, and all were deemed unfit for standard therapy by their treating physician. In this cohort, the CR rate was 18 % (6/34) with an additional 9 % (3/34) of patients achieving a CRi for an overall response rate of 26.5 % with 18 % (6/34) of patients alive at 1 year. Of the six patients alive at 1 year, five achieved either a CR or CRi. Median overall survival (OS) was 3.4 months (CI 1.54, 7.95). Of the nine patients achieving a CR or CRi, all but one had evidence of relapsed disease with one patient opting for hospice care after 12 cycles of decitabine who died without evidence of disease progression. Early mortality rates at 30 and 60 days were 15 and 38 %, respectively. Decitabine dose intensity did not appear to influence response in our small cohort as 26 % (5/19) of patients receiving 10-day decitabine achieved CR/CRi compared to 33 % (4/12) who received 5-day courses. None of the three patients who received azacitidine achieved a marrow response. Multiple courses of HAs were needed in all but two patients to achieve a CR or CRi, and the median number of cycles needed was 3.5 with one patient requiring 15 cycles (Table 2). Patients continued on HA therapy until evidence of disease progression or unacceptable toxicity (Table 3).
Several pretreatment variables have been suggested to influence response to therapy in AML such as blast percentage in the marrow, leukocytosis, and the presence of blasts in the peripheral blood [18]. Where data was available, we assessed the effect of these factors on HA response (Table 4).
Hypomethylating agents have a lower response rate in patients previously treated with standard chemotherapy
Since the hypomethylating agents act, in theory, predominantly through the hypomethylation of DNA and subsequent reexpression of tumor suppressor genes, it is unclear if previous exposure to standard DNA damaging agents would affect response. To address this question, we determined the outcome of patients who received hypomethylating agents for relapsed AML after their initial and/or relapsed disease was treated with standard chemotherapy. Among patients who received hypomethylating agents after relapse (salvage therapy), there was a low marrow response rate with only 1/28 patients achieving a CRi and none achieving a CR. This was significantly worse than the first-line cohort (3.6 versus 26.5 %, p = 0.0172). Despite this lack of marrow response, median OS was longer than in the frontline cohort at 8.2 months (CI 4.8, 10.3) vs 3.1 months (CI 1.3, 7.4) though this was not significant (p = 0.2967). The number of patients alive at 1 year from HA treatment was 25 % (7/28). Early all cause mortality rates were 7 % at day 30 and 14 % at day 60. HA was first-line salvage in 68 % (19/28) of patients, and for 93 % (26/28), it was either first- or second-line therapy. Almost all (27/28) patients received either 5- or 10-day decitabine with only one patient receiving azacitidine. The only marrow responder received 10-day decitabine as second-line therapy. That patient required six cycles of decitabine to achieve a CRi and ultimately went on to reduced-intensity allogeneic stem cell transplant. One additional salvage patient achieved a morphologically leukemic free state without count recovery and went on to allogeneic stem cell transplant.
Hypomethylating agents can be safely given to older AML patients in consolidation
The role of hypomethylating agents as therapy to prolong remission is currently being investigated in clinical trials. To assess the efficacy of the hypomethylating agents in this setting, we determined the outcomes for patients treated with these agents at our institution following standard induction therapy. These patients were deemed inappropriate for additional standard therapy at the time of consolidation. Among patients who received hypomethylating agents as consolidation therapy, the majority (11/13) received 5-day decitabine with a median of four cycles given. Of the 13 patients in this cohort, disease relapsed in ten with two patients dying in remission and one patient who remains alive and free of disease at the time of this analysis more than 30 months from diagnosis. Only one patient died within 60 days of initiation of therapy (on day 58). The median OS from time of HA treatment in this cohort was 11.4 months (CI 4.0, 17.7). When calculated from diagnosis, the median OS is 13.8 months (CI 8, 21.6). These data suggest that for patients unable to tolerate standard consolidation, the HAs may represent a viable alternative consolidation strategy.
Discussion
The optimal treatment of AML in older and unfit patients in all phases of the disease remains undefined, and these patients represent a significant clinical challenge. The hypomethylating agents have documented activity in these patients and may represent a less intensive therapeutic option; however, where they fit into the treatment algorithm is still evolving. In order to assess the activity of these agents over the course of the disease, we reviewed the outcomes of AML patients at our institution treated with hypomethylating agents as induction, salvage, or consolidation therapy.
As patients get older, the risk of toxicity increases while the ability to achieve a remission diminishes [9]. Indeed many elderly patients do not receive any AML-directed therapy following their diagnosis [19]. Recently, the use of hypomethylating agents in this population has increased, and several reports have documented their activity [13, 20, 21]. In fact, the NCCN guidelines now include recommendations for use of hypomethylating agents for elderly or unfit patients. However, the utility of this approach in the real-world setting has come into doubt with recent reports showing little activity in higher-risk patients [16, 17]. In the first-line setting at our institution, the hypomethylating agents showed a response rate of 26.5 % (CR + CRi). Interestingly, the dose intensity of decitabine did not seem to affect response with 26 % (5/19) of patients receiving 10-day decitabine achieving a CR/CRi compared to 33 % (4/12) who received 5-day courses. This is in disagreement with the higher response rate seen in previous studies of 10-day decitabine [13, 22] but agrees with previously observed rates with 5-day regimens [17, 20]. These results may be influenced by the fact that these patients were all considered not to be candidates for standard therapy and therefore likely had poor performance status and considerable comorbidities making the 10-day regimen more difficult to tolerate. Indeed, the first-line cohort had a significantly higher mean CCI score than the other two cohorts studied at 2.35. This is consistent with the short, 3.4-month (CI 1.54, 7.95) median OS of the cohort as a whole. Despite the overall poor characteristics of the cohort, 18 % of patients were alive at 1 year from diagnosis indicating that there are those even very poor-risk patients that can benefit from hypomethylating therapy. These results compare favorably with previously published results of low-intensity therapy (either hydrea or low-dose cytarabine) where only 13 % of patients were alive at 1 year [23]. Consistent with previous reports regarding the time to response in the HAs, most patients required more than one cycle to achieve a response with a median number of three cycles in the upfront setting and one patient who required 15 cycles to achieve their best response. This suggests that clinicians need to continue therapy in patients beyond one cycle to avoid discontinuing HAs in patients who would achieve a response if treated longer. Tumor burden also appeared to effect response in our small cohort with no patients with peripheral blasts or LDH >400 IU/L achieving a CR/CRi. Additionally, only one patient with a WBC >10,000 achieved a CR/CRi consistent with previous reports [24]. Marrow blast percentage did not appear to effect response with no difference in CR/CRi rate in patients with more or less than 30 % marrow blasts. This confirms the activity of the HAs in patients with >30 % blasts [25]. These data add to the efficacy of the hypomethylating agents and suggest that high-risk patients who refuse or are not candidates for standard induction therapy can be offered treatment with a hypomethylating agent as an alternative to best supportive care.
In the salvage setting, the hypomethylating agents showed a decreased response rate, with only 1/28 patients achieving a CR or CRi. This likely reflects the selection of resistant clones by the previously administered cytotoxic chemotherapy and suggests some degree of cross resistance. Despite this lack of marrow response, 21 of 28 patients received more than one cycle of a hypomethylating agent, and median overall survival from time of hypomethylating agent was 8.2 months (CI 4.8, 10.3) with 7 of 28 patients alive at 1 year. The survival result is consistent with a recent study of azacitidine as salvage in AML where a median survival of 9 months was observed although the response rate in that study was much higher with a 21 % CR rate [26]. In our study, 27/28 patients were treated with decitabine; thus, the difference in response rate may reflect the differential activity of the two agents in this setting. The median overall survival also compares well with published cytotoxic salvage regimens in AML [27, 28]; however, the lack of marrow response suggests this strategy is suboptimal in patients for whom a reduced-intensity allogeneic hematopoietic stem cell transplant (HSCT) is planned. Based on these data, hypomethylating agents, decitabine in particular, should be considered as salvage therapy for those patients who do not have the possibility of a HSCT. The utility of the hypomethylating agents as salvage therapy in AML is understudied and needs to be validated in a prospective clinical trial setting.
Consolidation for elderly and unfit patients is another area of clinical uncertainty. The goal of cytotoxic induction therapy is to achieve a complete remission. It has been known for nearly 30 years that patients who achieve a remission are not cured without additional therapy [29]. The optimal consolidation therapy in younger patients is better established with high-dose cytarabine being the current standard [30]. In elderly patients, the optimal consolidation therapy has not been well established. Less intense maintenance regimens as well as more intense regimens have both been shown to have activity [31], and significant controversy remains. At our institution, the use of hypomethylating agents as consolidation resulted in a median overall survival from time of hypomethylating agent treatment of 11.4 months (CI 4.0, 17.7) and when calculated from diagnosis was 13.8 months (CI 8, 21.6). This was an elderly cohort with a median age of 73, and the youngest patient was 58. In a similar age group, a median survival of 15 months was seen for patients who achieved a complete remission and went on to get cytotoxic consolidation chemotherapy [32]. This comparable survival is remarkable, given that our patients were deemed not to be candidates for further cytotoxic therapy because of poor performance status or previous complications during induction. The hypomethylating agents were well tolerated despite the high-risk features of the cohort with only one patient dying within 60 days of initiation of therapy. All patients but one were given hypomethylating agents until disease progression or death, and two patients died while in remission. The one surviving patient in this cohort stopped all therapy after three cycles of azacitidine consolidation and is now more than 2 years from diagnosis with no signs of disease. These data suggest that hypomethylating agents can prolong remission even in patients unfit for standard cytotoxic therapy. The activity of these agents in consolidation needs to be confirmed in prospective clinical trials.
The current study is limited in several ways. It is a single-institution retrospective study, and patient selection and institutional practices may bias the results. We could not collect functional status (either Eastern Cooperative Oncology Group Performance Status or Karnofsky Performance Status Scale) because it was not clearly or consistently documented in the patients’ electronic medical records. Finally, there is no direct comparison to control cohorts of patients treated with traditional chemotherapy. Despite these limitations, we believe the study suggests that the hypomethylating agents have activity in all phases of AML therapy. Additionally, this study represents real-world application of hypomethylating agents and is not constrained by prospective study restrictions making it more applicable to routine clinical practice. These results support the continued study of hypomethylating agents in AML treatment in prospective clinical trials.
References
Siegel R, Ward E, Brawley O, Jemal A (2011) Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin 61(4):212–236
Dohner H, Estey EH, Amadori S, Appelbaum FR, Buchner T, Burnett AK, Dombret H, Fenaux P, Grimwade D, Larson RA, Lo-Coco F, Naoe T, Niederwieser D, Ossenkoppele GJ, Sanz MA, Sierra J, Tallman MS, Lowenberg B, Bloomfield CD (2010) Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood 115(3):453–474
Tallman MS, Gilliland DG, Rowe JM (2005) Drug therapy for acute myeloid leukemia. Blood 106(4):1154–1163
Bennett JM, Young ML, Andersen JW, Cassileth PA, Tallman MS, Paietta E, Wiernik PH, Rowe JM (1997) Long-term survival in acute myeloid leukemia: the Eastern Cooperative Oncology Group experience. Cancer 80(11 Suppl):2205–2209
Kubal T, Lancet JE (2013) The thorny issue of relapsed acute myeloid leukemia. Curr Opin Hematol 20(2):100–106
Erba HP (2007) Prognostic factors in elderly patients with AML and the implications for treatment. Hematology Am Soc Hematol Educ Program 420–428.
Rollig C, Thiede C, Gramatzki M, Aulitzky W, Bodenstein H, Bornhauser M, Platzbecker U, Stuhlmann R, Schuler U, Soucek S, Kramer M, Mohr B, Oelschlaegel U, Stolzel F, von Bonin M, Wermke M, Wandt H, Ehninger G, Schaich M (2010) A novel prognostic model in elderly patients with acute myeloid leukemia: results of 909 patients entered into the prospective AML96 trial. Blood 116(6):971–978
Farag SS, Archer KJ, Mrozek K, Ruppert AS, Carroll AJ, Vardiman JW, Pettenati MJ, Baer MR, Qumsiyeh MB, Koduru PR, Ning Y, Mayer RJ, Stone RM, Larson RA, Bloomfield CD (2006) Pretreatment cytogenetics add to other prognostic factors predicting complete remission and long-term outcome in patients 60 years of age or older with acute myeloid leukemia: results from Cancer and Leukemia Group B 8461. Blood 108(1):63–73
Appelbaum FR, Gundacker H, Head DR, Slovak ML, Willman CL, Godwin JE, Anderson JE, Petersdorf SH (2006) Age and acute myeloid leukemia. Blood 107(9):3481–3485
Goldstone AH, Burnett AK, Wheatley K, Smith AG, Hutchinson RM, Clark RE (2001) Attempts to improve treatment outcomes in acute myeloid leukemia (AML) in older patients: the results of the United Kingdom Medical Research Council AML11 trial. Blood 98(5):1302–1311
Harousseau JL (1998) Acute myeloid leukemia in the elderly. Blood Rev 12(3):145–153
Aimiuwu J, Wang H, Chen P, Xie Z, Wang J, Liu S, Klisovic R, Mims A, Blum W, Marcucci G, Chan KK (2012) RNA-dependent inhibition of ribonucleotide reductase is a major pathway for 5-azacytidine activity in acute myeloid leukemia. Blood 119(22):5229–5238
Blum W, Garzon R, Klisovic RB, Schwind S, Walker A, Geyer S, Liu S, Havelange V, Becker H, Schaaf L, Mickle J, Devine H, Kefauver C, Devine SM, Chan KK, Heerema NA, Bloomfield CD, Grever MR, Byrd JC, Villalona-Calero M, Croce CM, Marcucci G (2010) Clinical response and miR-29b predictive significance in older AML patients treated with a 10-day schedule of decitabine. Proc Natl Acad Sci U S A 107(16):7473–7478
Slovak ML, Kopecky KJ, Cassileth PA, Harrington DH, Theil KS, Mohamed A, Paietta E, Willman CL, Head DR, Rowe JM, Forman SJ, Appelbaum FR (2000) Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood 96(13):4075–4083
Charlson M, Szatrowski TP, Peterson J, Gold J (1994) Validation of a combined comorbidity index. J Clin Epidemiol 47(11):1245–1251
Ozbalak M, Cetiner M, Bekoz H, Atesoglu EB, Ar C, Salihoglu A, Tuzuner N, Ferhanoglu B (2012) Azacitidine has limited activity in ‘real life’ patients with MDS and AML: a single centre experience. Hematol Oncol 30(2):76–81
Kantarjian HM, Thomas XG, Dmoszynska A, Wierzbowska A, Mazur G, Mayer J, Gau JP, Chou WC, Buckstein R, Cermak J, Kuo CY, Oriol A, Ravandi F, Faderl S, Delaunay J, Lysak D, Minden M, Arthur C (2012) Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol 30(21):2670–2677
Knipp S, Hildebrand B, Kundgen A, Giagounidis A, Kobbe G, Haas R, Aul C, Gattermann N, Germing U (2007) Intensive chemotherapy is not recommended for patients aged >60 years who have myelodysplastic syndromes or acute myeloid leukemia with high-risk karyotypes. Cancer 110(2):345–352
Menzin J, Lang K, Earle CC, Kerney D, Mallick R (2002) The outcomes and costs of acute myeloid leukemia among the elderly. Arch Intern Med 162(14):1597–1603
Cashen AF, Schiller GJ, O’Donnell MR, DiPersio JF (2010) Multicenter, phase II study of decitabine for the first-line treatment of older patients with acute myeloid leukemia. J Clin Oncol 28(4):556–561
Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Gattermann N, Germing U, Sanz G, List AF, Gore S, Seymour JF, Dombret H, Backstrom J, Zimmerman L, McKenzie D, Beach CL, Silverman LR (2010) Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J Clin Oncol 28(4):562–569
Blum W, Schwind S, Tarighat SS, Geyer S, Eisfeld AK, Whitman S, Walker A, Klisovic R, Byrd JC, Santhanam R, Wang H, Curfman JP, Devine SM, Jacob S, Garr C, Kefauver C, Perrotti D, Chan KK, Bloomfield CD, Caligiuri MA, Grever MR, Garzon R, Marcucci G (2012) Clinical and pharmacodynamic activity of bortezomib and decitabine in acute myeloid leukemia. Blood 119(25):6025–6031
Burnett AK, Milligan D, Prentice AG, Goldstone AH, McMullin MF, Hills RK, Wheatley K (2007) A comparison of low-dose cytarabine and hydroxyurea with or without all-trans retinoic acid for acute myeloid leukemia and high-risk myelodysplastic syndrome in patients not considered fit for intensive treatment. Cancer 109(6):1114–1124
Maurillo L, Venditti A, Spagnoli A, Gaidano G, Ferrero D, Oliva E, Lunghi M, D’Arco AM, Levis A, Pastore D, Di Renzo N, Santagostino A, Pavone V, Buccisano F, Musto P (2012) Azacitidine for the treatment of patients with acute myeloid leukemia: report of 82 patients enrolled in an Italian Compassionate Program. Cancer 118(4):1014–1022
van der Helm LH, Veeger NJ, Kooy M, Beeker A, de Weerdt O, de Groot M, Alhan C, Hoogendoorn M, Laterveer L, van de Loosdrecht AA, Koedam J, Vellenga E, Huls G (2013) Azacitidine results in comparable outcome in newly diagnosed AML patients with more or less than 30% bone marrow blasts. Leuk Res 37(8):877–882
Ivanoff S, Gruson B, Chantepie SP, Lemasle E, Merlusca L, Harrivel V, Charbonnier A, Votte P, Royer B, Marolleau JP (2013) 5-Azacytidine treatment for relapsed or refractory acute myeloid leukemia after intensive chemotherapy. Am J Hematol (in press)
Kohrt HE, Patel S, Ho M, Owen T, Pollyea DA, Majeti R, Gotlib J, Coutre S, Liedtke M, Berube C, Alizadeh AA, Medeiros BC (2010) Second-line mitoxantrone, etoposide, and cytarabine for acute myeloid leukemia: a single-center experience. Am J Hematol 85(11):877–881
Thomas X, Elhamri M, Chelghoum Y, Reman O, Arnaud P, Raffoux E, Le QH, Tavernier E, Dombret H, Michallet M (2005) Intensive chemotherapy with mitoxantrone administered as a single injection in patients with high-risk acute myeloid leukemia: results of the EMA 2000 trial. Ann Hematol 84(6):376–382
Cassileth PA, Harrington DP, Hines JD, Oken MM, Mazza JJ, McGlave P, Bennett JM, O’Connell MJ (1988) Maintenance chemotherapy prolongs remission duration in adult acute nonlymphocytic leukemia. J Clin Oncol 6(4):583–587
Mayer RJ, Davis RB, Schiffer CA, Berg DT, Powell BL, Schulman P, Omura GA, Moore JO, McIntyre OR, Frei E 3rd (1994) Intensive postremission chemotherapy in adults with acute myeloid leukemia. Cancer and Leukemia Group B. N Engl J Med 331(14):896–903
Itzykson R, Gardin C, Pautas C, Thomas X, Turlure P, Raffoux E, Terre C, Fenaux P, Castaigne S, Dombret H, Boissel N (2011) Impact of post-remission therapy in patients aged 65–70 years with de novo acute myeloid leukemia: a comparison of two concomitant randomized ALFA trials with overlapping age inclusion criteria. Haematologica 96(6):837–844
Baer MR, George SL, Sanford BL, Mrozek K, Kolitz JE, Moore JO, Stone RM, Powell BL, Caligiuri MA, Bloomfield CD, Larson RA (2011) Escalation of daunorubicin and addition of etoposide in the ADE regimen in acute myeloid leukemia patients aged 60 years and older: cancer and Leukemia Group B Study 9720. Leukemia 25(5):800–807
Acknowledgments
The authors would like to acknowledge Karen Klein for her critical reading of the manuscript; TSP is supported by NCI 1K08CA169809-01, SI is supported by NCI Cancer Center Support Grant (CCSG) P30CA012197, and HDK is supported by NIA K23AG038361.
Conflict of interest
The authors declare no competing financial interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tawfik, B., Sliesoraitis, S., Lyerly, S. et al. Efficacy of the hypomethylating agents as frontline, salvage, or consolidation therapy in adults with acute myeloid leukemia (AML). Ann Hematol 93, 47–55 (2014). https://doi.org/10.1007/s00277-013-1940-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-013-1940-9