Abstract
Temperate coastal lagoons are considered key habitats for several highly prized marine fishes, which colonise them as nurseries. Lagoons can, however, exhibit diverse abiotic and biotic conditions, with potential consequences for their quality as habitats. To investigate this, we compared size, body condition (Fulton’s condition factor K and lipid classes), past growth rate (from otoliths) and sources of food web organic matter (OM), among group 0 juveniles of the gilthead sea bream Sparus aurata L. 1758, captured at the end of their first summer of residence in four lagoons of the Gulf of Lions in the NW Mediterranean (Mauguio, Thau, Bages and Salses-Leucate). These lagoons have different environmental conditions and freshwater inputs. Although age was similar for all lagoons, juveniles from Mauguio and Bages were significantly larger and heavier than those from Thau and Salses-Leucate. They were also in better condition, with higher white muscle triacylglycerol/sterol ratio (mean ± SD 35.7 ± 20.1 in Mauguio and 23.2 ± 9.8 in Bages versus 15.1 ± 15.2 in Thau and 7.4 ± 7.9 in Salses-Leucate). All exhibited similar otolith growth rates for their larval marine phase (2.8 ± 0.4 μm day−1), but significant differences were found for the lagoon phase, with higher values in Mauguio and Bages (10.1 ± 0.9 and 9.7 ± 1.0 μm day−1, respectively) than those in Thau and Salses-Leucate (8.4 ± 1.2 and 8.9 ± 0.8 μm day−1, respectively). White muscle stable isotope analysis revealed that terrestrial carbon use by the juveniles was >33 % in Mauguio and <5 % in Salses-Leucate, with intermediate values (~15 %) in Thau and Bages. Although these effects on fish condition and growth rate may relate in part to differences in water salinity and dissolved oxygen in the four lagoons, it is probable that they are mostly related to differences in food web enrichment with terrestrial OM.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Understanding the life cycle of coastal marine fishes is essential for effective management of exploited stocks. This can, however, be remarkably difficult to achieve because many species have complex life histories (Forrester and Swearer 2002; Gillanders et al. 2003; Figueira 2009; Fromentin et al. 2009) often including migrations between offshore marine environments and inshore coastal habitats (Joyeux and Ward 1998; Day et al. 2012). For many species, early life stages occupy inshore nurseries and feeding grounds for several months to years, until they recruit to adult stocks (Beck et al. 2001; Able 2005; Vasconcelos et al. 2007). The condition and growth of juveniles in these areas are expected to influence their recruitment success to the adult population (van der Veer et al. 1990; Beverton and Iles 1992; Ciotti et al. 2014). Therefore, it is particularly important to understand the role, in overall metapopulation function, of the various coastal habitats colonized by the juveniles and to identify nursery grounds among them.
In this regard, temperate coastal lagoons have, so far, received little attention (Kjerfve 1994). Like estuaries, these transitional ecosystems may offer favourable developmental conditions with high primary productivity, lower salinity than seawater and some protection from predation (Claireaux and Lagardère 1999; Gillanders et al. 2003; Dahlgren et al. 2006; Stierhoff et al. 2009; Vasconcelos et al. 2010, 2011). Lagoons can, however, comprise a diverse mosaic of environments with different physico-chemical characteristics (Pauly and Yáñez-Arancibia 1994), and hence differ markedly in their suitability as habitats for early life stages of fishes. The Gulf of Lions, in the Northwestern Mediterranean, is a case in point (Mouillot et al. 2005), with a series of contiguous lagoons that are occupied seasonally by juveniles of numerous highly prized fish species (Quignard et al. 1984), among which the gilthead seabream Sparus aurata L. 1758 is an emblematic example (Audouin 1962; Lasserre 1976).
S. aurata is a euryhaline and eurythermal species found in both the open sea and brackish transitional waters along the coasts of the eastern Atlantic, the Mediterranean and the Black Sea (source FAO.org). In the Gulf of Lions, reproduction occurs in winter at sea and pelagic larvae drift for up to 3 months, their appearance inshore in spring coinciding with metamorphosis to the juvenile stage (Audouin 1962; Bodinier et al. 2010). The juveniles colonise sheltered areas, in particular the coastal lagoons, which they occupy massively over the summer, migrating out to sea in the autumn, when temperatures of the lagoons and open sea become similar (Audouin 1962; Lasserre 1976). Despite their apparent importance as juvenile habitats, the exact role of the various coastal lagoons in local S. aurata metapopulation function is unclear. In particular, the marked abiotic and biotic differences among them could be significant for their relative quality as nurseries. Indeed, there is evidence that a majority of the local adult population originates from relatively small, shallow, brackish lagoons (Mercier et al. 2012).
We focused on four lagoons of the Gulf of Lions with different environmental conditions and freshwater inputs, which harbour large populations of S. aurata juveniles each year. We investigated the hypothesis that shallow brackish lagoons would produce larger juveniles in better condition. To this end, we measured and compared various traits of body condition (Fraser 1989; Norton et al. 2001; Kerambrun et al. 2011) and otolith growth rates (Panfili et al. 2002) of juveniles captured at the end of their first summer of residence. These were then related to lagoon conditions, particularly temperature, salinity, phytoplankton biomass and dissolved oxygen. Terrestrial organic matter (OM) inputs to coastal ecosystems modify the composition, abundance and biomass of benthic prey (Drake et al. 2002; Salen-Picard et al. 2002; Nicolas et al. 2007; Kostecki et al. 2010), which subsequently influence growth and condition of juvenile fishes (Houde 1997). We therefore also investigated the hypothesis that the better habitat quality of brackish water lagoons would be explained, at least in part, by food web enrichment from terrestrial inputs in OM. We therefore analysed the δ13C and δ15N stable isotope signatures in muscle of the juveniles from the four lagoons and related these to signatures of major sources of OM in benthic food webs.
Materials and Methods
Study Area
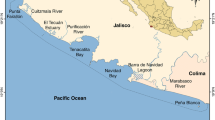
We focused on four lagoons of the Languedoc-Roussillon region (Mauguio, Thau, Bages and Salses-Leucate; Fig. 1), which all harbour large numbers of S. aurata juveniles each year (Audouin 1962; Lasserre 1976) although they differ in their morphological and physico-chemical characteristics (Table 1). Thau and Salses-Leucate are 2 to 4 m deep on average and exhibit water characteristics close to those of seawater, with low phytoplankton biomass and eutrophication. By contrast, Mauguio is shallow (0.8 m deep on average), brackish and eutrophic, with high phytoplankton biomass. Bages possesses intermediate depth and salinity values, with low phytoplankton biomass and eutrophication.
Fish Sampling
Using fyke nets, 140 juveniles were collected during the autumn of 2011, at the end of their first summer of residence in the four lagoons. To cover the full period of juvenile migration from each lagoon, the fish from Mauguio (N = 44) were collected between September and November, while those from Salses-Leucate (N = 30), Thau (N = 42) and Bages (N = 24) were captured only in October, November and December, respectively. On the day of capture, all fish were transported on ice to the laboratory, weighed to the nearest 0.1 g (total mass = Mt) and measured to the nearest mm (total length = Lt). Two portions of epaxial white muscle were collected and stored at −80 °C for subsequent analysis of lipid and stable isotope composition. Finally, the head of each fish was removed and stored at −20 °C for subsequent extraction of sagittal otoliths.
Condition Indices
Body condition was evaluated for all individuals by the Fulton’s condition factor (K; Bagenal and Tesch 1978), commonly used as an indicator of general well-being and calculated as follows:
where M t is the total mass (mg) and L t the total length (mm).
Condition was further investigated by determining total lipid content and concentrations of lipid classes in the muscle of 15 individuals per lagoon. Approximately 100 mg of muscle was ground on dry ice with a mixer mill MM400 (Retsch). Lipids were extracted with a Tenbroek Homogeniser in dichloromethane/methanol (2:1, v/v) (Folch et al. 1957), then spotted onto S-III chromarods (Iatron Laboratories) and separated into triacylglycerols (TAG), free fatty acids, free sterols (ST), sterol- and wax-esters, ketones, acetone mobile polar lipids and phospholipids (Parrish 1987). Chromarods were scanned by a flame ionization detection (FID) system (Iatroscan Mark-VI, Iatron Laboratories), and chromatograms were analyzed using integration software (Peak Simple version 3.2, SRI). Concentrations of lipid classes were estimated individually in μg mg−1 of wet muscle sample mass (w.m.), before calculating total lipid content as the sum of all lipid classes (in μg mg−1 w.m.). The TAG/ST ratio, which compares the concentrations of TAG, an indicator of reserve lipids, and ST, an indicator of structural lipids, was then used as an index of the immediate nutritional and energetic status of the juveniles (Håkanson 1993; Harding and Fraser 1999; Norton et al. 2001).
Otolithometry
Taking the same individuals used for lipid analyses, sagittal otoliths were extracted, washed and prepared for daily ring counts as described by Tomás and Panfili (2000). They were embedded in polyester resin and transverse sections of approximately 1-mm width, including the central nucleus, were made with a precision saw (Buehler, Isomet 1000). The sections were polished to approximately 10-μm depth with the nucleus exposed on the surface. They were photographed with a digital camera system (Olympus ProgRes C5) and ProRes Capture pro 2.5 software linked to a microscope (Olympus BX41, ×400). All pictures were imported into Perfect Image software.
Examination of the otolith sections revealed multiple concentric rings from the nucleus to the edge, clearly visible on all otoliths, in particular along the ventral axis, which was the maximum growth axis of the otolith in S. aurata. In accordance with previous literature (Morales-Nin et al. 1995; Panfili et al. 2002), the daily deposition of these increments was validated using five alizarine-marked S. aurata from an experimental study (Darnaude pers. comm.). Daily otolith rings were counted twice along the ventral axis by two independent readers, from the first visible increment corresponding to mouth opening, 3 days after hatch (Bodinier et al. 2010) to the edge of otolith, namely the capture date. Otoliths were read again if the two estimated ages in days differed by more than 5 %, to achieve a consensual age estimate between the two readers (Campana and Jones 1992). The two separate readings of otolith daily increments were compared by paired t tests after checking normality and homoscedasticity, and when there were non-significant differences, the mean reading was used.
Teleost otoliths can exhibit clear concentric marks (checks) that correspond to major life stage changes, such as metamorphosis from larva to post-larva, or major environmental changes (Campana 1992; Joh et al. 2011; Rey et al. 2012). In S. aurata, two successive checks were observed on the otoliths: (i) a band of five to 15 discontinuous and narrower increments at about 96 days after hatching, corresponding to metamorphosis (Bodinier et al. 2010), and (ii) a later single check mark followed by a clear change in the growth axis (appearance of an accessory primordium) on the ventral side. Because this latter check generally indicates a change of habitat in juvenile fish (Daverat et al. 2011; Feutry et al. 2012), it has provided a useful marker to delineate timing of migration from highly saline offshore areas to low-salinity estuaries (Hsu et al. 2009; Chang and Iizuka 2012). After validation of the presence of such a check on the edge of the otoliths of five of the smallest juveniles (Lt = 30–39 mm) captured in April–May in the four lagoons (two in Mauguio and one in the other three lagoons), we used it as the mark for the beginning of lagoon residence. This allowed estimation of the age at lagoon entrance (in days) and back-calculation of the date of lagoon entrance for each individual. Distances (in μm) were also measured along the maximum otolith growth axis between the nucleus and the check (i.e. larval marine life) and between the check and otolith edge (i.e. juvenile lagoon residence) to calculate absolute open-sea and lagoon daily growth rates (AGR, Panfili et al. 2002):
where, for each period of life, Sf and Si are the final and initial distances to the nucleus (in μm) measured along the maximum growth axis and Tf and Ti the corresponding final and initial ages estimated (in day).
Stable Isotope Analysis
Carbon (δ13C) and nitrogen (δ15N) stable isotope analysis constitutes a powerful tool for the description of the structure of coastal food webs and OM transfers through them (Carlier et al. 2008; Layman et al. 2012). Provided that distinct isotopic signatures can be identified for the primary producers, the method allows assessment of the main OM source(s) that sustain animal feeding (Kostecki et al. 2012). White muscle gives the most reliable results for predator isotopic signatures, with the lowest inter-individual variability (Pinnegar and Polunin 1999). Therefore, stable isotopic signatures in carbon (δ13C) and nitrogen (δ15N) were determined for this tissue in 34 juveniles, at least 8 per lagoon among the 15 individuals used for lipid and otolith analyses. Each muscle sample was freeze-dried separately and ground to a fine powder in a mortar and pestle. As total lipid content was low (i.e. <5 % dry mass), individual δ15N and δ13C values were determined on samples without lipid extraction (Bodin et al. 2009). Thus, 0.5 mg was weighed in a tin capsule and analysed with a continuous flow isotope-ratio mass spectrometer (Delta V Advantage, Thermo Scientific) coupled to an elemental analyzer (Flash EA1112 Thermo Scientific). Results were expressed as parts per thousand (‰) differences from international standard reference materials, i.e. the Vienna Pee Dee Belemnite (VPDB) for C and atmospheric N2 for N:
where X is the 13C or 15N, and R the corresponding ratio 13C/12C or 15N/14N.
Precision was assessed by repeated analysis of an internal laboratory standard (acetanilide) and was around 0.15 ‰ for both C and N.
Statistical Analyses
All statistical analyses in this work were carried out using R software (version 2.12.0, R Development Core Team 2010 ) taking α < 0.05 as the limit for statistical significance.
Inter-lagoon differences in fish biometry, condition, age, otolith growth rates and muscle isotopic signature were assessed after testing for data normality (Shapiro-Wilk test) and homoscedasticity (Bartlett test). When these conditions were met, one-way ANOVA were applied, followed by Tukey post hoc tests. Otherwise, data were compared by non-parametric Kruskal-Wallis tests followed by post hoc multiple comparison tests with Bonferroni correction.
To avoid size bias when comparing fish isotopic signature among lagoons, effect of fish size on muscle isotopic signature was evaluated separately for δ13C and δ15N, by Pearson correlation tests. Inter-habitat differences in the δ13C and δ15N of S. aurata muscle were interpreted in relation with isotopic signatures reported for the major potential OM sources at the base of the benthic food web in each lagoon, i.e. local macrophytobenthos (1) and microphytobenthos (2) and the marine (3) and terrestrial (4) particulate organic matters (POM) that end up mixed in the lagoon water column and sediment (Table 2). For each OM source, from 6 to 24 multiple-year estimates of δ13C and δ15N, signatures were compiled from the recent local literature, all specific to the study area and the four lagoons (Vizzini et al. 2005; Carlier et al. 2008; Harmelin-Vivien et al. 2008; Carlier et al. 2009; Pernet et al. 2012; Escalas et al. 2015). When possible, specific average values and standard deviations were calculated for each source and lagoon. Otherwise (i.e. for local marine and terrestrial POMs, microphytobenthos and the macroalgae of Thau and Bages), we used average signatures and standard deviations derived from the data available for all lagoons. Because, at this spatial scale, the variability in OM signatures among lagoons was always smaller than that observed among OM sources in all lagoons, at least for one of the two isotopes, we are confident that this did not bias identification of food source contributions to S. aurata growth. As normality and homoscedasticity were observed, differences in isotopic signatures among all potential major OM sources were tested for each lagoon by one-way ANOVAs, followed by multiple comparison Tukey post hoc tests (Table 2). The contributions to muscle composition of the four different OM sources were then estimated with mixing model analyses based on Bayesian methods (Parnell et al. 2010), using the R software package ‘stable isotope analysis in R’ (SIAR). A trophic level (TL) of 3.5 was considered for S. aurata (Froese and Pauly 2010). Thus, trophic enrichment factors (TEF) between OM sources (TL = 1) and S. aurata juveniles (TL = 3.5) in the model were calculated for δ13C and δ15N as follows:
where TEFmarine organism represents the average TEF value expected for marine organisms.
For this, TEFmarine organism for both δ13C and δ15N were obtained by averaging the TEF values listed for marine invertebrates and fish species in Caut et al. (2009), resulting in final values (±SD) of 1.26 ‰ (±0.67) (n = 35) and 2.26 ‰ (±0.75) (n = 42), for δ13C and δ15N, respectively. SIAR analyses using the TEFmodel derived from these two TEFmarine organism were run separately for each lagoon to estimate the respective contributions (medians and 50th Bayesian credibility intervals over 500,000 iterations) of the four OM sources to the final composition of muscle tissue.
Results
Size and Body Condition
Total length (Lt) and mass (Mt) of juveniles varied significantly among the four lagoons (Table 3). Individuals from Mauguio and Bages were significantly larger and heavier than those from Thau and Salses-Leucate (Kruskal-Wallis tests, H = 53.52, p value = 1.42 10−11 and H = 60.39, p value = 4.86 10−13, respectively). Fulton’s condition factor K was also significantly different among lagoons, the highest values being observed in Mauguio (Kruskal-Wallis test, H = 52.2, p value = 2.65 10−11).
Muscle total lipid content differed significantly among lagoons (Kruskal-Wallis test, H = 17.00, p value = 7.08 10−4, Table 3). However, although the highest mean value was in juveniles from Mauguio, post hoc multiple comparisons did not reveal significant differences among lagoons. The TAG/ST ratio was, however, significantly higher (Kruskal-Wallis test, H = 25.97, p value = 9.68 10−6) in Mauguio and Bages than that in Salses-Leucate, with Thau having an intermediate value (Table 3).
Age and Otolith Growth Rates
Ages at the time of sampling were not statistically different (ANOVA, F = 2.54, p value = 0.07) and were approximately 10 months for all fish (Table 3). Similarly, no significant difference was found among lagoons in the duration of either the offshore larval life (ANOVA, F = 0.66, p value = 0.58) or the inshore juvenile life (ANOVA, F = 1.22, p value = 0.31); all individuals had spent approximately 4 months (mean ± SD; 122.3 ± 9.6 days) at sea before entering the four lagoons and, then, approximately 6 months (182.4 ± 11.6 day) feeding and growing in these ecosystems before their capture in the autumn, before they could migrate back at sea. Back-calculation of hatching dates revealed that reproduction extended at least from December to February, and colonization of lagoons occurred at least from April to June (Fig. 2). The data indicated that the juveniles had entered the lagoons at different times (ANOVA, F = 36.46, p value = 4.14 10−13), mostly during April in Mauguio, from late April to early May in Salses-Leucate, during the second half of May in Thau and from June in Bages (Fig. 2).
Lagoon entrance dates (boxplot) for the S. aurata juveniles captured in the four lagoons during autumn (n = 15 per lagoon). The box for each lagoon contains 50 % of the data, with the thick horizontal line indicating the median; box whiskers (dotted lines) represent the first and fourth quartiles of the total range and circles represent extreme values. Letters indicate significant differences between lagoons (p < 0.05), when present
Growth during the marine larval phase did not differ among lagoons (Kruskal-Wallis, Fig. 3a), with a mean overall AGR of 2.8 ± 0.4 μm day−1. Growth during the lagoon phase was, however, significantly higher in Mauguio (9.6 ± 0.7 μm day−1) and Bages (9.3 ± 0.7 μm day−1) than that in Thau (8.5 ± 1.0 μm day−1) and Salses-Leucate (8.5 ± 0.7 μm day−1) (ANOVA, F = 10.05, p value = 2.22 10−5, Fig. 3b).
Organic Matter Sources Exploited for Growth
For the size range studied (153–205 mm), C and N isotopic ratios in muscle were not affected by fish size (Pearson correlation test: δ13C, t = −2.049, df = 29, p value = 0.05952, R 2 = 0.126; δ15N, t = 1.699, df = 29, p value = 0.09985, R 2 = 0.090). Muscle isotopic signatures varied according to the lagoons for both carbon (ANOVA: F = 28.03, p value = 1.90 10−8) and nitrogen (ANOVA: F = 31.56, p value = 5.63 10−9). Fish from Mauguio had significantly lower δ13C signatures (−19.86 ± 1.53 ‰) than those from Thau (−14.92 ± 0.52 ‰), Bages (−14.24 ± 0.91 ‰) and Salses-Leucate (−12.43 ± 0.48 ‰). On the other hand, muscle δ15N signatures were significantly higher in Mauguio (16.65 ± 0.74 ‰) and lower in Salses-Leucate (8.93 ± 0.22 ‰) compared to Thau (12.06 ± 0.72 ‰) and Bages (12.20 ± 0.65 ‰). This revealed three distinct trophic groups: one in Mauguio with high δ15N and depleted δ13C, one in Salses-Leucate with low δ15N and high δ13C and one in Bages and Thau with intermediate δ15N and high δ13C.
The mixing models revealed that this reflected significant differences among lagoons in the origin of the OM at the base of the sea bream food web, with Mauguio being particularly different from the other three (Table 4). In Mauguio, terrestrial POM and lagoon macroalgae represented 68 to 98 % of the OM exploited, with the former representing 33 to 49 %. Marine POM accounted for less than 9 % of OM exploited in Mauguio. In the three other lagoons, the two main sources of OM (47–93 %) were marine POM and microphytobenthos, with the former representing >20 % in all three lagoons. Terrestrial POM represented about 25 % in Thau and Bages but less than 5 % in Salses-Leucate.
Discussion
To our knowledge, the current data are the first to compare size and condition with accurate estimates of the age, lifetime growth rates and OM sources of juvenile fish among lagoon nursery grounds. Our results demonstrate that differences in the size and condition of S. aurata juveniles among lagoons at the end of the colonization period were due to differences in growth rate during the lagoon phase. This, in turn, was associated with different food webs and sources of OM. This therefore indicates that shallow brackish lagoons afford better growth rates and produce juveniles in better condition compared to deeper more saline lagoons.
Linking Size and Condition to age and Growth Rates
Previous studies on growth of S. aurata juveniles in the Gulf of Lions reached a similar conclusion when comparing Mauguio and Thau (Audouin 1962; Quignard et al. 1983), but not when comparing Thau and Bages, juveniles from Bages being smaller than Thau (Audouin 1962). In these earlier studies, however, the age of the fish was not known exactly. In the current study, we demonstrated that, after spending approximately 6 months in the lagoons, individuals from Mauguio and Bages were significantly bigger and heavier than those from Thau and Salses-Leucate. Values for the condition factor K in Thau, Bages and Salses-Leucate (1.4) were not significantly different to each other and similar to values reported for wild individuals in Spain and Greece (Laiz-Carrión et al. 2005). However, juveniles from Mauguio were in better ‘body condition’ with a K of 1.6 that is more similar to reports for individuals in aquaculture, which are typically characterised by higher K than wild fish (Arechavala-Lopez et al. 2011).
Direct biochemical condition indices are the most accurate means of measuring the energetic status of marine organisms (Vogt et al. 2002). In fish, lipids constitute the main energy source. The content and composition of tissue lipids can influence growth, reproduction, behaviour/movements, thermal acclimatisation and immune responses (Evans 1994). While total lipid content provides a good estimation of condition, separation and quantification of lipid classes reveal information about levels of metabolically available lipids (Sargent et al. 2002). In this study, the larger size of fish from Mauguio and Bages was generally correlated with a greater accumulation of storage lipid (TAG) and a higher TAG/ST ratio, which indicates larger reserves for use as energetic substrates or as structural components of new tissues.
The otolithometry analysis provided much novel information about the reasons for inter-lagoon differences. As in previous studies (Campana 1992; Joh et al. 2011; Rey et al. 2012), otolith reading revealed the timing of successive life stages in individual fish, indicating the durations of open-sea (larval) and lagoon (juvenile) phases and determining daily growth rates (Panfili et al. 2002). Otolith growth rate is directly related to somatic growth rate in fishes (Campana 1992); so, it is very interesting that the differences in body size and condition of the S. aurata juveniles among lagoons were not due to different ages or duration of larval phases, but to differences in growth rate in the lagoon phase. The date at which individuals entered the lagoons could also play a role in defining their final condition, if they encounter different intervals in the seasonal patterns of water temperature and productivity. The fact that larger fish were found in Mauguio and Bages, however, is not consistent with this theory because they entered first and last, respectively.
Environmental Influences on Condition
The condition and growth rate of juvenile fish depend on food availability (Fraser 1989). In this study, this likely contributed, at least in part, to the differences among lagoons. Indeed, the highest TAG/ST ratios and growth rates were observed in Mauguio and Bages, where the percentage of saturation in dissolved oxygen, often used as a proxy for productivity (Taylor et al. 1992; Bearzi et al. 2008), is maximal (Table 1). This boosts secondary production and probably increased prey availability for S. aurata, as the diet of this opportunistic predator is particularly flexible at the juvenile stage (Kraljevic and Dulčić 1997; Parra and Yufera 2000; Mariani et al. 2002; Tancioni et al. 2003; Chaoui et al. 2005). Digestibility and nutritional quality of the food might also play a role, since they both modulate growth and condition of S. aurata in aquaculture (Morais et al. 2006). However, the origin of OM in coastal food webs can affect the abundance and nutritional quality of the benthos (Kidd et al. 2001; Salen-Picard et al. 2002; Darnaude 2005; Vizzini et al. 2005; Gilliers et al. 2006); so, variation in food web carbon sources, revealed by the δ13C and δ15N isotope analyses, may have contributed significantly to the differences in growth and condition.
Small inaccuracies in the isotopic signatures of the OM sources used to parameterize the SIAR analyses cannot be fully excluded, mainly for Thau and Bages where site-specific signatures could not be found for all sources. We used, however, mean values from similar neighbouring lagoons influenced by the same watersheds (IFREMER 2011). Moreover, variations in OM source signatures among lagoons at our spatial scale were smaller than those observed between OM sources in all lagoons, with at least one of the two isotopes always allowing full separation of all sources. Therefore, we are reasonably confident that any bias in estimating contributions of the OM sources would not obscure the marked variation among lagoons, especially since the variance around the means was incorporated into the SIAR model. Another potential source of error is the kinetics of isotopic signature turnover in fish muscle. In juvenile fishes, growth is the main factor that causes turnover of muscle isotope signatures (Hesslein et al. 1993; MacAvoy et al. 2001; Maruyama et al. 2001; Sakano et al. 2005; Suzuki et al. 2005; Logan et al. 2006; Guelinckx et al. 2007); it takes anything from 2 to 7 months for juvenile fish muscle to reflect the isotopic signature of their food (Maruyama et al. 2001; Sakano et al. 2005; Guelinckx et al. 2007). The juveniles were captured in autumn as they migrated towards the sea to overwinter offshore. The otolith data indicate that they had been in the lagoons for at least 5 months; so, the isotopic signature of their muscle should reflect their lagoon food web.
In Mauguio, where juveniles grew the fastest and accumulated the most lipid reserves, food webs were highly dependent on terrestrial POM. That was not the case for the three other lagoons, where the major OM sources were lagoon microphytobenthos and marine POM. These results are in general agreement with the existing literature. Terrestrial POM uptake by coastal marine communities is generally weak (Deegan and Garritt 1997; Kwak and Zedler 1997; Paterson and Whitfield 1997; Vizzini and Mazzola 2003). In transitional systems under limited freshwater influence, in situ primary production is generally the major source of OM for growth of young fish (Boesch and Turner 1984; Melville and Connolly 2003; Kostecki et al. 2012; Le Pape et al. 2013), which was what we observed in Salses-Leucate and, to a lesser extent, in Thau and Bages. Many studies have, however, found significant incorporation of terrestrial OM into juvenile fish food webs in estuarine areas with major terrestrial inputs (Darnaude et al. 2004; Leakey et al. 2008; Pasquaud et al. 2008; Vinagre et al. 2008; Kostecki et al. 2010). This is the case in the Mauguio lagoon where, despite a high phytoplankton biomass and density (Henard and Vaulot 1979), the trophic importance of the pelagic pathway for benthic communities is limited and food webs are known to be primarily based on macroalgae and sedimentary OM (Vizzini et al. 2005). Because this latter, in turn, consists largely of terrestrial POM, our results corroborate previous observations and also indicate that, when present, OM of terrestrial origin in benthos is transferred up to seabream juveniles. Terrestrial OM use by marine fishes has already been demonstrated in several coastal and estuarine ecosystems (Darnaude 2005; Martinho et al. 2009; Kostecki et al. 2010; Le Pape et al. 2013), where freshwater inputs into nursery habitats can control both the availability of suitable prey for fish juveniles (Houde 1997; Darnaude et al. 2004) and their size (Le Pape et al. 2003). Studies in extensive aquaculture in brackish ponds have shown that supplementary feeding of S. aurata juveniles with high-quality food can increase their growth by 10 to 20 % (El-Ghobashy et al. 1993; Sadek et al. 2004). Therefore, taken together, it seems reasonable to speculate that the freshwater inputs into Mauguio, which are rich in terrestrial POM, contributed significantly to the higher growth and condition of resident seabream juveniles, through effects on prey availability and nutritional quality.
Abiotic conditions such as water temperature, salinity, dissolved oxygen and perhaps pollution can also influence growth and condition of fishes (Tandler et al. 1995a; Claireaux and Lagardère 1999; Boeuf and Payan 2001; Gilliers et al. 2006). In ectotherms, warmer temperatures generally promote growth by accelerating all metabolic processes (Claireaux and Lagardère 1999), including digestion and assimilation (McCue 2006). Temperature has a very marked influence on growth of S. aurata (Tandler et al. 1989), optimal rearing temperatures for the species’ post-larvae being between 20 and 28 °C (Requena et al. 1997). However, as the four lagoons provide temperatures in this range and have overlapping values, this abiotic factor does not seem to be responsible for the differences in fish growth and condition. On the contrary, differences in salinity or dissolved oxygen levels among lagoons might have partially modulated growth in S. aurata juveniles. Indeed, although S. aurata larvae grow well over a wide range of salinities (Tandler et al. 1995), including all those found during S. aurata presence in the four lagoons investigated, the optimal salinity for their growth is around 28 (Klaoudatos and Conides 1996; Conides et al. 1997; Laiz-Carrión et al. 2005). As shown in Table 1, salinities close to 28 are most often found in Bages, the waters in Mauguio typically being less saline and more saline in Thau and Salses-Leucate. This might have contributed to the differences in growth between Bages and Thau, but other environmental factors might be involved. For example, juvenile fishes are strongly affected by hypoxia, and their growth rate in coastal nurseries can be correlated with levels of dissolved oxygen (Stierhoff et al. 2009). Among the four lagoons, Thau is the worst in terms of water oxygenation (Table 1), with a mean dissolved oxygen of 6.2 mg L−1, due to frequent values between 2 and 5 mg L−1 during the summer (IFREMER 2012). Such low oxygen concentrations can have negative effects on fish growth and survival (Diaz and Rosenberg 2008; Stierhoff et al. 2009) and may therefore have contributed to the lower growth rates in this lagoon. Thus, the highest growth and the larger lipid reserves of juveniles in Bages are likely due to a combination of optimal salinities and oxygen availability.
Altogether, the data indicate that Mauguio was the best habitat for juvenile seabream in the particular year studied. This may not, however, always be the case. Among the four lagoons, Mauguio is the most susceptible to dystrophic crises that cause localized hypoxia (IFREMER 2010). No hypoxic events (i.e. oxygen concentration below 2 mg L−1) were recorded in any of the lagoons during the summer of this study (2011), but, because hypoxia can cause extensive mortality and inhibit growth rates of fishes (Diaz and Rosenberg 2008; Stierhoff et al. 2009), such events can be expected to have negative effects on fish growth and survival. Similarly, diverse pollutions could be associated with the freshwater inputs and thus alter the nursery function of lagoons. The 15N enrichment found in Mauguio sediments and primary producers has long been considered an indicator of wastewater pollution (e.g. McClelland and Valiela 1998). The Mauguio lagoon is also polluted by pesticides and persistent organic pollutants (POPs), mainly associated with the freshwater inputs (Souchu et al. 2010; Bec et al. 2011; Brehmer et al. 2011). For demersal fish, contamination of nursery areas can cause a decline in growth and population density (Gilliers et al. 2006). Previous studies have demonstrated a negative impact of contaminants on the development and recruitment of various aquatic organisms in Mauguio (Brehmer et al. 2011). The exceptional growth rates and body condition reached by S. aurata juveniles in Mauguio could therefore well be pondered by negative effects of freshwater inputs in terrestrial POM on fish physiological status and survival. Particular attention should therefore be paid to reduce pollution and control eutrophication in Mauguio to preserve its potential as nursery site for local S. aurata populations.
Implications for S. aurata Life Cycle and Metapopulation Function in the Gulf of Lions
Growth and body condition are primary criteria for classifying nursery habitats (Gilliers et al. 2006; Vasconcelos et al. 2009), indicating that Mauguio and, to a lesser extent, Bages might be key nursery sites for S. aurata in the Gulf of Lions. For many juvenile fishes, the first winter is a critical period (Miranda and Hubbard 1994; Fullerton et al. 2000; Jolley et al. 2013), with severe conditions in the open-sea such as low temperatures, limited food resources and an elevated risk of predation. According to the ‘bigger is better hypothesis’ (Miller et al. 1988), the largest juveniles with the best body condition may have increased chances of survival over winter. They may be less susceptible to predation due to gape limitations, and better able to resist starvation and tolerate physiological extremes, allowing them to retain condition and performance for a longer period (Sogard 1997; D’Alessandro et al. 2013) and so survive until the next spring.
The finding that shallow brackish lagoons produce larger and heavier juveniles than deeper ones with higher salinity is interesting in light of the recent findings on lifetime migrations of S. aurata adults in the Gulf of Lions (Mercier et al. 2012), which suggested that most of the S. aurata adults fished offshore originated from a shallow, brackish lagoon nursery. Juveniles from lagoons like Mauguio, in better condition after their first summer, may therefore recruit proportionally more to the adult population. Such differences in the contributions of lagoons to adult stocks, if confirmed, clearly are of profound consequence for local population dynamics and stock management strategies.
Conclusions and Perspectives
This study provided clear evidence that lagoons differ in their quality as juvenile fish habitats, which may result in large differences in the contribution of nursery areas to adult stocks (Mercier et al. 2012). Our results indicate that ongoing and future modifications of river inputs to the coastal zone in the NW Mediterranean, under the combined influence of climate change and anthropogenic freshwater use (Dolbeth et al. 2008), could profoundly alter the nursery function of transitional and coastal ecosystems. If enrichment of food webs in lagoons is altered, the consequences for fisheries resources could be dramatic. It is essential to have effective sustainable management plans for these fragile ecosystems, to ensure their water quality, and so preserve their carrying capacity as nurseries of highly prized coastal fish species.
References
Able, K.W. 2005. A re-examination of fish estuarine dependence: evidence for connectivity between estuarine and ocean habitats. Estuarine, Coastal and Shelf Science 64: 5–17.
Arechavala-Lopez, P., P. Sanchez-Jerez, J.T. Bayle-Sempere, D.G. Sfakianakis, and S. Somarakis. 2011. Morphological differences between wild and farmed Mediterranean fish. Hydrobiologia 679: 217–231.
Audouin, J. 1962. La daurade de l’étang de Thau Chrysophrys Aurata (Linné). Revue des Travaux l’Institut des Pêches Maritimes 26.
Bagenal, T.B., and F.W. Tesch. 1978. Age and growth. In Methods for assessment of fish production in fresh water, 3rd ed, ed. T.B. Bagenal. Oxford: Blackwell Scientific Publication.
Bearzi, G., A. Azzellino, E. Politi, M. Costa, and M. Bastianini. 2008. Influence of seasonal forcing on habitat use by bottlenose dolphins Tursiops truncatus in the Northern Adriatic Sea. Ocean Science Journal 43: 175–182.
Bec, B., Y. Collos, P. Souchu, A. Vaquer, J. Lautier, A. Fiandrino, L. Benau, V. Orsoni, and T. Laugier. 2011. Distribution of picophytoplankton and nanophytoplankton along an anthropogenic eutrophication gradient in French Mediterranean coastal lagoons. Aquatic Microbial Ecology 63: 29–45.
Beck, M.W., K.L. Heck, K.W. Able, D.L. Childers, D.B. Eggleston, B.M. Gillanders, B. Halpern, et al. 2001. The identification, conservation, and management of estuarine and marine nurseries for fish and invertebrates. BioScience 51: 633.
Beverton, R.J.H., and T.C. Iles. 1992. Mortality rates of 0-group plaice (Platessa platessa L.), dab (Limanda limanda L.) and turbot (Scophthalmus maximus L.) in European waters. Netherlands Journal of Sea Research 29: 61–79.
Bodin, N., H. Budzinski, K. Le Ménach, and N. Tapie. 2009. ASE extraction method for simultaneous carbon and nitrogen stable isotope analysis in soft tissues of aquatic organisms. Analytica Chimica Acta 643: 54–60.
Bodinier, C., E. Sucré, L. Lecurieux-Belfond, E. Blondeau-Bidet, and G. Charmantier. 2010. Ontogeny of osmoregulation and salinity tolerance in the gilthead sea bream Sparus aurata. Comparative Biochemistry and Physiology Part A 157: 220–228.
Boesch, D.F., and R.E. Turner. 1984. Dependence of fishery species on salt marshes: the role of food and refuge. Estuaries 7: 460–468.
Boeuf, G., and P. Payan. 2001. How should salinity influence fish growth? Comparative Biochemistry and Physiology 130: 411–423.
Brehmer, P., T. Do Chi, T. Laugier, F. Galgani, F. Laloë, A.M. Darnaude, A. Fiandrino, and D. Mouillot. 2011. Field investigations and multi-indicators for shallow water lagoon management: perspective for societal benefit. Aquatic Conservation: Marine and Freshwater Ecosystems 21: 728–742.
Campana, S.E. 1992. Measurement and interpretation of the microstructure of fish otoliths. In Otolith Microstructure Examination and Analysis, eds. Stevenson, D.K., Campana, S.E.: Canadian Special Publication of Fisheries and Aquatic Sciences, 59–71.
Campana, S.E. and C.M. Jones. 1992. Analysis of otolith microstructure data. In: Stevenson, D.K., Campana, S.E. (Eds.), Otolith Microstructure Examination and Analysis. Canadian Special Publication of Fisheries and Aquatic Sciences, 73–100
Carlier, A., P. Riera, J.-M. Amouroux, J.-Y. Bodiou, M. Desmalades, and A. Grémare. 2008. Food web structure of two Mediterranean lagoons under varying degree of eutrophication. Journal of Sea Research 60: 264–275.
Carlier, A., P. Riera, J.-M. Amouroux, J.-Y. Bodiou, M. Desmalades, and A. Grémare. 2009. Spatial heterogeneity in the food web of a heavily modified Mediterranean coastal lagoon: stable isotope evidence. Aquatic Biology 5: 167–179.
Caut, S., E. Angulo, and F. Courchamp. 2009. Variation in discrimination factors (δ15 N and δ13 C): the effect of diet isotopic values and applications for diet reconstruction. Journal of Applied Ecology 46: 443–453.
Chang, C.-W., and Y. Iizuka. 2012. Estuarine use and movement patterns of seven sympatric Mugilidae fishes: The Tatu Creek estuary, central western Taiwan. Estuarine, Coastal and Shelf Science 106: 121–126.
Chaoui, L., F. Derbal, M.H. Kara, and J.P. Quignard. 2005. Alimentation et condition de la dorade Sparus aurata (Teleostei: Sparidae) dans la lagune du Mellah (Algerie Nord-Est). Cahiers de Biologie Marine 46: 221–225.
Ciotti, B.J., T.E. Targett, R.D.M. Nash, and A.J. Geffen. 2014. Growth dynamics of European plaice Pleuronectes platessa L. in nursery areas: A review. Journal of Sea Research 90: 64–82.
Claireaux, G., and J.-P. Lagardère. 1999. Influence of temperature, oxygen and salinity on the metabolism of the European sea bass. Journal of Sea Research 42: 157–168.
Conides, A., A. Parpoura, and G. Fotis. 1997. Study on the effects of salinity on the fry of the euryhaline species gilthead sea bream (Sparus aurata L. 1758). Journal of Aquaculture in the Tropics 12: 297–304.
D’Alessandro, E.K., S. Sponaugle, and R.K. Cowen. 2013. Selective mortality during the larval and juvenile stages of snappers (Lutjanidae) and great barracuda Sphyraena barracuda. Marine Ecology Progress Series 474: 227–242.
Dahlgren, C.P., G.T. Kellison, A.J. Adams, B.M. Gillanders, M.S. Kendall, C.A. Layman, J.A. Ley, I. Nagelkerken, and J.E. Serafy. 2006. Marine nurseries and effective juvenile habitats : concepts and applications. Marine Ecology Progress Series 312: 291–295.
Darnaude, A.M. 2005. Fish ecology and terrestrial carbon use in coastal areas : implications for marine fish production. Journal of Animal Ecology 74: 864–876.
Darnaude, A.M., C. Salen-Picard, and M. Harmelin-Vivien. 2004. Depth variation in terrestrial particulate organic matter exploitation by marine coastal benthic communities off the Rhone River delta (NW Mediterranean). Marine Ecology Progress Series 275: 47–57.
Daverat, F., J. Martin, R. Fablet, and C. Pécheyran. 2011. Colonisation tactics of three temperate catadromous species, eel Anguilla anguilla, mullet Liza ramada and flounder Plathychtys flesus, revealed by Bayesian multielemental otolith microchemistry approach. Ecology of Freshwater Fish 20: 42–51.
Day, J.W., W.M. Kemp, A. Yáñez-Aran, and B.C. Crump. 2012. Estuarine Ecology, 2nd Edition. Edited by Wiley. Wiley-Blackwell. New York
Deegan, L., and R.H. Garritt. 1997. Evidence for spatial variability in estuarine food webs. Marine Ecology Progress Series 147: 31–47.
Diaz, R.J., and R. Rosenberg. 2008. Spreading dead zones and consequences for marine ecosystems. Science 321: 926–929.
Dolbeth, M., F. Martinho, I. Viegas, H. Cabral, and M.A. Pardal. 2008. Estuarine production of resident and nursery fish species: Conditioning by drought events? Estuarine, Coastal and Shelf Science 78: 51–60.
Drake, P., A.M. Arias, F. Baldó, J.A. Cuesta, A. Rodríguez, A. Silva-Garcia, I. Sobrino, D. García-González, and C. Fernández-Delgado. 2002. Spatial and temporal variation of the nekton and hyperbenthos from a temperate European estuary with regulated freshwater inflow. Estuaries 25: 451–468.
El-Ghobashy, A., E.Omar and A. Nour. 1993. Effect of supplementary feeding on survival rate, growth performance and yield of four marine fish species. D. labrax, S. aurata, M. cephalus, in: Aquaculture Symposium, Technology and Investment Opportunities. Riyadh, 537–549.
Escalas, A., F. Ferraton, C. Paillon, G. Vidy, F. Carcaillet, C. Salen-Picard, F. Le Loc'h, P. Richard, and A.M. Darnaude. 2015. Spatial variations in dietary organic matter sources modulate the size and condition of fish juveniles in temperate lagoon nursery sites. Estuarine, Coastal and Shelf Science 152: 78–90.
Evans, D. 1994. The physiology of fishes. Reviews in Fish Biology and Fisheries 4: 261–262.
Feutry, P., H. Tabouret, K. Maeda, C. Pécheyran, and P. Keith. 2012. Diadromous life cycle and behavioural plasticity in freshwater and estuarine Kuhliidae species (Teleostei) revealed by otolith microchemistry. Aquatic Biology 15: 195–204.
Figueira, W.F. 2009. Connectivity or demography: defining sources and sinks in coral reef fish metapopulations. Ecological Modelling 220: 1126–1137.
Folch, J., M. Lees, and G.H. Slone Stanley. 1957. A simple method for the isolation and purification of total lipides from animal tissues. The Journal of Biological Chemistry 226: 497–509.
Forrester, G., and S. Swearer. 2002. Trace elements in otoliths indicate the use of open-coast versus bay nursery habitats by juvenile California halibut. Marine Ecology Progress Series 241: 201–213.
Fraser, A.J. 1989. Triacylglycerol content as a condition index for fish, bivalve, and crustacean larvae. Canadian Journal of Fisheries and Aquatic Sciences 46: 1868–1873.
Froese, R. and D. Pauly. 2000. FishBase 2000: concepts, design and data sources.
Fromentin, J.-M., B. Ernande, R. Fablet, and H. de Pontual. 2009. Importance and future of individual markers for the ecosystem approach to fisheries. Aquatic Living Resources 22: 395–408.
Fullerton, A.H., J.E. Garvey, R.A. Wright, and R.A. Stein. 2000. Overwinter growth and survival of largemouth bass: interactions among size, food, origin, and winter severity. Transactions of the American Fisheries Society 129: 1–12.
Gillanders, B.M., K.W. Able, J.A. Brown, D.B. Eggleston, and P.F. Sheridan. 2003. Evidence of connectivity between juvenile and adult habitats for mobile marine fauna: an important component of nurseries. Marine Ecology Progress Series 247: 281–295.
Gilliers, C., O. Le Pape, Y. Désaunay, J. Morin, D. Guérault, and R. Amara. 2006. Are growth and density quantitative indicators of essential fish habitat quality? An application to the common sole Solea solea nursery grounds. Estuarine, Coastal and Shelf Science 69: 96–106.
Guelinckx, J., J. Maes, P. Van Den Driessche, B. Geysen, F. Dehairs, and F. Ollevier. 2007. Changes in δ13C and δ15N in different tissues of juvenile sand goby Pomatoschistus minutus: a laboratory diet-switch experiment. Marine Ecology Progress Series 341: 205–215.
Håkanson, J.L. 1993. Nutritional condition and growth rate of anchovy larvae (Engraulis mordax) in the California Current: two contrasting years. Marine Biology 115: 309–316.
Harding, G.C., and A.J. Fraser. 1999. Application of the triacylglycerol/sterol condition index to the interpretation of larval lobster Homarus americanus distribution in close proximity to Georges Bank, Gulf of Maine. Marine Ecology Progress Series 186: 239–254.
Harmelin-Vivien, M., V. Loizeau, C. Mellon, B. Beker, D. Arlhac, X. Bodiguel, F. Ferraton, R. Hermand, X. Philippon, and C. Salen-Picard. 2008. Comparison of C and N stable isotope ratios between surface particulate organic matter and microphytoplankton in the Gulf of Lions (NW Mediterranean). Continental Shelf Research 28: 1911–1919.
Henard, D. and D. Vaulot. 1979. Production primaire de l’étang de l’Or. Montpellier.
Hesslein, R.H., K.A. Hallard, and P. Ramlal. 1993. Replacement of sulfur, carbon, and nitrogen in tissue of growing broad Whitefish (Coregonus nasus) in response to a change in diet traced by δ34S, δ13C, and δ15N. Canadian Journal of Fisheries and Aquatic Sciences 50: 2071–2076.
Houde, E.D. 1997. Patterns and trends in larval-stage growth and mortality of teleost fish. Journal of Fish Biology 51: 52–83.
Hsu, C.-C., C.-W. Chang, Yoshiyuki Lizuka, and W.-N. Tzeng. 2009. A growth check deposited at estuarine arrival in otoliths of juvenile Flathead Mullet (Mugil cephalus L.). Zoological Studies 48: 315–324.
Ifremer. 2010. Réseau de Suivi Lagunaire du Languedoc-Roussillon: Bilan des résultats 2009. Rapport RSL-10/2010, 321p.
Ifremer. 2011. Réseau de Suivi Lagunaire du Languedoc-Roussillon: Bilan des résultats 2010. Rapport RSL-11/2011, 275p.
Ifremer. 2012. Réseau de Suivi Lagunaire du Languedoc-Roussillon: Bilan des résultats 2011. Rapport RSL-12/2012, 277p.
Joh, M., T. Matsuda, N. Satoh, N. Tanaka, and Y. Ueda. 2011. Otolith microstructure of brown sole Pseudopleuronectes herzensteini: validation of daily ring formation and the occurrence of microstructure denoting metamorphosis. Fisheries Science 77: 773–783.
Jolley, J.C., M.A. Kaemingk, D.W. Willis, and R.S. Holland. 2013. Overwinter mortality of sympatric juvenile Bluegill and Yellow Perch in mid-temperate Sandhill lakes, Nebraska, U.S.A. The Open Fish Science Journal 6: 58–70.
Joyeux, J.-C., and A.B.. Ward. 1998. Constraints on coastal lagoon fisheries. Advances in Marine Biology 34: 73–199.
Kerambrun, E., W. Sanchez, F. Henry, and R. Amara. 2011. Are biochemical biomarker responses related to physiological performance of juvenile sea bass (Dicentrarchus labrax) and turbot (Scophthalmus maximus) caged in a polluted harbour? Comparative Biochemistry and Physiology 154: 187–195.
Kidd, K.A., H.A. Bootsma, R.H. Hesslein, D.C.G. Muir, and R.E. Hecky. 2001. Biomagnification of DDT through the benthic and pelagic food webs of Lake Malawi, East Africa: importance of trophic level and carbon Source. Environmental Science & Technology 35: 14–20.
Kjerfve, Bjorn. 1994. Coastal lagoons processes. Amsterdam: Elsevier S. Ed.
Klaoudatos, S.D., and A.J. Conides. 1996. Growth, food canversion, maintenance and long-term survival of gilthead sea bream, Sparus auratus L., juveniles after abrupt transfer to low salinity. Aquaculture Research 27: 756–774.
Kostecki, C., F. Le Loc’h, J.-M. Roussel, N. Desroy, D. Huteau, P. Riera, H. Le Bris, and O. Le Pape. 2010. Dynamics of an estuarine nursery ground: the spatio-temporal relationship between the river flow and the food web of the juvenile common sole (Solea solea, L.) as revealed by stable isotopes analysis. Journal of Sea Research 64: 54–60.
Kostecki, C., J.-M. Roussel, N. Desroy, G. Roussel, J. Lanshere, H. Le Bris, and O. Le Pape. 2012. Trophic ecology of juvenile flatfish in a coastal nursery ground: contributions of intertidal primary production and freshwater particulate organic matter. Marine Ecology Progress Series 449: 221–232.
Kraljevic, M., and J. Dulčić. 1997. Age and growth of gilt-head sea bream (Sparus aurata L.) in the Mirna Estuary, Northern Adriatic. Fisheries Research 31: 249–255.
Kwak, T.J., and J.B. Zedler. 1997. Food web analysis of southern California coastal wetlands using multiple stable isotopes. Oecologia 110: 262–277.
Laiz-Carrión, R., S. Sangiao-Alvarellos, J.M. Guzmán, M.P. Martín del Río, J.L. Soengas, and J.M. Mancera. 2005. Growth performance of gilthead sea bream Sparus aurata in different osmotic conditions: Implications for osmoregulation and energy metabolism. Aquaculture 250: 849–861.
Lasserre, G. 1976. Dynamique des populations ichtyo-logiques lagunaires - application à Sparus aurata L. Université des Sciences et Techniques du. Montpellier: Languedoc.
Layman, C., M.S. Araujo, R. Boucek, C.M. Hammersclag-Peyer, E. Harrison, Z.R. Jud, P. Matich, A.E. Rosenblatt, J.J. Vaudo, L.A. Yeager, D.M. Post, and S. Bearshop. 2012. Applying stable isotopes to examine food-web structure: an overview of analytical tools. Biological Reviews 87: 545–562.
Le Pape, O., F. Chauvet, Y. Désaunay, and D. Guérault. 2003. Relationship between interannual variations of the river plume and the extent of nursery grounds for the common sole (Solea solea, L.) in Vilaine Bay. Effects on recruitment variability. Journal of Sea Research 50: 177–185.
Le Pape, O., J. Modéran, G. Beaunée, P. Riera, D. Nicolas, N. Savoye, M. Harmelin-Vivien, et al. 2013. Sources of organic matter for flatfish juveniles in coastal and estuarine nursery grounds: A meta-analysis for the common sole (Solea solea) in contrasted systems of Western Europe. Journal of Sea Research 75: 85–95.
Leakey, C.D.B., M.J. Attrill, S. Jennings, and M.F. Fitzsimons. 2008. Stable isotopes in juvenile marine fishes and their invertebrate prey from the Thames Estuary, UK, and adjacent coastal regions. Estuarine, Coastal and Shelf Science 77: 513–522.
Logan, J., H. Haas, L. Deegan, and E. Gaines. 2006. Turnover rates of nitrogen stable isotopes in the salt marsh mummichog, Fundulus heteroclitus, following a laboratory diet switch. Oecologia 147: 391–395.
MacAvoy, S.E., S.A. Macko, and G.C. Garman. 2001. Isotopic turnover in aquatic predators: quantifying the exploitation of migratory prey. Canadian Journal of Fisheries and Aquatic Sciences 58: 923–932.
Mariani, S., A. Maccaroni, F. Massa, M. Rampacci, and L. Tancioni. 2002. Lack of consistency between the trophic interrelationships of five sparid species in two adjacent central Mediterranean coastal lagoons. Journal of Fish Biology 61: 138–147.
Martinho, F., M. Dolbeth, I. Viegas, C.M. Teixeira, H.N. Cabral, and M.A. Pardal. 2009. Environmental effects on the recruitment variability of nursery species. Estuarine, Coastal and Shelf Science 83: 460–468.
Maruyama, A., Y. Yamada, B. Rusuwa, and M. Yuma. 2001. Change in stable nitrogen isotope ratio in the muscle tissue of a migratory goby, Rhinogobius sp. in a natural setting. Canadian Journal of Fisheries and Aquatic Sciences 58: 2125–2128.
McClelland, J.W., and I. Valiela. 1998. Changes in food web structure under the influence of increased anthropogenic nitrogen inputs to estuaries. Marine Ecology Progress Series 168: 259–271.
McCue, M.D. 2006. Specific dynamic action: A century of investigation. Comparative Biochemistry and Physiology Part A 144: 381–394.
Melville, A.J., and R.M. Connolly. 2003. Spatial analysis of stable isotope data to determine primary sources of nutrition for fish. Oecologia 136: 499–507.
Mercier, L., D. Mouillot, O. Bruguier, L. Vigliola, and A.M. Darnaude. 2012. Multi-element otolith fingerprints unravel sea−lagoon lifetime migrations of gilthead sea bream Sparus aurata. Marine Ecology Progress Series 444: 175–194.
Miller, T.J., L.B. Crowder, J.A. Rice, and E.A. Marschall. 1988. Larval size and recruitment mechanisms in fishes:toward a conceptual framework. Canadian Journal of Fisheries and Aquatic Sciences 45: 1657–1670.
Miranda, L.E., and W.D. Hubbard. 1994. Length-dependent winter survival and lipid composition of age-0 largemouth bass in Bay Springs Reservoir, Mississippi. Transactions of the American Fisheries Society 123: 80–87.
Morais, S., M. Torten, O. Nixon, S. Lutzky, L.E.C. Conceição, M.T. Dinis, and W. Koven. 2006. Food intake and absorption are affected by dietary lipid level and lipid source in seabream (Sparus aurata L.) larvae. Journal of Experimental Marine Biology and Ecology 331: 51–63.
Morales-Nin, B., E. Gutiérrez, and S. Massutí. 1995. Patterns of primary growth increments in otoliths of Sparus aurata larvae in relation to water temperature and food consumption. Scientia Marina 57–64.
Mouillot, D., S. Gaillard, C. Aliaume, M. Verlaque, and T. Belsher. 2005. Ability of taxonomic diversity indices to discriminate coastal lagoon environments based on macrophyte communities. Ecological Indicators 5: 1–17.
Nicolas, D., F. Le Loc’h, Y. Désaunay, D. Hamon, A. Blanchet, and O. Le Pape. 2007. Relationships between benthic macrofauna and habitat suitability for juvenile common sole (Solea solea, L.) in the Vilaine estuary (Bay of Biscay, France) nursery ground. Estuarine, Coastal and Shelf Science 73: 639–650.
Norton, E.C., R.B. MacFarlane, and M.S. Mohr. 2001. Lipid class dynamics during development in early life stages of shortbelly rockfish and their application to condition assessment. Journal of Fish Biology 58: 1010–1024.
Panfili, J., H. De Pontual, H. Troadec, and P.J. Wrigh. 2002. Manual of Fish Sclerochronology. Brest: Ifremer-IRD Coedition.
Parnell, A.C., R. Inger, S. Bearhop, and A.L. Jackson. 2010. Source partitioning using stable isotopes: coping with too much variation. PLoS ONE 5(3): e9672. doi:10.1371/journal.pone.0009672.
Parra, G., and M. Yufera. 2000. Feeding, physiology and growth responses in first-feeding gilthead seabream (Sparus aurata L.) larvae in relation to prey density. Journal of Experimental Marine Biology and Ecology 243: 1–15.
Parrish, C.C. 1987. Separation of aquatic lipid classes by chromarod thin-layer chromatography with measurement by latroscan flame ionization detection. Canadian Journal of Fisheries and Aquatic Sciences 44: 722–731.
Pasquaud, S., P. Elie, C. Jeantet, I. Billy, P. Martinez, and M. Girardin. 2008. A preliminary investigation of the fish food web in the Gironde estuary, France, using dietary and stable isotope analyses. Estuarine, Coastal and Shelf Science 78: 267–279.
Paterson, A.W., and A.K. Whitfield. 1997. A stable carbon isotope study of the food-web in a freshwater-deprived south African estuary, with particular emphasis on the ichthyofauna. Estuarine, Coastal and Shelf Science 45: 705–715.
Pauly, D., and A. Yáñez-Arancibia. 1994. Fisheries in coastal lagoons. In Coastal lagoon processes, 377–399. Amsterdam: Elsevier Science Publishers.
Pernet, F., N. Malet, A. Pastoureaud, A. Vaquer, C. Quéré, and L. Dubroca. 2012. Marine diatoms sustain growth of bivalves in a Mediterranean lagoon. Journal of Sea Research 68: 20–32.
Pinnegar, J.K., and N.V.C. Polunin. 1999. Differential fractionation of δ13C and δ15N among fish tissues: implications for the study of trophic interactions. Functional Ecology 13: 225–231.
Quignard, J.P., C. Mazoyer, R. Vianet, R. Man Wai, and K. Benharrat. 1983. Un exemple d’exploitation lagunaire en Languedoc : l’étang de l’Or (Mauguio) - Pêche et production halieutique. Science et Pêche 336: 3–23.
Quignard, J.P., R. Man Wai, and R. Vianet. 1984. Les poissons de l’étang de Mauguio (Hérault, France) inventaire, structure du peuplement, croissance et polymorphisme des tailles. Vie Milieu 34: 173–183.
Requena, A., J. Fernandez-Borras, and J. Planas. 1997. The effects of a temperature rise on oxygen consumption and energy budget in gilthead sea bream. Aquaculture International 5: 415–426.
Rey, J., L. Fernández-Peralta, A. Esteban, R. García-Cancela, F. Salmerón, M.Á. Puerto, and C. Piñeiro. 2012. Does otolith macrostructure record environmental or biological events? The case of black hake (Merluccius polli and Merluccius senegalensis). Fisheries Research 113: 159–172.
Sadek, S., M.F. Osman, and M.A. Mansour. 2004. Growth, survival and feed conversion rates of sea bream (Sparus aurata) cultured in earthen brackish water ponds fed different feed types. Aquaculture International 12: 409–421.
Sakano, H., E. Fujiwara, S. Nohara, and H. Ueda. 2005. Estimation of nitrogen stable isotope turnover rate of Oncorhynchus nerka. Environmental Biology of Fishes 72: 13–18.
Salen-Picard, C., A.M. Darnaude, D. Arlhac, and M. Harmelin-Vivien. 2002. Fluctuations of macrobenthic populations: a link between climate-driven river run-off and sole fishery yields in the Gulf of Lions. Oecologia 133: 380–388.
Sargent, J.R., R.J. Henderson, and D.R. Tocher. 2002. The lipids. In Fish nutrition, ed. J. Halver and H. RW, 153–218. London: London Academic Press.
Sogard, S.M. 1997. Size-selective mortality in the juvenile stage of teleost fishes: a review. Bulletin of Marine Science 60: 1129–1157.
Souchu, P., B. Bec, V.H. Smith, T. Laugier, A. Fiandrino, L. Benau, V. Orsoni, Y. Collos, and A. Vaquer. 2010. Patterns in nutrient limitation and chlorophyll a along an anthropogenic eutrophication gradient in French Mediterranean coastal lagoons. Canadian Journal of Fisheries and Aquatic Sciences 67: 743–753.
Stierhoff, K.L., T.E. Targett, and J.H. Power. 2009. Hypoxia-induced growth limitation of juvenile fishes in an estuarine nursery: assessment of small-scale temporal dynamics using RNA:DNA. Canadian Journal of Fisheries and Aquatic Sciences 66: 1033–1047.
Suzuki, K.W., A. Kasai, K. Nakayama, and M. Tanaka. 2005. Differential isotopic enrichment and half-life among tissues in Japanese temperate bass (Lateolabrax japonicus) juveniles: implications for analyzing migration. Canadian Journal of Fisheries and Aquatic Sciences 62: 671–678.
Tancioni, L., S. Mariani, A. Maccaroni, A. Mariani, F. Massa, M. Scardi, and S. Cataudella. 2003. Locality-specific variation in the feeding of Sparus aurata L.: evidence from two Mediterranean lagoon systems. Estuarine, Coastal and Shelf Science 57: 469–474.
Tandler, A., M. Har’el, M. Wilks, A. Levinson, L. Brickell, S. Christie, E. Avital, and Y. Barr. 1989. Effect of environmental temperature on survival, growth and population structure in the mass rearing of the gilthead seabream, Sparus aurata. Aquaculture 78: 277–284.
Tandler, A., F.A. Anav, and I. Choshniak. 1995. The effect of salinity on growth rate, survival and swimbladder inflation in gilthead seabream, Sparus aurata, larvae. Aquaculture 135: 343–353.
Taylor, A.H., A.J. Watson, and J.E. Robertson. 1992. The influence of the spring phytoplankton bloom on carbon dioxide and oxygen concentrations in the surface waters of the northeast Atlantic during 1989. Deep-Sea Research Part A. Oceanographic Research Papers 39: 137–152.
Tomás, J., and J. Panfili. 2000. Otolith microstructure examination and growth patterns of Vinciguerria nimbaria (Photichthyidae) in the tropical Atlantic Ocean. Fisheries Research 46: 131–145.
Van der Veer, H.W., L. Pihl, and M.J.N. Bergman. 1990. Recruitment mechanisms in North Sea plaice Pleuronectes platessa. Marine Ecology Progress Series 64: 1–12.
Vasconcelos, R.P., P. Reis-Santos, V. Fonseca, A. Maia, M. Ruano, S. França, C. Vinagre, M.J. Costa, and H. Cabral. 2007. Assessing anthropogenic pressures on estuarine fish nurseries along the Portuguese coast: a multi-metric index and conceptual approach. The Science of the Total Environment 374: 199–215.
Vasconcelos, R.P., P. Reis-Santos, V. Fonseca, M. Ruano, S. Tanner, M.J. Costa, and H.N. Cabral. 2009. Juvenile fish condition in estuarine nurseries along the Portuguese coast. Estuarine, Coastal and Shelf Science 82: 128–138.
Vasconcelos, R.P., P. Reis-Santos, A. Maia, V. Fonseca, S. França, N. Wouters, M.J. Costa, and H.N. Cabral. 2010. Nursery use patterns of commercially important marine fish species in estuarine systems along the Portuguese coast. Estuarine, Coastal and Shelf Science 86: 613–624.
Vasconcelos, R.P., P. Reis-Santos, M.J. Costa, and H.N. Cabral. 2011. Connectivity between estuaries and marine environment: Integrating metrics to assess estuarine nursery function. Ecological Indicators 11: 1123–1133.
Vinagre, C., J. Salgado, M.J. Costa, and H.N. Cabral. 2008. Nursery fidelity, food web interactions and primary sources of nutrition of the juveniles of Solea solea and S. senegalensis in the Tagus estuary (Portugal): a stable isotope approach. Estuarine, Coastal and Shelf Science 76: 255–264.
Vizzini, S., and A. Mazzola. 2003. Seasonal variations in the stable carbon and nitrogen isotope ratios (13C/12C and 15N/14N) of primary producers and consumers in a western Mediterranean coastal lagoon. Marine Biology 142: 1009–1018.
Vizzini, S., B. Savona, T.D. Chi, and A. Mazzola. 2005. Spatial variability of stable carbon and nitrogen isotope ratios in a Mediterranean coastal lagoon. Hydrobiologia 550: 73–82.
Vogt, A., T.R. Gormley, G. Downey, and J. Somers. 2002. A comparison of selected rapid methods for fat measurement in fresh herring (Clupea harengus). Journal of Food Composition and Analysis 15: 205–215.
Acknowledgments
This research was funded by the TOTAL Foundation (project LAGUNEX) and the CNRS (Project EC2CO METASPAR). The authors are grateful to local fishermen for fish collection, to Khady Diouf, Jacques Panfili and Charlotte Sirot for guidance in otolithometry, to Ève-Julie Pernet for assistance in the lipid analyses, Béatrice Bec for the very helpful comments and Valérie Derolez, Annie Fiandrino for providing environmental data from their lagoon monitoring programs.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Marco Bartoli
Rights and permissions
About this article
Cite this article
Isnard, E., Tournois, J., McKenzie, D.J. et al. Getting a Good Start in Life? A Comparative Analysis of the Quality of Lagoons as Juvenile Habitats for the Gilthead Seabream Sparus aurata in the Gulf of Lions. Estuaries and Coasts 38, 1937–1950 (2015). https://doi.org/10.1007/s12237-014-9939-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-014-9939-6