Abstract
Coastal estuaries provide essential juvenile habitat for many commercially and recreationally important fish, which may move between estuarine and coastal environments throughout their life. Identifying the most important estuarine nurseries that contribute to the broader stock can support targeted management of juvenile and spawning populations. The objective of this study was to (1) compare chemical fingerprints within sagittal otoliths of juvenile Mulloway (Argyrosomus japonicus) sampled from putative south-eastern Australian nurseries, (2) assess their potential as natural tags to distinguish nursery grounds for the broader coastal Mulloway stock and (3) assess the viability of otolith chemistry as a fisheries management tool when limited to opportunistic, fisheries-dependant, otolith sample collection from by-catch. Otoliths from juvenile Mulloway (0 to 3 years, 4 to 44.8 cm total length) were obtained from 8 major estuaries and 2 inshore ocean locations along coastal south-eastern New South Wales, Australia, from April 2015 to July 2018. Concentrations of Sr, Ba, Mg, Mn and Li in the otolith region corresponding to the juvenile nursery stage were determined using laser ablation-inductively coupled plasma-mass spectrometry (LA-ICP-MS). The element to Ca ratios of fish from coastal estuaries differed significantly among collection areas, based upon multivariate elemental fingerprints, with some exceptions. When the otoliths of fish were analysed in a multinomial logistic regression (MLR) classifier, there was an overall mean allocation success of 59% to the estuary of capture. This study highlights the use of otolith ‘fingerprints’ as natural tags in Mulloway, and contributes to progressive research in environmental reconstruction applications of otolith chemistry.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Estuaries are dynamic, productive systems and of direct importance to many aquatic species (Raoult et al. 2018; Rönnbäck 1999; Taylor et al. 2018). Specifically, these ecosystems provide food, habitat and refuge across a range of life stages (Able 2005), and many coastal estuaries provide essential nursery habitat for juvenile fish and crustaceans (Beck et al. 2001; Gunter 1967; Litvin et al. 2018). While some species are resident within estuaries for their entire life (Dolbeth et al. 2008; Potter and Hyndes 1999; Sakabe and Lyle 2010), other species utilise estuaries during their juvenile stage before migrating into nearby waters to complete their life cycle (Able 2005; Bode et al. 2006; Dias 1996; Gillanders et al. 2003). For these species, the contribution of juveniles to the broader stock may differ among estuaries, such that some estuarine nurseries are disproportionately more important to the overall stock than others.
In the context of fisheries, recruitment can be defined as the number of juvenile fish surviving to enter the fishery, or to some other life stage such as settlement or maturity. Studying the differential contribution of these estuarine nurseries to recruitment is essential for effective fisheries and ecosystem management (Nagelkerken et al. 2015). For example, distinguishing nursery grounds can help manage exploited populations (Thorrold et al. 2001), target conservation and repair efforts for essential habitat (Bertelli and Unsworth 2014; Boys and Pease 2017; Rose et al. 2015), support the design of spatial management measures (Liu et al. 2018) and inform other fishery management strategies such as stocking programs (Bell et al. 2008; Lorenzen 2008; Zohar et al. 2008). These studies generally involve tracing fish migration history and connectivity, to determine the contribution of particular areas to the broader stock (e.g. Fowler et al. 2016; Gillanders 2002a; Gillanders et al. 2003; Schilling et al. 2018; Taylor et al. 2006; Yamashita et al. 2000).
In recent decades otolith chemistry has become an increasingly popular ‘natural tag’ to determine connectivity between populations (DiBacco and Levin 2000; Fowler et al. 2016; Gibb et al. 2017; Gillanders and Kingsford 1996; Thorrold et al. 1998) and investigate life history in fish (Gillanders 2002a; Gillanders and Kingsford 2000; Marriott et al. 2016; Reis-Santos et al. 2012; Schilling et al. 2018). This tool is particularly useful in the context of early life history ‘patterns’ in fish, as conventional tagging techniques used for many marine organisms are impractical for small animals, are not ‘life-time’ tags, have high rates of mortality associated with tagging, and require sufficient rates of tag recovery (Gillanders 2002a). Calcified structures have been used to measure natural chemical markers in a range of organisms including bivalves, fish, sharks and corals (e.g. Gomes et al. 2016; Izzo et al. 2016; McMillan et al. 2017; Raybaud et al. 2017). The primary assumption of these studies is that elements within these structures are primarily sourced through absorption of minor and trace elements present in the ambient water (Elsdon et al. 2008; Izzo et al. 2018; Miller 2011).
A suite of elements are used in otolith (“earstone”) microchemical analyses as proxies for change in the physico-chemical properties of an individual’s environment (Thomas and Swearer 2019). For example, otolith strontium (Sr) and barium (Ba) concentrations vary strongly with environmental salinity and temperature (Barnes and Gillanders 2013; Elsdon and Gillanders 2002; Tsukamoto and Arai 2001), and manganese (Mg) is useful for tracking hypoxia (Limburg et al. 2011). Otoliths are acellular and metabolically inert structures (Thomas and Swearer 2019). Thus, incorporation of these elements can create a permanent record of the environmental conditions experienced by a fish, during a specific period of growth (Disspain et al. 2016; Sturrock et al. 2012), providing a sequence of location-specific fingerprints throughout a fish’s life history (Campana and Thorrold 2001; Grammer et al. 2017; Ruas and Vaz-dos-Santos 2017). Relationships are validated for many species using laboratory trials, and extrinsic and intrinsic factors (such as pH, water temperature, diet and genotype) may affect concentrations alongside salinity (Barnes and Gillanders 2013; Doubleday et al. 2013). For example, Sr, Ba and Li enrichment into the otolith is dependent on concentrations in the ambient environment (Thomas et al. 2017), whilst manganese (Mn) and magnesium (Mg) appear to be under strong physiological and growth rate control (Brophy et al. 2004; Hüssy et al. 2020; Limburg et al. 2018). These elemental ‘fingerprints’ or ‘signatures’ have effectively been used to classify nursery areas, identify nursery estuaries for adult fish (Gillanders 2002a; Gillanders and Kingsford 2000; Reis-Santos et al. 2013; Vasconcelos et al. 2011), discriminate among populations from different geographic locations (Tanner et al. 2016; Wells et al. 2015) and determine mixed-stock compositions (Campana et al. 2007; Geffen et al. 2011).
Mulloway (Argyrosomus japonicus) are a wide-ranging estuarine and coastal predator common to southern Australia, Africa, India, Pakistan, Korea, Japan and China (Silberschneider and Gray 2008). The early life history of Mulloway is poorly understood. In south-eastern Australia, particularly New South Wales (NSW), large summer rainfall events appear to trigger spawning aggregations around the mouths of estuaries, where larvae can disperse along the coast (see Taylor et al. 2014). Depending on the region, larvae return to and settle back into brackish regions of temperate estuaries (Gray and McDonall 1993; Griffiths 1996; Pursche et al. 2013; Taylor et al. 2006), where they remain during the first 3 to 4 years of life as sub-adults (Griffiths and Attwood 2005; Silberschneider and Gray 2008), utilising deep-hole habitats (Becker et al. 2017; Taylor et al. 2006). While juveniles are often highly associated with estuarine nursery habitats (Becker et al. 2017; Taylor et al. 2006), adults are known to undertake broader inter-estuarine (Hall 1984; Hall 1986) and coastal migrations (West 1992), potentially related to spawning (Barnes et al. 2019; Taylor et al. 2006) and food availability (Stewart et al. 2020). The extent of inter-estuarine connectivity is largely unknown for this species; however, it is thought that nursery areas are focused in larger estuaries, due to increased habitat, food and enhanced physico-chemical conditions to stimulate juvenile recruitment (Dahlgren et al. 2006; Taylor et al. 2014). In NSW, Australia, Mulloway mature at ~ 51 cm and ~ 67 cm (total length, male and female L50, respectively, Silberschneider and Gray 2005), and are heavily fished in both estuaries and inshore areas. Due to recent declines in the south-eastern Australian Mulloway stock, the species is subject to a recovery program in NSW (Earl et al. 2019).
Prior works on this species in Australia have employed techniques based on tag-recapture (Silberschneider and Gray 2008), telemetry (Barnes et al. 2019; Taylor et al. 2006) and genetics (Archangi 2008; Barnes et al. 2016; Farmer 2008) to define stock structure, habitat use and connectivity of Mulloway in Australian waters. Otolith elemental chemistry studies on Mulloway, however, are limited, particularly for the NSW Mulloway stock. Stock discrimination between adult Mulloway populations has been conducted in South Australia (i.e. the Western, Central and Eastern coast of SA) using otolith chemistry (Sr:Ca, Ba:Ca and Mg:Ca) and otolith morphometrics (Ferguson et al. 2011). However, the current study differs from previous analyses, as discrimination of juvenile Mulloway nursery estuaries using otolith chemistry has not yet been undertaken. Following recent laboratory and field otolith chemistry studies on Mulloway by Barnes and Gillanders (2013) and also Ferguson et al. (2011), otolith chemistry was evaluated as a natural tag to aid research on questions relating to stock structure and connectivity. In the current study, we assess the suitability of natural chemical tags to differentiate putative estuarine nurseries for juvenile Mulloway across south-eastern Australia. Specifically, the objectives were to:
-
1.
Evaluate geographic variation in the elemental chemistry of juvenile Mulloway across all major putative nursery estuaries and inshore locations (hereafter collectively referred to as locations)
-
2.
Determine if these differences in otolith elemental chemistry can support the classification of juvenile Mulloway back to the estuary of capture
-
3.
Assess the viability of otolith chemistry as a fisheries management tool when limited to opportunistic, fisheries-dependantFootnote 1 otolith sampling of by-catch.
Methods
Study Design and Fish Collection
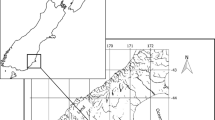
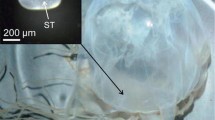
Juvenile Mulloway (n = 274, < 45 cm) were collected from 8 major estuaries and two inshore ocean locations along the south-eastern coast of Australia (Fig. 1, Table 1) during spring/summer in 2015–2018 (November to April, Fig. 2). These fish were collected in collaboration with commercial fishers from the NSW Estuary General Fishery and Ocean Prawn Trawl Fishery1, under NSW Department of Primary Industries Scientific Collection Permit P01/0059. Fish were captured using either gill nets or otter trawl, and sample size varied among locations due to availability (Table 1, Fig. 2). Chemical fingerprints were obtained from otolith edge ablations of juvenile Mulloway (Fig. 3) using a laser inductively coupled plasma-mass spectrometer (LA-ICP-MS). This ablation position was selected as elemental compositions acquired from the otolith edge are considered the most recently accrued otolith chemistry, and most likely to reflect the water chemistry of the estuary of capture (Campana 2013; Tanner et al. 2016). Subsequent otolith chemistry of fish collected from these putative coastal nursery areas was used to determine whether different estuaries had different elemental fingerprints.
Sample Preparation
All fish were frozen until dissection in the laboratory. Prior to processing, the fish were defrosted. Both sagittae were removed, cleaned in ultra-pure deionised water (Milli-Q) and then air-dried overnight. An otolith from each fish was embedded in indium-spiked (~ 40 ppm) two-part epoxy resin (Struers Epofix) and sectioned transversely (~ 300 μm wide) through the primordium (perpendicular to the long axis), using a low-speed saw (Buehler Isomet) and two spaced diamond blades. Milli-Q water was used to lubricate the blades during this process. Sections were then polished with 9-μm lapping paper. Otoliths from all collection locations were combined and arranged in random order to remove any systematic variation arising from possible instrumental drift between samples, and affixed to glass microscope slides using indium-spiked (~ 200 ppm) thermoplastic glue. Slides with mounted otoliths were then sonicated with Milli-Q water before being dried in a laminar-flow positive-pressure fume hood overnight and individually stored in plastic bags, ready for analysis using laser ablation-inductively coupled plasma-mass spectrometry (LA-ICP-MS). The age of individuals (Table 1) was estimated by counting opaque increments in the transversely sectioned otoliths under reflected light at a magnification of × 20 on a compound microscope. This process was carried out by two experienced readers, and where discrepancies occurred, a third, highly experienced otolith technician became the third reader. Uncertainties were handled by this third reader who made the final decision on the fish’s age. All fish aged 0+ to 3 years (n = 274) were selected for subsequent chemical analysis.
Analytical Methods
Transverse sections of juvenile A. japonicus otoliths were analysed at Adelaide Microscopy (Adelaide, SA, Australia) using a NewWave UP-213-nm laser ablation system connected to an Agilent 7500cs inductively coupled plasma-mass spectrometer (LA-ICP-MS). The ICP-MS instrument was run at a frequency of 5 Hz and fluency of 10 J cm−2, using a spot size of 60 μm. The sample to be analysed was placed in the ablation chamber and viewed remotely on a computer screen. The laser was focused on the intended spot position at the otolith edge and was fired through the microscope objective lens, essentially ‘drilling down’ vertically into the otolith edge. The laser operated with a dwell time of 40 s, and the mean ablation depth was 48 μm (SE ± 2.4, n = 10). Resultant ablated material from this single vertical spot on the otolith edge was entrained by argon and helium gas for analysis of 24Mg, 25Mg, 55Mn, 88Sr, 138Ba, 7Li, 23Na, 44Ca, 66Zn, 111Cd, 139La, 208Pb and 115In isotopes by ICP-MS. The element 115In was analysed to detect any contamination from resin or the thermoplastic glue, and calcium was the internal standard used to correct for any variations in ablation yield. Before data collection and each sample ablation, elemental background concentrations were determined by analysis of the chamber gases. After 20 to 30 s of the blank counts, a 3-s pre-ablation was conducted at the spot location to remove any surface contamination. This was immediately followed by a single 60-μm spot, ablated at the distal edge of each otolith along the proximal surface, beside the sulcal groove. This single spot location was selected as it is comprised of the material most recently incorporated into the otolith surface, and therefore the most useful composition to characterise the chemical fingerprint of the estuary from which the juvenile was captured. Certified reference materials (glass standard NIST 612 and carbonate standard MACS-3) were analysed every 10 to 12 samples, and a linear interpolation between the 2 consecutive sets of NIST 612 standards was made to correct for instrument drift, calibrate elemental concentrations, correct mass bias and assess external precision. Between each ablation, a 30-s washout delay was used to purge the chamber and prevent each sample from cross-contamination.
Background counts lasting 60 s were collected at the start and end of each day of analysis, with the variation among these counts used to calculate the limits of detection. In the few cases where data fell below the limit of detection (8% of samples with 2 samples being for the isotope 7Li and a further 18 samples for the isotope 55Mn), the recorded LOD values were used, since excluding or substituting values with an arbitrary number or zero has been shown to bias data owing to non-random patterns in the distribution of small values (Helsel 2006; Lazartigues et al. 2016; Schaffler et al. 2014). As outlined by Yoshinaga et al. (2000), calcium concentration was assumed from the stoichiometry of calcium carbonate as 38.8% and the concentrations of other elements (above the limits of detection) were estimated against the Ca concentration.
Mean estimates of precision (%RSD, relative standard deviation) based on a NIST 612 standard being treated as an unknown were 100% (Mg) and 99.95% (Mn, Sr, and Ba). Raw elemental count data were processed using the Iolite software plugin (Paton et al. 2011) for IgorPro (Wavemetrics) and sample measures were expressed as ratios to 44Ca (in μmol mol−1) to account for fluctuations in the ablation yield. For each session, baseline values were subtracted (step-forward integration) and 0.5 s was cropped from the start and finish of each measurement. Where the indium marker was detected (indicating ablation of the mounting material), individual measurements were further cropped. Output measurements were calibrated against the NIST612 measurements (Spline Smooth 7 integration) over the period of the run. All elemental data reduction was carried out manually using a spreadsheet program (Microsoft Excel) and involved determining the average of the background counts and subtracting this from the average of the sample counts. Data (counts s−1) were then converted to concentrations.
Statistical Methods
Analysis was undertaken in R v. 3.2.1 (R Development Core Team 2010). Although a larger suite of elements was initially measured using LA-ICP-MS, the elements selected for the final analyses were 88Sr, 138Ba, 25Mg, 55Mn and 7Li, since these produced readings above detection limits, provided the best precision (based on %RSD) and the best accuracy (percent recovery). Furthermore, 88Sr, 138Ba, 25Mg, 55Mn and 7Li are all influenced by environmental change (albeit influenced by intrinsic factors e.g. ontogeny, genetics and diet). Before data analyses, all raw data were checked for errors and outliers using box plots and Cleveland dot plots, as suggested by Zuur et al. (2010). Any erroneous data caused by machine error or spiked indium levels were removed (15 samples in total were removed, amounting to the loss of ~ 5% of samples).
A preliminary exploration of individual elements (88Sr, 138Ba, 25Mg, 55Mn, 7Li) was conducted to determine if there were differences between locations, using analysis of variance (ANOVA). To test the temporal and ontogenetic stability of the fingerprints, additional covariates were added to the initial model (e.g. element: Ca ~ estuary ∙ collection year + age + total length). There was an effect for age and the year of collection using multinomial logistic regression (MLR); however, there was only marginal (< 2%) improvement in allocation success after sub-setting the data to reduce these effects. Hence, all samples were retained for further analyses using multivariate techniques.
Differences between locations were evaluated for the multivariate data set (with a Euclidian distance matrix), using non-parametric permutational-ANOVA (PERMANOVA). Log10 transformation was applied to 138Ba and 55Mn data to address skewness and ensure assumptions of normality were met. A canonical variates analysis (CVA) was also used to visualise multivariate differences in elemental fingerprints among locations. Multinomial logistic regression (MLR) was used to determine if differences in otolith trace elements supported the back-classification of juvenile Mulloway to their capture location. The absence of collinearity is assumed for MLR; hence, all predictor variables were checked graphically before modelling (Zuur et al. 2010). MLR has been widely used (e.g. Wood 2017) including recent ecological applications (e.g. Bourel and Segura 2018). Briefly, a backward stepwise model variable selection process was used, with the lowest Akaike information criterion (AIC) used to select the most appropriate model variables from the five candidate elemental ratios (Wood 2017). The MLR categorical response variable was the location regressed against the continuous otolith elemental predictor variables.
Results
Univariate Comparisons of Individual Elements
Otolith elemental concentrations were significantly different among locations for all metals when tested individually (P < 0.05, Tables S1 and S2, Fig. S1). In particular, the two ocean locations had elevated concentrations of Li:Ca, Sr:Ca and Mn:Ca relative to other locations and Ba:Ca concentrations were greatest in the Clarence River, Hunter River and Hawkesbury River. Differences in Sr:Ca concentrations also occurred among locations with the Hunter, Hawkesbury and Clarence rivers having concentrations as high as the two ocean locations, Ballina and Yamba (Fig. S1).
Multivariate Comparisons and Classification
Non-parametric PERMANOVA showed there was significant variation in elemental fingerprints among locations (F9, 249 = 17.02, P = 0.005: Table 2, visualised by CVA in Fig. 4). Notably, fish collected from the Clarence River, Hunter River and Hawkesbury River had unique chemical fingerprints compared to all other locations. Using the MLR, juvenile Mulloway were back classified to their locations of capture with variable success (Table 3, Tables S3 and S4). As previously mentioned, chemical compositions of individuals collected from the Clarence River, Hunter River and Hawkesbury River were highly unique, and there were reasonable cross-validation scores for these groups when all locations were compared (64%, 61% and 62% respectively Table 3). The inshore ocean locations (Ballina and Yamba) showed the highest percent allocation (74% and 70% respectively), whereas in other locations, for example, the Macleay River and Shoalhaven River, classification success was comparatively poor (29% and 0%).
Other Influences on Otolith Chemical Fingerprints
To test for ontogenetic and temporal influences on elemental concentrations, additional analyses were conducted by adding the covariates age, total length and the year of sample collection to the initial ANOVA model. There was a significant effect for age and the year of collection (P < 0.05, df = 3 respectively, Table S1), although removing fish aged 0+ to 1 year only had marginal (< 2%) improvement in allocation success by the MLR. Similarly, inclusion of TL (which covaried with age) indicated that fish < 15 cm TL generally had elevated elemental fingerprints. Closer investigation of the data revealed that these fish were predominantly from the Clarence River. Subsequently, the MLR was again trialled on fish > 15 cm TL which caused the allocation success rate to decrease ~ 10%. There were no obvious patterns in classification success when compared among ages, locations or years of collection (Figs. 5, 6 and 7). Rather, misclassification appeared to be associated with similarities in elemental fingerprints among certain locations.
Discussion
Classification by Location Using Otolith Elemental Fingerprints
Our analyses detected substantial geographic variation in otolith elemental fingerprints for juvenile Mulloway, and for some locations, these differences supported a reasonable level of back classification of fish to their locations of capture. Fundamentally, this demonstrates the potential for such fingerprints to be used as natural tags for identification of nursery habitats and estuary of origin in Mulloway. The reasonable success of allocations of juvenile Mulloway in certain locations (e.g. Richmond River, Clarence River, Hawkesbury River and Hunter River, and Ballina and Yamba ocean locations) suggests a potential lack of mixing at the juvenile stage. This supports previous work using acoustic telemetry, which showed some estuary fidelity in juvenile Mulloway (Taylor et al. 2006).
Elemental fingerprints only differed significantly between some locations, and in some cases, there was considerable variability among individuals within locations. This concurs with previous research within the same study region for Pagrus auratus, Pelates sexlineatus and Pomatomus saltatrix, which also found homogeneity in otolith chemistry among locations (Gillanders 2002a; Sanchez-Jerez et al. 2002; Schilling et al. 2018). Juvenile Mulloway captured in the Shoalhaven River had poor back-classification success, which was similar to results found by Schilling et al. (2018) for juvenile P. saltatrix in this estuary. Similarity in elemental fingerprints among estuaries has been previously reported in many species (Gillanders 2002b; Marriott et al. 2016; Schilling et al. 2018), particularly in studies with large numbers of sample sites. It is conceivable that the lack of distinct elemental fingerprints among these estuaries may reflect a similar level of freshwater input (Table 1) or similarities in the geology of adjacent water catchments (Crook et al. 2016; Grimes and Kingsford 1996) (Table 4). Differences in freshwater inputs among estuaries arise through variable catchment sizes and the size and number of dams and impoundments on freshwater tributaries, which in turn may influence concentrations of certain elements in the estuaries. Often, mechanisms that drive differences in elemental chemistry are complex and not well understood (Gillanders and Kingsford 2000; Walther 2019), which makes it challenging to understand causative relationships. However, for this approach, it is not necessary to understand why differences occur just whether differences occur.
For samples collected at the Ballina and Yamba locations, elevated Sr:Ca and Li:Ca concentrations drove the differences from estuarine locations. This result is unsurprising, as elevated Sr:Ca is indicative of marine residence (Sturrock et al. 2012; Walther and Thorrold 2006), but concentrations can also be influenced by temperature and physiology (see Barnes and Gillanders 2013; Reis-Santos et al. 2018; Sturrock et al. 2014). However, fish collected from some of the estuaries had similar Sr:Ca concentrations to these oceanic locations (Fig. S1), and results for the Clarence River revealed simultaneously high ratios of Ba:Ca and Sr:Ca. Typically, most estuary mixing models show very little difference in water Sr:Ca at salinities above ~ 10 and the plateau in Sr:Ca values depends on endmember concentrations (Walther and Nims 2015), which may confound expected outcomes. While the positive relationship between otolith strontium and ambient salinity is widely assumed (see review: Secor and Rooker 2000), strontium concentrations in ambient water also contribute to increased otolith strontium (Bath et al. 2000; Elsdon and Gillanders 2003; Milton and Chenery 2001). Hence, depending on catchment mineralogy, it is possible for Sr:Ca ratios in freshwater to exceed that of marine waters and for mixed results for otolith strontium and salinity to occur (Gillanders 2005).
In contrast, elevated Ba:Ca observed in fish otoliths from the Clarence, Richmond, Hunter and Hawkesbury River are more typical of lower salinity and brackish water environments (Brown and Severin 2009; Elsdon and Gillanders 2005b; Gillanders et al. 2015; Walther and Limburg 2012). These larger systems also receive comparatively high freshwater inputs (Table 1), which will impact salinity levels throughout these estuaries, as well as delivering more dissolved minerals into the estuarine water. Certainly, Sr:Ca and Ba:Ca concentrations were elevated in these systems (Fig. S1). Specifically, Ba:Ca and Mn:Ca ratios were higher in fish otoliths collected from the Clarence River than from all other estuaries; this observation was also noted by Schilling et al. (2018) for juvenile Pomatomus saltatrix collected from the Clarence River. Rivers within the northern bioregion of NSW (i.e. the Clarence and Richmond rivers, Fig. 1) generally receive higher rainfall than the rest of the state and are more prone to flooding. Historical data reflects this trend, as average annual rainfall observations over the past 20 years are greater for the northern bioregion than the central and southern bioregions (1398 mm, 1076 mm, 712 mm respectively, based on mean weather data from 1998 to 2018) (Bureau of Meteorology 2019). In the current study, Mn:Ca and Mg:Ca ratios (along with Li:Ca) were highest in the Clarence River, followed by the inshore ocean groups (Ballina and Yamba) and the Hunter River (Fig. S1 and S2). This may be indicative of some ontogenetic (age at size) effect (see Hüssy et al. 2020), as the majority of juveniles sampled from these areas were age 0+ and age 1+ (Table 1). Broadly, concentrations of these elements were elevated in fish < 1 year of age (and < 15 cm total length). Similarly, Limburg et al. (2011) found elevated otolith Mn:Ca ratios in regions of cod (Gadus morhua) otoliths corresponding to their first year of life.
Although elemental fingerprints of fish showed some overlap among estuaries, an objective of our study was too assess the viability of otolith chemistry as a fisheries management tool, when limited to otoliths sourced entirely from commercial fishery by-catch. The elemental fingerprints of otolith samples derived from this source supported a reasonable level of classification to the location of capture (59% overall, Table 3). This result is comparable to or greater than those of other published otolith chemistry studies on Mulloway (in South Australia; see Ferguson et al. 2011) and for different species in similar environments (Bourret et al. 2014; Gahagan et al. 2012; Schilling et al. 2018). Otolith elemental fingerprints of some locations in this study thus represent a usable known-origin data set. Determining natal origins of exploited and threatened species is critical for their management and conservation (such as restocking efforts; see Pursche et al. 2013), particularly if source-sink dynamics are present, or natal homing occurs (Barnes et al. 2019). This study supports the application of otolith chemistry as a fisheries research tool for Mulloway, and suggests that samples collected opportunistically (i.e. using commercial fishing effort) may be suitable for this approach. However, greater sample numbers may be required to improve resolution of patterns in those locations for which there was poor classification.
Sources of Bias and Potential Impact on Classification
Otolith elemental concentrations are influenced by extrinsic factors, such as salinity and water temperature, as well as intrinsic factors such as genetics, diet and ontogeny (Barnes and Gillanders 2013; Limburg et al. 2018). Whilst it may be unnecessary to fully understand these effects when using elemental fingerprints as natural tags of nursery habitats (see Campana et al. 1994; and also Thorrold et al. 1998), the interaction of these factors can become potential sources of bias that contribute to variability in the data and resultant misclassification. Water chemistry can vary considerably within individual locations, both temporally and spatially, influenced by river and groundwater inflows (Elsenbeer et al. 1995), oceanographic connectivity and topographic forcing (e.g. currents, estuarine plumes and mixing; see Cooper et al. 2008; Walther et al. 2013), and the impact of urbanisation and industry (e.g. sewerage, drainage; see Miyan et al. 2016). Point sources of inflows may influence elemental chemistry, especially where fish are site attached or have small home ranges. Mulloway have relatively broad home ranges (7–30 km) within locations (Taylor et al. 2014), so small-scale variation is unlikely to have impacted results, but cannot be ruled out. Ontogeny can affect the stability of otolith fingerprints in some fish species (Grammer et al. 2017; Limburg et al. 2018). In the current study, there appeared to be some age and size effects, which were small relative to geographic differences; however, it is important to consider that some elements (especially Mn, Mg and Li) may be sensitive to growth rates in certain taxa (Thomas and Swearer 2019). Hence, caution may be needed when sampling very young juveniles, and excluding certain year-classes may be necessary. Broadly, elements that are not influenced by endogenous processes (Sr:Ca and Ba:Ca) are the most suitable candidates for use in environmental reconstructions; however, interactive effects between these two elements negate the use of Sr:Ca and Ba:Ca ratios exclusively, as environmental proxies (see De Vries et al. 2005). In the current study, when these two elements were tested exclusively, allocation success was reduced (from 59 to 45%).
Collection of environmental data was outside of the scope of the current study, due to the large number and spatial breadth of the estuaries involved, and the sample method employed (i.e. collection of individuals from commercial operators). A suite of environmental parameters (salinity and temperature) and water samples for analysis of elemental chemistry (see Dorval et al. 2007; Taddese et al. 2019) could be collected at the exact location and time of fish collection, if fishery-independent collection methodology is employed. The use of additional chemical (e.g. isotopes such as oxygen and/or carbon or eye lens carbon, nitrogen and sulfur (CNS) isotopes; see Hsieh et al. 2019; Trueman and St John Glew 2019) and non-chemical data may assist the interpretation of patterns in otolith chemistry. In addition, samples (fish and environmental data) from multiple sites along each estuary may provide a broader representation of otolith chemistry across each system, and thus better account for spatial and temporal biases. Sampling programs with a reasonable temporal period are recommended, as chemical fingerprints built over time for each estuary may be more robust to environmental fluctuations than elemental fingerprints of a single year-class of juveniles. It is also recommended that adults be matched back to the correct natal year; thus, the formation of a reference library is particularly important when using juvenile fingerprints to determine the natal estuary of a number of year-classes of adults (Gillanders 2002b). In the current study for this approach, otolith chemistry of individuals from some estuaries (i.e. Port Stephens and Hunter River) was stable through time, suggesting they may require less frequent sampling for a reference library. For others that were more variable through time, for example the Clarence River, annual collections are likely to be required. Although variations in fingerprints among the sample collection years were found (Fig. S3), they were not substantial enough to affect allocation results. However, interrogation of the data is important to ascertain inter-annual variability in otolith elemental fingerprints, to avoid temporal differences from confounding spatial interpretations (Gillanders 2002b).
Fish movements in and out of natal areas also present a potential source of error, which could contribute to poor classification success. Elsdon and Gillanders (2005a) have suggested that fish need to reside in an area for greater than 20 days to incorporate a measurable amount of the chemical fingerprints for that environment. While it is not known if the fish sampled in the current study were residing exclusively in the estuary of collection, it is known that juvenile Mulloway under 4 years of age (i.e. juveniles and sub-adults) are predominantly found in estuaries, depending on the region (Barnes et al. 2019). As these fish sampled were captured within estuaries, it is likely that their otolith chemistry reflects the water chemistry of the estuary of capture. It is under this assumption, therefore, that chemistry collected from the otolith edge could represent elemental fingerprints of known-origin fish, as a proxy for their juvenile chemical environment. We note that residence time is a potential source of bias that can occur alongside additional factors (e.g. time lag in the incorporation of elements). Additional experiments may be employed to overcome this bias, such as holding juvenile fish in cages within study estuaries for a period of time, such that migration history within the previous 20 days before analysis is known (e.g. Mohan et al. 2012). In situ caging was beyond the scope of the current study; however, such methods may also help to quantify small-scale temporal and spatial variation in elemental fingerprints as a function of variations in water chemistry, point sources of contaminants and/or fish movements.
Conclusion
In light of the array of potential confounding factors, employing otolith elemental chemistry as a proxy for fish habitat use remains challenging due to the dynamic nature of these coastal environments. The current study was essentially an allocation exercise, which incorporated an opportunistic sample collection approach and provided a satisfactory allocation result. The current suite of analysed elements, however, was not sufficient to fully distinguish all of the locations examined. For the locations with relatively high allocation success, the study provides a preliminary baseline of otolith fingerprints for juvenile Mulloway. This will support future resolution and interpretation of natal origins, estuary-to-ocean visitations, seasonal movements of Mulloway and identification of potential nurseries for adult fish. Improved sampling design, however, may be required for areas that had poor classification outcomes, such as the Macleay River and Shoalhaven River. When conducting future analyses, the numerous complexities and potential biases outlined above should be considered (e.g. temporal and ontogenetic stability of fingerprints), and additional chemical and non-chemical markers incorporated to better understand their influence. For Mulloway, establishing such linkages between estuarine nurseries and exploited size-classes will aid our understanding of the impact of habitat degradation and by-catch (Broadhurst and Kennelly 1994), and ultimately support targeted conservation efforts.
Notes
With the exception of Georges River, which is not a commercially fished estuary; samples for this estuary were obtained from independent mesh net sampling.
References
Able, K.W. 2005. A re-examination of fish estuarine dependence: evidence for connectivity between estuarine and ocean habitats. Estuarine, Coastal and Shelf Science 64 (1): 5–17.
Archangi, B. 2008. Levels and patterns of genetic diversity in wild and cultured populations of mulloway (Argyrosomus japonicus) using mitochondrial DNA and microsatellites. Queensland University of Technology.
Barnes, T.C., and B.M. Gillanders. 2013. Combined effects of extrinsic and intrinsic factors on otolith chemistry: implications for environmental reconstructions. Canadian Journal of Fisheries and Aquatic Sciences 70 (8): 1159–1166.
Barnes, T.C., C. Junge, S.A. Myers, M.D. Taylor, P.J. Rogers, G.J. Ferguson, J.A. Lieschke, S.C. Donnellan, and B.M. Gillanders. 2016. Population structure in a wide-ranging coastal teleost (Argyrosomus japonicus, Sciaenidae) reflects marine biogeography across southern Australia. Marine and Freshwater Research 67 (8): 1103–1113.
Barnes, T.C., P.J. Rogers, Y. Wolf, A. Madonna, D. Holman, G.J. Ferguson, W. Hutchinson, A. Loisier, D. Sortino, and M. Sumner. 2019. Dispersal of an exploited demersal fish species (Argyrosomus japonicus, Sciaenidae) inferred from satellite telemetry. Marine Biology 166 (10): 125.
Bath, G.E., S.R. Thorrold, C.M. Jones, S.E. Campana, J.W. McLaren, and J.W. Lam. 2000. Strontium and barium uptake in aragonitic otoliths of marine fish. Geochimica et Cosmochimica Acta 64 (10): 1705–1714.
Beck, M.W., K.L. Heck, K.W. Able, D.L. Childers, D.B. Eggleston, B.M. Gillanders, B. Halpern, C.G. Hays, K. Hoshino, T.J. Minello, R.J. Orth, P.F. Sheridan, and M.R. Weinstein. 2001. The identification, conservation, and management of estuarine and marine nurseries for fish and invertebrates. Bioscience 51 (8): 633–641.
Becker, A., A.K. Whitfield, P.D. Cowley, and V.J. Cole. 2017. Does water depth influence size composition of estuary-associated fish? Distributions revealed using mobile acoustic-camera transects along the channel of a small shallow estuary. Marine and Freshwater Research 68 (11): 2163–2169.
Bell, J.D., K.M. Leber, H.L. Blankenship, N.R. Loneragan, and R. Masuda. 2008. A new era for restocking, stock enhancement and sea ranching of coastal fisheries resources. Reviews in Fisheries Science 16 (1-3): 1–9.
Bertelli, C.M., and R.K.F. Unsworth. 2014. Protecting the hand that feeds us: seagrass (Zostera marina) serves as commercial juvenile fish habitat. Marine Pollution Bulletin 83 (2): 425–429.
Bode, M., L. Bode, and P.R. Armsworth. 2006. Larval dispersal reveals regional sources and sinks in the Great Barrier Reef. Marine Ecology Progress Series 308: 17–25.
Bourel, M., and A.M. Segura. 2018. Multiclass classification methods in ecology. Ecological Indicators 85: 1012–1021.
Bourret, S.L., B.P. Kennedy, C.C. Caudill, and P.M. Chittaro. 2014. Using otolith chemical and structural analysis to investigate reservoir habitat use by juvenile Chinook salmon Oncorhynchus tshawytscha. Journal of Fish Biology 85 (5): 1507–1525.
Boys, C.A., and B. Pease. 2017. Opening the floodgates to the recovery of nektonic assemblages in a temperate coastal wetland. Marine and Freshwater Research 68 (6): 1023–1035.
Broadhurst, M., and S. Kennelly. 1994. Reducing the by-catch of juvenile fish (mulloway Argyrosomus hololepidotus) using square-mesh panels in codends in the Hawkesbury River prawn-trawl fishery, Australia. Fisheries Research 19 (3-4): 321–331.
Brophy, D., T.E. Jeffries, and B.S. Danilowicz. 2004. Elevated manganese concentrations at the cores of clupeid otoliths: possible environmental, physiological, or structural origins. Marine Biology 144 (4): 779–786.
Brown, R.J., and K.P. Severin. 2009. Otolith chemistry analyses indicate that water Sr: Ca is the primary factor influencing otolith Sr: Ca for freshwater and diadromous fish but not for marine fish. Canadian Journal of Fisheries and Aquatic Sciences 66 (10): 1790–1808.
Bureau of Meteorology. 2019. Climate data online. http://www.bom.gov.au/climate/data/: accessed 11 September 2019.
Campana, S. 2013. Otolith elemental as a natural marker of fish stocks. Stock Identification Methods: Applications in Fishery Science: 227–245.
Campana, S.E., and S.R. Thorrold. 2001. Otoliths, increments, and elements: keys to a comprehensive understanding of fish populations? Canadian Journal of Fisheries and Aquatic Sciences 58 (1): 30–38.
Campana, S.E., A.J. Fowler, and C.M. Jones. 1994. Otolith elemental fingerprinting for stock identification of Atlantic Cod (Gadus morhua) using laser ablation ICPMS. Canadian Journal of Fisheries and Aquatic Sciences 51 (9): 1942–1950.
Campana, S.E., A. Valentin, J.-M. Sévigny, and D. Power. 2007. Tracking seasonal migrations of redfish (Sebastes spp.) in and around the Gulf of St. Lawrence using otolith elemental fingerprints. Canadian Journal of Fisheries and Aquatic Sciences 64 (1): 6–18.
Cooper, L., J. McClelland, R. Holmes, P. Raymond, J. Gibson, C. Guay, and B. Peterson. 2008. Flow-weighted values of runoff tracers (δ18O, DOC, Ba, alkalinity) from the six largest Arctic rivers. Geophysical Research Letters 35.
Crook, D.A., K. Lacksen, A.J. King, D.J. Buckle, S.J. Tickell, J.D. Woodhead, R. Maas, S.A. Townsend, and M.M. Douglas. 2016. Temporal and spatial variation in strontium in a tropical river: Implications for otolith chemistry analyses of fish migration. Canadian Journal of Fisheries and Aquatic Sciences 74: 533–545.
Dahlgren, C.P., G.T. Kellison, A.J. Adams, B.M. Gillanders, M.S. Kendall, C.A. Layman, J.A. Ley, I. Nagelkerken, and J.E. Serafy. 2006. Marine nurseries and effective juvenile habitats: concepts and applications. Marine Ecology Progress Series 312: 291–295.
De Vries, M.C., B.M. Gillanders, and T.S. Elsdon. 2005. Facilitation of barium uptake into fish otoliths: influence of strontium concentration and salinity. Geochimica et Cosmochimica Acta 69 (16): 4061–4072.
Dias, P.C. 1996. Sources and sinks in population biology. Trends in Ecology & Evolution 11 (8): 326–330.
DiBacco, C., and L.A. Levin. 2000. Development and application of elemental fingerprinting to track the dispersal of marine invertebrate larvae. Limnology and Oceanography 45 (4): 871–880.
Disspain, M.C.F., S. Ulm, and B.M. Gillanders. 2016. Otoliths in archaeology: methods, applications and future prospects. Journal of Archaeological Science-Reports 6: 623–632.
Dolbeth, M., F. Martinho, I. Viegas, H. Cabral, and M. Pardal. 2008. Estuarine production of resident and nursery fish species: conditioning by drought events? Estuarine, Coastal and Shelf Science 78 (1): 51–60.
Dorval, E., C.M. Jones, R. Hannigan, and J.v. Montfrans. 2007. Relating otolith chemistry to surface water chemistry in a coastal plain estuary. Canadian Journal of Fisheries and Aquatic Sciences 64 (3): 411–424.
Doubleday, Z.A., C. Izzo, S.H. Woodcock, and B.M. Gillanders. 2013. Relative contribution of water and diet to otolith chemistry in freshwater fish. Aquatic Biology 18 (3): 271–280.
Earl, J., D. Fairclough, J. Staunton-Smith, and J. Hughes. 2019. Mulloway (Argyrosomus japonicus). In In status of key Australian fish stocks 2018. Canberra: Fisheries Research and Development Corporation.
Elsdon, T.S., and B.M. Gillanders. 2002. Interactive effects of temperature and salinity on otolith chemistry: challenges for determining environmental histories of fish. Canadian Journal of Fisheries and Aquatic Sciences 59 (11): 1796–1808.
Elsdon, T.S., and B.M. Gillanders. 2003. Relationship between water and otolith elemental concentrations in juvenile black bream Acanthopagrus butcheri. Marine Ecology Progress Series 260: 263–272.
Elsdon, T., and B. Gillanders. 2005a. Strontium incorporation into calcified structures: separating the effects of ambient water concentration and exposure time. Marine Ecology Progress Series 285: 233–243.
Elsdon, T.S., and B.M. Gillanders. 2005b. Alternative life-history patterns of estuarine fish: barium in otoliths elucidates freshwater residency. Canadian Journal of Fisheries and Aquatic Sciences 62 (5): 1143–1152.
Elsdon, T.S., B.K. Wells, S.E. Campana, B.M. Gillanders, C.M. Jones, K.E. Limburg, D.H. Secor, S.R. Thorrold, and B.D. Walther. 2008. Otolith chemistry to describe movements and life-history parameters of fishes: hypotheses, assumptions, limitations and inferences. Oceanography and Marine Biology: An Annual Review 46: 297–330.
Elsenbeer, H., A. Lack, and K. Cassel. 1995. Chemical fingerprints of hydrological compartments and flow paths at La Cuenca, western Amazonia. Water Resources Research 31 (12): 3051–3058.
Farmer, B. 2008. Comparisons of the biological and genetic characteristics of the Mulloway Argyrosomus japonicus (Sciaenidae) in different regions of Western Australia. Murdoch University.
Ferguson, G.J., T.M. Ward, and B.M. Gillanders. 2011. Otolith shape and elemental composition: Complementary tools for stock discrimination of mulloway (Argyrosomus japonicus) in southern Australia. Fisheries Research 110 (1): 75–83.
Fowler, A.M., S.M. Smith, D.J. Booth, and J. Stewart. 2016. Partial migration of grey mullet (Mugil cephalus) on Australia’s east coast revealed by otolith chemistry. Marine Environmental Research 119: 238–244.
Gahagan, B.I., J.C. Vokoun, G.W. Whitledge, and E.T. Schultz. 2012. Evaluation of otolith microchemistry for identifying natal origin of anadromous river herring in Connecticut. Marine and Coastal Fisheries 4 (1): 358–372.
Geffen, A.J., R.D.M. Nash, and M. Dickey-Collas. 2011. Characterization of herring populations west of the British Isles: an investigation of mixing based on otolith microchemistry. ICES Journal of Marine Science 68 (7): 1447–1458.
Gibb, F.M., T. Régnier, K. Donald, and P.J. Wright. 2017. Connectivity in the early life history of sandeel inferred from otolith microchemistry. Journal of Sea Research 119: 8–16.
Gillanders, B.M. 2002a. Connectivity between juvenile and adult fish populations: do adults remain near their recruitment estuaries? Marine Ecology Progress Series 240: 215–223.
Gillanders, B.M. 2002b. Temporal and spatial variability in elemental composition of otoliths: implications for determining stock identity and connectivity of populations. Canadian Journal of Fisheries and Aquatic Sciences 59 (4): 669–679.
Gillanders, B.M. 2005. Otolith chemistry to determine movements of diadromous and freshwater fish. Aquatic Living Resources 18 (3): 291–300.
Gillanders, B.M., and M.J. Kingsford. 1996. Elements in otoliths may elucidate the contribution of estuarine recruitment to sustaining coastal reef populations of a temperate reef fish. Marine Ecology Progress Series 141: 13–20.
Gillanders, B.M., and M.J. Kingsford. 2000. Elemental fingerprints of otoliths of fish may distinguish estuarine ‘nursery’ habitats. Marine Ecology Progress Series 201: 273–286.
Gillanders, B.M., K.W. Able, J.A. Brown, D.B. Eggleston, and P.F. Sheridan. 2003. Evidence of connectivity between juvenile and adult habitats for mobile marine fauna: an important component of nurseries. Marine Ecology Progress Series 247: 281–295.
Gillanders, B.M., C. Izzo, Z.A. Doubleday, and Q. Ye. 2015. Partial migration: growth varies between resident and migratory fish. Biology Letters 11 (3): 20140850.
Gomes, I., L.G. Peteiro, R. Albuquerque, R. Nolasco, J. Dubert, S.E. Swearer, and H. Queiroga. 2016. Wandering mussels: using natural tags to identify connectivity patterns among marine protected areas. Marine Ecology Progress Series 552: 159–176.
Grammer, G.L., J.R. Morrongiello, C. Izzo, P.J. Hawthorne, J.F. Middleton, and B.M. Gillanders. 2017. Coupling biogeochemical tracers with fish growth reveals physiological and environmental controls on otolith chemistry. Ecological Monographs 87 (3): 487–507.
Gray, C.A., and V.C. McDonall. 1993. Distribution and growth of juvenile mulloway, (Argyrosomus hololepidotus) (Pisces: Sciaenidae), in the Hawkesbury River, South-Eastern Australia. Marine and Freshwater Research 44 (3): 401–409.
Griffiths, M.H. 1996. Life history of the dusky kob Argyrosomus japonicus (Sciaenidae) off the east coast of South Africa. South African Journal of Marine Science-Suid-Afrikaanse Tydskrif Vir Seewetenskap 17 (1): 135–154.
Griffiths, M., and C. Attwood. 2005. Do dart tags suppress growth of dusky kob Argyrosomus japonicus? African Journal of Marine Science 27 (2): 505–508.
Grimes, C.B., and M.J. Kingsford. 1996. How do riverine plumes of different sizes influence fish larvae: do they enhance recruitment? Marine and Freshwater Research 47 (2): 191–208.
Gunter, G. 1967. Some relationships of estuaries to the fisheries of the Gulf of Mexico. In Estuaries, ed. G.H. Lauff, 621–638. Washington, DC: American Association for the Advancement of Science.
Hall, D. 1984. The Coorong: biology of the major fish species and fluctuations in catch rates 1976-1983. Safic 8: 3–17.
Hall, D.A. 1986. An assessment of the mulloway (Argyrosomus hololepidotus) fishery in South Australia with particular reference to the Coorong Lagoon: a discussion paper: Department of Fisheries, South Australia.
Helsel, D.R. 2006. Fabricating data: How substituting values for nondetects can ruin results, and what can be done about it. Chemosphere 65 (11): 2434–2439.
Hsieh, Y., J.-C. Shiao, S.-w. Lin, and Y. Iizuka. 2019. Quantitative reconstruction of salinity history by otolith oxygen stable isotopes: an example of a euryhaline fish Lateolabrax japonicus. Rapid Communications in Mass Spectrometry 33 (16): 1344–1354.
Hüssy, K., K. Limburg, H. De Pontual, O. Thomas, P. Cook, Y. Heimbrand, M. Blass, and A. Sturrock. 2020. Trace element patterns in otoliths: the role of biomineralization. Reviews in Fisheries Science & Aquaculture: 1–33.
Izzo, C., C. Huveneers, M. Drew, C.J.A. Bradshaw, S.C. Donnellan, and B.M. Gillanders. 2016. Vertebral chemistry demonstrates movement and population structure of bronze whaler. Marine Ecology Progress Series 556: 195–207.
Izzo, C., P. Reis-Santos, and B.M. Gillanders. 2018. Otolith chemistry does not just reflect environmental conditions: a meta-analytic evaluation. Fish and Fisheries 19 (3): 441–454.
Lazartigues, A.V., S. Plourde, J.J. Dodson, O. Morissette, P. Ouellet, and P. Sirois. 2016. Determining natal sources of capelin in a boreal marine park using otolith microchemistry. ICES Journal of Marine Science 73 (10): 2644–2652.
Limburg, K.E., C. Olson, Y. Walther, D. Dale, C.P. Slomp, and H. Høie. 2011. Tracking Baltic hypoxia and cod migration over millennia with natural tags. Proceedings of the National Academy of Sciences of the United States of America 108 (22): E177–E182.
Limburg, K.E., M.J. Wuenschel, K. Hüssy, Y. Heimbrand, and M. Samson. 2018. Making the otolith magnesium chemical calendar-clock tick: plausible mechanism and empirical evidence. Reviews in Fisheries Science & Aquaculture 26 (4): 479–493.
Litvin, S.Y., M.P. Weinstein, M. Sheaves, and I. Nagelkerken. 2018. What makes nearshore habitats nurseries for nekton? An emerging view of the nursery role hypothesis. Estuaries and Coasts 41 (6): 1539–1550.
Liu, O.R., K.M. Kleisner, S.L. Smith, and J.P. Kritzer. 2018. The use of spatial management tools in rights-based groundfish fisheries. Fish and Fisheries 19 (5): 821–838.
Lorenzen, K. 2008. Understanding and managing enhancement fisheries systems. Reviews in Fisheries Science 16 (1-3): 10–23.
Marriott, A., I. McCarthy, A. Ramsay, and S. Chenery. 2016. Discriminating nursery grounds of juvenile plaice (Pleuronectes platessa) in the south-eastern Irish Sea using otolith microchemistry. Marine Ecology Progress Series 546: 183–195.
McMillan, M., C. Izzo, B. Wade, and B. Gillanders. 2017. Elements and elasmobranchs: hypotheses, assumptions and limitations of elemental analysis. Journal of Fish Biology 90 (2): 559–594.
Miller, J.A. 2011. Effects of water temperature and barium concentration on otolith composition along a salinity gradient: implications for migratory reconstructions. Journal of Experimental Marine Biology and Ecology 405 (1-2): 42–52.
Milton, D.A., and S.R. Chenery. 2001. Sources and uptake of trace metals in otoliths of juvenile barramundi (Lates calcarifer). Journal of Experimental Marine Biology and Ecology 264 (1): 47–65.
Miyan, K., M.A. Khan, D.K. Patel, S. Khan, and S. Prasad. 2016. Otolith fingerprints reveal stock discrimination of Sperata seenghala inhabiting the Gangetic river system. Ichthyological Research 63 (2): 294–301.
Mohan, J.A., R.A. Rulifson, D.R. Corbett, and N.M. Halden. 2012. Validation of oligohaline elemental otolith signatures of striped bass by use of in situ caging experiments and water chemistry. Marine and Coastal Fisheries 4 (1): 57–70.
Nagelkerken, I., M. Sheaves, R. Baker, and R.M. Connolly. 2015. The seascape nursery: a novel spatial approach to identify and manage nurseries for coastal marine fauna. Fish and Fisheries 16 (2): 362–371.
NSW Department of Planning Industry and Environment. 2020. SEED The Central Resource for Sharing and Enabling Environmental Data in NSW. https://datasets.seed.nsw.gov.au/dataset/estuary-catchment-streamflow-and-surface-runoff-1975-20078b0ae/resource/914d995f-c890-4847-bf5d-bb29a297b46d: accessed 6 July 2020.
Paton, C., J. Hellstrom, B. Paul, J. Woodhead, and J. Hergt. 2011. Iolite: freeware for the visualisation and processing of mass spectrometric data. Journal of Analytical Atomic Spectrometry 26 (12): 2508–2518.
Potter, I., and G. Hyndes. 1999. Characteristics of the ichthyofaunas of southwestern Australian estuaries, including comparisons with holarctic estuaries and estuaries elsewhere in temperate Australia: a review. Australian Journal of Ecology 24 (4): 395–421.
Pursche, A.R., I.M. Suthers, and M.D. Taylor. 2013. Post-release monitoring of site and group fidelity in acoustically tagged stocked fish. Fisheries Management and Ecology 20 (5): 445–453.
R Development Core Team. 2010. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.
Raoult, V., T.F. Gaston, and M.D. Taylor. 2018. Habitat–fishery linkages in two major south-eastern Australian estuaries show that the C4 saltmarsh plant Sporobolus virginicus is a significant contributor to fisheries productivity. Hydrobiologia 811 (1): 221–238.
Raybaud, V., S. Tambutté, C. Ferrier-Pagès, S. Reynaud, A.A. Venn, É. Tambutté, P. Nival, and D. Allemand. 2017. Computing the carbonate chemistry of the coral calcifying medium and its response to ocean acidification. Journal of Theoretical Biology 424: 26–36.
Reis-Santos, P., B.M. Gillanders, S.E. Tanner, R.P. Vasconcelos, T.S. Elsdon, and H.N. Cabral. 2012. Temporal variability in estuarine fish otolith elemental fingerprints: implications for connectivity assessments. Estuarine, Coastal and Shelf Science 112: 216–224.
Reis-Santos, P., S.E. Tanner, R.P. Vasconcelos, T.S. Elsdon, H.N. Cabral, and B.M. Gillanders. 2013. Connectivity between estuarine and coastal fish populations: contributions of estuaries are not consistent over time. Marine Ecology Progress Series 491: 177–186.
Reis-Santos, P., R.P. Vasconcelos, S.E. Tanner, V.F. Fonseca, H.N. Cabral, and B.M. Gillanders. 2018. Extrinsic and intrinsic factors shape the ability of using otolith chemistry to characterize estuarine environmental histories. Marine Environmental Research 140: 332–341.
Rönnbäck, P. 1999. The ecological basis for economic value of seafood production supported by mangrove ecosystems. Ecological Economics 29 (2): 235–252.
Rose, K.A., S. Sable, D.L. DeAngelis, S. Yurek, J.C. Trexler, W. Graf, and D.J. Reed. 2015. Proposed best modeling practices for assessing the effects of ecosystem restoration on fish. Ecological Modelling 300: 12–29.
Roy, P.S., R.J. Williams, A.R. Jones, I. Yassini, P.J. Gibbs, B. Coates, R.J. West, P.R. Scanes, J.P. Hudson, and S. Nichol. 2001. Structure and function of south-east Australian estuaries. Estuarine Coastal and Shelf Science 53 (3): 351–384.
Ruas, L.C., and A.M. Vaz-dos-Santos. 2017. Age structure and growth of the rough scad, Trachurus lathami (Teleostei: Carangidae), in the southeastern Brazilian bight. Zoologia 34: 11.
Sakabe, R., and J.M. Lyle. 2010. The influence of tidal cycles and freshwater inflow on the distribution and movement of an estuarine resident fish Acanthopagrus butcheri. Journal of Fish Biology 77 (3): 643–660.
Sanchez-Jerez, P., B. Gillanders, and M. Kingsford. 2002. Spatial variability of trace elements in fish otoliths: comparison with dietary items and habitat constituents in seagrass meadows. Journal of Fish Biology 61 (3): 801–821.
Schaffler, J., T. Miller, and C. Jones. 2014. Spatial and temporal variation in otolith chemistry of juvenile Atlantic Menhaden in the Chesapeake Bay. Transactions of the American Fisheries Society 143(4): 1061–1071
Schilling, H.T., P. Reis-Santos, J.M. Hughes, J.A. Smith, J.D. Everett, J. Stewart, B.M. Gillanders, and I.M. Suthers. 2018. Evaluating estuarine nursery use and life history patterns of Pomatomus saltatrix in eastern Australia. Marine Ecology Progress Series. 598: 187–199.
Secor, D.H., and J.R. Rooker. 2000. Is otolith strontium a useful scalar of life cycles in estuarine fishes? Fisheries Research 46 (1-3): 359–371.
Silberschneider, V., and C.A. Gray. 2005. Arresting the decline of the commercial and recreational fisheries for mulloway (Argyrosomus japonicus). NSW DPI Fisheries Final Report Series No. 82. New South Wales Fisheries, Cronulla.
Silberschneider, V., and C. Gray. 2008. Synopsis of biological, fisheries and aquaculture-related information on mulloway Argyrosomus japonicus (Pisces: Sciaenidae), with particular reference to Australia. Journal of Applied Ichthyology 24: 7–17.
Stewart, J., J.M. Hughes, C. Stanley, and A.M. Fowler. 2020. The influence of rainfall on recruitment success and commercial catch for the large sciaenid, Argyrosomus japonicus, in eastern Australia. Marine Environmental Research 157: 104924.
Sturrock, A.M., C.N. Trueman, A.M. Darnaude, and E. Hunter. 2012. Can otolith elemental chemistry retrospectively track migrations in fully marine fishes? Journal of Fish Biology 81 (2): 766–795.
Sturrock, A.M., C.N. Trueman, J.A. Milton, C.P. Waring, M.J. Cooper, and E. Hunter. 2014. Physiological influences can outweigh environmental signals in otolith microchemistry research. Marine Ecology Progress Series 500: 245–264.
Taddese, F., M.R. Reid, and G.P. Closs. 2019. Direct relationship between water and otolith chemistry in juvenile estuarine triplefin Forsterygion nigripenne. Fisheries Research 211: 32–39.
Tanner, S.E., P. Reis-Santos, and H.N. Cabral. 2016. Otolith chemistry in stock delineation: a brief overview, current challenges and future prospects. Fisheries Research 173: 206–213.
Taylor, M., S. Laffan, S. Fielder, and I. Suthers. 2006. Key habitat and home range of mulloway Argyrosomus japonicus in a south-east Australian estuary: finding the estuarine niche to optimise stocking. Marine Ecology Progress Series 328: 237–247.
Taylor, M.D., D.E. van der Meulen, M.C. Ives, C.T. Walsh, I.V. Reinfelds, and C.A. Gray. 2014. Shock, stress or signal? Implications of freshwater flows for a top-level estuarine predator. PLOS ONE 9.
Taylor, M.D., T.F. Gaston, and V. Raoult. 2018. The economic value of fisheries harvest supported by saltmarsh and mangrove productivity in two Australian estuaries. Ecological Indicators 84: 701–709.
Thomas, O.R.B., and S.E. Swearer. 2019. Otolith biochemistry—a review. Reviews in Fisheries Science & Aquaculture 27 (4): 458–489.
Thomas, O.R., K. Ganio, B.R. Roberts, and S.E. Swearer. 2017. Trace element–protein interactions in endolymph from the inner ear of fish: implications for environmental reconstructions using fish otolith chemistry. Metallomics 9 (3): 239–249.
Thorrold, S.R., C.M. Jones, P.K. Swart, and T.E. Targett. 1998. Accurate classification of juvenile weakfish Cynoscion regalis to estuarine nursery areas based on chemical signatures in otoliths. Marine Ecology Progress Series 173: 253–265.
Thorrold, S.R., C. Latkoczy, P.K. Swart, and C.M. Jones. 2001. Natal homing in a marine fish metapopulation. Science 291 (5502): 297–299.
Trueman, C.N., and K. St John Glew. 2019. Chapter 6 - Isotopic tracking of marine animal movement. In Tracking animal migration with stable isotopes, ed. K.A. Hobson and L.I. Wassenaar, 2nd ed., 137–172. Academic Press.
Tsukamoto, K., and T. Arai. 2001. Facultative catadromy of the eel Anguilla japonica between freshwater and seawater habitats. Marine Ecology Progress Series 220: 265–276.
Vasconcelos, R.P., P. Reis-Santos, M.J. Costa, and H.N. Cabral. 2011. Connectivity between estuaries and marine environment: integrating metrics to assess estuarine nursery function. Ecological Indicators 11 (5): 1123–1133.
Walther, B.D. 2019. The art of otolith chemistry: interpreting patterns by integrating perspectives. Marine and Freshwater Research 70 (12): 1643.
Walther, B., and K. Limburg. 2012. The use of otolith chemistry to characterize diadromous migrations. Journal of Fish Biology 81 (2): 796–825.
Walther, B.D., and M.K. Nims. 2015. Spatiotemporal variation of trace elements and stable isotopes in subtropical estuaries: I. freshwater endmembers and mixing curves. Estuaries and Coasts 38 (3): 754–768.
Walther, B.D., and S.R. Thorrold. 2006. Water, not food, contributes the majority of strontium and barium deposited in the otoliths of a marine fish. Marine Ecology Progress Series 311: 125–130.
Walther, B.D., M.J. Kingsford, and M.T. McCulloch. 2013. Environmental records from Great Barrier Reef corals: inshore versus offshore drivers. PLoS One 8 (10): e77091.
Wells, R.J.D., M.J. Kinney, S. Kohin, H. Dewar, J.R. Rooker, and O.E. Snodgrass. 2015. Natural tracers reveal population structure of albacore (Thunnus alalunga) in the eastern North Pacific. ICES Journal of Marine Science 72 (7): 2118–2127.
West, R.J. 1992. Mulloway. The Australian Anglers Fishing World 1992: 84–85.
Wood, S.N. 2017. Generalized additive models: an introduction with R. Chapman and Hall/CRC.
Yamashita, Y., T. Otake, and H. Yamada. 2000. Relative contributions from exposed inshore and estuarine nursery grounds to the recruitment of stone flounder, Platichthys bicoloratus, estimated using otolith Sr:Ca ratios. Fisheries Oceanography 9 (4): 316–327.
Yoshinaga, J., A. Nakama, M. Morita, and J.S. Edmonds. 2000. Fish otolith reference material for quality assurance of chemical analyses. Marine Chemistry 69 (1-2): 91–97.
Zohar, Y., A.H. Hines, O. Zmora, E.G. Johnson, R.N. Lipcius, R.D. Seitz, D.B. Eggleston, A.R. Place, E.J. Schott, J.D. Stubblefield, and J.S. Chung. 2008. The Chesapeake Bay blue crab (Callinectes sapidus): a multidisciplinary approach to responsible stock replenishment. Reviews in Fisheries Science 16 (1-3): 24–34.
Zuur, A.F., E.N. Ieno, and C.S. Elphick. 2010. A protocol for data exploration to avoid common statistical problems. Methods in Ecology and Evolution 1 (1): 3–14.
Acknowledgments
The authors wish to thank the NSW Fishermen’s Co-operatives and NSW commercial fishers who contributed to the project. We thank Adelaide Microscopy (AM) and Microscopy Australia, with special thanks to S. Gilbert and B. Wade at the LA-ICP-MS unit (AM) for technical assistance. Many thanks go to the NSW Recreational Anglers Program, and A. Gould, J. Hughes, C. Stanley and A. Pidd. Animal handling was permitted under the University of Adelaide’s Animal Ethics Approval (S-2018-014) and Animal Research Authority NSW DPI 07/03. This project was supported by the Fisheries Research and Development Corporation on behalf of the Australian Government (project 2016/020), and financial support was provided to A.L.R. through an Australian Government Research Training Program Scholarship at the University of Adelaide.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Henrique Cabral
Electronic Supplementary Material
ESM 1
(DOCX 118 kb)
Rights and permissions
About this article
Cite this article
Russell, A.L., Gillanders, B.M., Barnes, T.C. et al. Inter-estuarine Variation in Otolith Chemistry in a Large Coastal Predator: a Viable Tool for Identifying Coastal Nurseries?. Estuaries and Coasts 44, 1132–1146 (2021). https://doi.org/10.1007/s12237-020-00825-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-020-00825-x