Abstract
Cilostazol is practically insoluble in water and thus results in poor bioavailability. Only a few approaches have been reported for improving the bioavailability of cilostazol. Solid dispersion technique via solvent evaporation method was applied to improve the solubility and dissolution of cilostazol. Various polymers, mixture of polymer and surfactant, and mixture of polymers were screened as a carrier for the solid dispersion. Solubility of cilostazol was improved significantly when Eudragit® L100 was used as a carrier. However, addition of surfactant to Eudragit® L100 decreased the solubility slightly. Whereas, the mixture of Eudragit® L100 and Eudragit® S100 as a carrier system further increased the solubility. Based on the highest solubility obtained among the carriers screened, 1:1 ratio of Eudragit® L100 and Eudragit® S100 was selected as a carrier, and drug to carrier ratio was optimized to 1:5. Differential scanning calorimetry and X-ray diffraction studies showed that the characteristic peak of cilostazol disappeared in the solid dispersion, indicating that cilostazol existed in amorphous form in this formulation. Spray drying method was superior to vacuum drying method in terms of dissolution rate. Meanwhile, it was observed that the disintegration rate and the concentration of polymer had some effect on the crystallization of cilostazol in dissolution medium. Tablet formulation containing spray dried solid dispersion showed significant improvement in dissolution as compared to the commercial tablet.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Solid dispersion is an important technique used in the pharmaceutical field to improve the solubility and dissolution rate of poorly water soluble drugs (Sekiguchi and Obi 1961). It has been used widely to improve the oral absorption and bioavailability of BCS class II drugs (Leuner and Dressman 2000; Amidon et al. 1995). Solid dispersion is defined as ‘a dispersion of drug molecules in an inert carrier or matrix in the solid state’ (Chiou and Riegelman 1971). According to the carrier systems used, solid dispersions can be classified into three generations: I. Crystalline carriers, II. Polymeric carriers, III. Surfactants, mixture of polymers and surfactants, and mixture of polymers (Vasconcelos et al. 2007). The enhanced dissolution characteristics of solid dispersion are due to reduction in the drug particle size and the consequent increase in the surface area (Yonemochi et al. 1999), and improved wettability resulting from intimate contact with a hydrophilic carrier (Chow et al. 1995). In addition, formation of a higher energy solid-state form (i.e., amorphous) of drug which doesn’t require energy to break up the crystal lattice of a drug during dissolution process (Yonemochi et al. 1997) may also improve the dissolution properties.
Hot melt and solvent methods have been developed to prepare solid dispersions (Leuner and Dressman 2000; Usui et al. 1998; Sekiguchi and Obi 1961; Chiou and Riegelman 1971). In hot melt method, drug and hydrophilic carrier are molten together to form a uniform mass. Miscibility gaps in the phase diagram and thermostability issues of drug and carrier are potential limitations of hot melt method. Solvent method involves solvent evaporation, solvent wetting and surface-attachment (Joe et al. 2010; Yamashita et al. 2003). In solvent evaporation method, both drug and carrier are dissolved in a volatile solvent and evaporated to form amorphous dispersion of the drug in the carrier. In solvent wetting method, solvent dissolves drugs only. The dissolved drugs are attached onto the surface of undissolved carriers and converted to amorphous state. Conversely, amorphous carriers are attached onto the surface of crystalline drugs in surface-attached method. Solvent wetting method and surface-attached method have advantages of using less and no organic solvents, respectively. However, it has been reported that the order of improvement in solubility and dissolution was solvent evaporation method > solvent wetting method > surface-attached method (Joe et al. 2010; Park et al. 2010).
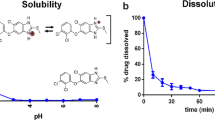
Peripheral arterial disease is common in about 20 % of the general population aged over 70 years (Dindyal and Kyriakides 2009). Especially, intermittent claudication is the most prominent symptom of peripheral arterial disease, affecting around 1–7 % of the population. Cramping pain in the legs or buttocks with muscle exertion occurs due to the poor circulation of blood in the arteries of the legs (Hamming 1959). Cilostazol (6-[4-(1-cyclohexyl-1H-tetrazol-5-yl)butoxy]-3,4-dihydro-2(1H)-quinolinone) is a cAMP phosphodiesterase III inhibitor approved for patients suffering from intermittent claudication. It increases cAMP in platelets and blood vessels, leading to inhibition of platelet aggregation and vasodilation (Kimura et al. 1985). Recent clinical trials have reported that cilostazol is also effective in preventing coronary artery restenosis post-endovascular treatments (Dindyal and Kyriakides 2009). It is marketed as oral tablet under the trade name Pletal®. The recommended dosage is 100 mg twice a day. Aqueous solubility of cilostazol is 3.34 µg/mL and its octanol/water partition coefficient is about 200 (Shimizu et al. 1985). According to Biopharmaceutics Classification System (BCS) (Amidon et al. 1995), cilostazol falls into Class II based on poor solubility and high permeability. Therefore, improving solubility would enhance the oral bioavailability of cilostazol.
Only a few approaches have been reported for improving the bioavailability of cilostazol hitherto. One study involved formation of inclusion complex with cyclodextrin (Patel and Rajput 2009), however, cyclodextrin is costly and the improvement in dissolution rate was only about twice that of pure cilostazol. Other approaches involve particle size reduction by Nanocrystal® and supercritical anti-solvent process (Jinno et al. 2006; Kim et al. 2007). Reducing the particle size of cilostazol increased the solubility, however, the extent of increment was not very significant. Therefore, present study aimed at improving dissolution rate of cilostazol using economical and simple solid dispersion process.
Materials and methods
Materials
Cilostazol was a generous gift from Korea United Pharm. Inc. (Seoul, Korea). Methacrylic acid-methyl methacrylate copolymer (1:1) (Eudragit® L100), Methacrylic acid-methyl methacrylate copolymer (1:2) (Eudragit® S100), basic butylated methacrylate copolymer (Eudragit® E100), ammonio methacrylate copolymer, type A (Eudragit® RL100) and ammonio methacrylate copolymer, type B (Eudragit® RS100) were obtained from Degussa (Rheinfelden, Germany). Polyvinylpyrrolidones (Kollidon® K-30 and Kollidon® K-90), Vinylpyrrolidone-vinyl acetate copolymer (Kollidon® VA64), macrogol 15 hydroxystearate (Solutol® HS 15) and Cremophor® ELP were obtained from BASF (Ludwigshafen, Germany). Carbopol 971NF was gifted from Korea United Pharm. Inc. (Seoul, Korea). Hydroxypropylmethyl cellulose (HPMC 2910) was obtained from Shin-Etsu Chemical Co. Ltd. (Tokyo, Japan). Polysorbate 80 (Tween® 80) and lecithin were purchased from Junsei Chemical Co. Ltd. (Tokyo, Japan). Stearoyl polyoxylglycerides (Gelucire® 50/13) was obtained from Gattefossé (Lyon, France). Sodium taurocholate, polyvinylacetate, polyethyleneglycol (PEG) 3400 were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Croscarmellose sodium was obtained from Whawon Co. (Seoul, Korea). Microcrystalline cellulose (Avicel® PH101) was obtained from Seoul Pharm Co. (Seoul, Korea). All other materials and reagents were of analytical grade and used as received without further purification.
Methods
Preparation of solid dispersion
Solid dispersion of cilostazol was prepared by solvent evaporation method. The carriers used in this system were polymers, surfactants and their combinations. Cilostazol and carrier were completely dissolved in methanol or methanol/dichloromethane (1:1) depending on carrier used. The organic solvent was evaporated by either spray dryer (B-290, Buchi, Switzerland) or in vacuum drying oven (NAPCO 5831, Fisher scientific, Waltham, MA, USA). Following parameters were used for spray drying process: spray-flow 538 L/h, feed flow 1.5 mL/min, inlet temperature 75 °C, and aspirator 75 %, and nozzle diameter 0.7 mm.
Solubility studies
Solid dispersion containing 2 mg of cilostazol was placed in 2 mL of pH 6.8 buffer and stirred at 500 rpm for 24 h at room temperature using Teflon coated magnetic bar. The samples were first centrifuged at 13,200 rpm for 10 min and filtered through 0.45 µm regenerated cellulose syringe filter (Target®, National scientific, USA). The first 1 mL of the filtrate was discarded. Samples were then suitably diluted with methanol and analyzed by high performance liquid chromatography (HPLC) system (Shimadzu Scientific Instrument, MD, USA).
Method of analysis
Cilostazol was determined by using a HPLC system, consisting of a UV detector (SPD-10A), a pump (LC-10AD) and an automatic injector (SIL-10A). The mobile phase consisted of water and acetonitrile (45:55) and the flow rate was 1.0 mL/min. The wavelength of the UV detector was set at 254 nm and a reversed-phase column (Gemini 5µ C18 110A, Phenomenex, USA) was used. The column temperature was maintained at 30 °C.
Differential scanning calorimetry (DSC)
Thermal analysis was carried out using DSC unit (Pyris 6 DSC, Perkin Elmer, Netherlands). Indium was used to calibrate the temperature scale and enthalpic response. Samples were placed in aluminum pans and heated at a scanning rate of 10 °C/min from 30 to 200 °C.
X-ray diffraction (XRD)
X-ray powder diffraction was obtained using X-ray diffractometer (X’Pert PRO MPD, PANalytical Co., Holland). The diffraction pattern was measured with a voltage of 40 kV and a current of 30 mA. 2θ range of 5–50o and step size of 0.03o were used at a scan speed of 1 s/step.
Scanning electron microscopy (SEM)
The morphology of the samples was examined by SEM (S4800, Hitachi, Japan). The samples were mounted onto an aluminum stub and sputter coated with platinum particles for 60 s in an argon atmosphere.
Preparation of solid dispersion tablet
Solid dispersion of cilostazol was prepared as explained in “Preparation of solid dispersion” section. The film-like product resulting from vacuum drying was ground by mortar and pestle and subsequently sieved through 70 mesh sieve. Solid dispersion equivalent to 100 mg of cilostazol was mixed with excipients (Avicel® PH 101, magnesium stearate) and compressed in pilot press tablet machine (Chamunda Machinery Private Ltd. Ahemdabad. India). To prepare tablets containing superdisintegrant or gelling agent, Avicel® PH 101 was replaced with predetermined amount of the agent with fixed tablet weight of 800 mg.
Dissolution studies
Dissolution studies of pure cilostazol, Pletal®, physical mixture and solid dispersion formulations were performed using dissolution apparatus (DST-810 and DS-600A, Labfine Inc., Suwon, Korea) at 37 ± 0.5 °C and the paddle was rotated at 75 rpm. The medium used for the study include 500 mL of pH 6.8 buffer and fasted state simulated intestinal fluid (FaSSIF). FaSSIF contains 3 mM sodium taurocholate and 0.75 mM lecithin and pH was adjusted to 6.5 (Marques 2004). All the tested formulations contain 100 mg of cilostazol. Pure cilostazol and physical mixture were placed in tea-bag, whereas tablet was placed in the sinker during the dissolution study. At the predetermined time intervals, 2 mL of the samples were withdrawn and the equal volume of fresh medium was replaced. The collected samples were filtered through regenerated cellulose syringe filters (Target®, National scientific, USA). Samples were then suitably diluted with methanol and analyzed by HPLC.
Results and discussion
Screening of carriers
Polymeric carrier
Various hydrophilic polymers were screened as a carrier for cilostazol solid dispersion. Solubility of cilostazol obtained using various hydrophilic polymers to prepare solid dispersion are summarized in Table 1. Majority of the solid dispersions showed significant improvement in solubility as compared to that of pure cilostazol (3.34 µg/mL). The highest solubility was achieved from the solid dispersion prepared with Eudragit® L100, where improvement was almost 10 fold. Solid dispersions prepared with Eudragit® S100 and Eudragit® E100 also showed significant increase in the solubility of cilostazol. This improvement in solubility could be due to the conversion of crystalline structure of the drug to amorphous form. In crystalline form, the crystal lattice has to be broken for the drug to dissolve, whereas only short range intermolecular interactions are present in amorphous form (Taylor and Zografi 1997). The conversion of crystalline form to amorphous form was confirmed by DSC and XRD studies which will be discussed in the later section. It is well known that amorphous form of drug may lead to supersaturated state when exposed to aqueous medium (Hasegawa et al. 1988). However, the supersaturated state of drug has a tendency to nucleate and precipitate from the solution (Konno et al. 2008). In this regard, Konno et al. discussed that polymers would be able to inhibit crystal growth and thereby maintain the supersaturated state in concentration to a level where nucleation is no longer spontaneous. The ability to stabilize the supersaturated solution by preventing crystallization depends on the nature of polymers (Konno et al. 2008; Yamashita et al. 2003). High solubility obtained with Eudragit® L100, Eudragit® S100, and Eudragit® E100 may also be attributable to their crystallization inhibition effect. Based on the highest solubility obtained, Eudragit® L100 was chosen as the carrier for further studies.
Mixture of polymer and surfactant
In an attempt to obtain further increase in solubility, the effect of adding various surfactants to Eudragit® L100 on the solubility of cilostazol was investigated. Incorporation of surfactants in the formulations containing polymeric carrier is known to promote wetting and facilitate solubilization (Leuner and Dressman 2000; Serajuddin et al. 1990). However, contrary to expectations, the addition of surfactants did not result in additional increase in the solubility, but slightly decreased the solubility (Table 2). We were not able to clearly elucidate the reason why added surfactant resulted in slight decrease in the solubility of cilostazol. The plasticization of polymer by surfactant could be one of the reasons for the lower solubility obtained by including surfactant in the present study. It was reported that change in polymeric properties could alter the stability of amorphous drug distributed in the matrix (Pouton 2006; Vasconcelos et al. 2007). Studies have shown that the incorporation of surfactant can decrease the glass transition temperature (Tg) of the polymer used in solid dispersion (Ghebremeskel et al. 2007). This may consequently increase the chain mobility, allowing the adjacent drug molecules to nucleate and crystallize to some extent.
Mixture of polymers
As another attempt to further improve solubility of cilostazol, the effect of using polymeric combination was studied. The total amount of polymers used in combination was kept constant in each formulation tested. Combination of Eudragit® L100 and Eudragit-®- S100 with Eudragit® E100 formed complex when their solutions were mixed together. The ionic interaction between carboxylate groups of Eudragit® L100 and Eudragit-®- S100, and dimethylamino groups of Eudragit® E100 induced the complexation (Moustafine et al. 2005). The complex formed did not dissolve along with the drug, thus solid dispersion with this combination was not studied. Combinations of Eudragit® L100 with HPMC 2910 and Kollidon® VA64 showed lower solubility than Eudragit® L100 alone (Table 2). Synergistic effect in terms of improvement in solubility was observed with combinations of Eudragit® L100:Eudragit® S100 and Eudragit® S100:Kollidon® VA64. More than 1.5 times increase in solubility was obtained with solid dispersion prepared from the combination of Eudragit® L100 and Eudragit® S100 as compared to Eudragit® L100 alone. Thus, this combination was chosen as a carrier for further study.
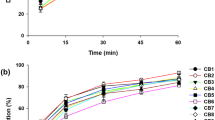
Optimization
Solubility study was carried out with solid dispersions containing various ratios of Eudragit® L100 and Eudragit® S100 at the drug and carrier ratio of 1:5. Based on the highest solubility obtained, 1:1 ratio of Eudragit® L100 and Eudragit® S100 was considered optimum (Fig. 1). Subsequently, the effect of various ratios of cilostazol and combination of Eudragit® L100 and Eudragit® S100 on the solubility was studied. The results were also compared with those obtained from physical mixtures. As shown in Fig. 2, the solubility values of cilostazol from solid dispersions were significantly higher than those from the physical mixtures at all ratios of drug and carrier. Increasing the proportion of carrier in the physical mixtures did not significantly increase the solubility of cilostazol. On the other hand, in the case of solid dispersion, solubility increased proportional to the amount of carrier used. The results indicated that the improvement in the solubility of cilostazol was due to the formation of solid dispersion rather than the wettability owing to the presence of hydrophilic polymer. Considering both the improvement in solubility and the tablet size, 1:5 ratio of cilostazol and combination of Eudragit® L100 and Eudragit® S100 was chosen for further study.
Characterization of solid dispersion
DSC study was performed to investigate the crystalline property of cilostazol in the formulations. As illustrated in Fig. 3, the DSC thermogram showed a characteristic peak of pure cilostazol at 159.62 °C. This peak was also observed in the physical mixture of cilostazol with Eudragit® L100 and Eudragit® S100. The calculated peak areas were 128.3, 114.99 mJ in pure cilostazol and physical mixture, respectively. Thus, it is clear that physical dispersion of cilostazol in polymers would not change the crystalline state of cilostazol. However, the characteristic peak of cilostazol disappeared in solid dispersions prepared by both vacuum drying and spray drying, indicating that cilostazol existed in amorphous form in these formulations. XRD analysis was also performed to confirm the results obtained from DSC study. XRD patterns of pure cilostazol confirmed its crystalline state (Fig. 4). Physical mixture of cilostazol and polymers also exhibited the characteristic peaks of cilostazol at 12.8°, 15.6° and 23.4° position of 2θ. These characteristic peaks were not observed in solid dispersions prepared by both spray drying and vacuum drying methods. Furthermore, SEM study was conducted to observe the morphology of solid dispersions. Figure 5a, b shows the morphology of pure cilostazol powder and mixture of Eudragit® L100 and Eudragit® S100, respectively. The morphology of cilostazol or polymers in physical mixture was similar to that observed separately (Fig. 5c). Whereas, this morphology of cilostazol was absent in both of solid dispersions prepared by vacuum drying (Fig. 5d) and spray drying (Fig. 5e) and cilostazol seemed to be well dispersed throughout polymer network.
Preparation of tablet and dissolution study
Tablets containing solid dispersion were prepared after adding various excipients to optimize dissolution characteristics. Dissolution study was conducted with tablets containing spray dried or vacuum dried solid dispersion and Avicel® PH 101 as a diluent, together with pure cilostazol, physical mixture and commercial tablet (Pletal®). As illustrated in Fig. 6, the release of cilostazol from the physical mixture did not show significant improvement over that of pure cilostazol which was consistent with the results obtained from solubility study (Fig. 2). It has been shown previously that mere presence of hydrophilic polymer did not improve the wettability of cilostazol significantly (Leuner and Dressman 2000). On the other hand, tablet containing solid dispersion of cilostazol showed significant improvement in dissolution rate of cilostazol. Even though the absolute extent of release was low, almost 5 and 3 times higher dissolution rate was observed from tablet containing spray dried and vacuum dried solid dispersion respectively, as compared to the commercial tablet. This could be due to persistence of supersaturated state by minimizing crystallization of cilostazol in the medium. Polymers in the solid dispersion could inhibit crystallization of amorphous drug and thereby stabilize the supersaturated state by reducing the mobility of drug (Yoshioka 1994). When solid dispersions prepared by spray drying and vacuum drying were compared by SEM, the size of spray dried solid dispersion particles was almost 40 times smaller than that of vacuum dried solid dispersion particles (Fig. 5d, e). Highly reduced size of spray dried solid dispersion particles seemed to cause higher extent of cilostazol release when compared to vacuum dried particles.
It was reported that incorporation of superdisintegrant into tablet containing solid dispersion could enhance the rate and extent of release by increasing the surface area of dissolving drug (Srinarong et al. 2009). Accordingly, superdisintegrant was incorporated to spray dried solid dispersion tablet to improve the dissolution rate of cilostazol. Croscarmellose sodium was used as the superdisintegrant owing to its high swelling pressure and swelling volume (Quadir and Kolter 2006). Dissolution study was carried out with solid dispersion tablets containing various amounts of croscarmellose sodium. As illustrated in Fig. 7, superdisintegrant increased initial dissolution rate and the maximum concentration of the drug in the medium. However, the concentration of cilostazol in the dissolution medium declined rapidly, and the rate was faster when more superdisintegrant was used. It is well known that the nucleation rate is strongly dependent on the degree of supersaturation. The higher degree of supersaturation caused faster crystallization of drug particles (Turnbull and Fisher 1949; Konno et al. 2008). The higher supersaturation level was obtained by the incorporation of superdisintegrant, however, it caused faster nucleation and crystallization. It was speculated that the level of attainable supersaturation may be a function of polymer concentration. Therefore, the effect of polymer concentration on the solubility of cilostazol was investigated. The solubility of cilostazol was assessed by adding various amounts of spray dried solid dispersion in the same volume of medium. Adding more solid dispersion in a fixed volume of medium will result in higher concentration of polymer. It was observed that the concentration of polymer in medium had a profound effect on the solubility of the cilostazol (Fig. 8). The results indicated that maintaining high polymer concentration is required to increase solubility of cilostazol. To control the disintegration rate and maintain the high polymer concentration in the vicinity of the dissolving tablet, effect of incorporating gelling agent in the tablet containing solid dispersion was examined. Figure 9 shows the dissolution profiles of tablets containing various gelling agents. On the contrary to our expectation, incorporation of gelling agent not only delayed disintegration time but decreased dissolution rate. The improved extent of dissolution was not attainable during the observation due to the excessive retarded release. Considering the transit time of cilostazol absorption site in the small intestine (3–4 h), the release of cilostazol beyond the last time point in the figure was thought to be irrelevant (Davis 1986).
Since the addition of superdisintegrant or gelling agent did not improve dissolution profile of cilostazol, tablet formulation containing spray dried solid dispersion and Avicel® PH 101 was chosen for further study. Since FaSSIF is known to better simulate intestinal fluid, dissolution study was done in this medium to compare the selected formulation with the commercial tablet (Fig. 10). Although the extent of dissolution was lesser than that obtained using pH 6.8 buffer as medium, significant improvement over commercial tablet was observed. Even though the FaSSIF solution contains surfactant (sodium taurocholate), the solubility of cilostazol in this medium was extremely low and the % released of cilostazol from Pletal as well as our product was low. Meanwhile, it can be observed that our product reached higher dissolution after 2 h. Therefore, it is highly possible that our product can improve the bioavailability of cilostazol through enhanced solubility and dissolution.
In conclusion, combination of polymers showed much higher improvement in solubility of cilostazol than when they were used alone. Optimized tablet formulation for cilostazol showed significant improvement in dissolution as compared to the commercial tablet. The developed dosage form could be beneficial in improving bioavailability. In addition to the improved solubility of cilostazol, retarded release profile owing to the nature of carrier polymer may allow us to develop sustained or prolonged release formulation of cilostazol. However, in vivo studies should be performed to confirm the results obtained in the present study.
References
Amidon, G.L., H. Lennernas, V.P. Shah, and J.R. Crison. 1995. A theoretical basis for a biopharmaceutic drug classification: The correlation of in vitro drug product dissolution and in vivo bioavailability. Pharmaceutical Research 12: 413–420.
Chiou, W.L., and S. Riegelman. 1971. Pharmaceutical applications of solid dispersion systems. Journal of Pharmaceutical Sciences 60: 1281–1302.
Chow, A.H.L., C.K. Hsia, J.D. Gordon, J.W.M. Young, and E.I. Vargha-Butler. 1995. Assessment of wettability and its relationship to the intrinsic dissolution rate of doped phenytoin crystals. International Journal of Pharmaceutics 126: 21–28.
Davis, S.S. 1986. Transit of pharmaceutical dosage forms through the small intestine. Gut 27: 886.
Dindyal, S., and C. Kyriakides. 2009. A review of cilostazol, a phosphodiesterase inhibitor, and its role in preventing both coronary and peripheral arterial restenosis following endovascular therapy. Recent Patents on Cardiovascular Drug Discovery 4: 6–14.
Ghebremeskel, A.N., C. Vemavarapu, and M. Lodaya. 2007. Use of surfactants as plasticizers in preparing solid dispersions of poorly soluble API: Selection of polymer-surfactant combinations using solubility parameters and testing the processability. International Journal of Pharmaceutics 328: 119–129.
Hamming, J.J. 1959. Intermittent claudication at an early age, due to an anomalous course of the popliteal artery. Angiology 10: 369.
Hasegawa, A., M. Taguchi, R. Suzuki, T. Miyata, H. Nakagawa, and I. Sugimoto. 1988. Supersaturation mechanism of drugs from solid dispersions with enteric coating agents. Chemical & Pharmaceutical Bulletin 36: 4941–4950.
Jinno, J., N. Kamada, M. Miyake, K. Yamada, T. Mukai, M. Odomi, H. Toguchi, G.G. Liversidge, K. Higaki, and T. Kimura. 2006. Effect of particle size reduction on dissolution and oral absorption of a poorly water-soluble drug, cilostazol, in beagle dogs. Journal of Controlled Release 111: 56–64.
Joe, J.H., W.M. Lee, Y.-J. Park, K.H. Joe, D.H. Oh, Y.G. Seo, J.S. Woo, C.S. Yong, and H.-G. Choi. 2010. Effect of the solid-dispersion method on the solubility and crystalline property of tacrolimus. International Journal of Pharmaceutics 395: 161–166.
Kim, M.-S., S. Lee, J.-S. Park, J.-S. Woo, and S.-J. Hwang. 2007. Micronization of cilostazol using supercritical antisolvent (SAS) process: Effect of process parameters. Powder Technology 177: 64–70.
Kimura, Y., T. Tani, T. Kanbe, and K. Watanabe. 1985. Effect of cilostazol on platelet aggregation and experimental thrombosis. Arzneimittel-Forschung 35: 1144–1149.
Konno, H., T. Handa, D.E. Alonzo, and L.S. Taylor. 2008. Effect of polymer type on the dissolution profile of amorphous solid dispersions containing felodipine. European Journal of Pharmaceutics and Biopharmaceutics 70: 493–499.
Leuner, C., and J. Dressman. 2000. Improving drug solubility for oral delivery using solid dispersions. European Journal of Pharmaceutics and Biopharmaceutics 50: 47–60.
Marques, M. 2004. Dissolution media simulating fasted and fed states. Dissolution Technologies 11: 16.
Moustafine, R.I., T.V. Kabanova, V.A. Kemenova, and G. Van Den Mooter. 2005. Characteristics of interpolyelectrolyte complexes of Eudragit E100 with Eudragit L100. Journal of Controlled Release 103: 191–198.
Park, Y.-J., D.-H. Oh, Y.-D. Yan, Y.-G. Seo, S.-N. Lee, H.-G. Choi, and C.-S. Yong. 2010. Surface-attached solid dispersion. Journal of Pharmaceutical Investigation 40: 103–112.
Patel, S.G., and S.J. Rajput. 2009. Enhancement of oral bioavailability of cilostazol by forming its inclusion complexes. AAPS PharmSciTech 10: 660–669.
Pouton, C.W. 2006. Formulation of poorly water-soluble drugs for oral administration: Physicochemical and physiological issues and the lipid formulation classification system. European Journal of Pharmaceutical Sciences 29: 278–287.
Quadir, A., and K. Kolter. 2006. A comparative study of current superdisintegrants. Pharmaceutical Technology 30: s38–s42.
Sekiguchi, K., and N. Obi. 1961. Studies on absorption of eutectic mixture. I. A comparison of the behavior of eutectic mixture of sulfathiazole and that of ordinary sulfathiazole in man. Chemical & Pharmaceutical Bulletin 9: 866–872.
Serajuddin, A.T.M., P.-C. Sheen, and M.A. Augustine. 1990. Improved dissolution of a poorly water-soluble drug from solid dispersions in polyethylene glycol: Polysorbate 80 mixtures. Journal of Pharmaceutical Sciences 79: 463–464.
Shimizu, T., T. Osumi, K. Niimi, and K. Nakagawa. 1985. Physico-chemical properties and stability of cilostazol. Arzneimittel-Forschung 35: 1117–1123.
Srinarong, P., J.H. Faber, M.R. Visser, W.L.J. Hinrichs, and H.W. Frijlink. 2009. Strongly enhanced dissolution rate of fenofibrate solid dispersion tablets by incorporation of superdisintegrants. European Journal of Pharmaceutics and Biopharmaceutics 73: 154–161.
Taylor, L.S., and G. Zografi. 1997. Spectroscopic characterization of interactions between PVP and indomethacin in amorphous molecular dispersions. Pharmaceutical Research 14: 1691–1698.
Turnbull, D., and J.C. Fisher. 1949. Rate of nucleation in condensed systems. Journal of Chemical Physics 17: 71–73.
Usui, F., K. Maeda, A. Kusai, M. Ikeda, K. Nishimura, and K. Yamamoto. 1998. Dissolution improvement of RS-8359 by the solid dispersion prepared by the solvent method. International Journal of Pharmaceutics 170: 247–256.
Vasconcelos, T., B. Sarmento, and P. Costa. 2007. Solid dispersions as strategy to improve oral bioavailability of poor water soluble drugs. Drug Discovery Today 12: 1068–1075.
Yamashita, K., T. Nakate, K. Okimoto, A. Ohike, Y. Tokunaga, R. Ibuki, K. Higaki, and T. Kimura. 2003. Establishment of new preparation method for solid dispersion formulation of tacrolimus. International Journal of Pharmaceutics 267: 79–91.
Yonemochi, E., S. Kitahara, S. Maeda, S. Yamamura, T. Oguchi, and K. Yamamoto. 1999. Physicochemical properties of amorphous clarithromycin obtained by grinding and spray drying. European Journal of Pharmaceutical Sciences 7: 331–338.
Yonemochi, E., Y. Ueno, T. Ohmae, T. Oguchi, S.-I. Nakajima, and K. Yamamoto. 1997. Evaluation of amorphous ursodeoxycholic acid by thermal methods. Pharmaceutical Research 14: 798–803.
Yoshioka, M. 1994. Crystallization of indomethacin from the amorphous state below and above its glass transition temperature. Journal of Pharmaceutical Sciences 83: 1700.
Acknowledgments
All authors (J.-H. Park and H.-K. Choi) declare that they have no conflict of interest. This study was supported by research funds from Chosun University, 2014.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, JH., Choi, HK. Enhancement of solubility and dissolution of cilostazol by solid dispersion technique. Arch. Pharm. Res. 38, 1336–1344 (2015). https://doi.org/10.1007/s12272-014-0547-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-014-0547-6