Abstract
The endofaunal assemblages associated with two species of sponge from the family Chalinidae (Haliclona sp. 1 and Haliclona sp. 2) were studied at four locations along the south west coast of Australia. The species have distinct morphologies and inhabit similar microhabitats; there is also considerable scientific interest in Haliclona sp. 1 (green Haliclona) due to the unique bioactive compound it produces. A total of 948 and 287 endofaunal individuals were found associated with 16 specimens of both the green Haliclona and Haliclona sp. 2 (brown Haliclona), respectively. Twenty-four endofaunal taxa were found (from mysid shrimps to teleost fish), with the brown Haliclona having a greater density of endofaunal species and individuals than the green Haliclona. The endofaunal assemblages of both species of sponge were significantly different, but only the endofaunal assemblage within the green Haliclona differed significantly among locations. Differences in the abundance and biomass of associated endofauna of each species of sponge can be related to differences in their morphologies, size and internal structure. In the green Haliclona, differences in endofaunal assemblages among locations are unlikely to be due to environmental influences as taxa discriminating each locations assemblage were common to both species of sponge. Numerous endofaunal individuals were found to be reproductively active, and it is clear that the species of sponge provide important habitats for their associated endofauna. This provision of habitat needs to be taken into account when harvesting green Haliclona biomass for supply of its target bioactive compound for further pharmaceutical development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heterogenous habitats have a positive influence on biodiversity, affecting species distributions, their persistence and resilience, and community composition (Hewitt et al. 2005). Habitat provided by both flora (e.g., sea grasses and algae) and fauna (e.g., corals and bivalves) form a major source of the habitat heterogeneity in the subtidal marine environment (Williams and Keck 2001; Connell 2007). These biogenic structures (i.e., produced by living organisms or biological processes) have direct and indirect effects through the provision of food and shelter (Ryer et al. 2004; Chapman et al. 2005; Dubois et al. 2006; People 2006; Connell 2007). For example, biogenic structures were found to play an important role in the ecology of juvenile flatfish by modifying predator prey interactions for the flatfish (Ryer et al. 2004), even shell debris has been found to increase and maintain diversity within soft sediment habitats by providing settlement surfaces for various flora and fauna (Hewitt et al. 2005).
Sponges are an important source of biogenic structure, particularly in temperate marine environments where they dominate the benthos (Fromont 1999). They have been found to provide shelter and food for many other organisms (Wendt et al. 1985; Duarte and Nalesso 1996; Wulff 2006), including amphipods (Serejo 1998), barnacles (Ilan et al. 1999), polychaetes (Neves and Omena 2003) and fish (Barthel 1997). The fauna associated with sponges can be found living on the surface of a sponge (e.g., hydrozoans: Puce et al. 2005), within the mesohyl (e.g., barnacles: Ilan et al. 1999), or within the internal spaces of the sponge (e.g., amphipods: Serejo 1998). The composition and abundance of associated fauna has been connected to various physical characteristics of the host sponge including availability of internal space (Koukouras et al. 1996), sponge morphology (Ribeiro et al. 2003) and bioactivity (Betancourt-Lozano et al. 1998; Skilleter et al. 2005). In addition, environmental factors such as depth and habitat type can also be influential in determining the composition of the fauna associated with sponges (Ribeiro et al. 2003).
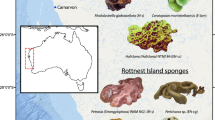
This research focuses on two currently undescribed species (Demospongiae; Haplosclerida; Chalinidae; Haliclona), which occur in shallow waters (3–30 m) and are often found within the same microhabitat (i.e., overhang or ledge habitat) of kelp dominated limestone reefs in southwest Australia. The formal description of the two sponges will be presented in a separate paper. They are both distributed along approximately 1,000 km of the south west coast of Australia (Fig. 1). Haliclona sp. 1 (hereafter green Haliclona; Western Australian Museum Voucher Z37485) has large chimney like oscules extending outwards at least 20 mm from the main sponge (Fig. 2a). These sponges have an amorphous shape, with varying numbers of oscules. Sponges of Haliclona sp. 2 (hereafter, brown Haliclona; Western Australian Museum Voucher Z37486) have a mound shaped body on a short basal stalk and have small slightly raised oscules which extend less than 5 mm above the surface of the sponge (Fig. 2b). Oscules are numerous in the brown Haliclona, and the sponges have a dense mesohyl with few internal canals. The green Haliclona has a less compact consistency and larger internal canals than the brown Haliclona. The green Haliclona produces the potent and unique anti-tumour compound Salicylihalamide A (Erickson et al. 1997), but the bioactivity of the brown Haliclona is currently unknown. Wild biomass supply of the species remains the current and sole source of salicylihalamide A leaving it vulnerable to over harvesting.
This research is part of a larger study examining the population ecology of these two sympatric species. The aim of this study is to understand the influence of the two species of sponge in the marine environment of the south west coast of Australia, and specifically to determine if they possess different endofaunal communities. It was hypothesized that the different morphologies of each species of sponge would be inhabited by different endofaunal assemblages. An assessment was also made as to whether the endofaunal communities associated with each species of sponge were consistent across the known distribution of the host sponges. The large geographical range of the host sponges (and associated environmental changes) was predicted to result in different endofaunal assemblages at each location for both species of sponge.
Materials and methods
Whole sponges (n = 4) from each Haliclona species were haphazardly collected on SCUBA from depths of 10–15 m from four locations (Bremer Bay, 34.45°S, 119.38°E; Hamelin Bay 34.20°S, 115.04°E; Rottnest Island 32.00°S, 115.52°E; and Jurien Bay 30.275°S, 115.02°E) along the south western coast of Western Australia (Fig. 1). All samples were collected during the Australian summer (December–March) of 2005/2006.
To determine the volume of each sponge, five stereo image pairs of each specimen were recorded using an underwater stereo camera prior to collection to obtain precise size estimates (Abdo et al. 2006). Each set of images (for each sponge collected) was later processed to obtain a volume estimate (Abdo et al. 2006).
Collections of endofauna (that is fauna found inhabiting the internal spaces of the sponge) from each sponge were made by covering the sponge with a plastic bag in situ, and severing the sponges from the substratum using a flat blade scraper.
In the laboratory the sponges were carefully dissected to collect any fauna associated with their internal spaces. Any water from the plastic bag was sieved (using a 1 mm sieve) to collect fauna, which may have left the sponge during transportation to the laboratory. All endofauna were counted, weighed and preserved in 70% ethanol pending taxonomic identification.
An estimate of the internal space available to associated endofauna was made by cutting a section of tissue from each host sponge and photographing each section. The total area of sponge tissue and area of open space was determined in Photoshop™. The percentage of internal space was determined by the following equation:
Statistical analyses
To determine if the volume of each species of sponge influenced their internal space, number of endofauna species and the total number of endofauna individuals, correlations between volume and each of the other variables was tested for using Pearson’s correlation coefficient (Zar 1999). If each of internal space, number of endofauna species and number of endofauna individuals were correlated to the volume of the sponge, data were converted to faunal density by dividing endofaunal abundance by the volume of each sponge (in litres) and used for all subsequent analyses (Betancourt-Lozano et al. 1998). Differences in internal space, number of endofauna species and number of endofauna individuals were then compared between species of sponge (fixed factor, 2 levels) and locations (random factor, 4 levels) using a crossed two-way analysis of variance (ANOVA). Data were checked for normality and homogeneity of variance using an Anderson-Darling and Bartlett’s tests respectively. Data not meeting assumptions were log transformed (Zar 1999), and rechecked for normality and homogeneity of variance.
Differences in faunal assemblages between the species of sponge and among locations were examined graphically with non-metric multidimensional scaling (nMDS), using the Bray-Curtis similarity measure on untransformed density (i.e., standardised for volume differences) data (Clarke 1993). To test for differences in the endofaunal assemblages of each species of sponge, and among the four locations a crossed mixed factor model (species of sponge (fixed): 2 levels, location (random): 4 levels) was analysed with a permutational multivariate analysis of variance (PERMANOVA). Data were untransformed and the Bray-Curtis similarity matrix was used during analysis (Anderson 2001). If locations significantly differed, a posteriori PERMANOVA analyses (9999 permutations) were conducted separately for each species of sponge to test for differences among locations. If a significant difference was found, pairwise comparisons were made among locations during the a posteriori analyses using PERMANOVA (9999 permutations) to determine where the different assemblages were occurring. All a posteriori PERMANOVA analyses were conducted on untransformed data using the Bray-Curtis similarity matrix.
Similarity Percentages (SIMPER) analysis (untransformed data using Bray-Curtis similarity matrix) was used to determine which taxa contributed to the variation in endofaunal assemblages between each species of sponge, and among locations for each species of sponge (Clarke 1993). The differences in abundance and biomass of taxa discriminating between each species of sponge were analysed separately using one-way ANOVA. If a location difference was observed (for a species of sponge), differences in the abundance and biomass of discriminating taxa among locations were examined separately by one-way ANOVA. The normality and heterogeneity of variance in the data were checked prior to analysis using an Anderson-Darling and Bartlett’s tests, respectively.
Results
A total of 948 individuals were found associated with the green Haliclona and 287 individuals with the brown Haliclona. A total of 24 endofaunal taxa (from 5 phyla) were found in this study, of which 10 taxa were common to both species of sponge (Table 1). Green Haliclona contained a larger total biomass of endofauna (100.86 g wet weight) than the brown Haliclona (54.65 g wet weight). Endofauna of both species of sponge were dominated by crustaceans followed by echinoderms and molluscs (Fig. 3).
Internal space (r = 0.933, P < 0.001); number of endofaunal species (r = 0.745, P = 0.01); and total number of endofaunal individuals (r = 0.620, P = 0.010) were all significantly correlated with the volume of the green Haliclona. There were also significant correlations between the internal space (r = 0.966, P < 0.001); number of species (r = 0.0.858, P < 0.001); and total individuals (r = 0.722, P = 0.002) to the volume of the brown Haliclona.
As sponge volume was found to be significantly correlated to the amount of internal space, number of endofauna species and total number of individuals for both species of sponge, each of these variables was standardised by the volume of the host sponge (in litres). Both species of sponge differed significantly (P < 0.05) in internal space, number of endofaunal species and total number of endofaunal individuals (Table 2; Fig. 4). However, there was no significant variation in these variables among locations (Table 2; Fig. 4).
Mean differences between sponges (green Haliclona and brown Haliclona) and locations (BB Bremer Bay, HB Hamelin Bay, RI Rottnest Island, and JB Jurien Bay) for a percentage internal space, b number of endofauna species (adjusted by dividing by sponge volume), and c number of endofauna individuals (adjusted by dividing by sponge volume)
The endofaunal assemblages differed significantly between species of sponge, and among locations (Table 3; Fig. 5a). Whilst no significant difference in endofaunal assemblages was observed among locations for the brown Haliclona (Table 4; Fig. 5c), there was significant variation among locations in the green Haliclona (Table 4; Fig. 5b). Assemblages from southern locations (Bremer Bay and Hamelin Bay) did not differ significantly from each other and neither did assemblages from northern locations (Rottnest Island and Jurien Bay). In addition, Hamelin Bay (a southern location) assemblages did not significantly differ from Jurien Bay (a northern location) assemblages (Table 5).
MDS ordinations of the density of endofauna in green and brown Haliclona, from four locations along the south west coast of Australia. Ordination a compares both sponge species (where filled square green Haliclona and open square brown Haliclona) with samples pooled across sites. Ordination b compares samples of green Haliclona, and ordination c compares samples of brown Haliclona from four locations (where open square Bremer Bay, filled square Hamelin Bay, open triangle Rottnest Island, and filled triangle Jurien Bay). Data was untransformed and MDS performed using Bray-Curtis similarity matrix
Taxa such as amphipods (Paraleucothoe sp., 34%), barnacles (Acasta sp., 19%), and isopods (Cirolanidae 14%) contributed 67% of the significant variation in endofaunal composition between the two species of sponge. Four additional taxa the brittle stars Ophiothrix sp. and Clarkcoma sp. (7 and 3% respectively), the snapping shrimp Alpheus sp. 2 (6%), and the mysid shrimps (Paramesodopsis sp., 3%) also separated the two species of sponge with respect to their endofaunal assemblages. The amphipod (Paraleucothoe sp.) and isopod (Cirolanidae) had a significantly greater density within the brown Haliclona compared to the green Haliclona (Table 6; Fig. 6a). In addition, the mysid shrimps (Paramesodopsis sp.) were only recorded within the green Haliclona (Fig. 6a). Of the discriminating taxa, only the amphipod (Paraleucothoe sp.), brittle star (Ophiothrix sp.) and snapping shrimp (Alpheus sp. 2) had a significantly greater total biomass within the green Haliclona compared to brown Haliclona (Table 6; Fig. 6b).
Three taxa, the barnacles (Acasta sp.), amphipods (Paraleucothoe sp.) and brittle star (Clarkcoma sp.), consistently contributed to the difference in the endofaunal assemblages among locations for the green Haliclona. Of these, only the amphipod (Paraleucothoe sp.) showed significant differences (between Bremer Bay and Rottnest Island) in density (Table 7). None of the discriminating endofaunal taxa showed any significant differences (P > 0.05) in total biomass among locations.
Discussion
The two Haliclonid sponges examined support two distinct endofaunal assemblages. The number of species and individuals of endofauna differed significantly between the two species of sponge, and the endofaunal assemblage of the green Haliclona differed among locations. The endofaunal assemblages of both species of sponge were dominated by crustaceans, echinoderms and molluscs.
The richness of associated fauna found inhabiting the two Haliclonid sponges of this study lie at the lower end of that reported for sponges and other biogenic structures (e.g., kelp holdfasts). Previous studies on sponges have found 139 and 53 species of associated fauna within two Aplysina species from the Caribbean (Wulff 2006), and 75 species of associated fauna in a Mycale species in Brazil (Ribeiro et al. 2003). Other habitat forming organisms such as kelp holdfasts have been found to support 89 species of associated fauna (Norderhaug et al. 2002; Jorgensen and Christie 2003), and bio-constructions by the Sabellaria polychaete supported 66 species of epibionts (Dubois et al. 2006). Comparisons with the only other study in Australia by Skilleter et al. (2005) is difficult as the number of species associated with the two Haliclonid species examined were not discussed. However, the number of associated species found in this study compares favourably with that of the sponge Aulospongus from Bimini which harboured only three individuals (Pearse 1950) and Haliclona rubens where approximately 15 species of associated fauna (Pearse 1950).
Sponge morphology and the amount of available internal space are likely to account for the differences in the endofaunal assemblages between each species of sponge seen in this study. Larger oscular size and internal space may provide more shelter and protection from predation for the endofauna (Peattie and Hoare 1981; Wendt et al. 1985; Wulff 2006). These features occurred in the green Haliclona specimens examined here. It would be expected that morphology, volume and amount of internal space of the host sponge would be positively related with endofaunal abundance and composition (Pearse 1950; Duarte and Nalesso 1996; Koukouras et al. 1996; Neves and Omena 2003), but previous studies have reported exceptions. Sponge species with less volume and internal space (such as those with such as encrusting or branching morphologies) have been found to harbour more associated fauna (Ribeiro et al. 2003; Skilleter et al. 2005).
In this study there was a greater overall density of endofaunal taxa (particularly amphipods, isopods and barnacles) in the brown Haliclona compared to the green Haliclona. This suggests that the dense tissue and small internal spaces of the brown Haliclona are used by small taxa. Although lower in density, these same taxa had a greater biomass within the green Haliclona indicating they were of a larger size in this species. These results demonstrate that sponge morphology, volume and amount of internal space have an important influence on the endofaunal assemblages present.
The number of endofaunal species and number of individuals for both species of sponge did not vary between locations (at the scale of approximately 250 km), which is a similar pattern to that observed by Ribeiro et al. (2003). However, the endofaunal composition of the green Haliclona did vary among locations, unlike reports for Mycale microstigmatosa from Brazil (Ribeiro et al. 2003). Variation in the composition of associated fauna at this scale (i.e., approximately 250 km) was seen in a study on Zygomycale parishii also in Brazil (Duarte and Nalesso 1996). In this study it was anticipated that a difference in the endofaunal assemblages between locations would be observed for both species, due to the large distances and environmental gradient along the coastline. Populations in isolated habitats are linked through dispersal and migration, and an organisms dispersal ability will determine the influence of habitat isolation (Russell et al. 2005). In one study, polychaetes with limited dispersal were more affected by habitat isolation than copepods, which had a large dispersal ability (Russell et al. 2005). This suggests that the dispersal ability of the organisms may be contributing to the differences among locations for the green Haliclona. In addition, a 0.5°C water temperature change for every degree of latitude has been reported along the south west coast of Australia (Creswell and Golding 1980), giving a temperature difference of approximately 2–3°C between the most northern (Jurien Bay) and southern (Bremer Bay) locations in this study. However, the endofauna species driving the differences among locations were common to both species of sponge suggesting that the dispersal strategies and environmental differences between locations are not influencing the endofaunal patterns observed for the green Haliclona.
It is possible a predator/prey relationship may be influencing the endofaunal pattern seen here. Sponges are known to protect inhabitants from potential predators (Wulff 2006). A predator (e.g., Mysid shrimps) may be preying upon one of the discriminating endofauna. The predator may be able to more easily target its prey within the green Haliclona, because of the species morphology, larger size and internal spaces. Mysid shrimps were only found within the green Haliclona and are known predators of other invertebrates (Wooldridge and Webb 1988; Wilhelm et al. 2002; Chigbu 2004; Jumars 2006). This could account for the lower density of amphipods in the green Haliclona compared to the brown Haliclona (Table 1), as well as for the differences among locations for the green Haliclona. That is, post hoc analysis revealed a significant negative correlation between the density of amphipods and mysid shrimps (r = −0.392, P = 0.027).
It is clear that both Haliclonid sponges offer a suitable habitat for a range of organisms. The relationship between the host sponge and associated fauna can be involuntary, such as with bivalves and barnacles, to obligatory endobionts of sponges such as snapping shrimp (Wulff 2006). While both species of sponge studied here have numerous inhabitants, the inhabitant/host relationship for many of the endofauna needs further examination. Some generalisations can, however, be made. The sponges examined here may provide shelter from predation with the small internal space of the brown Haliclona providing greater protection for small crustaceans (e.g., amphipods) and consequently greater abundances. Both species of sponge may provide food for the endofauna, with many taxa known to consume sponges (e.g., snapping shrimps and amphipods: Wulff 2006) being abundant within the sponge species examined here. Both sponge species may also provide food for the endofauna (e.g., the barnacles and brittle stars) via their aquiferous systems. That is, small brittle stars (Ophiothrix fragilis) in the Mediterranean were found to gain a feeding advantage from the inhalant current of the host sponge (Turon et al. 2000). O. fragilis was also found to leave its host when it grew to have a disc diameter larger than 1 mm (Turon et al. 2000) suggesting the host sponges may act as nursery areas for some endofauna. The same genus of brittle star was found to be smaller and more abundant in the brown Haliclona than the green Haliclona, possibly indicating the brown Haliclona may be a nursery for Ophiothrix brittle stars. Moreover, in this study many endofauna were found to be reproductively active, supporting the suggestion that sponges may act as nurseries for endofaunal species.
The goal of this study was to understand the influence of two Haliclonid sponges along the southwest coast of Australia. It is clear from the results of the study that both sponges support a unique endofaunal assemblage. As the sponges are potentially exploitable, particularly the green Haliclona, future demands upon the biomass of the sponges for further pharmaceutical development of Salicylihalamide A, must proceed carefully. It is well documented that habitat loss (both in terrestrial and marine environments) is linked to a loss of diversity and the number of animals (Fahrig 2003). While further examination of other habitat forming organisms is needed for the marine environment of the south west of Australia to fully appreciate what the result of over harvesting the Haliclonid sponges would be. Mass and unchecked harvesting of important habitats like the two species of sponge studied here may have direct impacts on the diversity and abundance of fauna such as the small crustaceans and fish which inhabit the sponges, or use the sponges as refuge (Moran and Stephenson 2000; Ryer et al. 2004). The importance of sponge habitats has been documented in other regions where large scale harvesting of the seafloor (e.g., trawling), and/or loss of sponges and the habitats they create, has led to the loss of other species (Jackson 2001; Butler et al. 2005). Any harvesting of sponges needs to take account of the potential impacts on associated species that may occur through loss of habitat which this study has shown sponges clearly provide.
References
Abdo DA, Seager JW, Harvey ES, McDonald JI, Kendrick GA, Shortis MR (2006) Efficiently measuring complex sessile epibenthic organisms using a novel photogrammetric technique. J Exp Mar Biol Ecol 339:121–133
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Aust Ecol 26:32–46
Barthel D (1997) Fish eggs and pentacrinoids in Weddell Sea hexactinellids: further examples for the structuring role of sponges in Antarctic benthic ecosystems. Polar Biol 17:91–94
Betancourt-Lozano M, Gonzalez-Farias F, Gonzalez-Acosta B, Garcia-Gasca A, Bastida-Zavala JR (1998) Variation of antimicrobial activity of the sponge Aplysina fistularis (Pallas 1766) and its relation to associated fauna. J Exp Mar Biol Ecol 223:1–18
Butler MJ, Dolan TW, Hunt JH, Rose KA, Herrnkind WF (2005) Recruitment in degraded marine habitats: a spatially explicit, individual-based model for spiny lobster. Ecol Appl 15:902–918
Chapman MG, People J, Blockley D (2005) Intertidal assemblages associated with natural corallina turf and invasive mussel beds. Biodiversity Conserv 14:1761–1776
Chigbu P (2004) Assessment of the potential impact of the mysid shrimp, Neomysis mercedis, on Daphnia. J Plankton Res 26:295–306
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Aust J Ecol 18:118–127
Connell SD (2007) Subtidal temperate rocky habitats: habitat heterogeneity at local to continental scales. In: Connell SD, Gillanders BM (eds) Marine Ecology. Oxford University Press, Melbourne, pp 378–401
Creswell GR, Golding TJ (1980) Observations of a south flowing current in the southeastern Indian Ocean. Deep-Sea Res 27A:449–466
Duarte LFL, Nalesso RC (1996) The sponge Zygomycale parishii (Bowerbank) and its endobiotic fauna. Estuar Coast Shelf Sci 42:139–151
Dubois S, Commito JA, Olivier F, Retie’re C (2006) Effects of epibionts on Sabellaria alveolata (L.) biogenic reefs and their associated fauna in the Bay of Mont Saint-Michel. Estuar Coast Shelf Sci 68:635–646
Erickson KL, Beutler JA, Cardellina JH, Boyd MR (1997) Salicylihalamides A and B, Novel cytotoxic macrolides from the marine sponge Haliclona sp. J Org Chem 62:8188–8192
Fahrig L (2003) Effects of habitat fragmentation on biodiversity. Ann Rev Ecol Evol Syst 34:487–515
Fromont J (1999) Reproduction of some demosponges in a temperate Australian shallow water habitat. Mem Qld Mus 44:185–192
Hewitt JE, Thrush SF, Halliday J, Duffy C (2005) The importance of small-scale habitat structure for maintaining beta diversity. Ecology 86:1619–1626
Ilan M, Loya Y, Kolbasov GA, Brickner I (1999) Sponge-inhabiting barnacles on the Red Sea coral reefs. Mar Biol 1999:709–716
Jackson JBC (2001) What was natural in the coastal oceans? Proc Natl Acad Sci 98:5411–5418
Jorgensen NM, Christie H (2003) Diurnal, horizontal and vertical dispersal of kelp-associated fauna. Hydrobiologia 503:69–76
Jumars PA (2006) Habitat coupling by mid-latitude, subtidal, marine mysids: import-subsidized omnivores. Oceanogr Mar Biol Annu Rev 45: In Press
Koukouras A, Russo A, Voultsiadou-Koukoura E, Arvanitidis C, Stefanidou D (1996) Macrofuana associated with sponge species of different morphology. Mar Ecol 17:569–582
Moran MJ, Stephenson PC (2000) Effects of otter trawling on macrobenthos and management of demersal scalefish fisheries on the continental shelf of north-western Australia. ICES J Mar Sci 57:510–516
Neves G, Omena E (2003) Influence of sponge morphology on the composition of the polychaete associated fauna from Rocas Atoll, northeast Brazil. Coral Reefs 22:123–129
Norderhaug KM, Christie H, Rinde E (2002) Colonisation of kelp imitations by epiphyte and holdfast fauna: a study of mobility patterns. Mar Biol 141:965–973
Pearse AS (1950) Notes on the inhabitants of certain sponges at Bimini. Ecology 31:149–151
Peattie ME, Hoare R (1981) The sublittoral ecology of the Menai Strait: The sponge Halichondria panicea (Pallas) and its associated fauna. Estuar Coast Shelf Sci 13:621–635
People J (2006) Mussel beds on different types of structures support different macroinvertebrate assemblages. Aust Ecol 21:271–281
Puce S, Calcinai B, Bavestrello G, Cerrano C, Gravili C, Boero F (2005) Hydrozoa (Cnidaria) symbiotic with porifera: a review. Mar Ecol 26:73–81
Ribeiro SM, Omena EP, Muricy G (2003) Macrofauna associated to Mycale microstigmatosa (Porifera, Demospongia) in Rio de Janeiro State, SE Brazil. Estuar Coast Shelf Sci 57:951–959
Russell BD, Gillanders BM, Connell SD (2005) Proximity and size of neighbouring habitat affects invertebrate diversity. Mar Ecol Prog Ser 296:31–38
Ryer CH, Stoner AW, Titgen RH (2004) Behavioural mechanisms underlying the refuge value of benthic habitat structure for two flatfishes with differing anti-predator strategies. Mar Ecol Prog Ser 268:231–243
Serejo CS (1998) Gammaridean and Caprellidean fauna (Crustacea) associated with the sponge Dysidea fragilis Johnston at Arraial do Cabo, Rio de Janeiro, Brazil. Bull Mar Sci 63:363–385
Skilleter GA, Russell BD, Degnan BM, Garson MJ (2005) Living in a potentially toxic environment: comparisons of endofauna in two congeneric sponges from the Great Barrier Reef. Mar Ecol Prog Ser 304:67–75
Turon X, Codina M, Tarjuelo I, Uriz MJ, Becerro MA (2000) Mass recruitment of Ophiothrix fragilis (Ophiuroidea) on sponges: settlement patterns and post settlement dynamics. Mar Ecol Prog Ser 200:201–212
Wendt PH, Van Dolah RF, O’Rourke CB (1985) A comparative study of the invertebrate macrofauna associated with seven sponge and coral species collected from the South Atlantic Bight. J Elisha Mitchell Sci Soc 101:187–203
Wilhelm FM, Hamann J, Burns CW (2002) Mysid predation on amphipods and Daphnia in a shallow coastal lake: prey selection and effects of macrophytes. Can J Fish Aquat Sci 59:1901–1907
Williams SL, Keck KL (2001) Seagrass community ecology. In: Bertness MD, Gaines SD, Hay ME (eds) Marine Community Ecology. Sinauer Associates Inc., Sunderland, pp 317–337
Wooldridge TH, Webb P (1988) Predator-prey interactions between two species of estuarine mysid shrimps. Mar Ecol Prog Ser 50:21–28
Wulff JL (2006) Ecological interactions of marine sponges. Can J Zool 84:146–166
Zar JH (1999) Biostatistical analysis. Prentice Hall, New Jersey
Acknowledgments
The editorial assistance of Dr Jane Fromont, Dr Gary Kendrick, Dr Justin McDonald and Stephen Whalan is greatly appreciated, as are the comments and suggestions of the anonymous reviewers. This research was supported by a University of Western Australia Postgraduate Award and complied with all current laws of Australia. Special thanks are given to Linda Heap, Caine Delacy, Diego Kendrick, David Gull and Craig Lebens (and Lebens Diving Services) for their help in the field.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S.D. Connell.
Rights and permissions
About this article
Cite this article
Abdo, D.A. Endofauna differences between two temperate marine sponges (Demospongiae; Haplosclerida; Chalinidae) from southwest Australia. Mar Biol 152, 845–854 (2007). https://doi.org/10.1007/s00227-007-0736-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-007-0736-7