Abstract
Sponges harbor great diversity and an abundance of organisms. Although this community can vary temporally, and between and within sponge species, it is not known how an induced change in habitat-specific environmental conditions might affect the structure of macroinvertebrate assemblages associated with sponges. Here, a reciprocal transplant experiment of individuals of the estuarine sponge Halichondria melanadocia was conducted between two neighboring habitats (seagrass and mangrove prop root habitats) in the Southern Gulf of Mexico. Transplanted sponges experienced different hydrodynamic and light conditions. Multivariate analyses showed differences in community structure of associated assemblages among treatments, which were mainly driven by changes in the abundance of common species. Sponges moved from seagrass to mangrove habitat experienced a significant increase in abundance and taxonomic richness of 88 and 35%, respectively. Given that changes in the volume and aquiferous system (oscula diameter and density) of the hosts and in salinity were also recorded during the study period, it is concluded that the structure of the H. melanadocia-associated assemblages was not only influenced by the habitat change but also by other factors such as host morphology and short-term variations in the population abundance of associated organisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Because of their three-dimensional architecture, the presence of oscular openings and multiple channels that interconnect in their interior, marine sponges have often been regarded as ecosystem engineers. They can offer a suitable microhabitat for either temporary or permanent residents, alter and ameliorate physical conditions, and affect biological interactions (Sara and Vacelet 1973; Miller et al. 2012; Gerovasileiou et al. 2016). Likewise, depending on the type of interaction, the associated organisms (epi- and endobionts) can receive benefit from the sponge host by obtaining substrate, structural support, food and protection against desiccation, wave action, and predation (Frith 1976; Vance 1978; Bloom 1981; Uriz et al. 1992; Henkel and Pawlik 2005; Ďuriš et al. 2011).
Diversity and abundance of sponge-inhabiting organisms are known to vary in relation to the host size and morphology (internal and external). In many case studies, the sponge volume has positively correlated with the abundance and diversity of associated organisms (Koukouras et al. 1992; Ribeiro et al. 2003). Likewise, sponge species with relatively more complex forms (massive, branched, etc.) generally harbor greater abundance of associated fauna than less complex ones (encrusting growth forms), due to the availability of a greater number of microrefuges (e.g., Pearse 1932; Frith 1976; Koukouras et al. 1985; Klitgaard 1995; Ribeiro et al. 2003; Neves and Omena 2003). A number of studies have also documented the influence of local environmental conditions (e.g., physical: depth, light availability, substrate type, hydrodynamic regime and sedimentation rate; biological: predation, competition for space, food availability and algal cover) on spatio-temporal variability in the assemblage structure of sponge-associated organisms (e.g., Pearse 1950; Peattie and Hoare 1981; Biernbaum 1981; Voultsiadou-Koukoura et al. 1987; Koukouras et al. 1996; Çinar et al. 2002; Ribeiro et al. 2003; Ávila and Ortega-Bastida 2015; Leite et al. 2016). For example, temperature may indirectly or directly influence faunal assemblages (Costa et al. 2015). Indirectly, temperature may lead to expansion of the area available for occupation and fixation and increase the availability of food and shelter, which may improve the survival of the associated fauna. Also, temperature (Clarke 1990) and other factors like reproduction (Sainte-Marie 1991) may directly influence the physiological processes of organisms.
So far, very few studies have successfully employed transplant experiments to test the role of environmental conditions on the sponge-associated organisms. While some studies have dealt with changes in species composition, abundance, morphology, size, metabolic integration, and physiological responses of associated organisms (e.g., cyanobacteria: Wilkinson and Vacelet 1979; microbes: Thoms et al. 2003; seaweeds: Enríquez et al. 2009; zooxanthellae: Weisz et al. 2010), the short-term effects of environmental change on the assemblage of sponge-associated macroinvertebrates are unclear. This is relevant not only to investigate the habitat-specific environmental conditions that can have an influence on the assemblage structure of sponge-associated macroinvertebrates but also to better understand the potential effects of a habitat change on the biodiversity associated with sponges.
A recent study conducted on the sponge Halichondria (Halichondria) melanadocia de Laubenfels, 1936, from a Southern Gulf of Mexico coastal lagoon, revealed that individuals of this species collected from seagrass beds (with massive-ramose form) had a significantly lower density of associated macroinvertebrates than individuals from adjacent mangrove prop root habitats (with thickly-encrusting form) (Ávila and Ortega-Bastida 2015). This work highlighted that, despite both habitats being adjacent, there were differences in the taxa composition of associated macroinvertebrate assemblages between habitats. This suggests that in addition to differences in sponge morphology, local environmental factors (such as those mentioned above) may influence the taxonomic composition of macroinvertebrate assemblages (Ávila and Ortega-Bastida 2015).
The aim of the present study was to perform a reciprocal transplant experiment of individuals of the sponge H. melanadocia between mangrove prop roots habitats and adjacent seagrass beds to test the hypothesis that habitat type leads to significant changes in the assemblage structure (e.g., species composition and abundance) of the macroinvertebrates associated with this sponge. The relative importance of different environmental variables (such as hydrodynamism, sedimentation rate, and light availability) and the phenotypic plasticity (oscula density and diameter) of the sponge in driving these differences are discussed.
Materials and Methods
Study Area
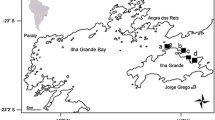
The study area is located within the Natural Protected Area Laguna de Terminos, at the southwestern Yucatan Peninsula, Mexico (Fig. 1). This is an estuarine-lagoon system with an average depth of 4 m and two mouths that are permanently connected to the Gulf of Mexico. Within this system, the sponge Halichondria melanadocia is commonly found in the prop root habitats of mangrove (Rhizophora mangle) and seagrass beds (composed of a mix of Thalassia testudinum/Halodule wrightii), mainly in the northern part of the lagoon. Individuals of H. melanadocia generally develop encrusting to massive forms when they live attached to mangrove roots, while in adjacent seagrass beds, individuals have a densely branched morphology (Ávila and Ortega-Bastida 2015). Based on these observations, a study area (about 1200 m2) was selected (18° 43′ 52.36″ N, 91° 34′ 40.69″ W) where H. melanadocia was abundant in adjacent seagrass bed and mangrove prop root habitats (Fig. 1).
Transplanting Experiment
To assess the influence of habitat-related environmental factors on the assemblage structure of macroinvertebrates associated with H. melanadocia, a reciprocal transplant experiment was conducted between the seagrass bed and adjacent mangrove root habitats (Carballo et al. 2006). Thirty individuals of this sponge of different sizes were randomly collected from each habitat. Individuals were detached from their base with a knife and carefully placed in plastic containers with seawater. Every specimen was labeled and measured using the fluid displacement method (Rützler 1978) to determine its initial volume (mL). Also, a quadrant of 5 cm × 5 cm was placed once on the surface of each specimen to determine the density of oscula (number of oscula per 25 cm2). The diameters (mm) of five oscula were measured with a ruler in order to obtain an average (± standard deviation) osculum diameter per specimen. In the case of the volume and density of oscula, an average (± SD) value was obtained (n = 30 specimens).
Each specimen was then attached to an artificial surface (20 cm × 20 cm square of rigid plastic mesh) using plastic cable ties. Twenty specimens from the seagrass bed were transplanted to the adjacent mangrove root habitat (treatment SM) and 20 specimens from mangrove habitat were transplanted to seagrass bed (treatment MS) (Fig. 2). In each habitat, the sponge individuals were placed 1 m apart from each other and the distance between each habitat was 50 m. As a control for transplant effects, ten specimens from each habitat had the same treatment (removed from their original substrate, volume measurement, attached to the artificial substrate and transportation), but they were placed back in their original habitat (seagrass bed habitat: control S and mangrove root habitat: control M) (Fig. 2). During collection, measurement of volume, and attachment to substrate, specimens were carefully manipulated and exposed to air for as short a period as possible (< 5 s at a time). Transplant of specimens was conducted underwater by snorkeling. In both habitats, transplants and controls were placed horizontally at about 5 cm above the bottom and between 0.8 and 1.0 m depth (Fig. 2). This experiment was conducted between April and July 2013 and lasted 108 days.
Inter-habitat reciprocal transplant experiment design, where individuals of H. melanadocia were transplanted from seagrass bed to adjacent mangrove prop root habitat and vice versa. Specimens transplanted from mangrove roots to seagrass habitat (MS) and from seagrass bed to mangrove root habitat (SM); control of mangrove (Control M) and seagrass bed (Control S)
Species Composition and Abundance of Associated Fauna
Ten individuals of H. melanadocia were collected from each habitat (IS = sponges collected from the seagrass bed, IM = sponges collected from the mangrove root habitat) to quantify the composition and abundance of the macroinvertebrate assemblage. This method was necessary, since separation of macroinvertebrates generally involves a complete dissection of sponge individuals, so it could not be done directly on the specimens that were used for transplants. In this case, each sponge individual was first covered with a plastic bag, detached from substratum, and then the bag was closed immediately to avoid the escape of fast-moving macroinvertebrates (Ribeiro et al. 2003). In the laboratory, the sponge volume and the oscula density and diameter were measured following the previously described methods.
Macroinvertebrates were separated from the sponge under a stereomicroscope (Stemi 305, Carl Zeiss Microscopy GmbH), and the seawater in the bags was filtered through a 0.5-mm sieve to recover any macroinvertebrates that might have detached from the sponge during transport (Ribeiro et al. 2003). Animals were fixed with 4% formaldehyde for 24 h before being transferred to 70% alcohol for preservation. The macroinvertebrates were identified to the lowest taxonomic level possible, individuals of each species were counted, and the average (± SD) abundance was expressed as the number of individuals per liter of sponge volume and total number of individuals per sponge. The number of species (or taxa) per sponge individual was also determined. At the end of the experiment, the macroinvertebrate assemblage was assessed using the same method. In addition, final measurements of the average (± SD) density (number of oscula per 25 cm2) and diameter (mm) of oscula were collected from transplanted individuals at the conclusion of the experiment.
Environmental Parameters
Water temperature (°C) was recorded daily during the study period at 12:00 hours by using a temperature sensor (HOBO Water Temperature Pro v2 Data Logger - U22-001), which was placed at the study site at 20 cm from the bottom. Salinity was recorded monthly using a YSI-63 multi-parameter meter. Sedimentation/resuspension rate in each habitat (seagrass bed and mangrove prop root habitat) was measured using a trap system consisting of four sets of plastic bottles of 23 cm height and 2.2 cm internal diameter (Blomquist and Kofoed 1981), with openings vertically positioned at 30 cm from the bottom. These sediment traps remained in each habitat for 12 days. The trapped material was repeatedly rinsed with distilled water to remove salts and dried at 60 °C for 24 h before weighing (see detailed method in Carballo et al. 2006). The total amount of sediment collected was then expressed as average (± SD) kg dry weight m−2 day−1 for each habitat.
Hydrodynamism was estimated using the “plaster dissolution” method, which measures loss in weight by plaster spheres during a determined period of time (see details in Gambi et al. 1989). At each site, four plaster spheres 5 cm in diameter were placed at about 20 cm above the bottom over 7 days (Naranjo et al. 1996). It has been suggested that loss of mass of each sphere is independent of flow direction and speed fluctuations (Denny 1988), but is linearly related with flow speed (Muus 1968; Komatsu and Kawai 1992; Maldonado and Young 1996) and water temperature. The hydrodynamism was expressed as the average (± SD) dissolution rate (% day−1) at each site.
Light intensity was measured (on cloudless days) using two sensors (HOBO Pendant Temperature/Light Data Logger) which were placed simultaneously in each habitat at 30 cm from the bottom. Both sensors remained for 5 days with light measurements (lx) recorded every hour, and average (± SD) daytime light intensity (07:00–19:00 hours) calculated for each habitat. These measurements (hydrodynamism, sedimentation/resuspension rate, and light intensity) were collected for comparative purposes and carried out simultaneously at each site. Given the proximity of the experimental sites of both habitats, temperature and salinity were measured in the central part of the study area to describe any variability during the experiment.
Data Analysis
Shapiro-Wilk’s and Levene’s tests were used to test for normality and homogeneity of the data (volume of sponges before and after the transplant, oscula density and diameter, abundance of associated macroinvertebrates, taxa richness, and number of taxa per sponge individual). Data of volume of sponges and abundance of macroinvertebrates had to be ln-transformed to meet criteria of normality and homoscedasticity. In order to determine whether the volume of sponge individuals showed significant variations among treatments and controls, one-way analyses of variance (one-way ANOVA) were performed before and after the transplant. To determine whether there was significant difference between the initial and final volume of sponges, oscula density and diameter, abundance of the associated macroinvertebrates, and taxa richness (number of higher taxa/sponge) in both treatments and controls, Student’s t tests were performed. In the case of the number of taxa per sponge individual, the data did not meet the criteria of normality and homogeneity of the variances, so to determine whether this variable varied between treatments, a Kruskal-Wallis test was performed.
To compare the taxonomic richness of the sponge-associated macroinvertebrates in individuals collected initially (IS vs. IM) and in those that were transplanted (SM vs. MS), Czekanowsky’s coefficient (Cz) was used: Cz = (2 W/A + B) 100 (Westinga and Hoetjes 1981), in which W is the number of shared taxa between sponges A and B. In the first case, A is the total number of taxa in sponges collected initially from the seagrass bed (IS), and B is the total number of taxa in sponges collected initially from the mangrove roots (IM). In the second case, A is the total number of taxa in sponges of treatment MS, and B is the total number of taxa in sponges of treatment SM. Cz may range between 0 and 100%, and at 100%, the taxonomic richness of sponges A and B is identical (Dauer and Simon 1975). Multivariate techniques were also used to evaluate changes in the structure of macroinvertebrate assemblages due to the transplant. A non-metric multidimensional scaling (nMDS) ordination was used to observe variability in the macroinvertebrate assemblages within and between the different experimental situations. A Bray-Curtis similarity matrix was calculated from abundance data for each macroinvertebrate species in each sponge (Bray and Curtis 1957), having been previously square root transformed (Field et al. 1982). K-dominance curves were used for comparing diversity of associated macroinvertebrates between sponges of each treatment (Lambshead et al. 1983). Analysis of similarity (ANOSIM) was performed to test differences between treatments. Also, a similarity percentage analysis (SIMPER) was used to explain aspects of the similarity between groups and to show those species that are responsible for the differences between groups (Clarke 1993). Multivariate analyses were performed using the PRIMER v6 software package (PRIMER-E Ltd., Plymouth, UK).
Finally, Student’s t tests were performed to determine whether there was a significant difference in the sedimentation/resuspension rate, the percentage of dissolution of plaster spheres, and light intensity, between the mangrove and seagrass habitats.
Results
Changes in Volume and in the Oscula Density and Diameter
The initial and final average volumes of sponges of each treatment were as follows: SM = 72.6 ± 31.7 mL and 30.3 ± 16.7 mL; MS = 89.4 ± 52.6 mL and 41.9 ± 25.4 mL; control S = 76 ± 31 mL and 91 ± 38.4 mL; control M = 125 ± 114.6 mL and 35 ± 47.7 mL. Sponges showed a significant decrease in volume for both treatments (SM: t 21.2 = 4.5, P < 0.01, MS: t 24.5 = 3.4, P < 0.01), but there were no significant changes in the volume of control sponges. At the end of the experiment, there was no significant (t = − 1.51, P > 0.05) difference in the final volume between treatments. Also, at the conclusion of the experiment, the final volume of control treatment S was significantly greater than treatment SM (t 23 = 5.43, P < 0.01), but there was no significant difference between control M and the treatment MS (t 19 = −0.38, P > 0.05).
Sponges collected from the seagrass bed (at the beginning of the experiment) had a mean oscula density of 2.5 ± 1.18 oscula per 25 cm2 and a mean diameter of 3.2 ± 1.4 mm, and those collected from the mangrove root habitat had a density of 1.4 ± 1.25 oscula per 25 cm2 and diameter of 1.9 ± 1.83 mm. There was a significant increase (t 26 = 1.73, P < 0.05) in the average oscula density of sponges in treatment MS (2.3 ± 1.3 oscula per 25 cm2, Fig. 3). Conversely, the oscula density of treatment SM (1.1 ± 1.3 oscula per 25 cm2) significantly decreased during the experiment (t 23 = 2.66, P < 0.05; Fig. 3). The oscula density was significantly higher (t 31 = −2.6, P < 0.05) in individuals of treatment MS than in those of treatment SM, and was significantly lower and higher than control treatments for MS (t 19 = −2.17, P < 0.05) and SM (t 23 = 2.54, P < 0.05), respectively (Fig. 3). Although the oscula diameter varied in a similar way, there were no significant differences between the initial and final data for either treatment (Fig. 3).
Average (± SD) oscula density and diameter in the individuals of H. melanadocia transplanted and in their respective controls before and after the experiment. Initial specimens collected from seagrass bed (IS) and mangrove roots (IM); specimens transplanted from mangrove roots to seagrass habitat (MS) and from seagrass bed to mangrove root habitat (SM); control of mangrove (Control M) and seagrass bed (Control S)
Changes in the Associated Macroinvertebrate Assemblages
A total of 39 species of macroinvertebrates (64% vagile and 36% sessile) were found in 66 individuals of H. melanadocia belonging to six higher taxonomic groups (13 Mollusca, 11 Annelida, 11 Arthropoda, 2 Echinodermata, 1 Porifera, and 1 Chordata; Table 1). At the beginning of the study, arthropods (subphylum Crustacea) were the most abundant group (42% of the total) in specimens collected from both habitats. Amphipods were the most abundant taxa within the arthropods (Fig. 4a), and polychaetes of the family Syllidae were the most abundant taxa within the annelids (13% in sponges from seagrass bed and 0.44% in sponges from mangrove root habitat). Poriferans and chordates (ascidians) were the least abundant groups (< 5%).
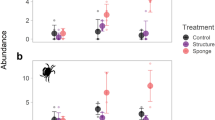
a Proportion of the major taxonomic groups associated with H. melanadocia before (in initial samples) and after the transplant (in treatments and controls). b Average abundance (± SD) and total number of taxa found in the individuals of H. melanadocia. Initial specimens collected from seagrass bed (IS) and mangrove roots (IM); specimens transplanted from mangrove roots to seagrass habitat (MS) and from seagrass bed to mangrove root habitat (SM); control of mangrove (Control M) and seagrass bed (Control S)
At the end of the experiment, a replacement of the dominant group was evident in both treatments. Echinoderms (mainly ophiuroids) replaced crustaceans as the dominant group, accounting for 44% in individuals of treatment SM and 45% in those of treatment MS. Besides the crustaceans, the relative abundance of polychaetes also showed a significant decrease in both treatments (18% in treatment SM and 72% in treatment MS). Chordates and poriferans remained as the least abundant groups (0.35 and 0.17%, respectively), without showing obvious changes in their abundance in relation to their initial values. Although these two groups are common in Rhizophora mangle roots, H. melanadocia was rarely overgrown by these macroinvertebrates. In the control S, crustaceans continued as the most abundant group (69%), but amphipods were replaced by isopods as the most abundant arthropod taxon. In control M, annelid polychaetes were the most abundant group (45%) and molluscs the least abundant (1%; Fig. 4a).
The overall mean abundance (expressed as individuals per liter of sponge, and hereafter referred to as ind L−1) of macroinvertebrates and the total taxonomic richness of sponge individuals collected initially was 1661 ± 2010 ind L−1 across 19 taxa in mangrove roots, and 205 ± 231.2 ind L−1 and 15 taxa in seagrass (Fig. 4b), with the abundance significantly higher in mangrove roots (t 18 = 2.4, P < 0.05). At the end of the experiment, the abundance of macroinvertebrates had increased significantly in treatment SM (t 23 = 3.8, P < 0.01) and control S (t 18 = −4.09, P < 0.01). The species driving this increase included Doris kyolis, Syllis prolifera, Ophiactis savignyi, Synaptula hydriformis, Elasmopus sp., and Ligia cf. exotica. Overall abundance did not change significantly in treatment MS. At the end of the transplant, the mean abundance of macroinvertebrates and the total taxonomic richness of sponges of treatment SM were 1788 ± 1519 ind L−1 and 18 taxa, respectively, whereas in individuals of treatment MS, the abundance and richness were 2260 ± 1532 ind L−1 and 31 taxa, respectively (Fig. 4b).
When the mean abundance of the macroinvertebrates was compared with initial values, changes were also recorded in some taxa in both treatments and controls, and some new species were found to colonize transplanted sponges (Table 1). Among the species whose abundance decreased were the amphipods Elasmopus sp. and Corophium sp. (in sponge individuals transplanted from mangrove to seagrass habitat), while those that remained constant were the polychaetes of families Serpullidae and Cirratullidae (also in individuals transplanted from mangrove to seagrass habitat) (Table 1). In treatment MS, 16% of the taxa increased in abundance, 13% decreased, 19% remained similar, and 52% were species not recorded prior to transplant. In treatment SM, 33% of the taxa increased in abundance, 6% decreased, and 61% were species not recorded prior to transplant (Table 1). In both treatments, the number of taxa gained exceeded the number of taxa lost (MS: gained 16 taxa and lost 4; SM: gained 11 and lost 8) (Fig. 5), whereas in control S, the gain and loss were equal (4 taxa) and in control M, there was only loss of taxa (11 taxa).
There was no clear pattern in the habits and preferences of the taxa that were lost. Among those that disappeared (11 taxa), the ratio of sessile to vagile was 9%:91%, among those that colonized (12 taxa) the ratio was 50%:50%, and among those that remained (16 taxa) the ratio was 44%:56%.
In addition to abundance, a significant increase (t 26 = −3.08, P < 0.05) in the number of higher taxa (phyla) per sponge individual was also found in treatment SM in relation to the initial value (IS) from 2.4 ± 1.3 taxa per sponge to 3.7 ± 1.0 taxa per sponge. In the case of sponges of treatment MS, the number of higher taxa per sponge decreased (although not significantly, t 23 = 0.31, P > 0.05) in relation to the initial value (IM) from 3.3 ± 0.8 to 3.2 ± 0.4 taxa per sponge. In both controls (control S and control M), the number of higher taxa per sponge individual did not change significantly in relation to the respective initial values (IS and IM).
When comparing the abundance of associated macroinvertebrates between treatments and their respective controls, there were only significant differences (t 23 = 3.6, P < 0.05) detected between treatment SM and control S. Moreover, Czekanowsky’s coefficient indicated a moderate similarity in the taxonomic richness of macroinvertebrates, both at the beginning (between IS and IM = 53%) and at the end of the experiment (between treatments SM and MS = 57%).
Based on macroinvertebrate species abundance data, the nMDS ordination indicated there were two assemblage groups (similarity 35%, stress 0.01). Group A consisted of samples of initial IS and control M and group B of samples of control S, treatment SM, treatment MS, and initial M; Fig. 6a). The ANOSIM test indicated that there are significant differences (global R = 0.857) at community level between these two groups. Likewise, the k-dominance curves indicated that samples of group A had the highest (initial IS) and lowest (control M) diversity and evenness, while those of group B had intermediate values (Fig. 6b). Results of the SIMPER analysis indicated that the species that most contributed to similarity within group A were S. prolifera (36.9%), D. kyolis (17.7%), O. savignyi (14.5%), Elasmopus sp. (10.2%), and L. cf. exotica (10.2%), and within group B, these were Elasmopus sp. (22.4%), S. prolifera (18.0%), O. savignyi (17.8%), and L. cf. exotica (12.5%). The species that most contributed to dissimilarity between groups A and B were Elasmopus sp. (13.9%), L. cf. exotica (13.5%), O. savignyi (13.1%), S. prolifera (7.5%), and Corophium sp. (5.9%). Only one species occurred exclusively in group A whereas there were 18 such in group B, implying that the differences in community structure among the two groups mainly resulted from changes in the abundance of common species.
a Non-metric multidimensional scaling ordination of macroinvertebrate abundance data for samples of H. melanadocia from the different experimental situations. b K-dominance curves for the different experimental situations of the transplant experiment. Initial specimens collected from seagrass bed (IS) and mangrove roots (IM); specimens transplanted from mangrove roots to seagrass habitat (MS) and from seagrass bed to mangrove root habitat (SM); control of mangrove (Control M) and seagrass bed (Control S)
Environmental Parameters
The water temperature remained relatively constant over the course of the study period (from 29.3 °C in April to 30.3 °C in July). Salinity decreased slightly from 39 to 34 during this same period. The sedimentation/resuspension rate did not differ significantly (t 6 = 0.16, P > 0.05) between both habitats (mangrove habitat = 0.35 ± 0.21 kg m−2 day−1; seagrass bed = 0.38 ± 0.16 kg m−2 day−1). Percentage dissolution of plaster spheres was significantly (t 4 = 6.99, P < 0.01) higher in the mangrove habitat (8.9 ± 0.7% day−1) than in the seagrass bed (5.1 ± 0.6% day−1). Contrarily, light intensity was significantly (t 8 = 2.29, P < 0.05) higher in the seagrass bed (131.6 ± 54.9 lx) than in the mangrove root habitat (33.8 ± 17.4 lx). In summary, sponges in the seagrass bed were exposed to 75% more illumination, but to a lesser (42%) hydrodynamic regime than those of the mangrove habitat.
Discussion
The taxonomic richness and abundance of macroinvertebrates recorded in H. melanadocia were within the range reported in other sponge species (Koukouras et al. 1985; Abdo 2007), which confirms the species’ functional role as host to a diverse and abundant community assemblage in this estuarine system. Regarding the inter-habitat differences found (both at the beginning and at the end of the experiment) in the abundance and taxonomic composition of associated macroinvertebrates, our data agree with a previous study conducted in H. melanadocia (Ávila and Ortega-Bastida 2015) and reports in other sponge species (Pearse 1950; Duarte and Nalesso 1996; Çinar et al. 2002; Ribeiro et al. 2003; Gerovasileiou et al. 2016). For example, variations were reported in sponge macroinvertebrate community composition between sampling sites in both Spheciospongia vesparia (Lamarck, 1814) (from Dry Tortugas and Bimini) and Mycale microsigmatosa Arndt, 1927 (from Rio de Janeiro State, SE Brazil), (Pearse 1950; Ribeiro et al. 2003). In the study on S. vesparia, sampling sites corresponded to coral reef habitats with different depths, whereas in M. microsigmatosa the habitat was rocky but with a different degree of exposure to the open sea, pollution levels, and primary productivity. Despite this variability in species composition, the diversity, abundance, richness, and dominance patterns of the highest taxa associated with M. microsigmatosa did not vary significantly among collection sites (Ribeiro et al. 2003). Nevertheless, other studies have shown that variation between sampling sites in the composition and abundance of associated macroinvertebrates may also depend on the sponge species (Abdo 2007), and within the same sponge species, on the study location (Westinga and Hoetjes 1981; Voultsiadou-Koukoura et al. 1987; Gerovasileiou et al. 2016).
Among the main changes found at the conclusion of the H. melanadocia transplanting experiment was the replacement of some dominant groups, changes in the abundance of some taxa, and changes in taxonomic richness. These findings are consistent with the results of other studies using transplant experiments to evaluate the response of macroinvertebrate assemblages to different environmental conditions (e.g., in freshwater [Graça et al. 2002] and terrestrial ecosystems [Nooten et al. 2014]). In this study, the changes recorded in the macroinvertebrate assemblages associated with H. melanadocia could be partially explained by the differing degrees of exposure to hydrodynamic conditions. This physical factor may cause changes in the aquiferous system (oscula and other piping elements) of sponges (Carballo et al. 2006), which in turn influences the structure of the associated assemblages (Koukouras et al. 1996; Duarte and Nalesso 1996; Henkel and Pawlik 2005; Abdo 2007). The oscula diameter, for example, may limit the size of endobionts and potential predators entering the sponge, and a greater number of these openings per surface unit of sponge can increase the chances for entry of endobionts. Here, H. melanadocia of both treatments showed an inverse change in the oscula density and diameter (although the latter was not significant), which was likely associated to a reorganization of its aquiferous system due to inter-habitat differences in hydrodynamic conditions (Carballo et al. 2006). In this sense, it is well-known that the exposition of sponges to a different hydrodynamic regime may cause changes in the aquiferous system, so that they can pump water through their body for feeding and breathing (Wilkinson and Vacelet 1979; Palumbi 1986; McDonald et al. 2002).
Although transplant experiments have not been performed to investigate the influence of environmental conditions on the sponge-associated macroinvertebrate assemblages, there are related studies on symbiotic microorganisms. For example, Wilkinson and Vacelet (1979) transplanted sponges with (Petrosia ficiformis [Poiret] and Chondrilla nucula Schmidt) and without cyanobacterial symbionts (Verongia aerophoba [Schmidt], V. cavernicola Vacelet, and Chondrosia reniformis Nardo) to different conditions of light and current (in a depth gradient). Their study found a differential effect in the growth of these sponge species and confirmed the hypothesis that the growth of sponges with symbiotic cyanobacteria would be enhanced by light, whereas the opposite would occur in sciaphilic sponges without cyanobacteria (Wilkinson and Vacelet 1979). These results are consistent with those of the present study, as changes in the structure of the macroinvertebrate community occurred alongside changes in the volume of sponges transplanted to different hydrodynamic and light conditions.
Sponge volume showed a decrease in almost all the explants, but despite this, there was a significant increase in the abundance of macroinvertebrates in the individuals transplanted from seagrass to mangrove habitat and in the control of seagrass (control S). This increase in abundance may be due to a cumulative effect of the number of associated individuals due to transplant (i.e., those that the sponge already had from the seagrass habitat plus those acquired from the mangrove habitat). In addition, the increase in abundance suggests a possible seasonal increase in the populations of some associated species. In the case of sponges of treatment MS, the overall abundance of macroinvertebrates did not change significantly with respect to the initial (IM) and the control (control M). Although the total number of taxa in this treatment also increased, the overall abundance of macroinvertebrates did not increase significantly, probably due to a decrease in the abundance of some dominant species such as the polychaete Syllis prolifera and the amphipods Elasmopus sp. and Corophium sp. This differential response among taxa to changes in local environmental conditions may be related to their relative tolerance to certain environmental factors and their adaptive capacity (Sanford and Kelly 2011).
The decrease in the volume of H. melanadocia during this time of year has already been documented and is thought to be due to a decrease in salinity during the rainy season (June–October; Ávila and Ortega-Bastida 2015; Ávila et al. 2015). Although during this study the salinity decreased by 5, it was not possible to demonstrate a direct effect of this parameter on the H. melanadocia-associated macroinvertebrates (Edgar and Barrett 2002; Teske and Wooldridge 2003).
In addition to the influence of environmental factors and sponge morphology, other biological factors (such as the macroinvertebrates of the surrounding environment, and predation) may have influenced the changes observed (Ávila and Ortega-Bastida 2015). Although the effect of predation was not examined here, it is well-known that this biological factor may also have a strong influence on the distribution patterns of littoral assemblages (Heck and Thoman 1981; Orth et al. 1984) and can differ between the mangrove and seagrass habitats. Therefore, the influence of predation (or even being exposed to different predators) on the macroinvertebrate assemblage remains a possibility (Hill and Hill 2002).
In addition to the effect of transplant, there is also evidence that shifts in abundance of some macroinvertebrates could be due to seasonal changes in extrinsic (e.g., temperature, salinity, nutrient availability) or intrinsic (e.g., recruitment events, mortality) factors, as has been documented in the associated assemblages of other sponge species (Duarte and Nalesso 1996; Costa et al. 2015). The replacement of crustaceans by echinoderms as the dominant group in both treatments may in part be due to this seasonal shift. This replacement was mainly due to an increase in the relative abundance of the ophiuroid Ophiactis savignyi Müller and Troschel, 1842 and the holothurian Synaptula hydriformis Lesueur, 1824 during the study period. However, given that the abundance of these species also increased in controls, this could be indicative of a recruitment event during the study period (as was documented in the sponge Tedania ignis by McGovern 2002), rather than a response to transplant. The species O. savignyi has also been reported as abundant in other sponge species (e.g., Spirastrella inconstans and Sarcotragus muscarum from the Red Sea and Aegean Sea, respectively; Fishelson 1962; Çinar et al. 2002), and it has been suggested that an increase in its population density may cause a decrease in the diversity of other associated taxa (Çinar et al. 2002).
There is some evidence that shifts in the dominance and richness of higher taxa (number of higher taxa per sponge individual) were due to the loss or gain of certain taxa, including both sessile and motile species, as well as to the changes in their relative abundance during the study period. For example, the presence of the gastropod Cerithium eburneum Bruguière, 1792 only in the individuals that were in the seagrass bed (IS, control S, and MS) and its absence in the individuals that were transplanted to the mangrove habitat (SM) suggest an effect of transplant on the taxa richness and species composition. This may be because this gastropod (which feeds on detritus and microalgae) is usually more abundant in the seagrass habitat than in mangrove roots (Layman and Silliman 2002). Moreover, multivariate analyses showed a clear separation of macroinvertebrate assemblages into two main groups, which according to the k-dominance curves are separated by their diversity and evenness values. When examining the species of associated macroinvertebrates that most contributed to the similarity and dissimilarity between groups, it was found that they were practically the same species, including Elasmopus sp., L. cf. exotica, O. savignyi, S. prolifera, and Corophium sp. (all with vagile lifestyle). These results showed also that differences in community structure among the two groups mainly resulted from changes in the abundance of common species.
In summary, this study reaffirms that H. melanadocia can host a high diversity and abundance of macroinvertebrates and, like in other sponge species, the abundance, taxonomic composition, and the overall taxa richness of associated organisms vary between sites due to differences in environmental conditions. Through a reciprocal transplant of individuals of this sponge between seagrass bed and mangrove root habitats, it was possible to detect changes in the macroinvertebrate assemblages (abundance, dominance, total taxa richness, and richness of higher taxa/sponge), and some physico-chemical variables showed small-scale spatial (hydrodynamism and light availability) and temporal (salinity) variations, which together with biological factors (e.g., predation, competition, and the surrounding fauna) may contribute to the differences observed in the assemblages.
References
Abdo, D.A. 2007. Endofauna differences between two temperate marine sponges (Demospongiae; Haplosclerida; Chalinidae) from southwest Australia. Marine Biology 152 (4): 845–854. https://doi.org/10.1007/s00227-007-0736-7.
Ávila, E., and A.L. Ortega-Bastida. 2015. Influence of habitat and host morphology on macrofaunal assemblages associated with the sponge Halichondria melanadocia in an estuarine system of the southern Gulf of Mexico. Marine Ecology 36 (4): 1345–1353. https://doi.org/10.1111/maec.12233.
Ávila, E., A.K. Ávila-García, and J.A. Cruz-Barraza. 2015. Temporal and small-scale spatial variations in abundance and biomass of seagrass-dwelling sponges in a tropical estuarine system. Marine Ecology 36 (3): 623–636. https://doi.org/10.1111/maec.12171.
Biernbaum, C.K. 1981. Seasonal changes in the amphipod fauna of Microciona prolifera (Ellis and Solander) (Porifera: Demospongia) and associated sponges in a shallow salt-marsh creek. Estuaries 4 (2): 85–96. https://doi.org/10.2307/1351671.
Blomquist, S., and K. Kofoed. 1981. Sediment trapping. A subaquatic in situ experiment. Limnology and Oceanography 26 (3): 585–590. https://doi.org/10.4319/lo.1981.26.3.0585.
Bloom, S.A. 1981. Specialization and noncompetitive resource partitioning among sponge-eating dorid nudibranchs. Oecologia 49 (3): 305–315. https://doi.org/10.1007/BF00347590.
Bray, J.R., and J.T. Curtis. 1957. An ordination of the upland forest communities of southern Wisconsin. Ecological Monographs 27 (4): 325–349. https://doi.org/10.2307/1942268.
Carballo, J.L., E. Ávila, S. Enríquez, and L. Camacho. 2006. Phenotypic plasticity in a mutualistic association between the sponge Haliclona caerulea and the calcareous macroalga Jania adherens induced by transplanting experiments. I: morphological responses of the sponge. Marine Biology 148 (3): 467–478. https://doi.org/10.1007/s00227-005-0104-4.
Çinar, M.E., T. Katagan, Z. Ergen, and M. Sezgin. 2002. Zoobenthos-inhabiting Sarcotragus muscarum (Porifera: Demospongiae) from the Aegean Sea. Hydrobiologia 482 (1/3): 107–117. https://doi.org/10.1023/A:1021260314414.
Clarke, A. 1990. Temperature and evolution: Southern Ocean cooling and the Antarctic marine fauna. In Antarctic ecosystems, ed. K.R. Kerry and G. Hempel, 9–22. New York: Springer-Verlag. https://doi.org/10.1007/978-3-642-84074-6_2.
Clarke, K.R. 1993. Non-parametric multivariate analyses of changes in community structure. Australian Journal of Ecology 18: 117–143. https://doi.org/10.1111/j.1442-9993.1993.tb00438.x.
Costa, M.F.B., K.F.R. Mansur, and F.P.P. Leite. 2015. Temporal variation of the gammaridean fauna (Crustacea, Amphipoda) associated with the sponge Mycale angulosa (Porifera, Demospongiae) in southeastern Brazil. Nauplius 23 (1): 79–87. https://doi.org/10.1590/S0104-64972015002312.
Dauer, D.M., and J.L. Simon. 1975. Repopulation of the polychaete fauna of an intertidal habitat following natural defaunation: species equilibrium. Oecologia 22: 99–117.
Denny, M.W. 1988. Biology and mechanics of the wave swept environment. Princeton: Princeton University Press, Princeton. https://doi.org/10.1515/9781400852888.
Duarte, L., and R. Nalesso. 1996. The sponge Zygomycale parishii (Bowerbank) and its endobiotic fauna. Estuarine, Coastal and Shelf Science 42 (2): 139–151. https://doi.org/10.1006/ecss.1996.0011.
Ďuriš, Z., I. Horka, P.J. Juračka, A. Petrusek, and F. Sandford. 2011. These squatters are not innocent: the evidence of parasitism in sponge-inhabiting shrimps. PLoS One 6 (7): e21987. https://doi.org/10.1371/journal.pone.0021987.
Edgar, G.J., and N.S. Barrett. 2002. Benthic macrofauna in Tasmanian estuaries: scales of distribution and relationships with environmental variables. Journal of Experimental Marine Biology and Ecology 270 (1): 1–24. https://doi.org/10.1016/S0022-0981(02)00014-X.
Enríquez, S., E. Ávila, and J.L. Carballo. 2009. Phenotypic plasticity induced in transplant experiments in a mutualistic association between the red alga Jania adhaerens (Rhodophyta, Corallinales) and the sponge Haliclona caerulea (Porifera: Haplosclerida): morphological responses of the alga. Journal of Phycology 45 (1): 81–90. https://doi.org/10.1111/j.1529-8817.2008.00640.x.
Field, J.G., K.R Clarke, and R.M. Warwick. 1982. A practical strategy for analysing multispecies distribution patterns. Marine Ecology Progress Series 8: 37–52. https://doi.org/10.3354/meps008037.
Fishelson, L. 1962. Spirastrella inconstans Dendy (Porifera) as an ecological niche in the littoral zone of the Dahlak Archipelago (Eritrea). Bulletin of the Sea Fisheries Research Station Israel 41: 17–25.
Frith, W. 1976. Animals associated with sponges at North Hayling, Hampshire. Zoological Journal of the Linnean Society 58 (4): 353–362. https://doi.org/10.1111/j.1096-3642.1976.tb01005.x.
Gambi, M.C., M.C. Buia, E. Casola, and M. Scardi. 1989. Estimates of water movement in Posidonia oceanica beds: a first approach. In II International Workshop of Posidonia Beds, eds. C.F. Boudouresque, A. Meinesz, E. Fresi, and V. Gravez, 2: 101–112. Marseille: GIS Posidonie Publisher.
Gerovasileiou, V., C.C. Chintiroglou, D. Konstantinou, and E. Voultsiadou. 2016. Sponges as “living hotels” in Mediterranean marine caves. Scientia Marina 80 (3): 279–289. https://doi.org/10.3989/scimar.04403.14B.
Graça, M.A., A. Rodrígues-Capítulo, C. Ocón, and N. Gómez. 2002. In situ tests for water quality assessment: a case study in Pampean rivers. Water Research 36 (16): 4033–4040. https://doi.org/10.1016/S0043-1354(02)00132-X.
Heck, K.L., Jr., and T.A. Thoman. 1981. Experiments on predator-prey interactions in vegetated aquatic habitats. Journal of Experimental Marine Biology and Ecology 53 (2-3): 125–134. https://doi.org/10.1016/0022-0981(81)90014-9.
Henkel, T.P., and J.R. Pawlik. 2005. Habitat use by sponge-dwelling brittlestars. Marine Biology 146 (2): 301–313. https://doi.org/10.1007/s00227-004-1448-x.
Hill, M.S., and A.L. Hill. 2002. Morphological plasticity in the tropical sponge Anthosigmella varians: responses to predators and wave energy. The Biological Bulletin 202 (1): 86–95. https://doi.org/10.2307/1543225.
Klitgaard, A.B. 1995. The fauna associated with outer shelf and upper slope sponges (Porifera, Demospongiae) at the Faroe Islands, northeastern Atlantic. Sarsia 80 (1): 1–22. https://doi.org/10.1080/00364827.1995.10413574.
Komatsu, T., and H. Kawai. 1992. Measurements of time-averaged intensity of water motion with plaster balls. Journal of Oceanography 48 (4): 353–365. https://doi.org/10.1007/BF02234014.
Koukouras, A., E. Voultsiadou-Koukoura, H. Chintiroglou, and C. Dounas. 1985. Benthic bionomy of the North Aegean Sea. III. A comparison of the macrobenthic animal assemblages associated with seven sponge species. Cahiers de Biologie Marine 26: 301–319.
Koukouras, A., A. Russo, E. Voultsiadou-Koukoura, C. Dounas, and C. Chintiroglou. 1992. Relationship of sponge macrofauna with the morphology of their hosts in the North Aegean Sea. International Review of Hydrobiology 77 (4): 609–619. https://doi.org/10.1002/iroh.19920770406.
Koukouras, A., A. Russo, E. Voultsiadou-Koukoura, C. Arvanitidis, and D. Stefanidou. 1996. Macrofauna associated with sponge species of different morphology. Marine Ecology 17 (4): 569–582. https://doi.org/10.1111/j.1439-0485.1996.tb00418.x.
Lambshead, P.J.D., H.M. Platt, and K.M. Shaw. 1983. The detection of differences among assemblages of marine benthic species based on an assessment of dominance and diversity. Journal of Natural History 17 (6): 859–874. https://doi.org/10.1080/00222938300770671.
Layman, C.A., and B.R. Silliman. 2002. Preliminary survey and diet analysis of juvenile fishes of an estuarine creek on Andros Island, Bahamas. Bulletin of Marine Science 70: 199–210.
Leite, F.P., L. Pavani, and M.O. Tanaka. 2016. Temporal variation of epi-and endofaunal assemblages associated with the red sponge Tedania ignis on a rocky shore (São Sebastião Channel), SE Brazil. Iheringia Série Zoologia 106: e2016007.
Maldonado, M., and C.M. Young. 1996. Effects of physical factors on larval behavior, settlement and recruitment of four tropical demosponges. Marine Ecology Progress Series 138: 169–180. https://doi.org/10.3354/meps138169.
McDonald, J.I., J.N.A. Hooper, and K.A. McGuinness. 2002. Environmentally influenced variability in the morphology of Cinachyrella australiensis (Carter 1886) (Porifera: Spirophorida: Tetillidae). Marine and Freshwater Research 53 (1): 79–84. https://doi.org/10.1071/MF00153.
McGovern, T.M. 2002. Patterns of sexual and asexual reproduction in the brittle star Ophiactis savignyi in the Florida Keys. Marine Ecology Progress Series 230: 119–126. https://doi.org/10.3354/meps230119.
Miller, R.J., J. Hocevar, R.P. Stone, and D.V. Fedorov. 2012. Structure-forming corals and sponges and their use as fish habitat in Bering Sea submarine canyons. PLoS One 7 (3): e33885. https://doi.org/10.1371/journal.pone.0033885.
Muus, B.J. 1968. A field method for measuring “exposure” by means of plaster balls: a preliminary account. Sarsia 34 (1): 61–68. https://doi.org/10.1080/00364827.1968.10413371.
Naranjo, S.A., J.L. Carballo, and J.C. García-Gómez. 1996. Effects of environmental stress on ascidian populations in Algeciras Bay (southern Spain). Possible marine bioindicators? Marine Ecology Progress Series 14: 119–131.
Neves, G., and E. Omena. 2003. Influence of sponge morphology on the composition of the polychaete associated fauna from Rocas Atoll, northeast Brazil. Coral Reefs 22 (2): 123–129. https://doi.org/10.1007/s00338-003-0295-4.
Nooten, S.S., N.R. Andrew, and L. Hughes. 2014. Potential impacts of climate change on insect communities: a transplant experiment. PLoS One 9 (1): e85987. https://doi.org/10.1371/journal.pone.0085987.
Orth, R.J., K.L. Heck Jr., and J. van Montfrans. 1984. Faunal communities in seagrass beds: a review of the influence of plant structure and prey characteristics on predator-prey relationships. Estuaries 7 (4): 339–350. https://doi.org/10.2307/1351618.
Palumbi, S.R. 1986. How body plans limit acclimation: responses of a demosponge to wave force. Ecology 67 (1): 208–214. https://doi.org/10.2307/1938520.
Pearse, A.S. 1932. Inhabitants of certain sponges at Dry Tortugas. Papers from the Tortugas Laboratory. Carnegie Institution of Washington 28: 117–124.
Pearse, A.S. 1950. Notes on the inhabitants of certain sponges at Bimini. Ecology 31 (1): 149–151. https://doi.org/10.2307/1931369.
Peattie, M., and R. Hoare. 1981. The sublittoral ecology of the Menai Strait. II. The sponge Halichondria panicea (Pallas) and its associated fauna. Estuarine Coastal and Shelf Science 13 (6): 621–635. https://doi.org/10.1016/S0302-3524(81)80044-8.
Ribeiro, S.M., E.P. Omena, and G. Muricy. 2003. Macrofauna associated to Mycale microsigmatosa (Porifera, Demospongiae) in Rio de Janeiro State, SE Brazil. Estuarine, Coastal and Shelf Science 57 (5-6): 951–959. https://doi.org/10.1016/S0272-7714(02)00425-0.
Rützler, K. 1978. Sponges in coral reefs. In Coral reefs: research methods. Monographs on Oceanographic Methodology, eds. D.R. Stoddart, and R.E. Johannes, 5: 209–313. Paris: UNESCO.
Sainte-Marie, B. 1991. A review of the reproductive bionomics of aquatic gammaridean amphipods: variation of life history traits with latitude, depth, salinity and superfamily. Hydrobiologia 223 (1): 189–227. https://doi.org/10.1007/BF00047641.
Sanford, E., and M.W. Kelly. 2011. Local adaptation in marine invertebrates. Annual Review of Marine Science 3 (1): 509–535. https://doi.org/10.1146/annurev-marine-120709-142756.
Sara, M., and J. Vacelet. 1973. Ecologie des Demosponges. In Traite de Zoologie. Anatomie, Systematique, Biologie, ed. P.P. Grassé, 462–576. Paris: Masson et Cie.
Teske, P.R., and T.H. Wooldridge. 2003. What limits the distribution of subtidal macrobenthos in permanently open and temporarily open/closed South African estuaries? Salinity vs. sediment particle size. Estuarine Coastal and Shelf Science 57 (1-2): 225–238. https://doi.org/10.1016/S0272-7714(02)00347-5.
Thoms, C., M. Horn, M. Wagner, U. Hentschel, and P. Proksch. 2003. Monitoring microbial diversity and natural products profiles of the sponge Aplysina cavernicola following transplantation. Marine Biology 142 (4): 685–692. https://doi.org/10.1007/s00227-002-1000-9.
Uriz, M.J., D. Rosell, and M. Maldonado. 1992. Parasitism, commensalism or mutualism? The case of Scyphozoa (Coronatae) and horny sponges. Marine Ecology Progress Series 81: 247–255. https://doi.org/10.3354/meps081247.
Vance, R.R. 1978. A mutualistic interaction between a sessile marine clam and its epibionts. Ecology 59 (4): 679–685. https://doi.org/10.2307/1938770.
Voultsiadou-Koukoura, H.E., A. Koukouras, and A. Eleftheriou. 1987. Macrofauna associated with the sponge Verongia aerophoba in the North Aegean Sea. Estuarine, Coastal and Shelf Science 24 (2): 265–278. https://doi.org/10.1016/0272-7714(87)90069-2.
Weisz, J.B., A.J. Massaro, B.D. Ramsby, and M. Hill. 2010. Zooxanthellar symbionts shape host sponge trophic status through translocation of carbon. The Biological Bulletin 219 (3): 189–197. https://doi.org/10.1086/BBLv219n3p189.
Westinga, E., and P.C. Hoetjes. 1981. The intrasponge fauna of Spheciospongia vesparia (Porifera, Demospongiae) at Curacao and Bonaire. Marine Biology 62 (2-3): 139–150. https://doi.org/10.1007/BF00388176.
Wilkinson, C.R., and J. Vacelet. 1979. Transplantation of marine sponges to different conditions of light and current. Journal of Experimental Marine Biology and Ecology 37 (1): 91–104. https://doi.org/10.1016/0022-0981(79)90028-5.
Acknowledgements
This study was funded by the Universidad Nacional Autónoma de México (internal project no. 618), with complementary funding from the project PAPIIT-IB200712. We thank Hernán Alvarez-Guillén, Andres Reda-Deara, and Alejandro Gomez-Ponce for their assistance with field samplings.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Paul A. Montagna
Rights and permissions
About this article
Cite this article
Ávila, E., Briceño-Vera, A.E. A Reciprocal Inter-habitat Transplant Reveals Changes in the Assemblage Structure of Macroinvertebrates Associated with the Sponge Halichondria melanadocia . Estuaries and Coasts 41, 1397–1409 (2018). https://doi.org/10.1007/s12237-017-0359-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-017-0359-2