Abstract
To assess the potential for habitat isolation effects on estuarine nekton, we used two species with different dispersal abilities and life history strategies, mummichog (Fundulus heteroclitus) and pinfish (Lagodon rhomboides) to examine: (1) distribution trends among estuarine shallow-water flat and various intertidal salt marsh habitats and (2) the influence of salt marsh habitat size and isolation. Collections were conducted using baited minnow traps set within nonisolated interior marshes (interior), nonisolated fringing marshes (nonisolated), isolated island marshes (isolated), and shallow-water flat habitats (flat) that were adjacent to isolated and nonisolated marshes. Size range of individuals collected included juvenile and adult F. heteroclitus (20–82-mm standard length) and L. rhomboides (22–151-mm standard length). During high tide, F. heteroclitus exclusively used marsh habitats, particularly high marsh, whereas L. rhomboides used marshes and flats. F. heteroclitus abundance followed an interior > nonisolated > isolated pattern. L. rhomboides abundance patterns were less consistent but followed a nonisolated > isolated > interior pattern. A size-dependent water depth relationship was observed for both species and suggests size class partitioning of marsh and flat habitats during high tide. Minimum water depth (~31 cm) restricted L. rhomboides populations in marshes, while maximum water depth (~69 cm) restricted F. heteroclitus population use of marshes and movement between marsh habitats. Disparities in F. heteroclitus young of year contribution between isolated compared to nonisolated and interior marsh types suggests isolated marshes acted as population sinks and were dependent on adult emigrants. Resident and transient salt marsh nekton species utilize estuarine habitats in different ways and these fundamental differences can translate into how estuarine landscape might affect nekton.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Habitat size and location should have significant affects on population patterns for dispersal-limited species based upon predicted consequences of colonization and recruitment patterns outlined by island biogeography and metapopulation theories (MacArthur and Wilson 1967; Simberloff and Wilson 1969; Rieman and McIntyre 1995; Harrison and Taylor 1997). Predictively, habitat size and location might also affect the functional role a habitat provides (Pulliam 1988; Dunning et al. 1992; Roberts and Rahel 2008). Faunal populations occurring in isolated habitat patches that lay beyond typical dispersal ranges should be particularly susceptible to extinction events, while populations occurring in habitats with high connectivity should be less susceptible to extinctions (Fahrig and Merriam 1985) or recover more quickly from local extirpation. Such habitat size and connectivity patterns have been noted for bull trout (Salvelinus confluentus) in fresh water streams, where movement among suitable habitats was reduced due to isolation by expanses of unsuitable habitat, and population resilience was directly related to habitat size (Rieman and McIntyre 1995). After extinction events, the ability of a species to recolonize infrequently connected habitats might be a prime factor in determining species assemblage structures, as has been observed for fish assemblages within infrequently flood-connected estuarine ponds (Sheaves and Johnston 2008). Similarly, boundary delineation of distinct source and sink larval supply habitat, as has been identified in sections of the Great Barrier Reef for reef fish, is vitally important to improve effectiveness of population maintenance and conservation efforts (Bode et al. 2006). Dispersal boundaries and habitat attributes might similarly influence populations of common estuarine species known for habitat specificity. Salt-marsh-dependent residents might be significantly affected by dispersal limitation, particularly the mummichog (Fundulus heteroclitus) (Abrams 1985; Sogard and Able 1994; Teo and Able 2003). Naturally or anthropogenically derived isolation of resident salt marsh faunal populations by surrounding expanses of open-water habitat can create the potential for restricting faunal immigration and emigration among source and sink habitats due to predation vulnerability (Heck and Thoman 1981). With increases in habitat fragmentation, reduction in essential habitat patch encounter rates within the inhospitable matrix habitat could also reduce immigration success (Dunning et al. 1995). However, populations of more transient species, such as the pinfish (Lagodon rhomboides) (Hettler 1989), which utilize not only salt marsh but multiple shallow-water estuarine habitats (Muncy 1984), might be little affected by such habitat isolation events.
Empirical evidence suggests that habitat patch size can directly affect a resident species’ population size (Andren 1994; Hanski 1994; Hokit and Branch 2003) and density (Eggleston et al. 1998; Hokit and Branch 2003; Long and Burke 2007). Further, resident species might be affected by minimal habitat size thresholds (Harrison et al. 1988; Rieman and McIntyre 1995; With and Crist 1995). Should these patterns hold in estuarine environments, species resident to habitats in which they are susceptible to landscape level or temporal isolation events, particularly salt marshes, could be adversely affected while little or no effect might be noted for transient species. Adverse effects related to isolation and habitat size might be particularly evident for created or restored salt marsh habitats, which can be initially simple in terms of habitat complexity and faunal diversity (Minello and Zimmerman 1992; Sacco et al. 1994; Levin et al. 1996).
To better understand estuarine habitat landscape affects on nekton distribution and utilization trends, two common codominant species within coastal salt marshes of the USA South Atlantic region, F. heteroclitus and L. rhomboides (Hettler 1989; Meyer 2006), were studied as representatives of differing life history strategies. F. heteroclitus is a benthic-oriented estuarine species that ranges from the Gulf of Saint Lawrence to northeastern Florida (Abrams 1985) and is common to intertidal salt marsh habitats (Kneib 1984, 1986; Abrams 1985; Halpin 1997, 2000; Rozas and Zimmerman 2000). F. heteroclitus spawn from midspring through midsummer in salt marsh habitats during high spring tides (Taylor et al. 1979). A restricted range of movement during summer time periods (<400 m; Lotrich 1975; Teo and Able 2003) and high site fidelity (Teo and Able 2003) has been observed for F. heteroclitus. However, a wider range (almost 2,000 m) of upstream fall migratory movement for a population within a marsh creek has been observed (Fritz et al. 1975). Adult and juvenile F. heteroclitus are typically known to move with the tide onto the marsh surface during flood tides and into shallow sublittoral habitats during ebb tides (Rozas and Odum 1987; Ruiz et al. 1993). F. heteroclitus larvae have typically been observed to be restricted to marsh habitats, in particular the intertidal marsh zone, and utilize shallow marsh pools as refuges (Taylor et al. 1979; Able and Hagan 2000). These characteristics make F. heteroclitus a good model species representing restricted habitat preferences and limited colonization potential. L. rhomboides occurs within benthic mesohaline estuarine and marine habitats in temperate (Hettler 1989) and subtropical (Paperno et al. 2001) regions of the USA South Atlantic coast and ranges from Massachusetts and Bermuda through the Gulf of Mexico (Hoese et al. 1977). L. rhomboides is a pelagic ocean spawner (Muncy 1984), with larvae moving into coastal estuaries during winter months and peak recruitment occurring from January through March (Warlen and Burke 1990). Once within estuaries, L. rhomboides larvae and subsequent life history stages utilize various benthic habitats, including seagrass (Meyer et al. 1999; Paperno et al. 2001; King and Sheridan 2008), salt marsh (Hettler 1989; Meyer 2006), and oyster reef (Wenner et al. 1996) and are not constrained by the lack of any one particular habitat type. L. rhomboides can attain 250-mm standard length (SL) (Hoese et al. 1977) compared to ~100-mm SL for F. heteroclitus (Kneib and Stiven 1978).
This study compares habitat use by juvenile and adult F. heteroclitus (20–82-mm SL) and L. rhomboides (22–151-mm SL) of existing natural isolated island fringing salt marshes (isolated), nonisolated fringing salt marshes (nonisolated), nonisolated interior salt marshes (interior), and shallow-water flat habitats (flat) adjacent to isolated and nonisolated salt marshes. The objectives of this study were to: (1) examine the distribution of F. heteroclitus and L. rhomboides populations within coastal estuarine shallow-water flat and intertidal salt marsh habitats and (2) examine the influence of salt marsh habitat size and isolation on F. heteroclitus and L. rhomboides populations.
Materials and Methods
Sites
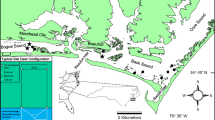
Six isolated and nonisolated salt marsh site pairs, located within Bogue, Back, and Core Sounds, North Carolina, USA, were sampled (Fig. 1, Table 1). A pair consisted of isolated and nonisolated sites within ~1.0 km of one another. Isolated salt marsh sites ranged from ~400 to 10,000 m−2 in size and were separated by >400 m of open water from the nearest salt marsh. Nonisolated salt marsh sites similarly bordered open-water habitats but were contiguous to other salt marsh habitats and were a minimum of 76,000 m−2 in size. Both isolated and nonisolated sites lacked dendritic channel development and the distance from the lower marsh edge to the upland fringe (nonisolated) or island center (isolated) did not exceed 24 m. All isolated and nonisolated sites were adjacent to flats that contained a mosaic of unvegetated bottom and submerged aquatic vegetation, including shoal grass (Halodule wrightii), widgeon grass (Ruppia maritima), and eel grass (Zostera marina). In addition to these paired sites, three interior salt marsh sites, located adjacent to three of the nonisolated sites, one each in Bogue, Back, and Core Sounds, were similarly sampled. Interior salt marsh sites were located near the headwaters of upland enclosed salt marsh creeks (Fig. 1). All sites contained low salt marsh areas (salt marsh areas typically flooded during diurnal neap tides), and high salt marsh areas (salt marsh habitat flooded only during spring or astronomically high tides and observed to contain high-marsh vegetation species) were common to all but two isolated sites.
Distribution Pattern Assessment
From November 2003 through September 2004, high- and ebb-tide distribution patterns for F. heteroclitus and L. rhomboides were examined through bimonthly minnow trap collections (Halpin 1997, 2000; Kneib and Craig 2001). Baited minnow traps were preferred over unbaited because of their higher catch attraction potential (Reebs et al. 1995) and retention rates (Whitelaw et al. 1991). Minnow traps were 80 cm in length and 22.5 cm in diameter, constructed of 0.5-cm bar mesh, and had conical capture ends that were positioned inward with 6 cm long by 3 cm wide capture openings (Halpin 1997, 2000; Kneib and Craig 2001). Minnow trap mesh size was capable of capturing and retaining a wide range of size classes from young of year (YOY) for both F. heteroclitus (down to 20-mm standard length) and L. rhomboides (down to 22-mm standard length) to larger mature individuals for each species.
During high tide at each isolated and nonisolated marsh, minnow traps were set at the high salt marsh (high marsh) (or the highest elevation point for the two isolated sites that contained no high marsh), 2 m inside the marsh edge (−2 m), at the salt marsh edge (0 m), and 5, 25, and 100 m seaward of the salt marsh edge on the flat (Fig. 2). Minnow traps were also placed at the midpoint between each isolated and nonisolated salt marsh pair. At interior salt marshes, minnow traps were set at the high marsh, −2, 0 m, and within a deep area of the salt marsh creek (creek) that fed interior salt marsh sites (Fig. 2). Minnow traps were set to maximize distance between sample locations (at least nine linear meters) to reduce potential capture interference. During high-tide collections, minnow traps were sufficiently baited (with 210 g of dry dog food) so that bait would not be exhausted during a set. Minnow traps during high-tide collections were only allowed to fish for a 1.0-h maximum duration, based on cautions from Kneib and Craig (2001) on long-duration retention estimations using unbaited minnow traps. All high-tide minnow trap collections were fished within a 3-h time window, 1.5 h before to 1.5 h after high tide. Relative abundance was examined based on catch per hour (CPH) fished for each minnow trap (Kneib and Craig 2001).
Ebb-tide distributions for F. heteroclitus and L. rhomboides were sampled for locations that would not be exposed during typical low tides (Halpin 1997, 2000). These locations included 25, 100 m, and midpoint locations for isolated and nonisolated sites and creek locations for interior sites. Ebb-tide distribution assessments for F. heteroclitus and L. rhomboides began with set during tidal ebb and ended with collection during the following tidal flood (~10 h later). Because minnow traps for ebb-tide distribution were fished >1 h, comparisons involved only presence (1) and absence (0) information, not CPH, due to increased escape potential associated with prolonged soak times (Whitelaw et al. 1991; Kneib and Craig 2001). During ebb-tide distribution assessments, each minnow trap was baited with 315 g of dry dog food to ensure that the bait supply was not exhausted prior to collections and to improve catch retention (Whitelaw et al. 1991).
For each minnow trap, nekton were identified to species, measured, and enumerated. If numerically abundant (>60 individuals), a randomly selected subsample of at least 30 individuals for each fish species was measured (SL). Individuals collected were released live back at the collection point.
Physical Parameters
Salt marsh vertical range was measured at the sediment base of the vegetation for each site using a laser level and stadia rod with a detector sensor (Laser MarkFootnote 1 LM 500 series, model 4910-20671; accuracy = ±5 mm at 310 m; Meyer et al. 1997). The vertical distance between the lowest elevation occurrence of salt marsh vegetation and the highest point measured at a site was considered to be the vertical range for a site.
Salt marsh site areas were estimated using a submeter (Trimble (see footnote 1) model TSC1 PN 29673-50) global positioning system (GPS). For each isolated site, total salt marsh, low salt marsh, and high salt marsh areas were delineated. The occurrence of high-marsh floral species, including glass wort (Salicornia spp.) (Nixon 1982), salt meadow hay (Spartina patens), and salt grass (Distichlis spicata) (Nixon 1982; Bertness 1991), was used to delineate high from low salt marsh habitats. Due to the relatively large size and interconnectedness of the nonisolated salt marshes, nonisolated salt marsh habitat size is presented as minimal values based on the smallest site sampled, and interior sites were included within nonisolated estimates. Distances from isolated sites to the nearest salt marsh were measured using GPS while distances for nonisolated and interior sites were not assessed due to their connection with adjacent salt marsh areas.
Environmental Parameters
High-tide salinity and water temperature were measured at each salt marsh site during each collection date. Additionally, for each salt marsh site, minnow trap set and retrieval times were recorded, as were water depths for each location during set and retrieval using the water surface as a level (Meyer 1994).
Statistical Analysis
Comparisons of F. heteroclitus and L. rhomboides high-tide CPH among the different salt marsh types for each minnow trap position and between minnow trap positions within a salt marsh type utilized the Kruskal–Wallis test (Sokal and Rohlf 1981). The Kruskal–Wallis test was also used to compare ebb-tide minnow trap collections based on time of year.
Linear regression analysis (regression analysis) examined F. heteroclitus and L. rhomboides CPH compared to water depth during each collection period, for the year as a whole per salt marsh type and for all salt marsh types combined. Regression analysis also tested mean F. heteroclitus and L. rhomboides size per minnow trap versus water depth using combined collections from the interior and nonisolated habitats. These two “expansive” salt marsh types were combined in this size–water depth regression analysis based on similarities of habitat scale and the nonisolated status for both habitat types with regard to their landscape level position to other adjacent salt marsh habitats (Fig. 1). A minimum criterion of three individuals for a species per minnow trap was necessary for inclusion in size–water depth analysis. Regression analysis data were tested for normality using the Shapiro–Wilk test (Shapiro and Wilk 1965; Sen et al. 2003). If data were not normal, they were ln(x + 1)-transformed and again tested to assure data conformity.
Regression analysis was also used to estimate maximum or minimum critical water depths (based on the y-intercept) at which population abundances of F. heteroclitus and Lagodon rhomboides would approach zero (critical water depth). While this application of regression analysis reverses the dependent and independent variables (a violation of regression analysis), such data manipulations were only applied to obtain y-intercepts for water depth, not to examine a relationship. Critical water depth analyses combined data for all salt marsh types from November 2003 and May, July, and September 2004. January and March 2004 data were excluded from these analyses due to temporal scarcity of both species. Data were analyzed using both all minnow trap locations (including flat locations) and locations only within salt marsh habitats. y-intercepts were estimated only for data combinations observed to have had significant regressions of CPH versus water depth.
Mean standard length of F. heteroclitus and L. rhomboides from high-tide collections during each collection period was compared among both locations within a salt marsh type and among isolated, nonisolated, and interior salt marsh types using the Kruskal–Wallis test (Sokal and Rohlf 1981). The capture of each individual was considered to be an independent event and n was considered to be the total number of individuals collected (Kneib and Wagner 1994; Kneib and Craig 2001). For these size comparisons, fish collected at the 0- and −2-m locations were pooled and considered to be low salt marsh due to the elevation similarity of habitat type fished and to improve replication level.
The Kruskal–Wallis test was also used to compare salt marsh physical parameter averages measured for each site and sample location. This test was also used to examine differences in environmental parameters between salt marsh types, including salinity, water temperature, and average collection water depth per distance location for the year as a whole. For all statistical analyses, minimal significance level was p = 0.05.
Results
Physical and Environmental Parameters
The amount of total, low, and high salt marsh areas significantly differed among nonisolated and isolated salt marsh types (Table 1). Approximately 600-m separated isolated salt marshes from other salt marshes while nonisolated and interior salt marsh types were contiguous to other expansive salt marsh areas (Table 1). High-tide salinities and temperatures measured did not significantly differ among marsh types (Table 1). Measured minnow trap water depths for comparable sampling locations showed a pattern of shallower depths within the interior compared to both nonisolated and isolated, and nonisolated compared to isolated salt marsh types. Further, low salt marsh habitat (0- and −2-m locations) of the interior was significantly shallower (26.5 cm) than that of nonisolated (38.8 cm) and isolated salt marsh types (43.2 cm) (Table 1).
Distribution Pattern Assessment
While low catch during January 2004 and March 2004 made distribution patterns difficult to assess and are not included in data presentations that follow, catches during November 2003 and May, July, and September 2004 indicated distribution differences. Consistent distribution patterns for both F. heteroclitus and L. rhomboides were evident among the different salt marsh types. For F. heteroclitus, location-based CPH typically followed an interior > nonisolated > isolated pattern (Fig. 3). For L. rhomboides, location-based CPH typically followed a nonisolated > isolated > interior pattern (Fig. 4).
Catch per hour abundance of F. heteroclitus for interior (INT), nonisolated (NON), and isolated (ISO) salt marshes based on shallow-water flat and marsh sample locations. Location mean comparisons between marsh types, for each month, that are significantly different (p ≤ 0.05) from one another are indicated by a different symbol type. Low catch during January and March 2004 collections made comparisons unsound and are not shown. High marsh is indicated as HM and N indicates that no samples were collected for that marsh type at specified locations. One standard error is indicated by the error bars
Catch per hour abundance of L. rhomboides for interior (INT), nonisolated (NON), and isolated (ISO) salt marshes based on shallow-water flat and marsh sample locations. Location mean comparisons between marsh types, for each month, that are significantly different (p ≤ 0.05) from one another are indicated by a different symbol type. Low catch during January and March 2004 collections made comparisons unsound and are not shown. High marsh is indicated as HM and N indicates that no samples were collected for that marsh type at specified locations. One standard error is indicated by the error bars
During high-tide collections, F. heteroclitus were exclusively collected within the salt marsh habitat, including high marsh, −2 and 0 m for isolated, nonisolated, and interior salt marsh types, and within the creek of interior salt marsh (Fig. 5). Among the salt marsh locations, F. heteroclitus consistently followed a high marsh > −2 m > 0 m catch pattern for the nonisolated and isolated salt marsh types (Fig. 5). Within the interior marsh type, CPH patterns changed over time. Only during September 2004 was a significant difference for CPH observed among high-marsh and both −2- and 0-m interior salt marsh locations, which was also the only time period in which the CPH pattern for interior salt marsh was similar to those observed for both nonisolated and isolated salt marsh types (Fig. 5). Few L. rhomboides were collected from November 2003 through March 2004, reducing potential to assess distribution trends during those time periods. High CPH occurred during July and September 2004, with consistent location distribution trends observed for both nonisolated and isolated salt marsh locations (Fig. 6). Generally, low salt marsh areas and intermediate distance flat locations had the highest CPH of L. rhomboides, including 0- and −2-m locations for salt marsh habitats and 100- and 25-m locations for flat habitats. For nonisolated and isolated salt marsh sites, midpoint, 5-m, and high-marsh locations tended to have lower L. rhomboides CPH compared to other locations (Fig. 6). For interior salt marshes, though the high-marsh location consistently had lower comparative L. rhomboides CPH than other locations, no significant differences in catch based on location were apparent during any collection period.
Catch per hour abundance comparisons of F. heteroclitus by salt marsh and shallow-water flat sample location, for each marsh type and month. Sample location comparisons for each marsh type that are significantly different (p ≤ 0.05) from one another are indicated by a different symbol type. Low catch during January and March 2004 collections made comparisons unsound and are not shown. High marsh is indicated as HM; midpoint is indicated by MP and creek is indicated by MC. N indicates that no samples were collected for that marsh type at specified locations. One standard error is indicated by the error bars
Catch per hour abundance comparisons of L. rhomboides by salt marsh and shallow-water flat sample location, for each marsh type and month. Sample location comparisons for each marsh type that are significantly different (p ≤ 0.05) from one another are indicated by a different symbol type. Low catch during January and March 2004 collections made comparisons unsound and are not shown. High marsh is indicated as HM; midpoint is indicated by MP and creek is indicated by MC. N indicates that no samples were collected for that marsh type at specified locations. One standard error is indicated by the error bars
Water Depth Effects
When regression analysis included all sampling distance locations, significant negative linear regressions were observed for F. heteroclitus CPH versus water depth for all individual salt marsh types and for all salt marsh types combined. Similar negative linear regressions were observed for L. rhomboides CPH versus water depth using all sampling distance locations for nonisolated, isolated, and all salt marsh types combined. Within interior salt marsh types, an opposite significant relation was observed with increased L. rhomboides CPH versus increasing water depth (November 2003) (Table 2).
Regression analysis that included only within-marsh sample locations comparing F. heteroclitus CPH versus water depth revealed significant negative linear regressions for individual salt marsh types and for all salt marsh types combined. However, significant positive relationships were observed between L. rhomboides CPH versus increasing water depth for nonisolated, isolated, and for all salt marsh types combined and for F. heteroclitus CPH versus increasing water depth for interior salt marshes (May 2004) (Table 2).
Regression analysis including all salt marsh types combined for the year revealed significant relationships for water depth versus F. heteroclitus CPH, with maximum critical water depths that ranged from 69.1 cm, for all minnow trap sample locations (Fig. 7a), to 36.4 cm, when only within salt marsh locations were used in the regression analysis (Fig. 7c). Based on regression analysis including all salt marsh types and sample locations combined for the year, no significant relationship for water depth versus L. rhomboides CPH was observed to indicate a possible maximum critical water depth (Fig. 7b). However, regression analysis using only sample locations within salt marshes for all salt marsh types combined for the year produced a significant regression showing a trend opposite that for F. heteroclitus, with L. rhomboides CPH positively influenced by increasing water depth and approaching zero at an estimated critical minimum water depth of 30.9 cm (Fig. 7d).
Linear regression analyses, for all salt marsh types combined, to determine critical water depths based on water depth versus minnow trap catch per hour for F. heteroclitus and L. rhomboides. Critical water depths at which the fish populations could be predicted to reach zero were estimated for all placement locations and those only within salt marsh. Combined data include collections from November 2003 and May, July, and September 2004. Data from January and March 2004 collections were excluded from these analyses due to F. heteroclitus and L. rhomboides scarcity during those months
Size Distribution
Size differences were evident for both F. heteroclitus and L. rhomboides between salt marsh types based on comparable sample locations (Figs. 8 and 9). Larger fish mean standard length for both F. heteroclitus and L. rhomboides per sample location generally followed an isolated > nonisolated > interior trend (Figs. 8 and 9). The lack of YOY contribution to F. heteroclitus populations at isolated versus nonisolated and interior salt marsh types during the summer (July and September 2004) was evidenced by significantly larger mean standard lengths of F. heteroclitus within high-marsh locations at isolated compared to nonisolated and interior salt marsh types (Fig. 8).
Mean standard length of F. heteroclitus for interior (INT), nonisolated (NON), and isolated (ISO) salt marshes based on shallow-water flat and marsh sample locations. Location mean comparisons between marsh types, for each month, that are significantly different (p ≤ 0.05) from one another are indicated by a different symbol type. Low catch during January and March 2004 collections made comparisons unsound and are not shown. High marsh is indicated as HM; −2- and 0-m sample locations were combined in these analyses and represented as low marsh (LM). N indicates that no samples were collected for that marsh type at specified locations. One standard error is indicated by the error bars
Mean standard length of Lagodon rhomboides for interior (INT), nonisolated (NON), and isolated (ISO) salt marshes based on shallow-water flat and marsh sample locations. Location mean comparisons between marsh types, for each month, that are significantly different (p ≤ 0.05) from one another are indicated by a different symbol type. Low catch during January and March 2004 collections made comparisons unsound and are not shown. High marsh is indicated as HM; −2- and 0-m sample locations were combined in these analyses and represented as low marsh (LM). N indicates that no samples were collected for that marsh type at specified locations. One standard error is indicated by the error bars
Regression analysis of F. heteroclitus and L. rhomboides mean standard length relative to water depth used the combined catch of nonisolated and interior (expansive nonisolated salt marshes). Scarcity of F. heteroclitus at the isolated salt marshes precluded inclusion of data collected from these sites. Significant positive regressions relative to fish mean standard length and water depth were observed for F. heteroclitus during the four collection periods (November 2003 and May, July, and September 2004) when individuals of both species were abundant and during two of the four collection periods (July and September 2004) for L. rhomboides (Figs. 10a–d).
Linear regression analyses for “expansive” nonisolated salt marshes (nonisolated and interior salt marshes combined) comparing fish mean standard length to average minnow trap water depth for all placement locations during the four collection time periods (November 2003 and May, July, and September 2004) when F. heteroclitus (upper left equations) and L. rhomboides (lower right equations) were abundant. Regression slope lines are included for significant (p ≤ 0.05) relationships only
Ebb-Tide Distribution
Comparisons examining F. heteroclitus time-of-year occurrence among sample locations during ebb tide revealed higher occurrence at nonisolated 25- and 100-m locations during November 2003 and March 2004 than during January, May, July, and September 2004. Significantly higher ebb-tide occurrences of F. heteroclitus at the nonisolated sites were observed at the 25-m location during November 2003 and March 2004 compared to both May and September 2004. For L. rhomboides, a significantly higher occurrence frequency was apparent at 25- and 100-m locations for both nonisolated and isolated sites during November 2003 and May, July, and September 2004 compared to January and March 2004 (Table 3). While F. heteroclitus ebb-tide occurrence at the interior creek did not show significant differences between sample time periods, L. rhomboides had higher ebb-tide occurrence during the November 2003 and May, July, and September 2004 compared to both January and March 2004 time periods (significantly so compared to September 2004 time period) (Table 3).
Discussion
F. heteroclitus exclusively utilized salt marsh habitats during high tide and not adjacent shallow-water flats. This specificity demonstrates the dependence of F. heteroclitus on salt marsh habitats. Even less optimal salt marsh habitat types, such as those encompassed by isolated island marshes, can act as discrete oases for F. heteroclitus in a matrix of less suitable shallow-water habitat just as coral patches in reef lagoons do for cryptic fishes (Alevizon et al. 1985), and seagrasses do for fishes and shrimps (Fonseca et al. 1993). While the dependence of F. heteroclitus on intertidal salt marsh has been observed by numerous investigators (Able and Castanga 1975; Kneib and Stiven 1978; Kneib 1984; McIvor and Odum 1986; Hettler 1989; Halpin 1997), the specificity of this salt marsh habitat dependence, in the presence of other habitats, had not been tested.
Nonisolated and isolated salt marshes consistently had higher F. heteroclitus abundance within high compared to low salt marsh zones, which was opposite the pattern observed by Kneib and Wagner (1994) within a Georgia, USA, salt marsh creek complex, representative of our interior salt marshes. While the F. heteroclitus summer distribution pattern we observed for the interior followed that noted by Kneib and Wagner (1994) during their May–August collections (higher F. heteroclitus utilization within lower salt marsh component relative to the high salt marsh), we observed a distribution shift during the late summer (outside of the Kneib and Wagner (1994) study temporal window), with F. heteroclitus abundance higher in the high salt marsh compared to the low salt marsh. The late summer time period coincided with significant YOY contribution to the sampled population. This suggests that F. heteroclitus not only shift habitat use based on physical setting of the salt marsh habitat but that predominant utilization patterns also shift due to recruitment-based temporal factors.
Similar to Hettler (1989), we noted that F. heteroclitus abundance was lower in salt marshes that had better access to open water (isolated and nonisolated) than in salt marshes that were more restricted to open-water access (interior), while the reverse was observed for L. rhomboides. Greater predation risk for marsh residents has been associated with marsh areas adjacent to large creeks compared to smaller creeks (Rozas and Odum 1987; Hettler 1989). Habitat use differences between F. heteroclitus and L. rhomboides might be partially explained by the smaller body size of F. heteroclitus compared to L. rhomboides, which might make it more susceptible to aquatic predators than L. rhomboides (Bretsch and Allen 2006). Pattern differences in habitat use might also be partially explained by increased predation susceptibility of larger-bodied fishes, including L. rhomboides, to wading and diving birds (Harvey and Stewart 1991) in shallow water. The dependence of F. heteroclitus on salt marsh habitats while seagrass and sand flat habitats were available suggests that competition or predation displacement might have influenced specific habitat use patterns of F. heteroclitus and L. rhomboides. The F. heteroclitus distribution patterns we observed in interior, nonisolated, and isolated salt marshes were similar to the predation displacement patterns observed by Posey and Hines (1991) for grass shrimp (Palaemonetes pugio). P. pugio shift their distribution to occupy shallow-water habitat areas in attempt to reduce predation threat when aquatic predators (in this case, F. heteroclitus) are present (Posey and Hines 1991). The shallower water depths over the entirety of interior compared to the nonisolated and isolated salt marshes provide a predation refuge for resident marsh nekton through the restriction of large-bodied predators (Posey and Hines 1991; Ruiz et al. 1993) and limits on predator foraging time. Restricted access to interior salt marsh for aquatic predators, compared to the less restrictive access from open water to the nonisolated and isolated salt marshes, should differentially affect predation pressure on resident marsh species. This might include predation by L. rhomboides, which is known to ontogenetically shift from a predominantly carnivorous to omnivorous feeding mode with increasing size and age (Carr and Adams 1973; Stoner 1980). Meyer (2006) noted a direct sequential increase in predation potential associated with perimeter to salt marsh area ratios, which in our case would follow an interior to nonisolated to isolated pattern for increased predation potential. Similar predation potential patterns were observed in bay scallops (Argopecten irradians) within continuous versus fragmented seagrass habitats (Irlandi 1994). An effect of reduced predation pressure should be a more even dispersion of forage species (as represented by F. heteroclitus) throughout a habitat, as seen in interior, and less inclination to seek the shallows of the high salt marsh as F. heteroclitus were observed to do in both nonisolated and isolated salt marshes.
Our results showing that F. heteroclitus populations in isolated island habitats are smaller than expansive nonisolated habitats are consistent with observations of the direct effect of salt marsh island size on salt marsh resident nekton population size and density (Meyer 2006) and consistent with island biogeography theory (MacArthur and Wilson 1967; Rieman and McIntyre 1995). Further, the limited occurrence of F. heteroclitus within isolated salt marsh islands is similar to the occurrence pattern observed for Bachman’s sparrow (Aimophila aestivalis) which did not occupy some suitable habitat patches due to dispersal barriers (Dunning et al. 1995). This suggests that mixing of local F. heteroclitus populations could be limited by their dependence on shallow-water salt marsh habitat, creating many small isolated subpopulations defined by individual salt marsh patches. Conversely, because of the transient widely spread distributional occurrence of L. rhomboides throughout the shallow-water flats and low salt marsh habitats, population division for this species into distinct subpopulations within an estuary would not be expected.
L. rhomboides is a significant component of intertidal salt marsh (Hettler 1989; Meyer 2006) and shallow-water seagrass (Fonseca et al. 1993, 1996; King and Sheridan 2008) nekton communities. The bimodal distribution of L. rhomboides we observed in the low salt marsh and interior seagrass–sand flat (25 to 100 m away from the salt marsh fringe) reflects ubiquitous shallow-water habitat use. Engrained within this pattern might also be size-based shifts of smaller L. rhomboides to shallow water, where they might experience lower predation, while larger size classes might have reached size refuge from predation (Harter and Heck 2006). Salt marsh habitats are facultatively used by L. rhomboides and are not as essential for L. rhomboides as they are for F. heteroclitus populations. Estuarine L. rhomboides distribution patterns suggest substantial local population mixing creating one large well mixed population. Population maintenance for such transient species might depend more on the occurrence of expansive shallow-water estuarine flats than upon low salt marsh. Low salt marsh represents a marginal habitat utilized by small L. rhomboides individuals, primarily as a shallow-water predation refuge (Posey and Hines 1991; Ruiz et al. 1993). High salt marsh habitat, especially within interior salt marshes, might act as low-quality habitats for such transient species.
Shallow water might be essential for F. heteroclitus population persistence, and reduction in the amount of high salt marsh refuge has been suggested to proportionally and directly affect abundance for both adults and juveniles (Meyer 2006). In fringing salt marshes, we observed that F. heteroclitus utilized high salt marsh over low salt marsh and did not occur in adjacent shallow-water flats. A maximum water depth also restricts F. heteroclitus distribution within salt marsh habitats. We estimated the maximum water depth for F. heteroclitus to be ~69 cm, within the 1-m critical water depth estimate hypothesized by Ruiz et al. (1993). The amount of shallow-water habitat deep enough to allow juvenile F. heteroclitus use, yet shallow enough to restrict predator incursion into salt marsh habitat, is suspected to be a primary factor contributing to the consistent F. heteroclitus abundance patterns we observed.
In contrast with F. heteroclitus water depth distribution patterns, L. rhomboides were restricted to areas of >31-cm water depth. Although L. rhomboides frequent many estuarine habitat types (Hettler 1989; Wenner et al. 1996; Paperno et al. 2001; Meyer 2006), habitat use is limited by minimal water depths. The bimodal L. rhomboides distribution pattern, with significant abundances observed in both the low salt marsh fringe, and the deep seagrass–sand flat interior suggests size- and life-history-based habitat segregation, which might be amplified by predator avoidance (Harvey and Stewart 1991; Bretsch and Allen 2006).
The size–water depth relationships observed for F. heteroclitus and L. rhomboides suggests a size-dependent partitioning of salt marsh habitat by both species and adjacent shallow-water flats by L. rhomboides. It is evident that seasonally prevalent YOY F. heteroclitus utilize shallow salt marsh areas and larger adults the deeper low salt marsh habitat areas, including interior salt marsh creeks. Similarly, Bretsch and Allen (2006) noted water-depth-dependent movement for F. heteroclitus and L. rhomboides into marsh creeks that not only paralleled our observed water-depth-dependent species distribution but also size-class-dependent distribution based on water depth for both species, effectively partitioning creek use based on water depth. Size-class-specific habitat partitioning has been noted for other estuarine habitats, including intertidal oyster reefs by xanthid crab species (Meyer 1994). The size–water depth distribution patterns for F. heteroclitus initially appear contrary to observations by Kneib and Wagner (1994) that larger F. heteroclitus individuals ventured far into the salt marshes they sampled (which were similar to our interior salt marshes), while smaller individuals occurred near the salt marsh–salt marsh creek interface. While it is likely that larger F. heteroclitus also ventured into our marsh shallows to spawn, the reason for perceived discrepancies between Kneib and Wagner (1994) and our study regarding within marsh, fish size to water depth distribution patterns might be based on the scale of the salt marsh habitat sampled. Kneib and Wagner (1994) did not examine adjacent marsh creek use as did we and focused on vegetated salt marsh locations. Hence, our fish size to water depth patterns for F. heteroclitus and L. rhomboides might have been undetectable for Kneib and Wagner (1994).
Movement corridors among fragmented patches of pine woodlands have been shown to be vitally important for Bachman’s sparrow (A. aestivalis) population maintenance in terrestrial environments (Dunning et al. 1995) and for fantail darter (Etheostoma flabellare) and riverweed darter (Etheostoma podostemone) among suitable habitat patches within freshwater streams (Roberts and Angermeier 2007). Movement corridors might be similarly important for estuarine species, such as F. heteroclitus, to disperse through inhospitable habitats. F. heteroclitus high-tide distributions indicate maximum water depth restrictions (Ruiz et al. 1993). Water-depth-based movement restrictions and distance between patches would have the potential to affect F. heteroclitus immigration and emigration among salt marsh habitats (Simberloff and Wilson 1969). Because F. heteroclitus exclusively use shallow-water habitat, movement corridors among salt marsh habitats are likely restricted to low-tide retreats associated with shoals and banks. Movement of F. heteroclitus between salt marshes along postulated shallow-water movement corridors is likely to occur in late fall to early spring. This is supported by observations by Fritz et al. (1975) on the seasonal migratory movements of F. heteroclitus of almost 2,000 m during the late fall within a salt marsh creek. Intra-annual differences in F. heteroclitus ebb-tide distribution indicate that the extent of movement out from salt marsh with the tidal prism (Brutner and Brattstrom 1960; Kneib 1984; Kneib and Wagner 1994; Bretsch and Allen 2006) is seasonally dependent (Fritz et al. 1975), with the greatest F. heteroclitus lateral movement during late fall and early spring. Late fall and early spring also corresponded to annual periods dominated by astronomically low tides (Hutchinson and Sklar 1993) and time periods when estuarine predator concentrations in North Carolina estuaries have been recorded to be at annual lows (Meyer 2006). Periodic opening and closing of movement corridors between salt marshes might occur seasonally for F. heteroclitus based on tidal and predator abundance variation (Meyer 2006). F. heteroclitus population mean standard lengths at isolated, nonisolated, and interior sites, during the midspring, were initially similar. Based on these similarities, it is surmised that colonization of all salt marsh habitats might have occurred by the previous years’ cohort during the late fall to early spring (Meyer 2006). Following these initial similarities among all salt marsh types, the consistent increase in size disparity between isolated compared to nonisolated and interior salt marsh types suggests that the populations of isolated salt marshes were not self-sustaining but were dependent upon adult emigrants. The apparent lack of YOY contribution to isolated salt marsh populations was particularly striking during late summer when YOY should have significantly contributed to isolated salt marsh populations, causing a temporal shift towards smaller mean standard lengths, as observed for nonisolated and interior salt marsh types (also see Meyer 2006). The isolated sites might have acted as sinks for F. heteroclitus populations. Hence, a mainland–island metapopulation maintenance pattern (Harrison and Taylor 1997) might best explain the F. heteroclitus population pattern for isolated salt marshes.
Though data for this study encompassed a single year, the consistency of utilization trends we observed and their similarity to trends observed for these species from other studies imply general species-specific trends. It is evident that resident salt marsh nekton species, as represented by F. heteroclitus, and transient salt marsh nekton species, as represented by L. rhomboides, utilize different estuarine habitats in different ways. The distribution and utilization patterns for these two species suggest that their fundamental differences may translate into estuarine landscape impacts on the ecology of nekton species. Because nekton life history attributes can significantly influence their use of particular habitats, management needs to consider the effects of landscape ecology, metapopulation, island biogeography, patch dynamics, and migration corridor theories to enhance potential for success when planning habitat preservation and restoration efforts. Although habitats contain elements considered appropriate to target nekton, habitat size or location might not be sufficient for optimal nekton use. Strategic clustering of preserved or restored habitat within a specific area or placement of restored habitats near other existing habitats might increase potential overall success for species resident to a particular habitat type (Dunning et al. 1995).
Notes
Product listing does not infer the endorsement of National Oceanic and Atmospheric Administration or University of North Carolina Wilmington.
References
Able, K.W., and M. Castanga. 1975. Aspects of an undescribed reproductive behavior in Fundulus heteroclitus (Pisces: Cyprinodontidae) from Virginia. Chesapeake Science 16: 282–284. doi:10.2307/1350946.
Able, K.W., and S. Hagan. 2000. Effects of common reed (Phragmites australis) invasion on marsh surface macrofauna: Response of fishes and decapod crustaceans. Estuaries 23: 633–646. doi:10.2307/1352890.
Abrams, B. J. 1985. Species profiles: Life histories and environmental requirements of coastal fishes and invertebrates (Mid-Atlantic)—mummichog and striped killifish. US Fish and Wildlife Service Biological Report 82(11.40). US Army Corps of Engineers, TR EL-82-4.
Alevizon, W., R. Richardson, P. Pitts, and G. Serviss. 1985. Coral zonation and patterns of community structure in Bahamian reef fishes. Bulletin of Marine Science 36: 304–318.
Andren, H. 1994. Effect of habitat fragmentation on birds and mammals in landscapes with different proportions of suitable habitat: A review. Oikos 17: 355–366. doi:10.2307/3545823.
Bertness, M.D. 1991. Interspecific interactions among high marsh perennials in a New England salt marsh. Ecology 72: 125–137. doi:10.2307/1938908.
Bretsch, K., and D.M. Allen. 2006. Tidal migrations of nekton in salt marsh intertidal creeks. Estuaries and Coasts 29: 474–486.
Brutner, A., and B.H. Brattstrom. 1960. Local movements in Menidia and Fundulus. Copeia 1960: 139–141. doi:10.2307/1440210.
Bode, M., L. Bode, and P.R. Armsworth. 2006. Larval dispersal reveals regional source and sinks in the Great Barrier Reef. Marine Ecology Progress Series 308: 17–25. doi:10.3354/meps308017.
Carr, W.E.S., and C.A. Adams. 1973. Food habits of juvenile marine fishes occupying seagrass beds in the estuarine zone near Crystal River, Florida. Transactions of the American Fisheries Society 102: 511–540. doi:10.1577/1548-8659(1973)102<511:FHOJMF>2.0.CO;2.
Dunning Jr., J.B., R. Borgella Jr., K. Clements, and G.K. Meffe. 1995. Patch isolation, corridor effects, and colonization by a resident sparrow in a managed pine woodland. Conservation Biology 9: 542–550. doi:10.1046/j.1523-1739.1995.09030542.x.
Dunning, J.B., B.J. Danielson, and H.R. Pulliam. 1992. Ecological processes that affect populations in complex landscapes. Oikos 65: 169–175. doi:10.2307/3544901.
Eggleston, D.B., L.L. Etherington, and W.E. Elis. 1998. Organism response to habitat patchiness: Species and habitat-dependent recruitment of decapod crustaceans. Journal of Experimental Marine Biology and Ecology 223: 111–132. doi:10.1016/S0022-0981(97)00154-8.
Fahrig, L., and G. Merriam. 1985. Habitat patch connectivity and population survival. Ecology 66: 1762–1768. doi:10.2307/2937372.
Fonseca, M.S., W.J. Kenworthy, D.R. Colby, K.A. Rittmaster, and G.W. Thayer. 1993. Comparison of fauna among natural and transplanted eelgrass (Zostera marina) meadows: Criteria for mitigation. Marine Ecology Progress Series 65: 251–264. doi:10.3354/meps065251.
Fonseca, M.S., D.L. Meyer, and M.O. Hall. 1996. Development of planted seagrass beds in Tampa Bay, Florida, USA. II. Faunal components. Marine Ecology Progress Series 132: 141–156. doi:10.3354/meps132141.
Fritz, E.S., W.H. Meredith, and V.A. Lotrich. 1975. Fall and winter movements and activity levels of the mummichog, Fundulus heteroclitus, in a tidal creek. Chesapeake Science 16: 211–215. doi:10.2307/1350898.
Halpin, P.M. 1997. Habitat use patterns of mummichog, Fundulus heteroclitus, in New England. I. Intramarsh variation. Estuaries 20: 616–625. doi:10.2307/1352619.
Halpin, P.M. 2000. Habitat use by intertidal salt-marsh fish: Trade-offs between predation and growth. Marine Ecology Progress Series 198: 203–214. doi:10.3354/meps198203.
Hanski, I. 1994. Patch-occupation dynamics in fragmented landscapes. Trends in Ecology and Evolution 9: 131–135. doi:10.1016/0169-5347(94)90177-5.
Harrison, S., D.D. Murphy, and P.R. Ehrlich. 1988. Distribution of the bay checkerspot butterfly, Euphydryas editha bayensis: Evidence for a metapopulation model. American Naturalist 132: 360–382. doi:10.1086/284858.
Harrison, S., and A.D. Taylor. 1997. Empirical evidence for metapopulation dynamics. In Metapopulation Biology: Ecology, Genetics, and Evolution, ed. I.A. Hanski, and M.E. Gilpin, 27–42. San Diego: Academic.
Harter, S.L., and K.L. Heck Jr. 2006. Growth rates of juvenile pinfish (Lagodon rhomboides): Effects of habitat and predation risk. Estuaries and Coasts 29: 316–327. doi:10.1007/BF02782000.
Harvey, B.B., and A.J. Stewart. 1991. Fish size and habitat depth relationships in headwater streams. Oecologia 87: 336–342. doi:10.1007/BF00634588.
Heck, K.L., and T.A. Thoman. 1981. Experiments on predator–prey interactions in vegetated aquatic habitats. Journal of Experimental Marine Biology and Ecology 53: 125–134. doi:10.1016/0022-0981(81)90014-9.
Hettler Jr., W.F. 1989. Nekton use of regularly-flooded salt marsh cordgrass habitat in North Carolina, USA. Marine Ecology Progress Series 56: 111–118. doi:10.3354/meps056111.
Hoese, H.D., R.H. Moore, and V.F. Sonnier. 1977. Fishes of the Gulf of Mexico, Texas, Louisiana, and Adjacent Waters. College Station: Texas A&M University Press.
Hokit, D.G., and L.C. Branch. 2003. Habitat patch size affects demographics of Florida scrub lizard (Sceloporus woodi). Journal of Herpetology 37: 257–265. doi:10.1670/0022-1511(2003)037[0257:HPSADO]2.0.CO;2.
Hutchinson, S.E., and F.H. Sklar. 1993. Lunar periods as grouping variables for temporally fixed sampling regimes in a tidally dominated estuary. Estuaries 16: 789–798. doi:10.2307/1352437.
Irlandi, E.A. 1994. Large- and small-scale effects of habitat structure on rates of predation: how percent cover of seagrass affects rates of predation and siphon nipping on an infaunal bivalve. Oecologia 98: 176–183. doi:10.1007/BF00341470.
King, S.P., and P. Sheridan. 2008. Nekton of new seagrass habitats colonizing a subsided salt marsh in Galveston Bay, Texas. Estuaries 29: 286–296.
Kneib, R.T. 1984. Patterns of utilization of intertidal salt marsh by larvae and juveniles of Fundulus heteroclitus (Linnaeus) and Fundulus luciae (Baird). Journal of Experimental Marine Biology and Ecology 83: 41–51. doi:10.1016/0022-0981(84)90116-3.
Kneib, R.T. 1986. The role of Fundulus heteroclitus in salt marsh trophic dynamics. American Zoologist 26: 259–267.
Kneib, R.T., and A.H. Craig. 2001. Efficacy of minnow traps for sampling mummichogs in tidal marshes. Estuaries 24: 884–893. doi:10.2307/1353179.
Kneib, R.T., and A.E. Stiven. 1978. Growth, reproduction, and feeding of Fundulus heteroclitus (L) in a North Carolina salt marsh. Journal of Experimental Marine Biology and Ecology 31: 121–140. doi:10.1016/0022-0981(78)90125-9.
Kneib, R.T., and S.L. Wagner. 1994. Nekton use of vegetated marsh habitats at different stages of tidal inundation. Marine Ecology Progress Series 106: 227–238. doi:10.3354/meps106227.
Levin, L.A., D. Talley, and G. Thayer. 1996. Succession of macrobenthos in a created salt marsh. Marine Ecology Progress Series 141: 67–82. doi:10.3354/meps141067.
Long, W.C., and R.P. Burke. 2007. Habitat size, flora, and fauna: Interactions in a tidal saltwater marsh. Journal of Experimental Marine Biology and Ecology 353: 80–88. doi:10.1016/j.jembe.2007.09.004.
Lotrich, V.A. 1975. Summer home range and movements of Fundulus heteroclitus (Pisces: Cyprinodontidae) in a tidal creek. Ecology 56: 191–198. doi:10.2307/1935311.
MacArthur, R.H., and E.O. Wilson. 1967. The Theory of Island Biogeography. Princeton: Princeton University Press.
McIvor, C.C., and W.E. Odum. 1986. The flume net: A quantitative method for sampling fishes and macrocrustaceans on tidal marsh surfaces. Estuaries 9: 219–224. doi:10.2307/1352133.
Meyer, D.L. 1994. Habitat partitioning between the xanthid crabs Panopeus herbstii and Eurypanopeus depressus on intertidal oyster reefs (Crassostrea virginica) in Southeastern North Carolina. Estuaries 17: 674–679. doi:10.2307/1352415.
Meyer, D. L. 2006. A comparison of nekton utilization of smooth cordgrass (Spartina alterniflora) marsh based on marsh size and degree of isolation from like habitat: Do size and site location matter? Ph.D. Dissertation, University of North Carolina Wilmington, Wilmington, North Carolina.
Meyer, D.L., M.S. Fonseca, P.L. Murphey, R.H. McMichael, M.M. Byerly, M.W. LaCroix, P.E. Whitfield, and G.W. Thayer. 1999. Effects of live-bait shrimp trawling on seagrass beds and fish by-catch in Tampa Bay, Florida. Fishery Bulletin 97: 193–199.
Meyer, D.L., E.C. Townsend, and G.W. Thayer. 1997. Stabilization and erosion control value of oyster cultch for intertidal marsh. Restoration Ecology 5: 93–99. doi:10.1046/j.1526-100X.1997.09710.x.
Minello, T.J., and R.J. Zimmerman. 1992. Utilization of natural and transplanted Texas salt marshes by fish and decapod crustaceans. Marine Ecology Progress Series 90: 273–285. doi:10.3354/meps090273.
Muncy, R. J. 1984. Species profiles: Life histories and environmental requirements of coastal fishes and invertebrates (Gulf of Mexico) pinfish. US Fish and Wildlife Service Biological Report 82(11.26). US Army Corps of Engineers, TR EL
Nixon, S.W. 1982. The ecology of New England high salt marshes: A community profile. Washington DC: US Fish and Wildlife Service, Office of Biological Services.
Paperno, R., K.J. Mille, and E. Kadison. 2001. Patterns of species composition of fish and selected invertebrate assemblages in estuarine subregions near Ponce de Leon Inlet, Florida. Estuarine Coastal and Shelf Science 52: 117–130. doi:10.1006/ecss.2000.0732.
Posey, M.H., and A.H. Hines. 1991. Complex predator–prey interactions within an estuarine benthic community. Ecology 72: 2155–2169. doi:10.2307/1941567.
Pulliam, H.R. 1988. Sources, sinks, and population regulation. The American Naturalist 132: 652–661. doi:10.1086/284880.
Reebs, S.G., L. Boudreau, P. Hardie, and R.A. Cunjak. 1995. Diel activity patterns of lake chubs and other fishes in a temperate stream. Canadian Journal of Zoology 73: 1221–1227. doi:10.1139/z95-146.
Rieman, B.E., and J.D. McIntyre. 1995. Occurrence of bull trout in naturally fragmented habitat patches of varied size. Transactions of the American Fisheries Society 124: 285–296. doi:10.1577/1548-8659(1995)124<0285:OOBTIN>2.3.CO;2.
Roberts, J.H., and P.L. Angermeier. 2007. Movement responses of stream fishes to introduced corridors of complex cover. Transactions of the American Fisheries Society 136: 971–978. doi:10.1577/T06-215.1.
Roberts, J.J., and F.J. Rahel. 2008. Irrigation canals as sink habitats for trout and other fishes in a Wyoming drainage. Transactions of the American Fisheries Society 137: 951–961. doi:10.1577/T07-058.1.
Rozas, L.P., and W.E. Odum. 1987. Use of tidal freshwater marshes by fishes and macrofaunal crustaceans along a marsh stream-order gradient. Estuaries 10: 36–43. doi:10.2307/1352023.
Rozas, L.P., and R.J. Zimmerman. 2000. Small-scale patterns of nekton use among marsh and adjacent shallow nonvegetated areas of the Galveston Bay Estuary, Texas (USA). Marine Ecology Progress Series 193: 217–239. doi:10.3354/meps193217.
Ruiz, G.M., A.H. Hines, and M.H. Posey. 1993. Shallow water as a refuge habitat for fish and crustaceans in non-vegetated estuaries: An example from Chesapeake Bay. Marine Ecology Progress Series 99: 1–16. doi:10.3354/meps099001.
Sacco, J.N., E.D. Seneca, and T.R. Wentworth. 1994. Infaunal community development of artificially established salt marshes in North Carolina. Estuaries 17: 489–500. doi:10.2307/1352678.
Sen, P.K., J. Jureckova, and J. Picek. 2003. Goodness-of-fit test for Shapiro–Wilk type with nuisance regression and scale. Australian Journal of Statistics 32: 163–177.
Shapiro, S.S., and M.B. Wilk. 1965. An analysis of variance test for normality (complete samples). Biometrika 52: 591–611.
Sheaves, M., and R. Johnston. 2008. Influence of marine and freshwater connectivity on the dynamics of subtropical estuarine wetland fish metapopulations. Marine Ecology Progress Series 357: 225–243. doi:10.3354/meps07292.
Simberloff, D.S., and E.O. Wilson. 1969. Experimental zoogeography of islands: The colonization of empty islands. Ecology 50: 278–296. doi:10.2307/1934856.
Sogard, S.M., and K.W. Able. 1994. Diel variation in immigration of fishes and decapods crustacean to artificial seagrass habitat. Estuaries 17: 622–630. doi:10.2307/1352410.
Sokal, R.R., and F.J. Rohlf. 1981. Biometry, 2nd ed. San Francisco: Freeman.
Stoner, A.W. 1980. Feeding ecology of Lagodon rhomboides (Pisces: Sparidae): variation and functional responses. Fishery Bulletin 78: 337–352.
Taylor, M.H., G.J. Leach, L. DiMichele, W.M. Levitan, and W.F. Jacob. 1979. Lunar spawning cycle in the mummichog, Fundulus heteroclitus (Pisces: Cyprinodontidae). Copeia 1979: 291–297. doi:10.2307/1443417.
Teo, S.L.H., and K.W. Able. 2003. Habitat use and movement of the mummichog (Fundulus heteroclitus) in a restored salt marsh. Estuaries 26: 720–730. doi:10.1007/BF02711983.
Warlen, S.M., and J.S. Burke. 1990. Immigration of larvae of fall/winter spawning marine fishes into a North Carolina estuary. Estuaries 13: 453–461. doi:10.2307/1351789.
Wenner, E., H.R. Beatty, and L. Coen. 1996. A method for quantitatively sampling nekton on intertidal oyster reefs. Journal of Shellfish Research 15: 769–775.
Whitelaw, A.W., K.J. Sainsbury, G.J. Dews, and R.A. Campbell. 1991. Catching characteristics of four fish-trap types on the North West Shelf of Australia. Australian Journal of Marine and Freshwater Research 42: 369–382. doi:10.1071/MF9910369.
With, K.A., and T.O. Crist. 1995. Critical thresholds in species’ responses to landscape structure. Ecology 76: 2446–2459. doi:10.2307/2265819.
Acknowledgments
This work was accomplished by the selfless dedication of many individuals. Thank you for advice and consultations to: C. Hackney, T. Lankford, L. Leonard, F. Scharf, and G. Thayer. Thank you to the individuals who provided much appreciated help including: T. Alphin, R. Cheshire, P. Hansen, M. LaCroix, A. Powell, B. Teer, and S. Viehman. Thank you to G. Bath-Martin, V. McDonough, P. Hay, A. Hohn, and P. Marraro and two anonymous reviewers for comments and insights that significantly improved this manuscript. Thank you to the NOAA/NMFS Restoration Center (especially C. Doley, P. Gayaldo, and R. Bellmer) and the NOAA Beaufort Laboratory (especially M. Fonseca) for grant and project support. This manuscript is dedicated to the memory of Lillian Cranfill Howell (1928–2005), who showed me that all life is precious and to be respected.
Disclaimer
The findings and conclusions in this paper are those of the authors and do not necessarily represent the views of NOAA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Meyer, D.L., Posey, M.H. Effects of Life History Strategy on Fish Distribution and Use of Estuarine Salt Marsh and Shallow-Water Flat Habitats. Estuaries and Coasts 32, 797–812 (2009). https://doi.org/10.1007/s12237-009-9164-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-009-9164-x