Abstract
Coastal lagoons are known to host numerous resident and migrant fish species. Spatio-temporal variation in abiotic and biotic conditions in these ecosystems results, however, in a mosaic of microhabitats that could differently affect juvenile growth and survival. To deepen our understanding of juvenile fish habitat requirements and their spatio-temporal use of lagoons, microhabitat characteristics and fish assemblages were monitored jointly in a small temperate lagoon (the Prévost lagoon), from March to October 2019. A total of 2206 juvenile fishes belonging to 22 species were collected. Resident lagoon species, especially Atherina boyeri, dominated the assemblage (74%), while, among migrant species, Sparus aurata (8%) and Liza aurata (5%) were the most represented. Changes in overall juvenile abundance were mainly temporal, following the seasonal shifts in water temperature, salinity, and chlorophyll a concentration (44.9% of the co-inertia). However, our results revealed that distinct types of microhabitats exist in small lagoons and that juvenile fish distribution among them is non-random. Indeed, fish species richness mainly differed among sampling sites in relation to their distance from the inlet and the complexity of the three-dimensional habitat structure (36.5% of the co-inertia). Juveniles preferentially selected microhabitats with medium to high structural complexity, which were essentially created by macroalgae. However, microhabitat preferences were both species and ontogenetic stage dependent, with more contrasting microhabitat requirements in young juveniles. These results underline the need for conservation measures to consider each lagoon as a dynamic mosaic of microhabitats with radically different importance for the juveniles of the various fish species that colonize them.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Located at the land-sea interface, coastal lagoons are recognized as highly productive habitats (Kennish and Paerl 2010), supporting multiple ecosystem services, including fish production (Levin et al. 2001; Elliott and Hemingway 2002). They often host numerous resident fish and are colonized by the juveniles of varied migrant species (e.g., Ribeiro et al. 2012; Bruno et al. 2013; Rodríguez-Climent et al. 2013; Verdiell-Cubedo et al. 2013). Indeed, the high productivity and macrophyte cover of most coastal lagoons provide optimum food and shelter for juvenile fish (Levin et al. 2001), and their lower salinities can reduce osmoregulation costs to the benefit of growth (Potter et al. 1986). Therefore, many coastal lagoons match the definition of fish nursery habitats (Beck et al. 2001), including species of high commercial value (Franco et al. 2006b; Dufour et al. 2009; Grati et al. 2013; Isnard et al. 2015). This makes them key environments for the conservation of coastal fish populations. However, environmental conditions in these transitional ecosystems are highly disparate. For example, lagoon waters vary from oligohaline to hypersaline depending on the weather (rainfall, evaporation) and the intensity of local freshwater inputs or marine water intrusions (Barnes 1980; Alongi 1998; Tagliapietra et al. 2009). Water temperature is also highly variable in lagoons (Tagliapietra et al. 2009; Ifremer 2014) and often reaches extreme values that threaten local fauna and flora at some times of the year (Kennish et al. 2014). Given this variability, sustainable management of the fish populations that depend on lagoon ecosystems requires deepening our understanding of the relationship between lagoon environmental conditions and juvenile fish habitat requirements.
This is particularly true for the north-western Mediterranean lagoons, as most measures aimed at identifying, protecting, and managing key coastal ecosystems in the European Union consider them as a single homogeneous type of habitat (European Commission DG Environment 2013) while they differ highly, not only in terms of marine and terrigenous inputs (Fiandrino et al. 2017) but also in terms of substrate types, depths, macrophyte covers, and anthropogenic pressures (Pérez-Ruzafa and Marcos 2012; Sfriso et al. 2017). This leads to marked inter-lagoon differences in contamination levels, productivity, and eutrophication status (Pérez-Ruzafa et al. 2007a; Souchu et al. 2010; Munaron et al. 2012; Derolez et al. 2019). In addition, the environmental parameters (e.g., temperature, salinity, oxygen) in each lagoon tend to vary considerably throughout the year, with anoxic events and extremely high temperatures frequently observed in summer, and particularly low temperatures in winter (Christia and Papastergiadou 2007; Como and Magni 2009).
Ichthyofauna diversity and abundance differ among lagoons and throughout the year (Koutrakis et al. 2005; Manzo et al. 2016; Franco et al. 2019; Selfati et al. 2019). These variations are not only due to inter-specific differences in spawning periods (Tsikliras et al. 2010; Manzo et al. 2011), but also depend on the suitability of local environmental conditions for the needs and tolerances of the juveniles of each species (Pérez-Ruzafa et al. 2004, Rountree and Able 2007). To fully understand the relationship between environmental characteristics and juvenile fish densities in coastal lagoons, this suitability, and its temporal changes have to be studied. However, to go beyond the knowledge gathered so far (e.g.,Yáñez-Arancibia et al. 1994; Marshall and Elliott 1998; Cuadros et al. 2017), this has to be done at a much finer spatial scale than that of the whole lagoon. Indeed, most lagoon ecosystems consist of a dynamic mosaic of interconnected yet different micro-habitats (Nagelkerken et al. 2015). This is particularly true in Mediterranean lagoons where marked physico-chemical gradients and the alternation of patches of seagrass bed or macroalgae on varied types of substrates form a highly heterogeneous “seascape” (Le Fur et al. 2018; Menu et al. 2019). This fine-scale variability in environmental conditions modulates the composition and productivity of local prey communities and also affects juvenile fish physiology, behavior, and physical condition (Peterson et al. 2000, Pichavant 2001, Como et al. 2014), with potential consequences on their survival and growth rates (Bouchereau et al. 2000; Vasconcelos et al. 2010; Isnard et al. 2015). For example, the three-dimensional structure resulting from the presence of certain types of substrate (e.g., rocks) or macrophytes can, not only provide shelter from predation for juvenile fish (Thiriet 2014; Whitfield 2017), but also attract specific benthic invertebrates that they exploit as prey (Mistri et al. 2000; Woodland et al. 2019). As a result, the response of juvenile fish to habitat quality is species-dependent (Colombano et al. 2020) but also evolves with changes in the nutritional and protective needs of juveniles during growth (Dahlgren and Eggleston 2000; Félix-Hackradt et al. 2014). This implies both interspecific differences and intraspecific changes in the preferred microhabitats of juvenile fish (Vigliola and Harmelin-Vivien 2001).
To investigate these differences and deepen our understanding of the relationship between lagoon environmental conditions and the habitat requirements of juvenile fish, the present study simultaneously monitored fine spatio-temporal changes in environmental characteristics and the composition of juvenile fish assemblage within a small but typical Mediterranean lagoon. By characterizing the main drivers of juvenile fish abundance in this heterogeneous ecosystem and identifying the preferred lagoon microhabitats of juveniles for several fish species at different ontogenetic stages, we hoped to gather valuable information for species conservation and lagoon management, both at local and regional scales.
Material and Methods
Study Area
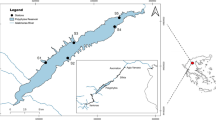
For this study, we chose the Prévost lagoon (43°30′N, 3°54′E), a small (2.4 km2 area) and shallow (1.5 m deep) expanse of water of 2.7 Mm3 permanently connected to the sea by a 30-m large, 393-m long, and 1.7-m deep straight canal located on its southern shore (Fig. 1). The mean daily volume of marine water entering the lagoon is about 0.6 Mm3 (Fiandrino et al. 2012). The average daily net balance of water is, however, negative (− 0.15 Mm3) due to continuous inputs of brackish water from the Rhône-to-Sète canal, through three channels located on the northern and eastern sides of the lagoon. Local water salinities and temperatures vary from 25 to 39 and from 8 to 24 °C, respectively, with minima generally observed in the winter and maxima in the summer for both factors (Ifremer 2014). Although relatively small, the Prévost lagoon displays heterogeneous environmental conditions: in its western part, water salinity is highly variable and substrate composition ranges from sand to mud, whereas in its eastern part, the salinity is quite stable and muddy bottoms dominate (Menu et al. 2019). Even if water quality in the lagoon has improved over the last decade (Leruste et al. 2016; Derolez et al. 2019), anoxic events are still frequently observed, especially during the warmer summer months (Bachelet et al. 2000). Eutrophication in the lagoon results in a general dominance of opportunistic green macroalgae on the bottom (Bachelet et al. 2000; Le Fur et al. 2018), but red and brown macroalgae and seagrass meadows are also present in certain areas (Cimiterra et al. 2020).
To date, 61 fish species, all commonly found in French Mediterranean lagoons at the adult and/or juvenile stages, have been reported in the lagoon (Kara and Quignard 2018d). Among them, three residents (Atherina boyeri, Pomatoschistus microps, Pomatoschistus minutus) and two migratory species (Anguilla anguilla and Engraulis encrasicolus) represent about 90% of both the local juvenile and adult abundances (Bouchoucha et al. 2012). Although no specific studies have been conducted on fish juvenile assemblages, the other fish species reported are mainly migratory ones (Kara and Quignard 2018d) which essentially occupy the lagoon from early spring to late autumn each year (Quignard et al. 1984).
2.2 Sampling
To cover the main period for juvenile fish recruitment and lagoon use in the area (Quignard et al. 1984; Aliaume et al. 1993; García-Rubies and Macpherson 1995), sampling was carried out from March to October 2019. This allowed us to capture the early juvenile stages of most local fish species, including those that recruit in the second half of the winter (Kara and Quignard 2018a, b, c). Sampling was carried out at least once, if possible twice, per 2-month periods (period 1 = March–April, period 2 = May–June, period 3 = July–August, period 4 = September–October), at six sites distributed either along the shoreline (NW, SW, SE, and NE) or in the central part (MW and MI) of the lagoon (Fig. 1). These sampling sites were positioned to reflect not only the west–east salinity gradient of the lagoon, but also local differences in the nature of the substrate (in relation to shoreline use) and the distribution of macrophytes (Menu et al. 2019).
Microhabitat Characterization
For each sampling site, potential accessibility for marine migrants was defined as the shorter distance from the sea inlet when following the edges of the lagoon (DIST). It was assessed prior to sampling, using the Quantum GIS 3.2 software (QGIS.org 2021). On each sampling date, water temperature (TEMP in °C) and salinity (SALI) were measured using a digital multiparameter meter (Multi 3430 WTW) coupled with a standard IDS conductivity cell probe (TetraCon® 925, WTW), and 2 L of water were collected in a plastic bottle and stored immediately in a cool box to assess local chlorophyll a concentrations (CHLA). Water depth (DEPT in cm) and seafloor type cover (i.e., rocks -ROCK-, bare sediment -SEDI-, pebbles -PEBB-, and/or bivalve shells -SHEL- in %) were estimated within nine quadrats (of 0.2 m2 each) distributed at 3, 6, and 9 m from the shore along three parallel linear transects. These later were positioned perpendicular to the shoreline, approximately 50 m apart, in order to limit disturbance during monitoring and to account for the spatial variability of the habitat at each site. All the macrophytes species present were also sampled after evaluating their respective spatial coverage (MCOV, %) and the overall canopy height (HEIG in cm) in each quadrat. Macrophytes were kept frozen for later identification in the laboratory.
Fish Sampling
To avoid scaring them away during microhabitat description and reduce sampling bias, juvenile fish were always collected before habitat description, between 10 and 12 am. Sampling at each site was done along the three parallel transects described above. To limit sampling bias linked to differences in gear selectivity and provide a more comprehensive image of the juvenile fish assemblage, notably in sites with non-uniform substrates (macrophytes, rocks), three sampling gears were combined (Bryan and Scarnecchia 1992; Franco et al. 2012). For each transect, an 8-m-long beach seine (mesh size: 4 mm), covering an area of 28 m2 per haul and targeting benthic and demersal juveniles, was first deployed once, perpendicularly to the shore. Then a 1.5-m diameter cast net (mesh size: 3 mm) targeting demersal and pelagic species was cast three times, at 3, 6, and 9 m from the shore. Finally, a dip net (opening: 0.07 m2, mesh size: 1 mm) was used to catch the smallest fish from the boat and the bank (five attempts at different depths per transect, targeting visible juveniles when present).
Laboratory Analyses
For each site and sampling date, water chlorophyll a concentration (in µg L−1) was determined using 1 L of water prefiltered through a 0.47 µm glass microfiber filter GF/F (Whatman®). For this, chlorophyll a was extracted in 90% acetone, gently mixed, stored at 6 °C for a minimum of 6 h in the dark, and centrifuged before analysis by spectrophotometry (Aminot and Kérouel 2004).
Macrophytes were identified down to the species level when possible, and the number of taxa corresponded to the macrophyte richness (MRICH). Their respective weights (MBIOM) were measured after drying them in an oven at 60 °C for at least 48 h and until a constant weight was reached.
Juvenile fish were identified down to the species level and measured to the nearest 0.1 mm. Mugilids were identified using the caeca dissection method of Farrugio (1975) and the melanophore patterns method (Minos et al. 2002) for individuals with total lengths between 20 and 30 mm. Species of the Pomatoschistus genus were distinguished according to scale arrangement (Kovačić 2020). For resident species, individual fish were considered as juveniles only when their total length was below that reported for sexual maturity in the area (see Annex 1 in the Appendix section).
Data Analyses
All data analyses were performed in R (in particular the packages Ade4, car, factoextra4, labdsv, Stats, and vegan), using 5% as the threshold for statistical significance. Transects were considered replicates for each sampling site. The number of replicates for each site per sampling period thus ranged from three, when only one sampling could be carried out in the corresponding 2 months (this was the case for MI during period 1, MW for all periods, and all sites during period 4), to six, when two samplings were successfully completed per period. To reduce the list of variables used for characterizing microhabitat diversity, a Pearson correlation test was applied to the 13 variables initially investigated (sediment cover, rock cover, pebble cover, shell cover, macrophyte cover, macrophyte richness, macrophyte canopy height, macrophyte biomass, depth, distance from the outlet, temperature, salinity, chlorophyll a concentration) and redundant ones (macrophyte canopy height, depth, pebble cover, shell cover) were removed from all analysis when Pearson correlation coefficient was superior to 0.7.
Univariate (ANOVAs) and two-way (ANOVA III type for unbalanced designs) analyses of variance, followed by Tukey’s post hoc tests, were used to test for temporal and spatio-temporal differences in environmental variables. To approach the normal distribution for this, cover variables were transformed using a sqrt(x) – sqrt(1 − x) transformation, except for macrophyte coverage, for which a log(x + 1) transformation was applied. To explore environmental variability and describe spatial and temporal environmental gradients in the lagoon, a principal component analysis (PCA) was performed on the mean values per site and per period of the variables describing the microhabitats. This multivariate method allows for the identification of the variables that contribute the most to dataset variability and their synthesis into new orthogonal variables called principal components (Abdi and Williams 2010). Due to the lack of salinity data for the stations MI and MW during period 1, their position in the PCA was estimated using the mean value of the other four other stations during this period. A post-PCA ascendant hierarchical classification based on Ward’s clustering method (Ward 1963) was also performed to assess the number of distinct microhabitat types encountered by juvenile fish in the lagoon during the study period, i.e., the number of groups of sampling events (period-site pair) with similar environmental conditions. This was achieved using the “silhouette” method (Charrad et al. 2014). To be retained as a distinct microhabitat type, each group had to gather at least three sampling events. ANOVAs and Tukey’s post hoc tests were used to compare environmental characteristics between microhabitat types.
Differences in juvenile fish assemblages were investigated using three parameters: species richness, global fish abundance, and relative species abundances. For this, species richness was estimated from the total number of species captured with the three fishing gears. However, because the abundance of fish in the dip net catches varied greatly depending on fishing conditions and the local presence or absence of juvenile schools at the time of sampling, global and relative abundances were derived only from beach seine and cast net captures only. Fish abundances were originally expressed as catch per unit effort (CPUE) by transect and sampling date, with each value corresponding to the total number of fish caught by the beach seine haul and the three net casts applied on each transect. However, species’ relative abundances for each site and period were calculated by grouping data from all transects. The variability in fish assemblage composition among sampling periods and sites was first tested by performing a multivariate analysis (PERMANOVA) on species abundances and considering both factors (period and site) as fixed. Then, two-way analyses of variance (ANOVA III type for unbalanced designs) were used to assess spatio-temporal variations in both the species richness and the global abundance of the juvenile fish assemblage in the lagoon. Again, both factors (period and Site) were considered fixed for this and variables were log 10 (x + 1)-transformed to approach the normality, if necessary. The effect of the sampling site was further investigated for each period separately, using univariate ANOVAs followed by Tukey’s post hoc tests.
Species responses to environmental variation were investigated with a co-inertia analysis comparing mean juvenile abundances per site and period with concomitant mean values of all environmental variables. This multivariate method, commonly used to study species-environment relationships, compares faunistic and environmental data by analyzing the co-structure between them. This approach allows the identification of plans which optimize co-variance between species abundances and environmental variables. The more faunistic and environmental data have similar structures, the more the output of the co-inertia analysis (RV coefficient of similarity) is close to 1. This analysis is recommended when a large number of variables are used in comparison to the number of samples (Dolédec and Chessel 1994), as is the case in the present work. To limit bias in our results, rare fish species (i.e., those with less than five individuals collected over the whole study period) were not included in this analysis. Fish data collected at MI and MW stations during period 1 were excluded from this analysis due to the lack of salinity data.
Species’ habitat preferences were investigated based on the list of distinct microhabitat types defined from the hierarchical clustering. For this, we used the Indicator Value (Indval) index (Dufrène and Legendre 1997), which considers both the selectivity and the fidelity of a species to a type of microhabitat:
\({\mathrm{Indval}}_{ij}={A}_{ij}\times {B}_{ij}\times 100\); where:
Aij = average abundance of species i across microhabitats of type j / average abundance of species i across all types of microhabitats (relative abundance across types of microhabitats).
Bij = number of microhabitats of type j where species i is present / number of microhabitats of type j (relative frequency across microhabitats of type j).
To account for interspecific differences in the time of lagoon use, the global Indval index for each species was calculated only for the microhabitat types available during the periods when its juveniles were captured in the lagoon. Fish data collected at MI and MW stations during period 1 were also excluded from this analysis as in the previous analysis on the species responses to environmental variation. When possible, this index was also used to assess ontogenetic changes in lagoon microhabitat preferences. For this, the juveniles of each species were split into up to three size classes (J1 to J3, hereafter referred to as “ontogenetic stages”), based on the available knowledge of the changes in habitat requirements (temperature and salinity ranges, preferred substrate and position in the water column) and diet type (e.g., benthivore, planktivore, detritivore, omnivore, piscivore) reported for its juveniles in the literature. Following the protocol from previous studies (e.g., Cattin et al. 2003; Van Hadler et al. 2007; Podani and Csányi 2010), rare species (i.e., with less than five individuals collected) were not included in this analysis and only ontogenetic stages with a minimum of three individuals were retained for the calculation of the Indval index per juvenile stage.
Results
In total, 2206 fish juveniles, with sizes ranging from 8.0 to 88.0 mm TL (except for Anguilla anguilla whose sizes ranged from 52.2 to 200.0 mm), were collected over our 8-month survey in the Prévost lagoon (Table 1). They belonged to at least 22 different species in Mediterranean coastal lagoons, classified as resident, migratory, or occasional. Among them, 41 juveniles from the genus Liza spp. were impossible to identify down to the species level due to their small size. As they were only captured with the dip net, they were not included in abundance estimates. This was also the case for the only juvenile from Belone belone captured and for some of the juveniles of varied species captured with all sampling gears (e.g., Liza aurata, Liza saliens, Chelon labrosus, Sparus aurata).
Juvenile abundance in the catches with the beach seine and cast net differed highly according to the species. The juveniles from occasional marine stragglers (Chelidonichthys lucerna, Engraulis russoi, Sardina pilchardus) represented only 1.0% of the total catch. The large majority of the juveniles caught in the lagoon, therefore, belonged to lagoon resident (N = 6) or marine migratory (N = 13) species, which represented 74.2% and 24.8% of the total abundance, respectively. Surprisingly, six species alone accounted for more than 90% of the total catch. The lagoon resident species Atherina boyeri in particular represented 69.9% of the total juvenile fish abundance, followed by five marine migratory species of commercial importance: the sparid S. aurata (8.1%) and the mugilids L. aurata (4.8%), Mugil cephalus (3.6%), L. saliens (3.0%), and Liza ramada (2.5%).
Variation in the Composition of the Juvenile Fish Assemblage
The composition of the juvenile fish assemblage in the lagoon varied highly with both the site (PERMANOVA, p = 0.010) and the sampling period (PERMANOVA, p = 0.010), with a significant interaction between the two factors (PERMANOVA, p = 0.010). This was largely due to significant spatial variation in species richness (ANOVA, p < 0.001, Fig. 2a), but also to marked temporal changes (ANOVA, p < 0.001) in the abundance of juvenile fish (Fig. 2b). Overall, juvenile fish abundance in the lagoon was minimum (4.6 ± 1.0 ind. transect−1) in period 2 (Tukey’s test, p < 0.010), and maximum (53.1 ± 17.4 ind. transect−1) in period 4 (Tukey’s test, p < 0.001). The values for periods 1 and 3 are similar and intermediate (Fig. 2b). Nonetheless, juveniles from migrant species were mostly captured during the first two sampling periods, accounting for 64.3% of the total catch in period 1, and 42.1% in period 2 (Table 1; Fig. 2c). Their proportion in the fish assemblage decreased in periods 3 (18.1%) and 4 (8.5%) when juvenile fish in the lagoon essentially belonged to resident species. As a result, the global composition of the juvenile fish assemblage differed markedly between sampling periods.
a Mean species richness, b mean total abundance (ind. transect-1), and c taxonomic composition of the juvenile fish assemblages caught with the beach seine and the cast net at each site (for sites codes and locations, see Fig. 1) during the four sampling periods (1, March–April; 2, May–June; 3, July–August; 4, September–October). Note that the MW station could not be sampled in period 3. It was therefore not included in the statistical tests, as signalized by the “*” symbol. In (a) and (b), error bars correspond to standard errors. Letters and p values indicate results of post hoc multiple comparisons (t-test performed after ANOVA and applied to species richness and fish abundance for each period) with p values inferior to 0.05 corresponding to significant differences. The absence of letters indicates when the variations found were not significant. In (c), “Others” gathers seven species rarely observed in the lagoon, at least at the juvenile stage: Diplodus puntazzo, Gobius niger, Sardina pilchardus, Sarpa salpa, Solea solea, Chelidonichthys lucerna
The spatial distribution of juvenile fish within the lagoon also differed from one period to the other (Fig. 2). In period 1, the total juvenile fish abundance was maximum (23.8 ± 6.5 ind. transect−1) at NW, where four species were caught, and minimum (0.7 ± 0.7 ind. transect−1) at MI, where only juveniles of the resident species Salaria pavo and the marine straggler S. pilchardus were captured (Fig. 2b, c). Juvenile abundance was also low (3.7 ± 2.0 ind. transect−1) at MW, where only migratory sparids were collected, with S. aurata representing 91% of the catches, and D. sargus 9%. Species richness was higher at the four other sites (Fig. 2a), but species composition varied markedly among them. For example, although NW and SE displayed comparable proportions of juvenile A. boyeri (48 and 50%, respectively), S. aurata (44% at both sites), and L. saliens (1% at both sites), L. aurata juveniles were only captured at NW, while those of D. sargus, S. pavo, and of the migratory sparid Sarpa salpa were only found at SE (Fig. 2c). At SW, S. aurata juveniles were the most abundant (46%), with a similar proportion as in the NW and SE, but A. boyeri only represented 22% of global abundance, against 30% for L. aurata and L. ramada. Lastly, NE was the only site where the juvenile fish assemblage was dominated (64%) by mugilids (L. aurata and L. ramada) and where juveniles of A. anguilla and Dicentrarchus labrax were captured. It was also, with SW, one of the only two sites where juveniles of S. solea were captured.
In period 2, when the average global juvenile fish abundance in the lagoon was minimal, juvenile abundances were again the lowest (0.3 ± 0.3 ind. transect−1) at MI (Fig. 2b), where only juveniles of the marine straggler C. lucerna were captured (Fig. 2c). Juvenile catches were the highest (14.0 ± 6.1 ind. transect−1) at MW (Fig. 2b), where A. boyeri specimens dominated (93%) but juvenile Syngnathus abaster and A. anguilla were also captured (Fig. 2c). Atherina boyeri was also the most abundant species (38 to 56%) at SE, NE, and NW, but the juvenile fish assemblages at these three sites had different compositions. Indeed, the next most represented species (> 10%) at these sites were D. labrax, L. aurata, and L. ramada for NW, L. aurata and S. aurata for SE, and D. labrax and Pomatoschistus microps for NE. Finally, the SW site exhibited a singular fish assemblage, dominated by juveniles of L. ramada (66%). This site was also the only one where the three species most commonly observed in the lagoon (A. boyeri, S. aurata, and L. aurata) represented less than 25% of the global juvenile abundance and where juveniles of the resident gobiids Pomatoschistus marmoratus and P. microps were present simultaneously.
In period 3, overall juvenile fish abundance was minimal (0.5 ± 0.2 ind. transect−1) at MI, where only specimens of P. marmoratus were captured (Fig. 2b and c). The NE and NW displayed significantly higher global juvenile abundances (37.5 ± 22.0 and 19.8 ± 9.3 ind. transect−1, respectively), while the other sites exhibited intermediate values (Fig. 2b). At NE, A. boyeri dominated (80%) the juvenile assemblage (Fig. 2c), with the other resident species (P. microps, P. marmoratus, and S. pavo) and the migratory sparid D. sargus accounting 17% and 3% of the total abundance, respectively. NW and SW shared the same species, but in different proportions: A. boyeri represented only 53% of the local juvenile catches at NW, against 77% at SW, and, while the rest of the catches mainly consisted of mugilids at both sites, L. saliens dominated at NW, and C. labrosus at SW. Lastly, besides A. boyeri juveniles (77%), the fish assemblage at SE essentially included juveniles of the two marine stragglers E. russoi (13%) and S. pilchardus (9%).
Finally, in period 4, when the average global juvenile fish abundance in the lagoon was maximal, local juvenile catches varied from 7.3 ± 7.3 ind. transect−1 at MW to 171.0 ± 64.1 ind. transect−1 at SW, but no significant spatial difference could be demonstrated (ANOVA, p = 0.075) due to the high inter-transect variability at most sites (Fig. 2b). Except for MI, A. boyeri largely dominated fish assemblages, accounting for 100% of total abundance at SW, MW, and SE, and 70% in the NW. This latter site was the only one where M. cephalus juveniles were captured in abundance (30%, Fig. 2c). Species richness at this period was thus low, particularly at MI where only juveniles of the resident gobiid P. marmoratus were captured (Fig. 2a and c). The only exception was NE (Fig. 2c), where the fish assemblage was dominated by A. boyeri (87%) but also included juveniles of other resident species (P. microps, S. pavo, S. abaster) and rare migratory species (Diplodus puntazzo, E. russoi).
Intra-lagoon Variation in Environmental Characteristics
During this 8-month study, the environmental conditions encountered in the Prevost lagoon were highly variable with, for example, local values ranging from 12.1 to 28.3 °C for water temperature, from 25.5 to 42.9 for water salinity, from 30 to 100%, and 0 to 48% for sediment and rock covers, respectively; from 0.5 to 5.5 µg L−1 for water chlorophyll a concentration; and from 2 to 100% for macrophyte cover. Despite this variability, the hierarchical clustering approach distinguished only three broad types of microhabitats in the lagoon (Fig. 3a; Table 2), whose respective extent and location varied over time due to the combined effects of spatial and seasonal variability of local environmental variables. Local differences in environmental conditions were primarily driven by inter-site variation in habitat characteristics, especially in three-dimensional (3D) habitat complexity (Table 2), which was essentially caused by spatial differences in local substrate type (e.g., % of sediment or rock cover, ANOVAs, p < 0.001) or in the percentage of macrophyte cover (ANOVA, p < 0.001). This is illustrated by the PCA on the environmental variables (Fig. 3b), where the first dimension (explaining 34.8% of the total variance) opposes sites with low 3D complexity (MI, SW, NE, and MW on the left), where sediment was the main substrate type (> 84% of average cover) and average macrophyte cover was low to medium (from 7 to 55%) in MI and NE, respectively, to sites with high 3D complexity (NW and SE on the right), characterized by the highest average rock (9–33%) and macrophyte (57–66%) covers (Table 3). However, annual seasonality also contributed significantly to the environmental variations in the lagoon. First, although macrophyte cover varied mainly by sampling site (ANOVA, p < 0.001), this parameter also showed some level of temporal variation at some locations (ANOVA, p < 0.001; Fig. 4). Macrophyte cover at SE was significantly the highest in periods 1 and 2 (with means of 80.3 ± 6.9 and 100.0 ± 0.0%, respectively), before decreasing in period 3 (to a minimal value of 23.3 ± 7.1%) and increasing again in period 4 (52.6 ± 1.7%). Although not significant due to high intra-site variability, temporal trends in macrophyte coverage were also observed for NW, MW, NE, and SW, with mean values ranging from 9 to 49% depending on the site (Fig. 4). When reflected in temporal fluctuations in macrophyte biomass (particularly marked at SE, MW, and SW, Table 3), these changes affected habitat characteristics by modulating habitat 3D structure. However, annual seasonality in the Prevost lagoon mostly contributed to environmental variation through temporal changes for the three water parameters investigated (Fig. 3). These changes were only statistically significant for temperature and chlorophyll a concentration (ANOVAs, p < 0.023), but salinities in the lagoon globally followed the same seasonal cycle as local temperatures, increasing from periods 1 to 3 and decreasing in period 4 (Fig. 5a and b). Temperature variations were most pronounced, ranging from 2.1 (at SW, in period 1) to 28.3 °C (at NW, in period 3), while local salinities varied mainly between 31.1 (at SW, in period 1) and 42.9 (at NE, in period 3) only, except in period 4 when a minimum value of 25.5 was observed at NW. Chlorophyll a concentrations also showed a clear temporal trend (ANOVA, p = 0.023; Fig. 5c), with values at most sites starting relatively low (between 0.5 and 1.5 µg L−1) in periods 1 and 2, then increasing significantly to values generally between 2.7 and 4.0 µg L−1 in period 3, and decreasing to values globally between 1.5 and 3.3 µg L−1 in period 4. The second dimension of the PCA (explaining 22.7% of the total variance) illustrates these temporal trends. Indeed, it contrasts the sampling events, mainly in periods 1 and 2, when the values for temperature, salinity, and chlorophyll a concentration were the lowest (Fig. 3b), with the sampling events in periods 3 and 4 with high values for all three water parameters (Fig. 3b).
a Hierarchical clustering of sampling events produced by Ward’s method depending on their environmental conditions and b biplot of the principal component analysis (PCA) investigating environmental variation in the Prévost lagoon. Codes for samples (in bold) include both the sampling period (1, March–April; 2, May–June; 3, July–August; 4, September–October) and the site name (see Fig. 1 for abbreviations and site locations). Grey shades on both figures illustrate the three distinct groups of microhabitats retained: L3D-LWP for “low 3-dimensional complexity and low water parameters,” H3D-VWP for “high 3-dimensional complexity and variable water parameters,” and V3D-HWP for “variable 3-dimensional complexity and high water parameters”. On (b), SALI, CHLA, TEMP, MBIOM, DIST, MCOV, MRICH, ROCK, and SEDI correspond to salinity, chlorophyll a concentration, water temperature, macrophyte biomass, distance to the inlet channel, macrophyte cover, rock coverage, and sediment coverage, respectively. Asterisk indicates the few sampling events for which the salinity data were missing. To allow positioning them on the PCA, these events were attributed to the mean overall salinity in the lagoon during this period. For period 3, the station MW was not sampled, so it does not appear on the graph. Salinity data were missing for the two sampling events marked by an asterisk, their position on the biplot was estimated using the mean value of the other four stations at this period
Mean macrophyte cover at each sampling site (for site location refer to Fig. 1) over the four periods sampled (1, March–April; 2, May–June; 3, July–August; 4, September–October). Error bars correspond to standard errors. Note that the MW station could not be sampled in period 3, and it was therefore not included in the statistical tests. Letters and p values indicate results of post hoc multiple comparisons (t-test performed after ANOVA and applied to macrophyte cover at each site) with p values inferior to 0.05 corresponding to significant differences. The absence of letters indicates when the variations found were not significant
Evolution of a water temperature, b salinity, and c chlorophyll a concentration over the four periods sampled (1, March–April; 2, May–June; 3, July–August; 4, September–October). In each box plot, the dark full line represents the median and the dark square represents the average value for all stations. Box delineations correspond to the 25th and 75th percentiles, and vertical bars to the 5th and 95th percentiles. When present, outliers are indicated by dark circles
As a result of these complex spatio-temporal variations in environmental conditions, some sites in the lagoon (e.g., NE, SW, MW) were assigned to a different microhabitat type during certain sampling periods (Fig. 3), and microhabitat type assignment remained stable over most of the survey period for SE, MI, NW, and NE only. The primary type of microhabitat available over the survey period (40% of sampling events, spread over the six study sites) was one with variable 3-dimensional complexity and high water parameters (V3D-HWP). It gathered all the sampling events from period 3, but also some from periods 2 and 4 (at MW and NE), characterized by the highest (Tukey’s test, p ≤ 0.04) water temperatures, salinities, and Chl a concentrations (Fig. 3; Table 2). Rock and macrophyte covers in this microhabitat type were variable but significantly lower (Table 2, Tukey’s test, p ≤ 0.03) than in the next most abundant (30% of sampling events, in all periods but only at three different sites) microhabitat type (characterized by a “high 3-dimensional complexity and variable water parameters” (H3D-VWP)). This latter grouped together all the sampling events where the highest values of 3-dimensional complexity were observed, i.e., most of those at NW and SE, plus those at MW during period 2, when the macrophyte cover at this site was the highest (Table 2; Fig. 3). Its values for water parameters were comparable (Tukey’s test, p > 0.640) to those observed in the last microhabitat type identified (for “low 3-dimensional complexity and low water parameters” (L3D-LWP)), but their variability was much higher (Table 2). The L3D-LWP microhabitat type also represented 30% of the sampling events, but only at four different sites and in three sampling periods. Indeed, it regrouped sampling events characterized by the lowest temperature, salinity, and chlorophyll a values (observed during periods 1 and 2, but also at MI in period 4), essentially at sites with very low 3D structure (Fig. 3; Table 2). This was particularly clear for MI, where the average macrophyte cover and biomass were the lowest of all sites, and for SW in periods 1 and 2, when the macrophyte cover was less than 25% (Table 3; Fig. 4). This was also the case for NE in period 1: although the local macrophyte cover was 82% in this period, the corresponding macrophyte biomass was low (6.3 g quadrat−1) as it was only due to the presence of flat green macroalgae (Ulva spp.) spread in a thin layer over the bottom.
Juvenile Fish Preferential Microhabitats
Our results revealed that juvenile fish distribution in the lagoon was non-random and largely resulted from variations in lagoon microhabitat preferences depending on the species. Due to the low abundance of juveniles in the captures at several sites and on many dates and to high inter-transect variability in the catches, variations in Indval indexes between microhabitat types could only be assessed for 16 species (Table 4). The corresponding results revealed that, among them, three species (E. russoi, M. cephalus, and S. pichardus) occurred in only one microhabitat type, seven species (C. labrosus, D. sargus, L. ramada, L. saliens, P. marmoratus, P. microps, and S. abaster) excluded one microhabitat, and six species (A. anguilla, A. boyeri, D. labrax, L. aurata, S. pavo, and S. aurata) frequented all three types of microhabitats. The maximum Indval values observed for each species suggested that V3D-HWP microhabitats were the most widely preferred in the Prevost lagoon. Indeed, they were preferentially used by C. labrosus, D. labrax, E. russoi, L. saliens, P. microps, S. pavo, and S. pilchardus, but also by A. boyeri, although the juveniles of this abundant resident species were also strongly associated with H3D-VWP microhabitats (Table 4). In comparison, only four of the 16 species (A. anguilla, L. aurata, L. ramada, and P. marmoratus) preferentially selected L3D-LWP microhabitats and two (M. cephalus and S. aurata) of those of the H3D-VWP type. Habitat preference for the juveniles of D. sargus and S. abaster was less clear, even though both species seemed to prefer V3D-HWP microhabitats to the H3D-LWP ones (Table 4).
The co-inertia analysis (RV = 0.3) allowed us to further specify the environmental parameters at the origin of microhabitat preference for each species (Fig. 6). Indeed, its first three dimensions explained 81.4% of the common variability between lagoon environmental parameters and species abundances. Dimension 1 (44.9% of the total inertia) reflected an increasing gradient for all water parameters (temperature, salinity, and chlorophyll a concentrations), whereas dimension 2 (20.6% of the total inertia) opposed microhabitats close to the sea outlet and was characterized by high sediment cover and low macrophyte richness to microhabitats far from the sea outlet and combining high rock cover and high macrophyte richness (Fig. 6a). Dimension 3 (15.9% of the total inertia) reflected a joint gradient of increasing macrophyte cover (and biomass) and increasing distance from the sea outlet (Fig. 6b). Confronting the positions of environmental variables and juvenile fish abundances on the co-inertia graphs showed that most of the species preferentially observed in the V3D-HWP microhabitat type mainly responded to high values for water parameters, although differently (Fig. 6a). The juveniles of A. boyeri were found to mainly respond to high chlorophyll a concentrations (correlation coefficient = 0.52) and those of S. pavo to high salinities (0.44). D. labrax and L. saliens juveniles were mainly associated with higher temperatures (0.28 and 0.52, respectively), although they also positively responded to high macrophyte cover (0.25 and 0.28, respectively, Fig. 6b). For P. microps, higher juvenile abundances were associated with joint increases in water salinity (0.40) and chlorophyll a concentration (0.38, Fig. 6a), while for E. russoi, they positively responded to joint increases in chlorophyll a concentration (0.27) and macrophyte richness (0.27, Fig. 6a and b). Only S. pilchardus exhibited a higher sensitivity to substrate type than to water parameters, avoiding microhabitats with important sediment covers (− 0.40, Fig. 6b). The distribution of the two species associated with H3D-VWP microhabitats, S. aurata and M. cephalus, was drawn by high habitat 3D complexity but mainly also by the distance to the sea outlet (0.21 and 0.33, respectively), and by low water parameters, comparable to those observed in L3D-LWP microhabitats (Fig. 6). The position for M. cephalus illustrated the affinity of its juveniles with microhabitats (like SE and MW) with important rock (0.15) and low sediment (− 0.19) covers, while that of S. aurata mainly reflected its affinity with high macrophyte covers (0.21) and diversity (0.19). M. cephalus responded negatively to high salinities (− 0.70) and S. aurata to high temperatures (− 0.61, Fig. 6a). Finally, juvenile distribution in the four species preferentially associated with L3D-LWP microhabitats was drawn by different environmental parameters. For both L. aurata and L. ramada, juveniles were found to be mainly sensitive to water parameters, but they mainly responded negatively to increasing temperature (− 0.37) or salinity (− 0.33), respectively (Fig. 6b). Conversely, the juveniles of P. marmoratus were preferentially attracted to microhabitats close to the sea outlet with a low macrophyte cover (− 0.36). Finally, the juveniles of A. anguilla seemed to globally prefer habitats with a significant macrophyte cover (0.46) but from only a few macroalgal species (of low 3D structure), as illustrated by their negative response to macrophyte richness (− 0.28).
Results from the co-inertia analysis confronting environmental variables (in bold) and juvenile fish abundances (in italic) for all periods grouped, projected along the first three dimensions: a plan formed by dimensions 1 and 2 and b plan formed by dimensions 2 and 3. Codes for environmental variables are indicated in Table 2. Colors refer to fish species’ preferential type of microhabitat from IndVal analysis (see Table 4). In (b), note that a gap was added on the axis for dimension 3 in order to allow fitting of the projection for P. marmoratus on the graph. Note that data for the MW site in period 3 and the MI and MW sites in period 1 were excluded from the analysis because they were missing or incomplete
Due to low sample sizes (see above), shifts in juvenile microhabitat preferences with growth could only be assessed for nine species. The corresponding results revealed different strategies for lagoon habitat use among them. Hence, only in two species (L. aurata and L. ramada) did the preferred microhabitat (L3D-LWP) remain unchanged across all the juvenile stages sampled (Indval = 6.7 to 18.3, Fig. 7). In all the remaining species, at least two different microhabitat types were successively preferred at the juvenile stage, but substantial differences in habitat preference were observed between species. The juveniles of D. sargus and L. saliens were found to both prefer V3D-HWP microhabitats at the J1 stage, and H3D-VWP microhabitats at later juvenile stages (Fig. 7). In D. labrax, although J2 and J3 juveniles were found in both H3D-VWP and V3D-HWP microhabitats, J3 juveniles exhibited a significant preference for the V3D-HWP microhabitat type (Indval = 55.6, Permutational test, p = 0.004, Fig. 7). In A. boyeri and S. pavo, the juveniles were largely restricted to V3D-HWP microhabitats at the J1 stage, but they then widened their environmental niche, using both V3D-HWP (Indval = 36.9 and Indval = 7.6, respectively) and H3D-VWP (Indval = 27.8 and Indval = 2.2, respectively) microhabitats at the J2 stage (Fig. 7). In S. aurata, all the juveniles preferred H3D-VWP microhabitats (Indval = 8.0–7.8), but their secondary preferential type of microhabitat shifted from L3D-LWP, at the J1 (Indval = 10.5) and J2 (Indval = 11.9) stages, to V3D-HWP, at the J3 stage (Indval = 5.1, Fig. 7). Finally, at least the J1 and J2 juveniles of A. anguilla clearly mainly used L3D-LWP microhabitats (Indval = 5.8 to 16.7), but only where significant macroalgal cover was observed. Overall, the J1 juveniles of most species seemed to prefer V3D-HWP (4 species) or L3D-LWP (4 species) microhabitats. H3D-VWP microhabitats were rather preferred by J2 (3 species) or J3 (2 species) juveniles, but sometimes alongside at least one other habitat (4 species).
Values for the Indval index illustrate the affinity of the successive juvenile stages (J1, J2, and J3) of different fish species for the three microhabitat types identified by hierarchical clustering (see codes in Fig. 2). For each species and ontogenetic stage, the number of individuals captured is indicated in parentheses. Abbreviations refer to species’ names: A.ang, Anguilla anguilla; A.boy, Atherina boyeri; D.lab, Dicentrarchus labrax; D.sar, Diplodus sargus; L.aur, Liza aurata; L.ram, Liza ramada; L.sal, Liza saliens; S.aur, Sparus aurata; S.pav, Salaria pavo. Note that data for the MW site in period 3 and the MI and MW sites in period 1 were excluded from this analysis because they were missing or incomplete
Discussion
This fine-scale study of the microhabitats and juvenile fish assemblages of the Prévost lagoon allowed clarification of the value of lagoon habitats as nursery sites for Mediterranean fish. While previous works had highlighted differences in environmental quality between lagoons for juvenile fish growth and survival (e.g., Vasconcelos et al. 2007; Chaoui et al. 2012; Isnard et al.2015), very few attempts had been made so far to explain these differences and relate them to differences in lagoon habitat characteristics (Franco et al. 2006a, 2010; Escalas et al. 2015). In this respect, our results demonstrate that juvenile fish are not randomly distributed in coastal lagoons and that their preferential microhabitats differ according to both the species and the ontogenetic stage. This supports the hypothesis that the value of Mediterranean lagoons as nursery habitats for fish is defined at the microhabitat scale, with important consequences for both coastal fish conservation and lagoon management.
Image of the Juvenile Fish Assemblage
The 22 fish species in our captures mainly belonged to nine families (Atherinidae, Moronidae, Anguillidae, Sparidae, Mugilidae, Gobiidae Blennidae, Soleidae, and Syngnathidae) commonly reported in Mediterranean lagoons (e.g., Pérez-Ruzafa et al. 2004; Verdiell-Cubedo 2009; Embarek and Amara 2017). This diversity was consistent with the recent results from 2-year monitoring of the lagoon’s fish assemblage using fyke nets (Bouchoucha et al. 2012) but represented only a small fraction of the total number of species (61) reported in the lagoon over the past 10 years (Kara and Quignard 2018d). This is not particularly surprising as our survey only targeted juvenile stages and several species only entered the lagoon as adults. Because our survey only lasted from March to October in 2019, we also probably missed several rare species and some migratory ones that only visit the lagoon during winter. Lastly, most of our catches were made in the shallower parts of the lagoon and with a small beach seine, which is most effective for sampling small, slow-swimming bentho-demersal fish (Franco et al. 2012). While small juvenile fish are known to concentrate along the shallow banks of coastal lagoons (Verdiell-Cubedo 2009), large fish tend to prefer deeper areas (Stoll et al. 2008). This partly explains the high numerical dominance of A. boyeri juveniles in our samples and the low representation of benthic species and large specimens in the catch. However, the selectivity of the beach seine may also be an important factor in this regard. Indeed, although this sampling gear provided the most comprehensive estimate of fish diversity at most sites, the use of cast and dip nets allowed us to obtain a more complete picture of the juvenile fish community at some sites. This confirmed the relevance of these two later fishing gears for sampling fish in the presence of rocks or thick macroalgal mats that reduce the efficiency of the beach seine (Říha et al. 2008). In particular, the dip net proved to be the most effective in catching the smallest juveniles of most migratory demersal species (e.g., Sparidae and Mugilidae), which usually live in small schools and flee or hide quickly at the slightest alarm. Unfortunately, the catches with this fishing gear were not standardized enough to be included in our quantitative analysis of fish preferential microhabitats. However, our results call for the combination of several fishing gears with different selectivity and operating modes when sampling lagoon juvenile assemblages, not only to provide a more realistic picture of the species present, but also to better evaluate the respective importance of lagoon microhabitats of contrasting structural complexity for the local juvenile ichthyofauna.
The choice of the spatio-temporal scale is central when studying variations in the characteristics of fish assemblages and trying to unveil fish habitat preferences in Mediterranean lagoons. Indeed, as shown, for example, in the Mar Menor (Spain), hydrological conditions, habitat productivity and structure, and fish assemblages in these shallow ecosystems can all exhibit high variability at different spatial and temporal scales (Pérez-Ruzafa et al. 2007b). Lagoon fish assemblage, in particular, can vary at small spatial scales, depending on substrate type, so sampling at the micro-habitat level is essential to properly describe the relationship between fishes and lagoon habitats, especially at the juvenile stage (Maci and Basset 2009). Lagoon fish assemblages also vary on a variety of temporal scales, with seasonal, monthly, but also fortnightly changes (Pérez-Ruzafa et al. 2007b). With this regard, our sampling strategy allowed us to capture most, but not all, of the temporal variability in fish distribution over the period surveyed. Indeed, in most sites, sampling was conducted on a monthly scale, but only during the day. As the catchability and distribution of fish can fluctuate in the course of a day according to species’ circadian rhythms and feeding periods (Thiel et al. 1995; Rountree and Able 2007), the spatial image of the juvenile assemblage provided here may not be fully accurate, especially for nocturnal species. This also likely biased the assessment of the value of microhabitats, at least for some species. For example, while the juveniles of A. anguilla are usually associated with substrates with high complexity (Table 5), we mainly captured them at sites where sediment (mud) dominated, although covered by dense mats of Ulva spp. Since A. anguilla is mainly nocturnal and generally displays cryptic behavior during the day (Neveu 1981; Baras et al. 1998), this habitat preference might reflect the need for its juveniles to hide in macroalgae mats to limit predation during their daily resting hours. Night sampling might have revealed a different pattern of habitat use for this species, but also for other nocturnal feeders such as S. solea and A. boyeri (Lagardère et al. 1998; Pulcini et al. 2008). A more comprehensive sampling at the nycthemeral scale would therefore allow us to considerably deepen our understanding of the value of the different types of lagoon microhabitats, notably by assessing whether fish juveniles preferentially use them when foraging or for protection (Nagelkerken et al. 2015).
Variations in the Global Juvenile Fish Assemblage
In spite of the sampling biases mentioned above, we are confident that our study provides relevant information on fine-scale spatio-temporal variations in juvenile fish assemblages and on juvenile microhabitat preferences for most fish species in the Prévost lagoon, at sizes below 10 cm. It is in this size range that the information is most valuable because the smallest size classes are the most critical for survival in fish and the most likely to be sensitive to microhabitat features, as small specimens have low swimming abilities and therefore require protection from predators (Dahlgren and Eggleston 2000; Nagelkerken et al. 2015).
From the temporal point of view, juvenile fish assemblages in Mediterranean lagoons are commonly characterized by strong variability due to the seasonality of lagoon use by juvenile fish (Aliaume et al. 1993; Malavasi et al. 2004; Maci and Basset 2010). This seasonality is thought to be mainly driven by species-specific responses and adaptability to environmental factors, notably water temperature and phytoplanktonic production (Marshall and Elliott 1998; Pérez-Ruzafa et al. 2004), or salinity (Drake and Arias 1991; Marshall and Elliott 1998). However, cycles of migration and reproduction of migrant and resident fish species, which reflect their evolutionary adaptations to exploit environmental conditions favoring the growth and survival of their early life stages (Marshall and Elliott 1998), can sometimes prevail on seasonal patterns in local environmental parameters (Potter et al. 1986). This is apparently the case in the Prévost lagoon. Thus, the abundant juvenile catches observed despite the drops in both water temperatures and chlorophyll a concentration in period 4, were due to the massive recruitment of most local resident species, in particular A. boyeri, S. pavo (Table 5), P. marmoratus, P. microps, and S. abaster (Franzoi et al. 1993; Malavasi et al. 2005; Leitão et al. 2006) in the late summer-early autumn. Conversely, migratory species like S. aurata, L. ramada, L. aurata, and D. labrax, which all recruit from early spring to early summer in the Mediterranean (Koutrakis et al. 1994; Mariani 2006; Martinho et al. 2008), dominated in the comparatively small catches of periods 1 and 2.
Besides these biological considerations, disentangling the respective roles of water temperature, primary production, and salinity in the spatio-temporal evolution of the juvenile fish assemblage in the Prévost lagoon is complicated. Temperature is known to have a major influence on fish physiology and life cycle (Beitinger and Fitzpatrick 1979), so juvenile fish assemblage composition primarily depends on species’ optimal thermal ranges. Here though, average temperatures in the lagoon only varied from 12.1 to 28.3 °C, remaining within the tolerance range of most of the species captured (Table 5). Therefore, even if water temperature probably affects the global list of species found in the lagoon, it was not the primary direct driver for the observed temporal changes in juvenile fish abundance. Nonetheless, in estuarine and lagoon systems, the primary productivity cycle coincides with that of temperature, usually peaking in the summer (Murrel and Lores 2004; Bertolini et al. 2021), and fish abundances, notably at the larval and juvenile stages, usually follow this annual cycle (Pérez-Ruzafa et al. 2004; Kristiansen et al. 2011). Our study tends to confirm this coupling since the global juvenile fish abundance in the Prevost lagoon was higher during periods 3 and 4 when primary productivity was at its highest. However, this trend was primarily due to temporal fluctuations in the abundance of juvenile A. boyeri, which largely dominated the local catches. Because this pelagic species is primarily planktivorous (Table 5), it is not surprising that the evolution of its abundance follows that of lagoon planktonic productivity. However, it is possible that the dominance of A. boyeri in the catches partly masked the contrasted response of other, less abundant, species to temporal variations in temperature, at least in certain parts of the lagoon. Notably, the fact that S. aurata is sensitive to high temperatures (Heather et al. 2018) probably explains their lower abundance in period 2 at the only site where temperatures > 25 °C were recorded (MW).
Disentangling the respective roles of temperature and salinity is also complicated because the two factors globally co-varied over much of the period studied. In estuarine environments with strong haline gradients, salinity is the main driver for species’ distribution, depending on their respective tolerance ranges (Gordo and Cabral 2001; Maci and Basset 2010; Rodríguez-Climent et al. 2013). However, in the Prévost lagoon, the salinity range during our survey (25.5–42.9) was within the tolerance limits for most species (Table 5), and spatial salinity gradients were globally weak irrespective of the sampling period. Therefore, temporal changes in salinity probably only influenced the composition of the juvenile fish assemblage in certain parts of the lagoon, where extreme values were recorded. Notably, the fact that M. cephalus juveniles are attracted to lower salinities (Cardona 2006) probably explains their exclusive presence in period 4 at the NW site, where the minimum salinity in our survey (25.5) was measured. Differences in the spatial distribution of the two resident Gobiidae species captured within the Prévost lagoon in period 3 can also partly be attributed to salinity: at this time of the year, P. microps, which has higher osmoregulatory abilities than P. marmoratus (Rigal et al. 2008), was observed at the NE site (where the salinity was above 40), whereas P. marmoratus was exclusively observed at MI (where the salinity was close to that of the sea).
The limited spatial differences in water parameters in the lagoon during each sampling period allowed us to investigate the effect of microhabitat structural heterogeneity on juvenile fish abundance and diversity. Small-bodied aquatic organisms, such as macroinvertebrates and fish juveniles, are known to respond strongly to habitat complexity and heterogeneity, which provide physical structure for protection and offer a diversity of feeding grounds (e.g., Kingsford and Choat 1985; Verdiell-Cubedo 2009; Mercader et al. 2017; Ferrari et al. 2018). In the Prévost lagoon, the most complex and structured microhabitats (H3D-VWP and V3D-HWP types), characterized by heterogeneous substrates and/or the presence of macrophytes, were those with the highest juvenile fish diversity and abundance. In contrast, low values for these two parameters were observed in soft-bottom microhabitats, notably where the macrophyte canopy was reduced (i.e., in the L3D-LWP microhabitat type). Because the presence of rocks is scarce in the Prévost lagoon (they are only observed in the SE and NW), macrophyte cover can be considered the main local source of habitat structural complexity (Menu et al. 2019). In this regard, macroalgae were observed in most of the sites sampled, while seagrasses were only present within a small area surrounding the SW site. Seagrass meadows are known to provide refuge and host a diversity of prey for juvenile fish, so their contribution to lagoon nursery function is largely recognized (Thiriet 2014). This is less common for macroalgae beds (McDevitt-Irwin et al. 2016), which are considered unstable microhabitats because of their seasonal cycle (Holmquist 1997; Bachelet et al. 2000). In the present work, the impact of this seasonality on microhabitat structure was observed at various sites, notably at SE, where macrophyte cover varied markedly according to the period. Despite this variability, the attractiveness of macroalgae beds for juvenile fish was particularly clear. Indeed, maximum values for global fish abundance and species richness during the three first sampling periods were consistently observed at the NE, SE, and/or NW sites, where macrophyte cover and biomass were maximum. This confirmed previous suggestions that vegetated microhabitats globally attract higher numbers of fish juveniles than bare soft substrates (Verdiell-Cubedo 2009), and that macroalgae beds are good substitutes for seagrass meadows as nursery habitats for fish (Sogard and Able 1991). Even within seagrass beds, the presence of macroalgae can increase local fish diversity and abundance, as they also enhance protection from predators (Adams et al. 2004; Woodland et al. 2019) and attract many invertebrates (Diehl and Kornijów 1998; Nohrén and Odelgård 2010), thus reducing food competition between the juvenile fish that feed on epibenthic fauna.
Differences in Juvenile Microhabitat Preferences
This study confirmed that small-scale habitat use within Mediterranean lagoons largely differs between species depending on their respective morphology and ethology (Kara and Quignard 2018d). For example, the fact that S. abaster juveniles are almost exclusively found in the presence of dense macroalgae beds (mainly in Chaetomorpha sp. mats) is probably linked to their body shape, which allows them to easily camouflage within the macrophyte canopy (Malavasi et al. 2007; Selfati et al. 2019). Likewise, the fact that P. marmoratus juveniles showed a marked affinity for the MI and SW sampling sites, with high sediment but low macrophyte cover (L3D-HWP microhabitat type), is probably due to the fact that Gobiids are morphologically adapted to bare soft substrates, on which they can easily hide and camouflage (Anne-Marie et al. 1980). The same applies to the few juveniles of S. solea that we captured in this study (Post et al. 2017). However, species’ microhabitat preferences in the Prévost lagoon apparently also depended on their diet and feeding behavior. For example, the juveniles of L. aurata and L. ramada preferentially selected microhabitats with a bare soft substrate and low macrophyte cover (L3D-LWP microhabitat type), confirming previous similar observations in the Mar Menor and Venice lagoons (Franco et al. 2006a; Verdiell-Cubedo 2009). This is likely due to their diet, as the two species are partially detritivores and feed on the fine organic fraction of the sediment (Table 5). Similarly, the strong association of D. labrax juveniles with high macrophyte covers in our study (notably at the SE and NE sites) could be related to their diet (Table 5), as this species is known to mainly feed on the hyperbenthos (Ferrari and Chieregato 1981; Arias and Drake 1990; Franco et al. 2008), which usually thrive on macroalgae mats (Bachelet et al. 2000). So far, D. labrax juveniles have been reported in various types of microhabitats though, ranging from unvegetated mudflats to heterogeneous substrates with high macroalgae covers (Gordo and Cabral 2001; Malavasi et al. 2004; Verdiell-Cubedo 2009; Ribeiro et al. 2012). Such diversity might reflect local differences in biotic settings among locations, as juvenile distribution in fish often aims at avoiding interspecific competition for food (Rooper et al. 2006; Nunn et al. 2012). This latter strategy is also commonly observed in Gobiidae species with similar diets (Wilkins and Myers 1992; Leitão et al. 2006). In the Prévost lagoon, this could also partly explain, together with the interspecific differences in salinity tolerance mentioned above, why P. microps juveniles were essentially found in V3D-HWP microhabitats (NE), and not where the sediment cover was the highest, and P. marmoratus juveniles were mainly found (i.e., in L3D-HWP microhabitats).
Studies investigating ontogenetic changes in microhabitat preference during juvenile life are rare for Mediterranean lagoon fish and mostly limited to Diplodus species, for which juvenile ontogenetic stages are well described (Vigliola and Harmelin-Vivien 2001; Ventura et al. 2014). The present work thus provides new insights in this regard. It highlighted ontogenetic changes in microhabitat preferences for most of the fish species investigated. In some species, microhabitat changes were particularly subtle. For example, despite slightly extending their niche to V3D-HWP microhabitats with growth, the juveniles of A. boyeri exhibited a wide distribution across the lagoon (at all sites but MI) at all periods irrespective of the juvenile stage. They only noticeably avoided L3D-LWP microhabitats, probably because the low planktonic productivity associated with low chlorophyll a concentrations is detrimental to their zooplanktivorous diet (Table 5). Similarly, although S. pavo juveniles widened their environmental niche during growth, this shift in habitat preference was restricted to sites (NE and SE) characterized by the notable presence of rocks and macrophyte beds, reflecting the documented attraction to complex structures in Blennidae, which typically seek shelter in cavities (Orlando-Bonaca and Lipej 2007). In most species though, microhabitat changes with growth were more pronounced. Among all the species investigated, ontogenetic changes in microhabitat preferences were the most marked in D. sargus and L. saliens, which were both found to prefer V3D-HWP microhabitats at the J1 stage, and H3D-VWP microhabitats at later juvenile stages. In Diplodus species, these habitat shifts have been attributed to a progressive loss of the larval shoaling behavior and a morphological adaptation to the benthic habitat, with a strong preference for hard substrates (Vigliola and Harmelin-Vivien 2001; Ventura et al. 2014). For the other species, the shift in microhabitat preference with growth was less marked. In D. labrax, it only consisted of an increasing affinity for the V3D-HWP microhabitat type, probably due to the gradual change of the species’ diet, relying increasingly on benthic invertebrates and small fish (Table 5), which thrive in macroalgal beds (Bachelet et al. 2000). In S. aurata, the ontogenetic shift in microhabitat use was even more gradual, with juveniles widely distributed in the lagoon at most periods and using at least two different types of microhabitats regardless of the ontogenetic stage. As in the Venice lagoon, this progressive change in the species’ habitat could reflect its gradual colonization of the lagoon ecosystem inwards from the sea inlets (Redolfi Bristol 2019). However, the avoidance of the MI site by the juveniles of the species and their preference for H3D-VWP microhabitats at the J2 stage suggest that they are also increasingly seeking substantial algal cover, probably because macroalgae beds attract macroinvertebrates (Bachelet et al. 2000), which are increasingly dominant in their diet (Table 5). This corroborates previous observations in the Mar Menor, where the abundance of Sparidae juveniles (including S. aurata) is significantly lower on sand beaches than in vegetated habitats (Verdiell-Cubedo et al. 2007) and probably reflects the progressive shift from meiobenthic to macrobenthic prey in S. aurata diet (Table 5), as macroinvertebrates are more abundant in macroalgae beds (Bachelet et al. 2000).
Implication for Conservation and Management
The most recent definitions for fish nursery sites express the need to consider them not as unique optimized habitats but as mosaics of microhabitats with different but complementary functions for juvenile fish (Nagelkerken et al. 2015; Litvin et al. 2018). The overall value of lagoon habitats for a species’ recruitment, therefore, needs to incorporate the quality of each of the successive microhabitats used during the growth of its juveniles. This implies considering the spatial diversity of microhabitats and their connectivity but also their dynamics, which depend on both the seasonal environmental variability and the evolution of fish needs as they grow. By identifying the most attractive environments for different species and at different juvenile stages, our work provides valuable information on the microhabitat types to be targeted to preserve and improve the quality of Mediterranean lagoons as fish nursery sites. In this regard, our results highlight the primary importance of microhabitats with either high sediment (MI, SW, NE) or high macrophyte (SE, NW) cover. Indeed, all the main resident species of commercial interest in the lagoon (A. boyeri, P. marmoratus, and P. microps) and most of the migratory ones (e.g., S. aurata, A. anguilla, D. sargus, D. labrax, S. solea, L. aurata, L. ramada, and L. saliens) were found to use them preferentially, at least during one stage of their juvenile life. As these two types of habitats are common in Mediterranean lagoons, where they usually spread over a significant part of the surface area (Menu et al. 2019), it is likely that they highly contribute to the lagoon’s overall importance for the successful recruitment of coastal fishes (Sogar and Able 1991; Adams et al. 2004; Verdiell-Cubedo et al. 2013). In fish, the earliest juvenile stage is the most vulnerable, and many species tend to settle at sites of high structural complexity where they can easily hide from predators (Tupper and Boutilier 1995; Caddy 2008). However, as observed in the present work, this does not apply to species like gobiids or flatfishes whose protection and feeding rely on bare substrates (Le Luherne et al. 2016). Given the diversity of feeding guilds and the high number of species exploiting the same resources in the fish assemblage of the Prévost lagoon (Table 5), the overall diversity of the microhabitats and the high global productivity in this typical Mediterranean lagoon probably both contribute to reducing competition for food and sustaining high juvenile growth and body condition (Willemsem 1980; Isnard et al. 2015). However, even for a given species, the growth and body condition of the juveniles can vary greatly depending on their spatial distribution within the lagoons due to marked differences in the quality or quantity of local resources (Escalas et al. 2015). Furthermore, fish microhabitat preferences do not necessarily allow identification of where the best conditions for their growth are found. Indeed, fish distribution results from a mix of abiotic factors but also from complex inter- and intra-specific interactions. As shown for A. boyeri (Maci and Basset 2010), the earliest stages in fish can be preferentially observed at lagoon sites where environmental conditions (e.g., extreme salinities) exclude some predators but lower body conditions. Therefore, once preferred microhabitats are identified for a given species, we suggest that physiological stress and body condition should also be assessed against food availability, environmental parameters, and predation rates to conclude their potential quality as nursery sites for fish.
The results of the present work also highlight that the microhabitats of lagoon ecosystems are largely intertwined when considering their use as fish nurseries. This connectivity results both from the temporal variability in the extent and spatial location of microhabitat types within each lagoon and from ontogenetic changes in microhabitat preference during the juvenile life of fish. Given the economic importance of fish (e.g., Emiroglu and Tolon 2003) and the primary ecological role of their juveniles in lagoon food webs, the potential impact of human intervention in lagoons (even if minor and localized) on the overall quality of these ecosystems for juvenile fish should be carefully considered for the sustainable management of lagoons but also of coastal fisheries. For example, even in large lagoons, the mere creation of some sandy beaches along the shores has been shown to result in a significant loss of overall lagoon fish diversity, through the resulting homogenization of the environment and the reduction of the structural complexity of key shallow habitats (Pérez-Ruzafa et al. 2006; Verdiell-Cubedo et al. 2007). Meanwhile, the construction of breakwaters may locally increase the abundance and diversity of fish but alter the quality of the water and sediments in their area of influence (Pérez-Ruzafa et al. 2006). This calls for particularly high caution when planning the development and positioning of human activities in coastal lagoons. From another point of view, the local creation of artificial habitats adapted to the needs of juvenile fish but not adding pressure on the ecosystem could be considered, especially when the restoration of natural habitats is impossible or slow (Mercader et al. 2017). All these actions should be carried out considering local temporal fluctuations in the spatial extent and location of lagoon microhabitat types and their successive use at different ontogenetic stages by several fish species.
Conclusion
The present study provides valuable insights into the role of microhabitat diversity and variability in coastal lagoons in determining the final value of these heterogeneous ecosystems as nursery sites for fish. In particular, it highlights that even within small lagoons, differences in environmental characteristics are not only marked but also dynamic, with important consequences for the attractiveness of each lagoon area for young fish. Although an overall preference for lagoon areas with substantial macrophyte cover and three-dimensional habitat structure was observed, microhabitat preferences were found to be both species and ontogenetic stage dependent, with more contrasting environmental requirements in early juveniles. These findings are in line with recent clarifications of the fish nursery concept, which stress the importance of considering each nursery area as a landscape of (micro)habitats with potentially different but complementary ecological functions for juvenile fish (Nagelkerken et al. 2015; Litvin et al. 2018). For a full understanding of the value of coastal lagoons as nursery sites for fish, the information gathered here needs to be further developed by comparing the microhabitat preferences of juvenile fish between different lagoons, or even different types of coastal ecosystems, in the Mediterranean and beyond. This research should consider the effects of global change, as the impact of increasing climatic and anthropogenic pressures in the littoral zone threatens many coastal environments and will probably affect their quality as nursery sites for juvenile fish.
References
Abdi, H., and L.J. Williams. 2010. Principal component analysis. Wiley Interdisciplinary Reviews: Computational Statistics 2: 433–459. https://doi.org/10.1002/wics.101.
Adams, A.J., J.V. Locascio, and B.D. Robbins. 2004. Microhabitat use by a post-settlement stage estuarine fish: Evidence from relative abundance and predation among habitats. Journal of Experimental Marine Biology and Ecology 299: 17–33. https://doi.org/10.1016/j.jembe.2003.08.013.
Albertini-Berhaut, J. 1974. Biologie des stades juveniles de Teleosteens Mugilidae Mugil auratus Risso 1810, Mugil capito Cuvier 1829 et Mugil saliens Risso 1810. Aquaculture 4: 13–27. https://doi.org/10.1016/0044-8486(74)90015-5.
Aldanondo, N., U. Cotano, and E. Etxebeste. 2011. Growth of young-of-the-year European anchovy (Engraulis encrasicolus, L.) in the Bay of Biscay. Scientia Marina 75: 227–235.
Aliaume, C., C. Monteiro, M. Louis, T. Lam Hoai, and G. Lasserre. 1993. Organisation spatio-temporelle des peuplements ichtyologiques dans deux lagunes cotières: Au Portugal et en Guadeloupe. Oceanologica Acta 16: 291–301.
Alongi, D.M. 1998. Coastal ecosystem processes. New York: CRC Press.
Aminot, A., and R. Kérouel. 2004. Hydrologie des écosystèmes marins. Paramètres et analyses. Éd. Ifremer, 336p
Anne-Marie, E., G. Sundmark, and S. Thorman. 1980. The identification of Pomatoschistus pictus, P. microps, and P. minutus (Gobiidae, Pisces). Sarsia 65. Taylor & Francis: 239–242. https://doi.org/10.1080/00364827.1980.10431485.
Arias, A.M., and P. Drake. 1990. Estados juveniles de la ictiofauna en los caños de las salinas de la bahía de Cádiz. Acuicultura Intermareal. Cádiz: Consejo Superior de Investigaciones Científicas.
Bacha, M., and R. Amara. 2009. Spatial, temporal and ontogenetic variation in diet of anchovy (Engraulis encrasicolus) on the Algerian coast (SW Mediterranean). Estuarine, Coastal and Shelf Science 85: 257–264. https://doi.org/10.1016/j.ecss.2009.08.009.
Bachelet, G., X. De Montaudouin, I. Auby, and P.-J. Labourg. 2000. Seasonal changes in macrophyte and macrozoobenthos assemblages in three coastal lagoons under varying degrees of eutrophication. ICES Journal of Marine Science 57: 1495–1506.
Baras, E., D. Jeandrain, B. Serouge, and J .C. Philippart. 1998. Seasonal variations in time and space utilization by radio-tagged yellow eels Anguilla anguilla (L.) in a small stream. In Advances in Invertebrates and Fish Telemetry, ed. Claireaux G. Lagardère JP., Anras ML.B., 187–198. Dordrecht: Springer. https://doi.org/10.1007/978-94-011-5090-3_22.
Barnes, R.S.K. 1980. Coastal lagoons: The natural history of a neglected habitat. Cambridge: Cambridge University Press.
Beck, M.W., K.L. Heck, K.W. Able, D.L. Childers, D.B. Eggleston, B.M. Gillanders, B. Halpern, et al. 2001. The identification, conservation, and management of estuarine and marine nurseries for fish and invertebrates. BioScience 51: 633–641. https://doi.org/10.1641/0006-3568(2001)051[0633:TICAMO]2.0.CO;2.
Beitinger, T.L., and L.C. Fitzpatrick. 1979. Physiological and ecological correlates of preferred temperature in fish. American Zoologist 19: 319–329. https://doi.org/10.1093/icb/19.1.319.
Bertolini, C., E. Royer, and R. Pastres. 2021. Multiple evidence for climate patterns influencing ecosystem productivity across spatial gradients in the Venice lagoon. Journal of Marine Science and Engineering 9: 363. https://doi.org/10.3390/jmse9040363.
Bodinier, C., E. Sucré, L. Lecurieux-Belfond, E. Blondeau-Bidet, and G. Charmantier. 2010. Ontogeny of osmoregulation and salinity tolerance in the gilthead sea bream Sparus aurata. Comparative Biochemistry and Physiology Part a: Molecular & Integrative Physiology 157: 220–228. https://doi.org/10.1016/j.cbpa.2010.06.185.
Borme, D., V. Tirelli, S.B. Brandt, S. Fonda Umani, and E. Arneri. 2009. Diet of Engraulis encrasicolus in the northern Adriatic Sea (Mediterranean): Ontogenetic changes and feeding selectivity. Marine Ecology Progress Series 392: 193–209. https://doi.org/10.3354/meps08214.
Bouchereau, J-L., J.P. Quignard, J.C. Joyeux, and J.A. Tomasini. 1993. Structure du stock des géniteurs de la population de Pomatoschistus microps (Kroyer, 1838)(Gobiidae), dans la lagune de Mauguio, France. Cybium 17: 3–15.
Bouchereau, J.-L., O. Guelorget, Y. Vergne, and J.-P. Perthuisot. 2000. The fish fauna in the biological organization of a lagoonar paralic system: The Prevost-Arnel lagoonal complex (Languedoc, France). Vie Milieu 50: 19–27.
Bouchoucha, M., C. Aliaume, Y. Baldi, I. Lebel, Y. Abdallah, L. Thieulle, A. Sofianos, A. Courrat, and M. Lepage. 2012. Suivi des peuplements ichtyologiques dans les eaux de transition du bassin Rhône-Méditerranée et Corse : Campagne 2010 & 2011. IFREMER.
Bruno, D.O., S.A. Barbini, J.M. Díaz de Astarloa, and P. Martos. 2013. Fish abundance and distribution patterns related to environmental factors in a choked temperate coastal lagoon (Argentina). Brazilian Journal of Oceanography 61. SciELO Brasil: 43–53.
Bryan, M.D., and D.L. Scarnecchia. 1992. Species richness, composition, and abundance of fish larvae and juveniles inhabiting natural and developed shorelines of a glacial Iowa lake. Environmental Biology of Fishes 35: 329–341. https://doi.org/10.1007/BF00004984.
Bureau du Colombier, S., P. Lambert, and A. Bardonnet. 2008. Is feeding behaviour related to glass eel propensity to migrate? Estuarine, Coastal and Shelf Science 80: 323–329. https://doi.org/10.1016/j.ecss.2008.08.015.
Caddy, John F. 2008. The importance of “cover” in the life histories of demersal and benthic marine resources: a neglected issue in fisheries assessment and management. Bulletin of Marine Science 83. University of Miami-Rosenstiel School of Marine and Atmospheric Science: 7–52.
Cardona, L. 2006. Habitat selection by grey mullets (Osteichthyes: Mugilidae) in Mediterranean estuaries: The role of salinity. Scientia Marina 70: 443–455. https://doi.org/10.3989/scimar.2006.70n3443.
Cattin, M.F., G. Blandenier, C. Banasek-Richter, and L.F. Bersier. 2003. The impact of mowing as a management strategy for wet meadows on spider (Araneae) communities. Biological Conservation 113 (2): 179–188.
Chaoui, L., P.‐A. Gagnaire, B. Guinand, J.P. Quignard, C. Tsigenopoulos, M.H. Kara, and F. Bonhomme. 2012. Microsatellite length variation in candidate genes correlates with habitat in the gilthead sea bream Sparus aurata. Molecular Ecology 21. Wiley Online Library: 5497–5511.
Charrad, M., N. Ghazzali, V. Boiteau, and A. Niknafs. 2014. NbClust : An R Package for determining the relevant number of clusters in a data set. Journal of Statistical Software 61: 1–36.
Chervinski, J. 1977. Adaptability of Chelon labrosus (Risso) and Liza saliens (Risso) (Pisces, Mugilidae) to fresh water. Aquaculture 11: 75–79.
Christensen, M.S. 1978. Trophic relationships in juveniles of three species of sparid fishes in the south african marine littoral. Fishery Bulletin 76: 389–401.
Christia, C., and E.S. Papastergiadou. 2007. Spatial and temporal variations of aquatic macrophytes and water quality in six coastal lagoons of western Greece. Belgian Journal of Botany. JSTOR: 39–50.
Cimiterra, N., L. Massinelli, L. Dijoux, J. Oheix, and V. Derolez. 2020. Diagnostic du compartiment macrophyte dans 17 masses d’eau de transition lagunaires en 2016, 2017 et 2018. IFREMER.
Colombano, D.D., A.D. Manfree, T.A. O’Rear, J.R. Durand, and P.B. Moyle. 2020. Estuarine-terrestrial habitat gradients enhance nursery function for resident and transient fishes in the San Francisco Estuary. Marine Ecology Progress Series 637: 141–157. https://doi.org/10.3354/meps13238.
Como, S., C. Lefrancois, E. Maggi, F. Antognarelli, and C. Dupuy. 2014. Behavioral responses of juvenile golden gray mullet Liza aurata to changes in coastal temperatures and consequences for benthic food resources. Journal of Sea Research 92: 66–73. https://doi.org/10.1016/j.seares.2013.10.004.
Como, S., and P. Magni. 2009. Temporal changes of a macrobenthic assemblage in harsh lagoon sediments. Estuarine, Coastal and Shelf Science 83: 638–646. https://doi.org/10.1016/j.ecss.2009.05.024.
Compaire, J.C., R. Cabrera, C. Gómez-Cama, and M.C. Soriguer. 2016. Trophic relationships, feeding habits and seasonal dietary changes in an intertidal rockpool fish assemblage in the Gulf of Cadiz (NE Atlantic). Journal of Marine Systems 158: 165–172. https://doi.org/10.1016/j.jmarsys.2016.02.006.
Costalago, D., I. Palomera, and V. Tirelli. 2014. Seasonal comparison of the diets of juvenile European anchovy Engraulis encrasicolus and sardine Sardina pilchardus in the Gulf of Lions. Journal of Sea Research 89: 64–72. https://doi.org/10.1016/j.seares.2014.02.008.
Cuadros, A., J. Moranta, L. Cardona, P. Thiriet, J. Pastor, N.L. Arroyo, and A. Cheminée. 2017. Seascape attributes, at different spatial scales, determine settlement and post-settlement of juvenile fish. Estuarine, Coastal and Shelf Science 185: 120–129. https://doi.org/10.1016/j.ecss.2016.12.014.
Crean, S.R., J.T.A. Dick, D.W. Evans, R.S. Rosell, and R.W. Elwood. 2005. Survival of juvenile European eels (Anguilla anguilla), transferred among salinities, and developmental shifts in their salinity preference. Journal of Zoology 266: 11–14. https://doi.org/10.1017/S0952836905006539.
Dahlgren, C.P., and D.B. Eggleston. 2000. Ecological processes underlying ontogenetic habitat shifts in a coral reef fish. Ecology 81: 2227–2240. https://doi.org/10.1890/0012-9658(2000)081[2227:EPUOHS]2.0.CO;2.
Dendrinos, P., and J.P. Thorpe. 1985. Effects of reduced salinity on growth and body composition in the European bass Dicentrarchus labrax (L.). Aquaculture 49: 333–358. https://doi.org/10.1016/0044-8486(85)90090-0.
Derolez, V., B. Bec, D. Munaron, A. Fiandrino, R. Pete, M. Simier, P. Souchu, T. Laugier, C. Aliaume, and N. Malet. 2019. Recovery trajectories following the reduction of urban nutrient inputs along the eutrophication gradient in French Mediterranean lagoons. Ocean & Coastal Management 171: 1–10. https://doi.org/10.1016/j.ocecoaman.2019.01.012.
Diehl, S., and R. Kornijów. 1998. The influence of submerged macrophytes on trophic interactions among fish and macroinvertebrates. In The structuring role of submerged macrophytes in Lakes, ed. Ecological Studies (Analysis and Synthesis), 24–46. New York: Springer. https://doi.org/10.1007/978-1-4612-0695-8_2.
Dolédec, S., and D. Chessel. 1994. Co-inertia analysis: An alternative method for studying species-environment relationships. Freshwater Biology 31: 277–294. https://doi.org/10.1111/j.1365-2427.1994.tb01741.x.
Drake, P., and A.M. Arias. 1991. Ichthyoplankton of a shallow coastal inlet in south-west Spain: Factors contributing to colonization and retention. Estuarine, Coastal and Shelf Science 32: 347–364. https://doi.org/10.1016/0272-7714(91)90048-G.
Dufour, V., M. Cantou, and F. Lecomte. 2009. Identification of sea bass (Dicentrarchus labrax) nursery areas in the north-western Mediterranean Sea. Journal of the Marine Biological Association of the United Kingdom 89: 1367–1374. https://doi.org/10.1017/S0025315409000368.
Dufrène, M., and P. Legendre. 1997. Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecological Monographs 67: 345. https://doi.org/10.1890/0012-9615(1997)067[0345:SAAIST]2.0.CO;2.
Elliott, M., and F. Dewailly. 1995. The structure and components of European estuarine fish assemblages. Netherlands Journal of Aquatic Ecology 29: 397–417. https://doi.org/10.1007/BF02084239.
Elliott, M., and K. Hemingway. 2002. Fishes in estuaries. Oxford: Blackwell Science.
Embarek, R., and R. Amara. 2017. Fish assemblage structure in shallow waters of the Mellah Lagoon (Algeria): Seasonal and spatial distribution patterns and relation to environmental parameters. Acta Ichthyologica Et Piscatoria 47: 133–144. https://doi.org/10.3750/AIEP/02080.
Emiroglu, D., and T. Tolon. 2003. Fish production and marketing in the Mediterranean coastal lagoons. New Medit 2 (4): 59–62.
Escalas, A., F. Ferraton, C. Paillon, G. Vidy, F. Carcaillet, C. Salen-Picard, and F. Le Loc’h, P. Richard, and A.M. Darnaude. 2015. Spatial variations in dietary organic matter sources modulate the size and condition of fish juveniles in temperate lagoon nursery sites. Estuarine, Coastal and Shelf Science 152: 78–90. https://doi.org/10.1016/j.ecss.2014.11.021.
European Commission DG Environment. 2013. Interpretation manual of European Union Habitats-EUR 28. Brussels.
Farrugio, H. 1975. Les muges (poissons, téléosteens) de Tunisie-Répartition et pêche-Contribution à leur étude systématique et biologique. Thesis: Université des Sciences et Techniques du Languedoc.
Félix-Hackradt, F.C., C.W. Hackradt, J. Treviño-Otón, A. Pérez-Ruzafa, and J.A. García-Charton. 2014. Habitat use and ontogenetic shifts of fish life stages at rocky reefs in South-western Mediterranean Sea. Journal of Sea Research 88: 67–77. https://doi.org/10.1016/j.seares.2013.12.018.
Ferrari, I., and A.R. Chieregato. 1981. Feeding habits of juvenile stages of Sparus auratus L., Dicentrarchus labrax L. and Mugilidae in a brackish embayment of the Po River delta. Aquaculture 25: 243–257.
Ferrari, R., H.A. Malcolm, M. Byrne, A. Friedman, S.B. Williams, A. Schultz, R. Al, and Jordan, and W.F. Figueira. 2018. Habitat structural complexity metrics improve predictions of fish abundance and distribution. Ecography 41: 1077–1091. https://doi.org/10.1111/ecog.02580.
Fiandrino, A., A. Giraud, S. Robin, and C. Pinatel. 2012. Validation d’une méthode d’estimation des volumes d’eau échangés entre la mer et les lagunes et définition d’indicateurs hydrodynamiques associés.
Fiandrino, A., V. Ouisse, F. Dumas, F. Lagarde, R. Pete, N. Malet, S. Le Noc, and R. de Wit. 2017. Spatial patterns in coastal lagoons related to the hydrodynamics of seawater intrusion. Marine Pollution Bulletin 119: 132–144. https://doi.org/10.1016/j.marpolbul.2017.03.006.
Fouda, M.M. 1995. Life history strategies of four small-size fishes in the Suez Canal. Egypt. Journal of Fish Biology 46: 687–702. https://doi.org/10.1111/j.1095-8649.1995.tb01104.x.
Franco, T.P., L.M. Neves, and F.G. Araújo. 2019. Better with more or less salt? The association of fish assemblages in coastal lagoons with different salinity ranges. Hydrobiologia 828: 83–100. https://doi.org/10.1007/s10750-018-3804-8.
Franco, A., P. Franzoi, S. Malavasi, F. Riccato, and P. Torricelli. 2006a. Fish assemblages in different shallow water habitats of the Venice Lagoon. Hydrobiologia 555: 159–174. https://doi.org/10.1007/s10750-005-1113-5.
Franco, A., P. Franzoi, S. Malavasi, F. Riccato, P. Torricelli, and D. Mainardi. 2006b. Use of shallow water habitats by fish assemblages in a Mediterranean coastal lagoon. Estuarine, Coastal and Shelf Science 66. Elsevier: 67–83. https://doi.org/10.1016/j.ecss.2005.07.020.
Franco, A., P. Franzoi, and P. Torricelli. 2008. Structure and functioning of Mediterranean lagoon fish assemblages: A key for the identification of water body types. Estuarine, Coastal and Shelf Science 79: 549–558. https://doi.org/10.1016/j.ecss.2008.05.011.
Franco, A., R. Fiorin, M. Zucchetta, P. Torricelli, and P. Franzoi. 2010. Flounder growth and production as indicators of the nursery value of marsh habitats in a Mediterranean lagoon. Journal of Sea Research 64: 457–464. https://doi.org/10.1016/j.seares.2010.01.006.
Franco, A., A. Pérez-Ruzafa, H. Drouineau, P. Franzoi, E.T. Koutrakis, M. Lepage, D. Verdiell-Cubedo, et al. 2012. Assessment of fish assemblages in coastal lagoon habitats: Effect of sampling method. Estuarine, Coastal and Shelf Science 112: 115–125. https://doi.org/10.1016/j.ecss.2011.08.015.
Franzoi, P., R. Maccagnani, R. Rossi, and V.U. Ceccherelli. 1993. Life cycles and feeding habits of Syngnathus taenionotus and S. abaster (Pisces, Syngnathidae) in a brackish bay of the Po River Delta (Adriatic Sea). Marine Ecology Progress Series 97: 71–81. https://doi.org/10.3354/meps097071.
García-Rubies, A., and E. Macpherson. 1995. Substrate use and temporal pattern of recruitment in juvenile fishes of the Mediterranean littoral. Marine Biology 124: 35–42. https://doi.org/10.1007/BF00349144.
Gordo, L., and H. Cabral. 2001. The fish assemblage structure of a hydrologically altered coastal lagoon: The Óbidos lagoon (Portugal). Hydrobiologia 459: 125–133. https://doi.org/10.1023/A:1012510608231.
Grati, F., G. Scarcella, P. Polidori, F. Domenichetti, L. Bolognini, R. Gramolini, C. Vasapollo, et al. 2013. Multi-annual investigation of the spatial distributions of juvenile and adult sole (Solea solea L.) in the Adriatic Sea (northern Mediterranean). Journal of Sea Research 84: 122–132. https://doi.org/10.1016/j.seares.2013.05.001.
Heather, F.J., D. Childs, A.M. Darnaude and J. Blanchard. 2018. Using an integral projection model to assess the effect of temperature on the growth of gilthead seabream Sparus aurata. PloS ONE 13(5): e0196092.
Holmquist, J.G. 1997. Disturbance and gap formation in a marine benthic mosaic: Influence of shifting macroalgal patches on seagrass structure and mobile invertebrates. Marine Ecology Progress Series 158: 121–130. https://doi.org/10.3354/meps158121.
Ifremer. 2014. Réseau de Suivi Lagunaire du Languedoc-Roussillon : Bilan des résultats 2013. IFREMER.
Isnard, E., J. Tournois, D.J. McKenzie, F. Ferraton, N. Bodin, C. Aliaume, and A.M. Darnaude. 2015. Getting a good start in life? A comparative analysis of the quality of lagoons as juvenile habitats for the gilthead seabream sparus aurata in the Gulf of Lions. Estuaries and Coasts 38: 1937–1950. https://doi.org/10.1007/s12237-014-9939-6.
Joyeux, J-C., J.A. Tomasini, and J-L. Bouchereau. 1992. Modalités de la reproduction de Gobius niger (Teleostei, Gobiidae) dans une lagune méditerranéenne. Vie et Milieu/Life & Environment 1–13.
Kara, M.H., and J.P. Quignard. 2018a. Les poissons des lagunes et des estuaires de Méditerranée 2: Les poissons sédentaires. Great Britain: ISTE Group.
Kara, M.H., and J.P. Quignard. 2018b. Les poissons des lagunes et des estuaires de Méditerranée 3A: Les poissons migrateurs. Great Britain: ISTE Group.
Kara, M.H., and J.P. Quignard. 2018c. Les poissons des lagunes et des estuaires de Méditerranée 3B: Les poissons migrateurs. Great Brtitain: ISTE Group.
Kara, M.H, and J.P. Quignard. 2018d. Les poissons des lagunes et des estuaires de Méditerranée 1: Diversité, bio-écologie et exploitation. Great Britain: ISTE Group.
Kemp, J.O.G. 2009. Effects of temperature and salinity on resting metabolism in two south african rock pool fish: The resident Gobiid caffrogobius caffer and the transient sparid Diplodus sargus capensis. African Zoology 44: 151–158. https://doi.org/10.3377/004.044.0203.
Kennish, M.J., M.J. Brush, and K.A. Moore. 2014. Drivers of change in shallow coastal photic systems: An introduction to a special issue. Estuaries and Coasts 37: 3–19. https://doi.org/10.1007/s12237-014-9779-4.
Kennish, M.J., and H.W. Paerl. 2010. Coastal lagoons: Critical habitats of environmental change. Boca Raton: CRC Press.
Kiener, A., and C.J. Spillmann. 1969. Contribution à l’étude systématique et écologique des athérines des côtes françaises. Mémoires du Muséum national d’Histoire naturelle. Série a, Zoologie 60: 33–74.
Kingsford, M.J., and J.H. Choat. 1985. The fauna associated with drift algae captured with a plankton-mesh purse seine net. Limnology and Oceanography 30: 618–630. https://doi.org/10.4319/lo.1985.30.3.0618.
Kohler, A. 1976. Observations biologiques et biometriques sur Atherina boyeri Risso dans l’étang du Prévost à Palavas (Hérault). Vie et milieu 26: 157–174.
Koussoroplis, A.-M. 2010. Sources de carbone et d’acides gras essentiels pour les juvéniles de Liza saliens (Pisces, Mugilidae) dans le réseau trophique d’une lagune côtière méditerranéenne : Approches in situ par biomarqueurs lipidiques et isotopiques. Thesis: Université Blaise Pascal-Clermont-Ferrand II.
Koutrakis, E.T., A.I. Sinis, and P.S. Economidis. 1994. Seasonal occurrence, abundance and size distribution of gray mullet fry (Pisces, Mugilidae) in the Porto-Lagos lagoon and lake Vistonis (Aegean sea, Greece). Israeli Journal of Aquaculture/bamidgeh 46: 182–196.
Koutrakis, E.T., A.C. Tsikliras, and A.I. Sinis. 2005. Temporal variability of the ichthyofauna in a Northern Aegean coastal lagoon (Greece). Influence of Environmental Factors. Hydrobiologia 543: 245–257. https://doi.org/10.1007/s10750-004-7891-3.
Kovačić, M. 2020. Checklist of gobies (Teleostei: Gobiidae) of the Mediterranean Sea and a key for species identification. Zootaxa 4877: 75–101. https://doi.org/10.11646/zootaxa.4877.1.3.
Kristiansen, T., K.F. Drinkwater, R.G. Lough, and S. Sundby. 2011. Recruitment variability in North Atlantic cod and match-mismatch dynamics. PLoS ONE 6: e17456. https://doi.org/10.1371/journal.pone.0017456.
Kyprianou, T.-D., H.O. Pörtner, A. Anestis, B. Kostoglou, K. Feidantsis, and B. Michaelidis. 2010. Metabolic and molecular stress responses of gilthead seam bream Sparus aurata during exposure to low ambient temperature: An analysis of mechanisms underlying the winter syndrome. Journal of Comparative Physiology B 180: 1005–1018. https://doi.org/10.1007/s00360-010-0481-y.
Labelle, M., and J.R. Nursall. 1985. Some aspects of the early life history of the redlip blenny, Ophioblennius atlanticus (Teleostei: Blenniidae). Copeia: 39–49.
Laffaille, P., E. Feunteun, A. Baisez, T. Robinet, A. Acou, A. Legault, and S. Lek. 2003. Spatial organisation of European eel (Anguilla anguilla L.) in a small catchment. Ecology of Freshwater Fish 12: 254–264. https://doi.org/10.1046/j.1600-0633.2003.00021.x.
Lagardère, F., R. Amara, and L. Joassard. 1998. Vertical distribution and feeding activity of metamorphosing sole, Solea solea, before immigration to the Bay of Vilaine nursery (northern Bay of Biscay, France). In When do fishes become juveniles?, ed. Hensel K. Copp G.H., Kováč V., 213–228. Dordrecht: Springer. https://doi.org/10.1007/978-94-017-3678-7_15.
La Mesa, M., F. Donato, G. Giannetti, and E. Arneri. 2009. Growth and mortality rates of European anchovy (Engraulis encrasicolus) in the Adriatic Sea during the transition from larval to juvenile stages. Fisheries Research 96: 275–280. https://doi.org/10.1016/j.fishres.2008.12.011.
Lasserre, P., and J.-L. Gallis. 1975. Osmoregulation and differential penetration of two grey mullets, Chelon labrosus (Risso) and Liza ramada (Risso) in estuarine fish ponds. Aquaculture 5: 323–344. https://doi.org/10.1016/0044-8486(75)90053-8.
Le Luherne, E., E. Réveillac, A. Ponsero, A. Sturbois, S. Ballu, M. Perdriau, and O. Le Pape. 2016. Fish community responses to green tides in shallow estuarine and coastal areas. Estuarine, Coastal and Shelf Science 175: 79–92. https://doi.org/10.1016/j.ecss.2016.03.031.
Lecomte-Finiger, R., and J. Bruslé. 1984. L’Anguilla anguilla des lagunes du Languedoc-Roussillon: Intérêt biologique et valeur halieutique. Vie Et Milieu 34: 185–194.
Lee, J.Y., and C. Juge. 1965. Observations morphologiques et biologiques sur les anchois (Engraulis encrasicholus) du golfe du Lion. Rapports Et Procès-Verbaux Des Réunions De La Commission Internationale Pour L’exploration Scientifique De La Mer Méditerranée 18: 221–224.
Le Fur, I., R. De Wit, M. Plus, J. Oheix, M. Simier, and V. Ouisse. 2018. Submerged benthic macrophytes in Mediterranean lagoons: Distribution patterns in relation to water chemistry and depth. Hydrobiologia 808: 175–200. https://doi.org/10.1007/s10750-017-3421-y.
Leitão, R., F. Martinho, J.M. Neto, H. Cabral, J.C. Marques, and M.A. Pardal. 2006. Feeding ecology, population structure and distribution of Pomatoschistus microps (Krøyer, 1838) and Pomatoschistus minutus (Pallas, 1770) in a temperate estuary, Portugal. Estuarine, Coastal and Shelf Science 66: 231–239. https://doi.org/10.1016/j.ecss.2005.08.012.
Leruste, A., N. Malet, D. Munaron, V. Derolez, E. Hatey, Y. Collos, R. De Wit, and B. Bec. 2016. First steps of ecological restoration in Mediterranean lagoons: Shifts in phytoplankton communities. Estuarine, Coastal and Shelf Science 180: 190–203. https://doi.org/10.1016/j.ecss.2016.06.029.
Levin, L.A., D.F. Boesch, A. Covich, C. Dahm, C. Erséus, K.C. Ewel, R.T. Kneib, et al. 2001. The function of marine critical transition zones and the importance of sediment biodiversity. Ecosystems 4: 430–451. https://doi.org/10.1007/s10021-001-0021-4.
Litvin, S.Y., M.P. Weinstein, M. Sheaves, and I. Nagelkerken. 2018. What makes nearshore habitats nurseries for nekton? An emerging view of the nursery role hypothesis. Estuaries and Coasts 41: 1539–1550. https://doi.org/10.1007/s12237-018-0383-x.
Maci, S., and A. Basset. 2009. Composition structural characteristics and temporal patterns of fish assemblages in non-tidal Mediterranean lagoons: A case study. Estuarine Coastal and Shelf Science 83 (4): 602–612. https://doi.org/10.1016/j.ecss.2009.05.007.
Maci, S., and A. Basset. 2010. Spatio-temporal patterns of abundance, size structure and body condition of Atherina boyeri (Pisces: Atherinidae) in a small non-tidal Mediterranean lagoon. Estuarine, Coastal and Shelf Science 87: 125–134. https://doi.org/10.1016/j.ecss.2010.01.002.
Madeira, D. 2016. Effects of ocean warming throughout the life cycle of Sparus aurata : A physiological and proteomic approach. Thesis: Universidade Nova de Lisboa.
Madeira, D., L. Narciso, H.N. Cabral, and C. Vinagre. 2012. Thermal tolerance and potential impacts of climate change on coastal and estuarine organisms. Journal of Sea Research 70: 32–41. https://doi.org/10.1016/j.seares.2012.03.002.
Madeira, D., L. Narciso, H.N. Cabral, C. Vinagre, and M.S. Diniz. 2013. Influence of temperature in thermal and oxidative stress responses in estuarine fish. Comparative Biochemistry and Physiology Part a: Molecular & Integrative Physiology 166: 237–243. https://doi.org/10.1016/j.cbpa.2013.06.008.
Malavasi, S., R. Fiorin, A. Franco, P. Franzoi, A. Granzotto, F. Riccato, and D. Mainardi. 2004. Fish assemblages of Venice Lagoon shallow waters: An analysis based on species, families and functional guilds. Journal of Marine Systems 51: 19–31. https://doi.org/10.1016/j.jmarsys.2004.05.006.
Malavasi, S., A. Franco, R. Fiorin, P. Franzoi, P. Torricelli, and D. Mainardi. 2005. The shallow water gobiid assemblage of the Venice Lagoon: Abundance, seasonal variation and habitat partitioning. Journal of Fish Biology 67: 146–165. https://doi.org/10.1111/j.0022-1112.2005.00919.x.
Malavasi, S., A. Franco, F. Riccato, C. Valerio, P. Torricelli, and P. Franzoi. 2007. Habitat selection and spatial segregation in three pipefish species. Estuarine, Coastal and Shelf Science 75: 143–150. https://doi.org/10.1016/j.ecss.2007.02.022.
Manzo, C., C. Brugnano, A. Fabbrocini, L. Roselli, and R. D’adamo. 2011. Recruitment dynamics of young-of-the-year marine fish in the coastal lagoon of Lesina. In Marine Research at CNR, DTA/06–201, 2099–2114. Roma.
Manzo, C., A. Fabbrocini, L. Roselli, and R. D’Adamo. 2016. Characterization of the fish assemblage in a Mediterranean coastal lagoon: Lesina Lagoon (central Adriatic Sea). Regional Studies in Marine Science 8: 192–200. https://doi.org/10.1016/j.rsma.2016.04.003.
Mariani, S. 2001. Can spatial distribution of ichthyofauna describe marine influence on coastal lagoons? A central Mediterranean case study. Estuarine, Coastal and Shelf Science 52: 261–267. https://doi.org/10.1006/ecss.2000.0746.
Mariani, S. 2006. Life-history- and ecosystem-driven variation in composition and residence pattern of seabream species (Perciformes: Sparidae) in two Mediterranean coastal lagoons. Marine Pollution Bulletin 53. Elsevier: 121–127. https://doi.org/10.1016/j.marpolbul.2005.09.019.
Marshall, S., and M. Elliott. 1998. Environmental influences on the fish assemblage of the Humber estuary, U.K. Estuarine, Coastal and Shelf Science 46: 175–184. https://doi.org/10.1006/ecss.1997.0268.
Martinho, F., R. Leitão, J.M. Neto, H. Cabral, F. Lagardère, and M.A. Pardal. 2008. Estuarine colonization, population structure and nursery functioning for 0-group sea bass (Dicentrarchus labrax), flounder (Platichthys flesus) and sole (Solea solea) in a mesotidal temperate estuary. Journal of Applied Ichthyology 24: 229–237. https://doi.org/10.1111/j.1439-0426.2007.01049.x.
Mazzoldi, C., and M. B. Rasotto. 2001. Extended breeding season in the marbled goby, Pomatoschistus marmoratus (Teleostei: Gobiidae), in the Venetian Lagoon. Environmental Biology of Fishes 61: 175–183
McDevitt-Irwin, J.M., J.C. Iacarella, and J.K. Baum. 2016. Reassessing the nursery role of seagrass habitats from temperate to tropical regions: A meta-analysis. Marine Ecology Progress Series 557: 133–143. https://doi.org/10.3354/meps11848.
Menu, M., S. Vaz, T. Bajjouk, V. Derolez, A. Fiandrino, A. Giraud, P. Grillas, and V. Ouisse. 2019. Rapport final du projet CHAMILA (Cartographie des habitats en milieu lagunaire méditerranéen). IFREMER.
Mercader, M., A. Mercière, G. Saragoni, A. Cheminée, R. Crec’hriou, J. Pastor, M. Rider, et al. 2017. Small artificial habitats to enhance the nursery function for juvenile fish in a large commercial port of the Mediterranean. Ecological Engineering 105: 78–86. https://doi.org/10.1016/j.ecoleng.2017.03.022.
Minos, G., G. Katselis, I. Ondrias, and I.J. Harrison. 2002. Use of melanophore patterns on the ventral side of the head to identify fry of grey mullet (Teleostei: Mugilidae). The Israeli Journal of Aquaculture - Bamidgeh 54: 12–26.
Mistri, M., E.A. Fano, G. Rossi, K. Caselli, and R. Rossi. 2000. Variability in macrobenthos communities in the Valli di Comacchio, northern Italy, a hypereutrophized lagoonal ecosystem. Estuarine, Coastal and Shelf Science 51: 599–611. https://doi.org/10.1006/ecss.2000.0697.
Munaron, D., N. Tapie, H. Budzinski, B. Andral, and J.-L. Gonzalez. 2012. Pharmaceuticals, alkylphenols and pesticides in Mediterranean coastal waters: Results from a pilot survey using passive samplers. Estuarine, Coastal and Shelf Science 114: 82–92. https://doi.org/10.1016/j.ecss.2011.09.009.
Murrel, M.C., and E.M. Lores. 2004. Phytoplankton and zooplankton seasonal dynamics in a subtropical estuary: Importance of cyanobacteria. Journal of Plankton Research 26: 371–382. https://doi.org/10.1093/plankt/fbh038.
Nagelkerken, I., M. Sheaves, and Ronald. Baker, and R.M. Connolly. 2015. The seascape nursery: A novel spatial approach to identify and manage nurseries for coastal marine fauna. Fish and Fisheries 16: 362–371. https://doi.org/10.1111/faf.12057.
Neveu, A. 1981. Densité et microrépartition des différentes espèces de poissons dans la Basse-Nivelle, petit fleuve côtier des Pyrénées Atlantiques. Bulletin Français de Pisciculture: 86–102. https://doi.org/10.1051/kmae:1981021.
Nohrén, E., and E. Odelgård. 2010. Response of epibenthic faunal assemblages to varying vegetation structures and habitat patch size. Aquatic Biology 9: 139–148. https://doi.org/10.3354/ab00247.
Nunn, A.D., L.H. Tewson, and I.G. Cowx. 2012. The foraging ecology of larval and juvenile fishes. Reviews in Fish Biology and Fisheries 22: 377–408.
Orlando-Bonaca, M., and L. Lipej. 2007. Microhabitat preferences and depth distribution of combtooth blennies (Blenniidae) in the Gulf of Trieste (North Adriatic Sea). Marine Ecology 28: 418–428. https://doi.org/10.1111/j.1439-0485.2007.00185.x.
Pérez-Ruzafa, A., and C. Marcos. 2012. Fisheries in coastal lagoons: An assumed but poorly researched aspect of the ecology and functioning of coastal lagoons. Estuarine, Coastal and Shelf Science 110: 15–31. https://doi.org/10.1016/j.ecss.2012.05.025.
Pérez-Ruzafa, A., C. Marcos, I.M. Pérez-Ruzafa, E. Barcala, M.I. Hegazi, and J. Quispe. 2007a. Detecting changes resulting from human pressure in a naturally quick-changing and heterogeneous environment: Spatial and temporal scales of variability in coastal lagoons. Estuarine, Coastal and Shelf Science 75: 175–188. https://doi.org/10.1016/j.ecss.2007a.04.030.
Pérez-Ruzafa, A., J.A. Garcı́a-Charton, E. Barcala, C. Marcos. 2006. Changes in benthic fish assemblages as a consequence of coastal works in a coastal lagoon: The Mar Menor (Spain Western Mediterranean). Marine Pollution Bulletin 53(1-4): 107–120. https://doi.org/10.1016/j.marpolbul.2005.09.014.
Pérez-Ruzafa, A., J.I. Quispe-Becerra, J.A. Garcia-Charton, and C. Marcos. 2004. Composition, structure and distribution of the ichthyoplankton in a Mediterranean coastal lagoon. Journal of Fish Biology 64: 202–218. https://doi.org/10.1111/j.1095-8649.2004.00301.x.
Pérez-Ruzafa, A., M.C. Mompeán, and C. Marcos. 2007b. Hydrographic, geomorphologic and fish assemblage relationships in coastal lagoons. Hydrobiologia 577: 107–125. https://doi.org/10.1007/s10750-006-0421-8.
Peterson, M.S., C.F. Rakocinski, B.H. Comyns, and G.L. Fulling. 2000. Laboratory growth responses of juvenile Mugil sp. to temperature and salinity: Delineating optimal field growth conditions. Proceedings of the Gulf and Caribbean Fisheries Institute 51: 341–352.
Petitgas, P., A.D. Rijnsdorp, M. Dickey-Collas, G.H. Engelhard, Myron A. Peck, John K. Pinnegar, K. Drinkwater, M. Huret, and R.D.M. Nash. 2013. Impacts of climate change on the complex life cycles of fish. Fisheries Oceanography 22: 121–139. https://doi.org/10.1111/fog.12010.
Pichavant, K. 2001. Comparative effects of long-term hypoxia on growth, feeding and oxygen consumption in juvenile turbot and European sea bass. Journal of Fish Biology 59. Wiley Online Library: 875–883. https://doi.org/10.1006/jfbi.2001.1702.
Plaut, I. 1998. Comparison of salinity tolerance and osmoregulation in two closely related species of blennies from different habitats. Fish Physiology and Biochemistry 19: 181–188. https://doi.org/10.1023/a:1007798712727.
Podani, J., and B. Csányi. 2010. Detecting indicator species: Some extensions of the IndVal measure. Ecological Indicators 10. Elsevier: 1119–1124. https://doi.org/10.1016/j.ecolind.2010.03.010.
Post, P., E. Blom, C. Chen, L.J. Bolle, and M.J. Baptist. 2017. Habitat selection of juvenile sole (Solea solea L.): Consequences for shoreface nourishment. Journal of Sea Research 122. https://doi.org/10.1016/j.seares.2017.02.011.
Potter, I.C., P.N. Claridge, and R.M. Warwick. 1986. Consistency of seasonal changes in an estuarine fish assemblage. Marine Ecology Progress Series 32: 217–228.
Pulcini, D., C. Costa, J. Aguzzi, and S. Cataudella. 2008. Light and shape: A contribution to demonstrate morphological differences in diurnal and nocturnal teleosts. Journal of Morphology 269: 375–385. https://doi.org/10.1002/jmor.10598.
QGIS.org. 2021. QGIS Geographic Information System. QGIS Association.
Quignard, J.-P., A. Man-Wai, and R. Vianet. 1984. Les poissons de l’étang de Mauguio (Hérault, France) inventaire, structure du peuplement, croissance et polymorphisme des tailles. Vie Et Milieu/life & Environment 34: 173–183.
Redolfi Bristol, S. 2019. Nursery function of coastal lagoons: Implications of habitat connectivity for the management of lagoon habitats. Thesis: Università Ca’Foscari Venezia.
Ribeiro, J., G.M. Carvalho, J.M. Gonçalves, and K. Erzini. 2012. Fish assemblages of shallow intertidal habitats of the Ria Formosa lagoon (South Portugal): Influence of habitat and season. Marine Ecology Progress Series 446: 259–273. https://doi.org/10.3354/meps09455.
Rigal, F., T. Chevalier, C. Lorin-Nebel, G. Charmantier, J.-A. Tomasini, F. Aujoulat, and P. Berrebi. 2008. Osmoregulation as a potential factor for the differential distribution of two cryptic gobiid species, Pomatoschistus microps and P. marmoratus in French Mediterranean lagoons. Scientia Marina 72: 469–476. https://doi.org/10.3989/scimar.2008.72n3469.
Říha, M., J. Kubečka, T. Mrkvička, M. Prchalová, M. Čech, V. Draštík, J. Frouzová, et al. 2008. Dependence of beach seine net efficiency on net length and diel period. Aquatic Living Resources 21: 411–418. https://doi.org/10.1051/alr:2008061.
Rodríguez-Climent, S., N. Caiola, and C. Ibáñez. 2013. Salinity as the main factor structuring small-bodied fish assemblages in hydrologically altered Mediterranean coastal lagoons. Scientia Marina 77: 37–45. https://doi.org/10.3989/scimar.03698.26B.
Rooper, C.N., D.R. Gunderson, and D.A. Armstrong. 2006. Evidence for resource partitioning and competition in nursery estuaries by juvenile flatfish in Oregon and Washington. Fishery Bulletin 104: 616.
Rosecchi, E. 1987. L' alimentation de Diplodus annularis, Diplodus sargus, Diplodus vulgaris et Sparus aurata (Pisces, Sparidae) dans le Golfe du Lion et les lagunes littorales. Revue Des Travaux De L’institut Des Pêches Maritimes 49: 125–141.
Rountree, R.A., and K.W. Able. 2007. Spatial and temporal habitat use patterns for salt marsh nekton: Implications for ecological functions. Aquatic Ecology 41: 25–45. https://doi.org/10.1007/s10452-006-9052-4.
Ruchon, F. 1995. Lipophrys pavo (Risso 1810)(Pisces, Blenniidae). Biologie d’une population lagunaire (stratégies et tactiques adaptatives). Thesis: Univ Aix-Marseille.
Sadler, K. 1979. Effects of temperature on the growth and survival of the European eel, Anguilla anguilla L. Journal of Fish Biology 15: 499–507. https://doi.org/10.1111/j.1095-8649.1979.tb03633.x.
Selfati, M., N. El Ouamari, A. Franco, P. Lenfant, G. Lecaillon, A. Mesfioui, P. Boissery, and H. Bazairi. 2019. Fish assemblages of the Marchica lagoon (Mediterranean, Morocco): Spatial patterns and environmental drivers. Regional Studies in Marine Science 32: 100896. https://doi.org/10.1016/j.rsma.2019.100896.
Sfriso, A., A. Buosi, C. Facca, and A.A. Sfriso. 2017. Role of environmental factors in affecting macrophyte dominance in transitional environments: The Italian Lagoons as a study case. Marine Ecology 38: e12414. https://doi.org/10.1111/maec.12414.
Shahriari Moghadam, M., B. Abtahi, S. Mosafer Khorjestan, and M.A. Bitaab. 2013. Salinity tolerance and gill histopathological alterations in Liza aurata Risso, 1810 (Actinopterygii: Mugilidae) fry. Italian Journal of Zoology 80: 503–509. https://doi.org/10.1080/11250003.2013.853326.
Sogard, S.M., and K.W. Able. 1991. A comparison of eelgrass, sea lettuce macroalgae, and marsh creeks as habitats for epibenthic fishes and decapods. Estuarine, Coastal and Shelf Science 33: 501–519. https://doi.org/10.1016/0272-7714(91)90087-R.
Souchu, P., B. Bec, V.H. Smith, T. Laugier, A. Fiandrino, L. Benau, V. Orsoni, Y. Collos, and A. Vaquer. 2010. Patterns in nutrient limitation and chlorophyll a along an anthropogenic eutrophication gradient in French Mediterranean coastal lagoons. Canadian Journal of Fisheries and Aquatic Sciences 67: 743–753. https://doi.org/10.1139/F10-018.
Steendam, C., P. Verhelst, S. Van Wassenbergh, and J. De Meyer. 2020. Burrowing behaviour of the European eel (Anguilla anguilla): Effects of life stage. Journal of Fish Biology 97: 1332–1342. https://doi.org/10.1111/jfb.14481.
Stoll, S., P. Fischer, P. Klahold, N. Scheifhacken, H. Hofmann, and K.-O. Rothhaupt. 2008. Effects of water depth and hydrodynamics on the growth and distribution of juvenile cyprinids in the littoral zone of a large pre-alpine lake. Journal of Fish Biology 72: 1001–1022. https://doi.org/10.1111/j.1095-8649.2007.01780.x.
Tagliapietra, D., M. Sigovini, and A.V. Ghirardini. 2009. A review of terms and definitions to categorise estuaries, lagoons and associated environments. Marine and Freshwater Research 60: 497–509. https://doi.org/10.1071/MF08088.
Thiel, R., A. Sepulveda, R. Kafemann, and W. Nellen. 1995. Environmental factors as forces structuring the fish community of the Elbe Estuary. Journal of Fish Biology 46: 47–69. https://doi.org/10.1111/j.1095-8649.1995.tb05946.x.
Thiriet, P. 2014. Comparison of fish assemblage structure and underlying ecological processes, between Cystoseira forests and less structurally complex habitats in North-Western Mediterranean rocky subtidal rocky reefs. Thesis: Université Nice Sophia Antipolis.
Tomasini, J.A., D. Collart, and J.P. Quignard. 1996. Female reproductive biology of the sand smelt in brackish lagoons of southern France. Journal of Fish Biology 49 : 594–612.
Tomasini, J.A, J.P. Quignard, C. Capapé, and J-L. Bouchereau. 1991. Facteurs du succès reproductif de Syngnathus abaster Risso, 1826 (Pisces, Teleostei, Syngnathidae) en milieu lagunaire méditerranéen (lagune de Maugio, France). Acta oecologica 12: 331–355.
Tsikliras, A.C., E. Antonopoulou, and K.I. Stergiou. 2010. Spawning period of Mediterranean marine fishes. Reviews in Fish Biology and Fisheries 20: 499–538. https://doi.org/10.1007/s11160-010-9158-6.
Tupper, M., and R. G. Boutilier. 1995. Effects of habitat on settlement, growth, and postsettlement survival of Atlantic cod (Gadus morhua). Canadian Journal of Fisheries and Aquatic Sciences 52. NRC Research Press Ottawa, Canada: 1834–1841.
Van Halder, I., L. Barbaro, E. Corcket, and H. Jactel. 2007. Importance of semi-natural habitats for the conservation of butterfly communities in landscapes dominated by pine plantations. In: Brockerhoff E.G., Jactel H., Parrotta J.A., Quine C.P., Sayer J., Hawksworth D.L. (eds) Plantation Forests and Biodiversity: Oxymoron or Opportunity? Topics in Biodiversity and Conservation, vol 9. Springer, Dordrecht. https://doi.org/10.1007/978-90-481-2807-5_12.
Vasconcelos, R.P., P. Reis-Santos, V. Fonseca, A. Maia, M. Ruano, S. França, V. Vinagre, M.J. Costa, and H. Cabral. 2007. Assessing anthropogenic pressures on estuarine fish nurseries along the Portuguese coast: A multi-metric index and conceptual approach. Science of the Total Environment 374 (2–3): 199–215. https://doi.org/10.1016/j.scitotenv.2006.12.048.
Vasconcelos, R.P., P. Reis-Santos, A. Maia, V. Fonseca, S. França, N. Wouters, M.J. Costa, and H.N. Cabral. 2010. Nursery use patterns of commercially important marine fish species in estuarine systems along the Portuguese coast. Estuarine, Coastal and Shelf Science 86: 613–624. https://doi.org/10.1016/j.ecss.2009.11.029.
Velasco, E.M., M.C. Gómez-Cama, J.A. Hernando, and M.C. Soriguer. 2010. Trophic relationships in an intertidal rockpool fish assemblage in the gulf of Cádiz (NE Atlantic). Journal of Marine Systems 80: 248–252. https://doi.org/10.1016/j.jmarsys.2009.10.014.
Ventura, D., G. Jona Lasinio, and G. Ardizzone. 2014. Temporal partitioning of microhabitat use among four juvenile fish species of the genus Diplodus (Pisces: Perciformes, Sparidae). Marine Ecology 36: 1013–1032. https://doi.org/10.1111/maec.12198.
Verdiell-Cubedo, D. 2009. Ictiofauna de las zonas someras litorales del Mar Menor (SE Península Ibérica): Parámetros de su biología y relaciones con el hábitat. Thesis: Universidad de Murcia.
Verdiell-Cubedo, D., F.J. Oliva-Paterna, A. Ruiz-Navarro, and M. Torralva. 2013. Assessing the nursery role for marine fish species in a hypersaline coastal lagoon (Mar Menor, Mediterranean Sea). Marine Biology Research 9: 739–748. https://doi.org/10.1080/17451000.2013.765580.
Verdiell-Cubedo, D., F. Oliva Paterna, M. Andreu Soler, and M. Torralva Forero. 2007. Characterisation of the nursery areas for YOY Sparidae fish species in a Mediterranean coastal lagoon (SE Iberian Peninsula). Anales de Biología: 3–11.
Verdiell-Cubedo, D., F.J. Oliva Paterna, A. Egea Serrano, and M. Torralva Forero. 2008. Biología poblacional y asociaciones con el hábitat de las especies de peces bentónicas en las áreas someras de una laguna costera mediterránea (SE Península Ibérica). Scientia Marina 72: 319–328. https://doi.org/10.3989/scimar.2008.72n2319.
Vigliola, L., and M. Harmelin-Vivien. 2001. Post-settlement ontogeny in three Mediterranean reef fish species of the genus Diplodus. Bulletin of Marine Science 68: 271–286.
Ward, J.H. 1963. Hierarchical grouping to optimize an objective function. Journal of the American Statistical Association 58: 236–244. https://doi.org/10.1080/01621459.1963.10500845.
Westerberg, H., J. Haamer, and I. Lagenfeldt. 1993. A new method for sampling elvers in the coastal zone. In ICES, C.M. Anadromous and Catadromous Fish Committee 1993, M:5:10.
Whitehead, P.J.P. 1988. FAO species catalogue: An annotated and illustrated catalogue of the herrings, sardines, pilchards, sprats, shads, anchovies and wolf-herrings, vol. 7. Rome: Food & Agriculture Org.
Whitfield, A.K. 1999. Ichthyofaunal assemblages in estuaries: A South African case study. Reviews in Fish Biology and Fisheries 9: 151–186. https://doi.org/10.1023/A:1008994405375.
Whitfield, A.K. 2017. The role of seagrass meadows, mangrove forests, salt marshes and reed beds as nursery areas and food sources for fishes in estuaries. Reviews in Fish Biology and Fisheries 27: 75–110. https://doi.org/10.1007/s11160-016-9454-x.
Whitfield, A.K., R.H. Taylor, C. Fox, and D.P. Cyrus. 2006. Fishes and salinities in the St Lucia estuarine system—A review. Reviews in Fish Biology and Fisheries 16: 1–20. https://doi.org/10.1007/s11160-006-0003-x.
Wilkins, H.K.A., and A.A. Myers. 1992. Microhabitat utilisation by an assemblage of temperate Gobiidae (Pisces: Teleostei). Marine Ecology Progress Series 90: 103–112.
Willemsen, J. 1980. Fishery-aspects of eutrophication. Hydrobiological Bulletin 14. Springer: 12–21.
Woodland, R.J., F.Y. Warry, Y. Zhu, R. Mac Nally, P. Reich, G.P. Jenkins, D. Brehm, and P.L.M. Cook. 2019. Role of benthic habitat structure and riverine connectivity in controlling the spatial distribution and ecology of estuarine fish. Marine Ecology Progress Series 630: 197–214. https://doi.org/10.3354/meps13116.
Yáñez-Arancibia, A., A.L. Lara-Dominguez and D. Pauly. 1994. Coastal lagoons as fish habitats, Chapter 12:363–376 in Coastal Lagoon Processes, Oceanography Series 60, ed B. Kjerve (Amsterdam: Elsevier Science B.V.), 578
Acknowledgements
This work is part of the NURSE project funded by Office Français de la Biodiversité and Ifremer. M. Iotti was financially supported by the “Ministère de l’Agriculture et de l’Alimentation”. The authors would like to thank the LERLR team for their participation in field sampling, and the “Compagnons de Maguelone” for allowing access to the Prevost lagoon. The authors also thank the two anonymous referees whose comments greatly contributed to the improvement of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Henrique Cabral
Appendix
Appendix
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Iotti, M., Darnaude, A.M., Bouriat, A. et al. Spatio-temporal Variation of Shallow Microhabitats and Associated Juvenile Fish Assemblages in a Mediterranean Lagoon. Estuaries and Coasts 46, 198–226 (2023). https://doi.org/10.1007/s12237-022-01102-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-022-01102-9