Abstract
The mutualistic association between the sponge Haliclona caerulea and the calcareous red macroalga Jania adherens is conspicuous on shallow rocky regions of Mazatlán Bay (eastern tropical Pacific, Mexico). Transplanting experiments were carried out to examine the morphological responses of the association to an environmental depth gradient. Simultaneously, we conducted caging experiments to examine the effects of predation (mainly by angelfishes) on association morphology. For this, we transplanted specimens of the association from a control area at 3 m depth to depths of 1 and 5 m, and measured the morphological responses in the association (macro- and microstructure) from the three sites before and after 103 days. The association had the capacity to adjust both macro and micromorphologically, and both external morphology and body structure changed significantly with depth. The specimens grown at 1 m developed a larger surface area of attachment, higher organic density and higher inorganic content than the control specimens at 3 m, and the organisms grown at 5 m depth. We also detected significant differences in the aquiferous system of the sponge, which developed smaller and more numerous oscula at 1 m than at 5 m depth. These differences seem to be consistent with the wave movement as one of the main regulatory factors of the morphology of the association. However, the spicules from H. caerulea were most slender in shallow water, which is not consistent with increasing robustness in the face of greater wave force. The algal skeleton supplied up to 27% of the total inorganic structure of the association; thus, algal contribution significantly reduces the energy costs of spicule production, specifically under high wave exposure, when H. caerulea requires structural reinforcement relative to organic content. The contribution of the sponge to the association (as ratio Si to CaCO3) increased significantly from 3 to 5 m (12% in the uncaged specimens and 22% in the caged specimens), showing that the mutualistic relationship decreases with depth. The production of sponge branches in caged individuals was the most notable difference from uncaged morphs, which could suggest the effect of predators like angelfishes. However, branches could also be a response to the reduction in water movement and irradiance inside the cages. Sponges are known to show morphological acclimation in response to habitat variation, but this is the first study to show it in a sponge living in association with a macroalga.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The ability of an organism to respond appropriately to spatial and temporal environmental changes may regulate the local, regional and seasonal distribution of a species (Palumbi 1984, 1986; Woodin 1991; Maldonado and Young 1996). This ability, known as phenotypic plasticity (Meroz et al. 2001), may occur at different levels of organization in response to changes, from variations in physiology, to changes in the external morphology of the organism.
Phenotypic plasticity is particularly important for sessile organisms such as sponges, which cannot evade the new changing conditions by moving away (Sará and Vacelet 1973). However, even though intra-specific morphological variability does not occur in all the sponges (Carballo 1994), some species have the capacity to acclimate to a variable environment by means of continual remodeling processes (Palumbi 1984, 1986; Barthel 1989; Kaandorp 1999; Hill and Hill 2002).
In shallow marine habitats, environmental factors such as bottom topography, sediment characteristics and hydrodynamic processes are the main determinants of sponge morphology and distribution (Vogel 1981; Palumbi 1984; Carballo et al. 1996; Bell and Barnes 2000, among others). Morphological responses in sponges involve both spicule and gross morphology (mainly relating to body form and the aquiferous system) (Bell et al. 2002; McDonald et al. 2002). In fast current environments, sponges need to construct a more robust inorganic frame to minimize biomass losses from breakage (Vicente 1978; Meroz and Ilan 1995; Bell and Barnes 2000). Disadvantages of fast current flow include increased body damage produced by suspended material (Palumbi 1986) and increased body loss or mortality in the population (Bell 2002). Sedimentation also directly reduces sponge fitness by smothering or burying them, or clogging their filter-feeding systems (Sará and Vacelet 1973; Muricy 1991). Thus, species living in sedimentary environments have evolved morphological differentiations such as the production of branches that rise above the substrate (Carballo et al. 1996; Hill 1999).
The most successful resident in terms of abundance, biomass, distribution, and permanence in Mazatlán Bay (eastern tropical Pacific, Mexico) is the symbiotic association between the sponge Haliclona caerulea (Hechtel, 1965) and the red macroalga Jania adherens Lamouroux (Carballo and Ávila 2004). H. caerulea is a haplosclerid sponge, with sigmas (17.5–23.8 μm) and oxeas (83–210×2.5–11.3 μm) as spicular elements. The sponge has a unispicular ectosomal skeleton (Fig. 1a, b, f), formed by an isotropic tangential reticulation of oxeas. The choanosomal skeleton is a somewhat confused reticulation of uni-multispicular primary and secondary lines (Fig. 1d, e) that are difficult to appreciate because of their association with the alga (Fig. 1c). Jania adherens is an articulated erect red macroalga, which is repeatedly branched, with a calcified thallus. According to the basic categorization of life forms (Raven 1986), it is a haptophyte, because it needs to be attached to solid particles. In this association, the alga is entirely covered by the sponge, which also lives between the algal filaments, and very rarely does the sponge or the alga protrude over the surface of the association (Fig. 1a, b).
a, b Tangential view of the surface of the association showing the ectosomal skeleton of Haliclona caerulea formed by an isotropic tangential reticulation of oxeas. c Cross-section of choanosome showing the skeletal architecture of the sponge H. caerulea and the thallus of Jania adherens. The choanosomal skeleton is a somewhat confused reticulation of uni-multispicular primary and secondary lines because it partly replaces its skeleton with macroalgal thallus. d, e Typical reticulation of multispicular primary and secondary lines in the choanosome of H. caerulea living in isolation (see Discussion). f Detail of the unispicular ectosomal skeleton showing an arrangement in a perfect network
The association forms very stable populations in rocky ecosystems between 2 and 4 m depth affecting the abundance, survival and distribution of the two partners. J. adherens was found growing independently in the intertidal zone, which is out of range of distribution for the association, but we did not find sponges living in isolation, although in association it is one of the dominant members of the shallow rocky ecosystem in the Bay (Carballo and Ávila 2004). The association was highly specific; other coralline algae such as Amphiroa sp. occur in the same habitat, but H. caerulea associated with Amphiroa sp. was found in less than 3% of the samples studied (Carballo and Ávila 2004).
Detailed studies on symbioses involving sponges and macroalgae are very scarce (Palumbi 1985; Rützler 1990; Trautman and Hinde 2002), and only a few quantitative studies of their biomass or productivity (Trautman et al. 2000; Ávila and Carballo 2004), distribution and population dynamics (Trautman et al. 2003; Carballo and Ávila 2004), and metabolic relationships (Davy et al. 2002) as have been done. Therefore, much more research on these associations is necessary if we want to understand the capacity (i.e., acclimatory responses) of these organisms to adjust their morphology or physiology to local conditions. This is vital to understand the distribution limits of this association, and its role in natural assemblages.
Transplanting experiments allow us to examine the phenotypic plasticity of the association along and beyond s distribution range, and to compare the morphological changes observed against related environmental factors, such as light, sedimentation rates, hydrodynamic stress and predation.
In this study, we tested the association’s acclimation capacity when transplanted outside its normal range of distribution, by examining the morphological adjustments induced by local conditions (including spicule morphology). Other factors that may affect the population dynamics of the association and limits distribution in Mazatlán Bay, such as physiological adjustments, or larval dispersal and settlement, are not considered in this study.
This research is the first to examine the changes in the morphology of a sponge living in association with a macroalga along an environmental depth gradient.
Material and methods
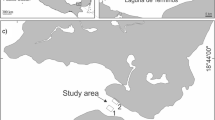
Study area
This study was carried out at a rocky coast located at Venados Island in the centre of Mazatlán Bay (Mexico, Pacific Ocean) (Fig. 2). Three depths were selected that are representative of three different environments. At 1 m depth, the bottom is rocky, and is mainly composed of large and medium-size boulders; at 3 m depth the bottom consists of a gently sloping mosaic of relatively flat boulders between patches of sediment; at 5 m depth the rocks disappear almost completely, and the bottom is mostly sediment.
Environmental factors
Environmental variability along depth was established based on the analysis of five abiotic variables (deposition of sediment, composition of the particles in the sediment, water movement, water temperature and irradiance). The deposition of sediment was measured using a trap system consisting of four sets of plastic bottles (1 l) (Carballo et al. 1996). The trapped material was repeatedly rinsed with distilled water to remove salts, and dried at 60°C for 24 h before weighing. The abundance and composition of the particles were quantitatively assessed, and the total amount of sedimented material was then expressed as kg m−2 day−1 . The deposition of sediment was monitored monthly during the whole experiment by changing the bottles. To estimate the water movement, we used the method of "plaster dissolution" (Gambi et al. 1989), consisting of four sets of plaster spheres 5 cm in diameter. The spheres remained at the bottom for 4 days each week during the whole sampling period (Naranjo et al. 1996). These measurements are useful for comparative purposes since they were measured simultaneously at each depth. The effect of water temperature on plaster spheres dissolution rates was corrected through a regression model previously calculated in the laboratory, which allows us to standardize the dissolution rates obtained from different water temperatures. Water temperature was measured monthly with a maximum/minimum thermometer. The trap system, the plaster spheres and the thermometer were placed in a permanent submerged structure at 1, 3 and 5 m depths on the seafloor. The irradiance was measured using a cosine corrected underwater light sensor (LI-192SA, Li-Cor, NE, USA) attached to a data-logger (LI-1400, Li-Cor, NE, USA).
Measurements of irradiance and water movement were done simultaneously outside and inside the cages described below. The results showed an average of 7% less water movement in the inside of the cages than outside them, and 20 to 40% less light depending on fouling.
Transplanting experiments: benign versus stressful environments
The association is resilient, adapting well to transplantation and experimental manipulations including brief periods of emersion (Ávila 2002).
Ninety intact specimens with a volume of 104–112 cm3 were collected on March 1, 2003 at 3 m depth (where the population reaches its maximum density). We collected specimens several meters apart to reduce the possibility of collecting any that were genetically identical. Once at the surface, every specimen was measured for volume using the fluid displacement method (Rützler 1978). Later, each one was attached to an artificial surface (10 cm×10 cm×0.5 cm CaCO3 squares) by immobilizing it with fishing line, and relocating it at 3 m depth for 15 days before the start of transplantation experiments. The specimens started to overgrow the surfaces quickly, and after 15 days, they had formed a secure attachment to the artificial surface. Once mortality was not detected, 15 specimens were slowly moved at the same time, completely submerged on a rigid plastic net at 1, 3 (control), and 5 m depths (45 specimens in total). To test different adaptations without the possible effect of predation by spongivore fishes (Camacho-Cruz 2004), a caging experiment was undertaken simultaneously. At each depth, 10 additional specimens were placed in cages (100 cm×20 cm×20 cm) constructed of metallic 5 mm wire and covered with plastic net with a mesh size of 1.5 cm. In April, we lost the cages deployed at 1 m due to high water movement. At the end of the experiment, only data from the cages at 3 and 5 m were obtained. The selection of the specimens to be placed in the environments was done totally at random.
The artificial surfaces (10 cm×10 cm×0.5 cm) were attached to several concrete surfaces (0.8 cm×0.6 cm×0.10 m, over 100 kg), which had been previously placed at the bottom at 1, 3 and 5 m depth using plastic cable ties.
The explants were grown for 103 days. After that, measurements of the morphology of the association were done in situ before moving the specimens to the laboratory for spicule measurements and body structure analyses.
Measurements of the morphology and body structure
The following external features were measured in situ: (1) specimen height (cm); (2) specimen coverage (cm2); (3) sponge branch production (number cm−2); (4) sponge branch length (mm); (5) oscula density (number cm−2); (6) osculum diameter (mm), and, finally, (7) algal height (mm).
Specimen height was measured vertically in four places for each individual. Specimen coverage or area of attachment (cm2) was obtained by measuring the length of each specimen at its widest part, and then measuring it again at a 90° angle (Stone 1970). By multiplying the two measurements, we obtained the area of coverage. This procedure provides a repeatable measurement easy to do in situ, and gives a basis for comparisons, as variations in size would probably affect these measurements. Sponge branch production (number cm−2) was measured by counting the total number of branches in small squares (9×9 cm−2), which were placed at the center of the surface of each organism. The length of the branches (mm) was measured as the distance between the tip of the branches and the body of the association. The density of oscula (number cm−2) and their diameter (mm) were measured in situ by counting the oscula on 9×9 cm−2 surfaces, and using a plastic caliber for diameter. The distance between the substrate and the highest point in the alga living in isolation in the intertidal zone, was taken as alga height. For J. adherens living in association, algal height was determined as the distance between the base of attachment and the top of the association.
Finally, the length and thickness (μm) of H. caerulea oxeas were determined in the laboratory. Each measurement consisted of five replicates per specimen (samples from different parts of each specimen), and for each replicate a minimum of 50 spicules was measured. The density of the association (g DW cm−3) was measured at the end of the experiment by excising five pieces per specimen, and measuring their volumes and mass weight. The pieces were previously washed with distilled water, and dried at 100°C during 24 h to determine the dry weight (DW). Later, 1 g of DW was burned for 24 h in a muffle furnace at 550°C to obtain the ash weight (AW). The organic weight expressed as ash-free dry weight (AFDW) was determined as: AFDW=DW−AW. AW represents the inorganic fraction and is commonly used to describe the amount of siliceous skeleton in sponges (Schönberg and Barthel 1997). However, taking into account that J. adherens is a calcareous alga, this procedure did not remove the CaCO3 from the alga. To obtain the ash-free CaCO3 fraction (AFCaCO3), the AW was burned again at 1,000°C for 24 h. The carbonate content was estimated by calculating the difference between the two dry weights (AW−AFCaCO3) (Ávila and Carballo 2004). The amounts were converted to CaCO3 and silica per g DW, and later all measurements were standardized to a known association volume.
Data analysis
Differences in gross morphology and body structure between different depths was analyzed by one-way ANOVA after verifying normality (Kolmogorov–Smirnov test) and variance homogeneity (Barlett test). The data were rank-transformed prior to the analysis when assumptions were not fulfilled. The significance of differences found between the treatments was tested using the Tukey test (Sokal and Rohlf 1981).
In order to determine significant differences between alga height living in isolation in the intertidal zone, and alga living in association with the sponge (at 1, 3, and 5 m depth, in caged and uncaged specimens) a t test was used (Palumbi 1984). A Spearman rank correlation was used to assess relationships between morphology and environmental variables.
Results
Environmental variables
Water movement was significantly higher at 1 m depth than at 3 and 5 m (Table 1). The rate of sedimentation/resuspension varied according to water movement, with higher values at 1 m depth. Water temperature increased at an average of 7.4°C, from March to June. The average monthly values measured between 11:00 a.m. and 12:00 noon were: 20.02°C in March; 23.7°C in April; 25.2°C in May, and 27.6°C in June. We did not obtain significant differences in water temperature among depths. However, we detected differences in the average monthly fluctuation (Table 1). Light availability in the water column was reduced during the experimental period from 70.5% of the surface irradiance (% Es) at 1 m to less than 20% Es at 5 m (Table 1).
Gross morphology
Significant morphological differences were observed in the association under different environmental conditions. The external morphology varied from flattened forms with a higher surface area of attachment at 1 m depth, to massive forms at 5 m depth (Fig. 3). Thus, the individuals grown at 1 m had a larger area of coverage per individual (144±15 cm2) than organisms grown at 5 m depth (83.7±12 cm2) (Tables 2, 3). We did not find significant differences in the mean height of the individuals among treatments (P>0.05), but at 5 m (5.1±0.2 cm) the specimens were higher than at 1 m (4.7±0.5 cm) (Tables 2, 3).
We also detected significant differences in the aquiferous system of the sponge (Table 3, Fig. 4). The individuals transplanted to shallower water developed significantly higher numbers of oscula per unit surface area (0.71±0.07 cm−2) than the control (0.4±0.03 cm−2) and specimens grown at 5 m depth (0.38±0.04 cm−2). They also had smaller diameter (3.7±0.1 mm) than the control individuals (4.8±0.35 mm) (Table 2).
J. adherens forms taller individuals growing in association with H. caerulea than living in isolation in the intertidal area. The maximum height of the intertidal forms living in isolation was 12±0.7 mm, whereas the individuals growing in association with H. caerulea at 1, 3 and 5 m depth reached a mean height of 47±5 mm (t=−13.59, df=23, P<0.0001), 40±3 mm (t=−11, df=27, P<0.0001), and 52±4 mm (t=−15.9, df=26, P<0.0001), respectively (Fig. 5).
Mean height (±SE) of the alga J. adherens free-living in the intertidal and living in association with the sponge Haliclona caeruela at 1, 3 and 5 m depth. The open bars show the results from the uncaged experiment, and the black bars that of the caged experiment. Transplanting experiment resulted in significant increase in the height of the alga living in association
The caged specimens at 5 m were less dense (135±15 mg cm−3) and less inorganic content (100±1.2 mg cm−3) than the uncaged specimens grown at the same depth (Tables 2, 4). The coverage/height relationship also showed that caged individuals reached a bigger size than uncaged individuals at the same depth. But in general, the caged individuals showed the same morphological variability as the controls (uncaged individuals at the same depth), and the only significant feature regarding the control was the production of sponge branches in the caged specimens at 3 m (0.1±0.1 branch cm−2 ; 7 mm length) and at 5 m (0.16±0.03 branch cm−2 ; 18±5.8 mm length) (Tables 4, 5). In the caged specimens, J. adherens was also significantly taller at 3 m (40±1 mm; t=−8.4, df=21, P<0.0001) and 5 m (56±5 mm; t=−12.5, df=27. P<0.0001) than living in isolation in the intertidal area (Fig. 5).
Body structure
Density and inorganic content decreased with depth, showing that the body is less dense at deeper waters (Table 2). Density decreased from 247±15 mg cm−3 (at 1 m depth) to 159±13 mg cm−3 (at 5 m depth). The inorganic content also decreased from 180±10 mg cm−3 (at 1 m depth) to 113±9 mg cm−3 at 5 m depth (Table 2), and both CaCO3 and silicate content varied significantly with depth (Table 3).
The reduction in density was not significant in the caged specimens at 5 m depth (135±15 mg cm−3) versus the specimens from the control (159±13 mg cm−3), which showed less inorganic content (100±1.2 mg cm−3) than the uncaged individuals grown at the same depth (113 ± mg cm−3) (Tables 4, 5)
Specimens at 1 and 3 m depth were composed of 73% silica and carbonate, but at 5 m depth the amount of inorganic content decreased to 71%. Silicate was the main constituent of the association; it accounted for 62% of the inorganic content at 1 and 3 m depth, and for 65.4% at 5 m depth. The contribution of the sponge to the association (as ratio Si to CaCO3) increased significantly from 3 to 5 m: 12% in the uncaged specimens (t=−2.43, P=0.03), and 22% in the caged specimens (t=−2.6, P=0.02), showing that the mutualistic relationship decreases with depth (Fig. 6).
The organic matter varied positively with the silicate (P<0.01), whereas density varied with carbonate (P<0.05), silicate (p<0.01), and organic matter (P<0.05) concentration.
The mean length of the oxeas increased with depth; the oxeas of the specimens transplanted at 1 m were significantly shorter (169.4±0.84 μm) than the oxeas of the control (172.7±1.01) and of the individuals transplanted at 5 m depth (173.1±1.4 μm) (Tables 2, 3). In contrast, the oxeas were significantly thicker at 5 m (6.13±0.08 μm) than at 1 m depth (5.92±0.05 μm).
Morphological variation as a function of environmental changes
Water movement was positively correlated with the coverage (r=0.9, P<0.05, n=5), with the number of oscula per unit surface area (r=0.9, P<0.01, n=5), with density (r=0.95, P<0.01, n=5), and with the inorganic content (r=0.9, P<0.01, n=5). Density and Si and CaCO3 content showed positive associations with irradiance (r=0.95, r=0.93, and r=0.90, P<0.01, respectively).
Discussion
Water movement is considered the primary environmental factor in shallow waters (Lewis 1968), and our transplanting experiment confirmed its importance in the regulation of sponge morphology (Kaandorp 1999).
The specimens grown at 1 m developed a larger surface of attachment with higher density than the control at 3 m and the organisms grown at 5 m depth. In addition, at 1 m depth H. caerulea developed smaller and more oscula per unit of surface area than at 3 and 5 m.
Water movement also regulates inorganic content and spicule morphology of sponges (Bell et al. 2002). The spicules are the main constituent of their skeletal material, along with collagen and spongin fibers, and the sponges exposed to high mechanical stress contain more spicules than sponges from habitats with medium hydrodynamism (Palumbi 1984, 1986). Increments in inorganic content may also be achieved by decreasing other organic skeletal components, such as spongin fibers (Stone 1970), or by increasing the thickness of the spicular elements for a larger surface area and greater support and structural integrity to the organic content within the sponge (Koehl 1982; Palumbi 1984, 1986; McDonald et al. 2002). The sponges Halichondria panicea (Palumbi 1986) and Cinachyrella australiensis (McDonald et al. 2002) developed significantly thicker oxeas in high wave environments to increase the stiffness of the body, but in contrast, the spicules of H. caerulea were shorter and more slender at 1 m, which is not consistent with wave force as a determining factor.
Although body resistance to breakage was not quantified in this study, increased density may increase body resistance to wave action. In fact, the stiffness of sponges increases with spicule concentration (Palumbi 1986). We found similar values in density at 1 and 3 m (247 mg cm−3 and 211 mg cm−3, respectively) as those reported for Halichondria panicea (210 mg cm−3) transplanted to high wave force environments (Palumbi 1986), and we found significant differences in the inorganic content over depth. However, in the H. caerulea/J. adherens association, the calcified branches of J. adherens accounted for 27.1% of the 73% inorganic content in the specimens grown at 1 and 3 m depth (Fig. 1 C). Similar values in the percentage of inorganic content have been reported for H. panicea (76%) (Palumbi 1986), and C. australiensis (78.2%) (McDonald et al. 2002) growing in high wave force environments; however, in these species the inorganic content is exclusively spicule silicate.
The use of different organisms as a substitute for skeletal fibers in sponges has been previously documented (Rützler 1990). Ours results show that the sponge utilizes the symbionts as a substitute skeleton (mainly as a substitute of the primary fibers). However, the benefits for the sponge seem to go beyond the stable substrate and structure offered by the alga, as it has also been suggested for the sponges Dysidea janiae and Strongylacidon osburnensis, which substitute or reinforce their own skeleton by living in association with calcified Rhodophyta alga of the genus Jania (Rützler 1990). We do not know if the sponge and alga derive mutual metabolic benefit by living in this particular symbiotic association, but H. caerulea replaces more than 27% of its skeleton at 1 m depth with macroalgal thallus reducing the investment in sponge spicule production. In fact, in experimental tanks, it was possible to grow the sponge without the algae; the sponge developed the typical reticulation of primary and secondary lines in the choanosome (Fig. 1d, e), which are partly substituted for the branches of J. adherens when both species live in association (Fig. 1 c) (unpublished).
The contribution of the sponge to the association increased significantly (between 12 and 22%) with depth. This data supports our preliminary observations that the mutualistic relationship decreases towards deeper water (Ávila and Carballo 2004), and they are also consistent with those of Palumbi (1985), which showed that the Halichondria panicea/Corallina vancouveriensis relationship varies over remarkably small spatial scales.
The morphological response of the sponge to high water movement requires additional energy to construct a more robust inorganic frame at the expense of softer organic mass. Moreover, the small pipes produced by H. caerulea under high hydrodynamism, impose higher resistance to water flow and concomitantly increase water-pumping costs (Vogel 1981). J. adherens supplies an important fraction of the structural content of the association thereby helping to reduce the energy costs of spicule synthesis significantly, especially when H. caerulea requires structural reinforcement under high water movement. The fact that spicules from H. caerulea were most slender in shallow water, which is inconsistent with increasing robustness in the face of greater water movement, partly supports our findings.
However, possible nutritional interactions, such as photosynthate translocation and nitrogen supply, could also explain part of the trends seen. We do not know if any metabolic relationships exist between H. caerulea and J. adherens as it seems to occur between the roots of the red mangrove (Rhizophora mangle), and the two common root-fouling sponges Tedania ignis and Haliclona implexiformis, where the roots obtain dissolved inorganic nitrogen from the sponges, and in return, the sponges obtain carbon from the mangrove roots (Ellison et al. 1996). However, in the case of a very similar association between Haliclona cymiformis (Esper) and the red macroalga Ceratodictyon spongiosum, the data suggest that while the alga may supply the sponge with some essential nutrients, the major source of organic carbon is the particulate and dissolved organic matter in the ambient seawater, making the primary role of the algal symbionts structural rather than nutritional (Davy et al. 2002; Trautman and Hinde 2002).
Branch productions were observed at 3 and 5 m depths in cage specimens as opposed to the control specimens (uncaged individuals at the same depth), which lacked branches entirely (Figs. 7, 8). This suggests that the exclusion of predators in situ also had an effect on sponge morphology. H. caerulea forms part of the diet of two species of angelfishes (Holacanthus passer and Pomacanthus zonipecthus) (Camacho-Cruz 2004) and of the nudibranch Discodoris keto (Padilla 2005). This branch morphotype could be more exposed to predation (Hill and Hill 2002) because the sponge branches do not contain calcified thallus of J. adherens. In fact, pieces of H. caerulea without algae were found in the contents in the stomachs of fishes and nudibranches, but traces of the calcareous algae were not found (Camacho-Cruz 2004; Padilla 2005). This can also be supported by the fact that the non-manipulated association in its natural habitat forms massive and compact structures, where sponge or macroalga protrudes only occasionally over the surface of the association (see images in previous papers; Ávila and Carballo 2004; Carballo and Ávila 2004).
a Uncaged specimen transplanted at 1 m depth. b The same specimen at the end of the experiment 103 days later. c Caged specimen transplanted to 3 m depth. d The same specimen at the end of the experiment. The most remarkable feature in the caged specimens was the production of small sponge branches (arrows). Scale bar (1 cm) is the same for the four images
a Caged specimen at 5 m depth. b The same specimen at the end of the experiment. c Lateral view of the same specimen. The most remarkable feature in the caged specimens transplanted at 5 m depth was the production of long branches in the sponge, which protruded the surface of the association. Scale bar (1 cm) is the same for the three images
Nevertheless, considering the 30% reduction in light availability inside the cages, and the 7% reduction in water movement, we cannot conclude that the lack of branches observed in situ in the non-manipulated population and in uncaged individuals (control) was only due to predation because (1) prolonged wave exposure probably breaks them, and (2) light limitation may favor sponge branching by reducing or even preventing J. adherens growth without causing a similar effect in H. caerulea. The branching morphotype of the H. caerulea/J. adherens association was more prevalent in the cages located at 5 m depth. It is probable that the balance between (1) branching formation, which is favored when H. caerulea growth exceeds J. adherens growth, and (2) branch breakage by waves or predators, regulates the relative importance of sponge branching.
Finally, it is known that a relatively large stagnant and diffusion-dominated region develops within a branching colony. Recently, simulation experiments and isotope analyses of Madracis mirabilis skeletons (scleractinian coral) have shown that external gradients of dissolved inorganic carbon (DIC) determine the morphogenesis of branching, phototrophic corals (Kaandorp et al. 2005). Thus, another possibility to explain these results could be that in the cage, and especially between protrusions and branches in the sponge, a stagnant diffusion-limited zone might occur, in which nutrients such as silicate could limit the growth process of the sponge and favor the formation of branches in a similar way as M. mirabilis.
Exposure to waves may dramatically increase biomass losses (Meroz and Ilan 1995; Bell and Barnes 2000), therefore, all phenotype adjustments such as (1) increased body resistance to breakage, and/or (2) increased resistance to the hydrodynamic force by development of larger attachment area, may enhance species survival in this environment. Associated with these morphological changes, oscula (and other piping elements) have to decrease in diameter to be able to pump water through a denser body for feeding and respiration (Wilkinson and Vacelet 1979; Palumbi 1986; McDonald et al. 2002, among others).
Adopting a specific growth form may increase the ecological fitness of a species under certain environments, while being suboptimal under other environmental conditions. The ecological benefit of being morphologically plastic is to have the ability to produce a broad diversity of growth forms or phenotypic adjustments to the environment. The results also show that the association is able to live outside its typical range of distribution (Ávila and Carballo 2004; Carballo and Ávila 2004). The fact that the free-living form is only present in the intertidal area suggests that it is in association with H. caerulea where J. adherens acquires the protection and/or the emergent properties that enhance its survival capacity with depth. Thus, we must consider other factors that may affect the population dynamics of the association and limit its distribution in depth, such as the survival of the settlers, appropriate habitat selection, availability of substrate, or mortality of adults, which are currently undertaken in other research (unpublished).
In summary, the association of H. caerulea with Jania adherens could be a mechanism to offer resistance to heavy flow, as it has been reported for the associations Haliclona cymiformis/ Ceratodictyon spongiosum (Davy et al. 2002), and Halichondria sp./ Corallina vancouveriensis (Palumbi 1985). This can be also supported by the fact that the sponge appears to be able to live without the alga in more sheltered habitats, like on mangrove roots in San Blas Island (Wulff, personal communication), and in a semi-enclosed coastal lagoon near the Bay of Mazatlán (personal observations). However, Wilkinson and Vacelet (1979) showed that the morphologies of several shallow-water sponges are primarily influenced not only by water flow but also by light regime. In fact, the increase in biomass seen in shallow water could also be linked to light regime, because of a faster growth rate in the alga in response to more light. Some of these questions are addressed in detail in another paper currently under revision (Enríquez et al. 2005).
References
Ávila E (2002) Dinámica poblacional de la asociación Haliclona caerulea (Hechtel, 1965) (Demospongiae, Haplosclerida) y algas rojas en la bahía de Mazatlán (México, Pacífico Oriental). MS dissertation, Instituto de Ciencias del Mar y Limnología, Universidad Nacional Autónoma de México, p 76
Ávila E, Carballo JL (2004) Growth and standing stock biomass of a mutualistic association between the sponge Haliclona caerulea and the red alga Jania adherens. Symbiosis 36(3):225–244
Barthel D (1989) Growth of the sponge Halichondria panicea in the North Sea habitat. Polish Academy of Sciences, Institute of Oceanology, pp 23–30
Bell JJ (2002) Regeneration rates of a temperate demosponge: the importance of water flow rate. J Mar Biol Assoc UK 82:169–170
Bell JJ, Barnes DKA (2000) The influences of bathymetry and flow regime upon the morphology of sublittoral sponge communities. J Mar Biol Assoc UK 80:707–718
Bell JJ, Barnes DKA, Turner JR (2002) The importance of micro and macro morphological variation in the adaptation of a sublittoral demosponge to current extremes. Mar Biol 140:75–81
Camacho-Cruz ML (2004) Estudio preliminar de la composición faunística de depredadores de esponjas en la bahía de Mazatlán (Sinaloa). Bs dissertation, Facultad de Ciencias del Mar, Universidad Autónoma de Sinaloa, p 53
Carballo JL (1994) Taxonomía, zoogeografía y autoecología de los Poríferos del Estrecho de Gibraltar. PhD dissertation, Facultad de Biología, Universidad de Sevilla, p 334
Carballo JL, Ávila E (2004) Population dynamics of a mutualistic interaction between the sponge Haliclona caerulea, and the red alga Jania adherens. Mar Ecol Prog Ser 279:93–104
Carballo JL, Naranjo SA, García Gómez JC (1996) The use of marine sponges as stress indicators in marine ecosystems at Algeciras Bay (Southern Iberian Peninsula). Mar Ecol Prog Ser 135:109–122
Davy SK, Trautman DA, Borowitzka MA, Hinde R (2002) Ammonium excretion by a symbiotic sponge supplies the nitrogen requirements of its rhodophyte partner. J Exp Biol 205:3505–3511
Ellison AM, Farnsworth EJ, Twilley RR (1996) Facultative mutualism between red mangroves and root-fouling sponges in belizean mangle. Ecology 77(8):2431–2444
Enríquez S, Ávila E, Carballo JL (2005) Phenotypic plasticity induced in transplanting experiments with a mutualistic association between the sponge Haliclona caerulea and the red macroalgae Jania adherens II Morphological responses of the algae. Oecologia (submitted)
Gambi MC, Buia MC, Casola E, Scardi M (1989) Estimates of water movement in Posidonia oceanica beds: a first approach. In: Boudouresque CF, Meinesz A, Fresi E, Gravez V (eds) International Workshop of Posidonia Beds, France 2:101–112
Hill MS (1999) Morphological and genetic examination of phenotypic variability in the tropical sponge Anthosigmella varians. Mem Qld Mus 44:239–247
Hill MS, Hill AL (2002) Morphological plasticity in the tropical sponge Anthosigmella varians: responses to predators and wave energy. Biol Bull 202:86–95
Kaandorp JA (1999) Morphological analysis of growth forms of branching marine sessile organisms along environmental gradients. Mar Biol 134:295–306
Kaandorp JA, Sloot PMA, Merks RMH, Bak RPM, Vermeij MJA (2005) Morphogenesis of the branching reef coral Madracis mirabilis. Proc R Soc B 272:127–133
Koehl MAR (1982) Mechanical design of spicule-reinforced connective tissue: stiffness. J Exp Biol 98:239–267
Lewis JR (1968) Water movement and their role in rocky shore ecology. Sarsia 34:13–36
Maldonado M, Young CM (1996) Effects of physical factors on larval behavior, settlement and recruitment of four tropical demosponges. Mar Ecol Prog Ser 138:169–180
McDonald JI, Hooper JNA, McGuinness KA (2002) Environmentally influenced variability in the morphology of Cinachyrella australiensis (Carter 1886) (Porifera: Spirophorida: Tetillidae). Mar Fresh Res 53:79–84
Meroz E, Ilan M (1995) Cohabitation of a coral reef sponge and a colonial scyphozoan. Mar Biol 124:453–459
Meroz E, Brickner I, Loya Y, Peretzman-Shemer A, Micha I (2001) The effect of gravity on coral morphology. Proc R Soc Lond B 269:717–720
Muricy G (1991) Structure des peuplements de spongiaires autour de l’égout de Cortiou (Marseille, France). Vie Milieu 41(4):205–221
Naranjo SA, Carballo JL, Garcìa Gómez JC (1996) The effects of environmental stress on ascidian populations in Algeciras Bay (Southern Spain). Possible Marine Bioindicators. Mar Ecol Prog Ser 144:119–131
Padilla C (2005) Composición faunística, y alimenticia de moluscos comedores de esponjas (Mollusca, Opistobranquia) en la bahía de Mazatlán (Sinaloa). Bs dissertation, Facultad de Ciencias del Mar, Universidad Autónoma de Sinaloa
Palumbi SR (1984) Tactics of acclimation: morphological changes of sponges in an unpredictable environ. Science 225:1478–1480
Palumbi SR (1985) Spatial variation in an alga-sponge commensalism and the evolution of ecological interactions. Am Nat 126:267–274
Palumbi SR (1986) How body plans limit acclimation: responses of a demosponge to wave force. Ecology 67(1):208–214
Raven ME (1986) Evolution of plant life forms. In: Givnish TJ (ed) On the economy of plant form and function. Cambridge University Press, Cambridge, pp 421–492
Rützler K (1978) Sponges in coral reefs. In: Stoddart DR, Johannes RE (eds) Coral reefs: research methods. UNESCO Monogr Oceanogr Methodol, Paris 5:299–313
Rützler K (1990) Associations between Caribbean sponges and photosynthetic organisms. In Rützler K (ed) New perspectives in sponge biology. Smithsonian Institution Press, Washington DC, pp 455–466
Sará M, Vacelet J (1973) Ecologie des Démosponges. In: Masson et Cie (eds) Traité de Zoologie, Anatomie, Sistématique, Biologie, vol III. Spongiaires, Paris, pp 472–576
Schönberg CHL, Barthel D (1997) Inorganic skeleton of the demosponge Halichondria panicea. Seasonality in spicule production in the Baltic Sea. Mar Biol 130:133–140
Sokal RR, Rohlf JJ (1981) Biometry. WH Freeman and Co, San Francisco
Stone AR (1970) Growth and reproduction of Hymeniacidon perleve (Montagu) (Porifera) in Langsone Harbour, Hampshire. J Zool 161:443–459
Trautman DA, Hinde R (2002) Sponge/algal symbioses: a diversity of associations. In: Seckbach J (ed) Symbiosis: mechanisms and model systems. Kluwer, Dordrecht, pp 523–537
Trautman DA, Hinde R, Borowitzka MA (2000) Population dynamics of an association between a coral reef sponge and a red macroalga. J Exp Mar Biol Ecol 244:87–105
Trautman DA, Hinde R, Borowitzka MA. (2003) The role of habitat in determining the distribution of a sponge-red alga symbiosis on a coral reef. J Exp Mar Biol Ecol 283:1–20
Vicente VP (1978) An ecological evaluation of the West Indian demosponge Anthosigmella varians (Hadromerida: Spirastrellidae). Bull Mar Sci 28:771–777
Vogel S (1981) Life in moving fluids-the physical biology of flow. Princeton University Press, Princeton, pp 1–467
Wilkinson CR, Vacelet J (1979) Transplantation of marine sponges to different conditions of light and current. J Exp Mar Biol Ecol 37:91–104
Woodin SA (1991) Recruitment of infauna: positive or negative cues? Am Zool 31:797–807
Acknowledgements
The study has been funded in part by the project "Ecología de la asociación entre la esponja Haliclona Caerulea y el alga roja Jania adherens”, (IX232004), funded by the PAPIIT (Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica, DGAPA). We thank the support of Postgrado de Ciencias del Mar y Limnología (UNAM), the support in funding the exchange of EA and SE. We also thank JM Geraldía (Servicio Central de Ciencia y Tecnología) of Universidad de Cádiz (Spain) for his help with the SEM photographs, C Ramírez Jáuregui, P. Allende and V. Montes (ICML–UNAM) for generous help with the literature and aerial images, G. Ramirez Reséndiz and C. Suarez (ICML-UNAM) for their computer assistance, and staff members at the ICML-UNAM (Mazatlán), C Vega Juarez, P Pérez and J Toto Fiscal, for their help in the sampling.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. W. Sammarco, Chauvin
Rights and permissions
About this article
Cite this article
Carballo, J.L., Ávila, E., Enríquez, S. et al. Phenotypic plasticity in a mutualistic association between the sponge Haliclona caerulea and the calcareous macroalga Jania adherens induced by transplanting experiments. I: morphological responses of the sponge. Marine Biology 148, 467–478 (2006). https://doi.org/10.1007/s00227-005-0104-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-005-0104-4