Abstract
Haploids have often been used to simplify the genetic complexity of potato due to its high heterozygosity and autotetraploidy. We maintained some haploid plants of wild potato species in vitro that were produced through anther culture almost a half century ago. Among them, a diploid Solanum bulbocastanum and its monohaploid, three monohaploids of S. verrucosum, a disomic tetraploid S. stoloniferum and its dihaploid (all Mexican species) and a dihaploid of the disomic tetraploid S. acaule (South American species) were analyzed morphologically, cytologically and by molecular markers. Their species identity and ploidy levels were confirmed to be correct except for those of a monoploid S. bulbocastanum, which had already experienced natural chromosome doubling. The monohaploids and chromosome-doubled monohaploid were all homozygous in 19,889 SNP loci surveyed. Two of three monohaploids of S. verrucosum were morphologically different but similar by SNP analysis. Most of the heterozygous loci of the tetraploid S. stoloniferum were in a duplex condition. The heterozygous loci of the dihaploid S. stoloniferum were mostly duplexed in the tetraploid S. stoloniferum, suggesting that the alleles are fixed in homoeologous loci. Therefore, each of these haploid-plant genomes is homozygous and is suitable for whole-genome sequencing.
Resumen
Los haploides se han utilizado con frecuencia para simplificar la complejidad genética de la papa debido a su alta heterocigosidad y autotetraploidía. Mantuvimos algunas plantas haploides de especies silvestres de papa in vitro que se produjeron a través del cultivo de anteras hace casi medio siglo. Entre ellas, un diploide de Solanum bulbocastanum y su monohaploide, tres monohaploides de S. verrucosum, un tetraploide disómico de S. stoloniferum y su dihaploide (todas especies mexicanas) y un dihaploide del tetraploide disómico S. acaule (especie sudamericana). Se analizaron morfológicamente, citológicamente y por marcadores moleculares. Se confirmó que su identidad de especie y sus niveles de ploidía eran correctos, excepto los de una monoploide de S. bulbocastanum, que ya había experimentado la duplicación natural de cromosomas. Los monohaploides y el monohaploide doblado cromosómico fueron todos homocigotos en 19,889 loci SNP analizados. Dos de los tres monohaploides de S. verrucosum eran morfológicamente diferentes pero similares según el análisis de SNP. La mayoría de los loci heterocigotos del tetraploide S. stoloniferum estaban en una condición de duplicidad. Los loci heterocigotos del dihaploide S. stoloniferum fueron en su mayoría duplicados en el tetraploide S. stoloniferum, sugiriendo que los alelos se fijan en loci homólogos. Por lo tanto, cada uno de estos genomas de plantas haploides es homocigoto y es adecuado para la secuenciación completa del genoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Due to its high heterozygosity and autotetraploidy, genetic analyses in potato (the tetraploid form of Solanum tuberosum L.) are difficult. To simplify this genetic complexity, haploids have often been used (Ortiz and Peloquin 1994). Many diploid haploids (dihaploids) have been produced from commercial varieties with relative ease by pollination with the pollen of specific clones of S. phureja Juz. et Buk., called haploid inducers (Hougas et al. 1964; Hermsen and Verdenius 1973). Female gametes (or eggs) grow to mature plants through parthenogenesis. Even monoploid haploids (monohaploids) have been produced using haploid inducers (van Breukelen et al. 1975; Uijtewaal et al. 1987). This haploidization method has been quite efficient in obtaining dihaploid S. tuberosum. However, this method is not applicable to a wide range of wild potato species (tuber-bearing Solanum species). A more general method for obtaining haploids is anther culture, which results in haploids originating from microspores (or pollen). In the 1970s and 1980s, monohaploids were generated from dihaploid S. tuberosum by anther culture (Foroughi-Wehr et al. 1977; Sopory et al. 1978; Mix 1983; Uhrig 1985). Breeding schemes to develop highly heterozygous tetraploid clones using monoploids have been proposed (Wenzel et al. 1979; Meyer et al. 1992). The first androgenetic monohaploids in the tuber-bearing Solanum species were generated from the Mexican diploid species S. verrucosum Schlechtd. (Irikura and Sakaguchi 1972). Irikura (1975a, b) further induced monohaploids from the diploid species S. bulbocastanum Dun., S. verrucosum, S. phureja, and S. stenotomum Juz. et Buk.; dihaploids from the tetraploid species S. fendleri Asa Gray, S. hjertingii Hawkes, S. polytrichon Rydb., and S. stoloniferum Schlechtd. et Bché.; and trihaploids from the hexaploid species S. demissum Lindl. and S. nigrum L. Since then, many androgenetic monohaploids have been induced from various species, such as S. chacoense Bitt. and S. phureja (Cappadocia et al. 1984; Veilleux et al. 1985). Chromosome doubling of a monohaploid can produce a completely homozygous diploid clone; one such clone generated from S. phureja (clonal identity of DM 1–3 516 R44, Lightbourn and Veilleux 2007) was used for whole-genome sequencing, resulting in a reference genome for potato (Potato Genome Sequencing Consortium 2011).
Dr. Yukio Irikura, who was the first to produce a monoploid tuber-bearing Solanum species (Irikura and Sakaguchi 1972), maintained anther culture-derived haploids and many somatic hybrids in vitro. After his death, they were maintained by the Hokuren Federation of Agricultural Cooperatives (Sapporo, Japan). Finally, in 2010, most of these materials were unable to be maintained and only some plants of particular importance were transferred to our laboratory. Thus, Irikura’s materials have been cultured in vitro for nearly a half century. The remaining materials included monohaploids of S. bulbocastanum and S. verrucosum and a dihaploid of S. stoloniferum. These three species are Mexican wild species (Hawkes 1990). The Mexican diploid species, except S. verrucosum, are strictly isolated by reproductive barriers from the South American species (Hawkes 1990). S. verrucosum (2n = 2x = 24), however, is only a diploid species, with a genome constitution of AA, from Mexico, and it is self-compatible and cross-compatible as a female parent with most South American species that consist of the A genome (Matsubayashi 1991; Eijlander et al. 2000). S. verrucosum is also crossable and functions as a bridge to some Mexican diploid species, such as S. bulbocastanum, S. cardiophyllum Lindl., S. jamesii Torr., S. pinnatisectum Dun., and S. trifidum Corr. (Hermsen and Ramanna 1976; Hamernik et al. 2001; Dinu et al. 2005; Jansky and Hamernik 2009; Bamberg et al. 2021). S. bulbocastanum (2n = 2x = 24) is well known for its resistance to late blight (caused by Phytophthora infestans), and the resistance genes Rpi-blb1/RB, Rpi-blb2, Rpi-blb3, and Rpi-bt1 have been cloned from this species (Lokossou et al. 2010). S. stoloniferum (2n = 4x = 48) is a tetraploid forming only bivalent chromosomes at meiosis, so it is considered to be an allotetraploid species with a genome formula of AABB (Matsubayashi 1955; Irikura 1976; Pendinen et al. 2008). The most likely maternal diploid ancestor and the A genome donor to S. stoloniferum is S. verrucosum (Spooner and Castillo 1997; Rodríguez and Spooner 2009; Sanetomo and Hosaka 2013). The B genome donor might be S. cardiophyllum, S. ehrenbergii (Bitter) Rydb., S. jamesii Torrey, or S. bulbocastanum (Irikura 1976; Pendinen et al. 2008; Wang et al. 2008; Rodríguez and Spooner 2009). A tetraploid species that forms only bivalent chromosomes at meiosis has also found in South America, S. acaule Bitt., which the most widely distributed species in the Andean highlands and a highly self-fertile species (Swaminathan 1954; Irikura 1976; Hawkes 1990). However, different understandings of the trivalent formation frequencies at meiosis in triploid hybrids between S. acaule and a diploid A-genome species lead to two hypotheses for the genome constitution: AAAaAa (segmental allopolyploidy, Hermsen and Ramanna 1969; Matsubayashi 1982; Camadro et al. 1992) or AABaBa (allopolyploidy, Irikura 1976).
In this study, Irikura’s haploids of S. bulbocastanum, S. verrucosum, and S. stoloniferum were analyzed morphologically, cytologically, and by molecular analyses, including the single nucleotide polymorphism (SNP) array method, and compared with haploids of the South American species S. phureja and S. acaule. The specific objectives are; 1) to investigate whether the original genotypes have been reliably maintained, 2) to reveal heterozygosity levels, and 3) to evaluate genetic relationships among these species. The usefulness of these materials for whole-genome sequencing is also discussed.
Materials and Methods
Plant Materials
The plant materials used in this study are listed in Table 1. Haploids of the Mexican diploid species S. bulbocastanum and S. verrucosum and a haploid of a Mexican tetraploid species, S. stoloniferum, were derived from anther culture (Irikura and Sakaguchi 1972; Irikura 1975b). ATDH-1 is a dihaploid clone induced by anther culture from a Peruvian tetraploid species, S. acaule (acl-T), at the Laboratory of Plant Breeding, Kobe University (Yamada et al. 1997). It has been maintained by tubers in our laboratory. Hereinafter, all the genotypes are represented by the codes shown in Table 1. Based on the Irikura’s notes provided in Table 1, we assumed that 2x blb was a source plant for the anthers, from which 1x blb was created. Similarly, 4x sto was assumed to be a source plant for 2x sto. The source plant (11H16) for 1x ver1, 1x ver2, and 1x ver3 was lost in our laboratory but is still available as PI 666966 from the US Potato Genebank at Sturgeon Bay, Wisconsin, USA. 2x pnt is a clone of a Mexican diploid species, S. pinnatisectum Dun. used as a control plant. DM is a chromosome-doubled clone of a monoploid plant derived from an anther culture of S. phureja (Lightbourn and Veilleux 2007) and is a source plant for the reference genome of potato (Potato Genome Sequencing Consortium 2011).
Ploidy Determination
The ploidy level was determined by flow cytometry (CyFlow Ploidy Analyzer, Sysmex Corporation, Kobe, Japan). Approximately 1 cm2 of fresh leaf was used for DAPI fluorescence staining according to the manufacturer’s instructions.
Determination of Cytoplasm Type
The cytoplasm type (A, D, P, M, T, or W) was determined using the procedure described by Hosaka and Sanetomo (2012).
Pollen Stainability
Pollen fertility was estimated by stainability with 1% acetocarmine. Over 300 pollen grains in each sample were counted under the microscope. The stainability percentage was calculated as 100 × (the number of stained pollen grains/the total number of pollen grains).
SNP Analysis
Total DNA was extracted from fresh leaves by the method described in Hosaka and Hanneman (1998). A total of 2.5 µg of dried DNA was sent to GeneSeek (Neogen Corporation, NE, USA) to obtain 31 K potato V4 SNP array data genotyping 30,991 SNP loci. Genotype calling was performed using a tetraploid model (i.e., AAAA, AAAB, AABB, ABBB, or BBBB). From the obtained data, heterozygous or no-call SNPs in DM (completely homozygous) and SNPs with > 10% missing values were first discarded. Then, ambiguous SNPs such as those suggested by Peterson et al. (2016) and those originating from chloroplast DNA or unknown locations were discarded. Further filtering was performed by considering ploidy levels; SNPs showing AAAB or ABBB for diploid or dihaploid plants and those showing AAAB, AABB, or ABBB for monohaploid or doubled monohaploid plants were discarded. All genotypes were represented as AA (identical to that of DM), AB, BB or NC (no calls = missing data). To evaluate similarities among samples, the AA, AB and BB genotypes were converted to numerical values of 3, 2 and 1, respectively. Then, a distance matrix was calculated, and principal coordinate analysis (PCoA) was performed using principal component analysis in JMP Pro 16.0.0 software (SAS Institute Inc.).
Results
Ploidy Levels
The ploidy level was determined for all the materials except 2x pnt and DM. The expected ploidy levels were confirmed for all these materials except 1x blb. As indicated by Irikura’s notes (Table 1), 1x blb had already become diploid and was considered to be a naturally doubled monohaploid.
Morphology, Cytoplasm Type, and Pollen Stainability
All the haploids exhibited species-specific characteristics shown by the parental species and described in the literature (Hawkes 1990; Spooner et al. 2004), although they had smaller flowers and narrower leaflets than the parental species (Fig. 1). Unlike most of the other wild species, 2x blb and 1x blb had simple leaves characteristic of S. bulbocastanum (Hawkes 1990). Both clones were diploid, as mentioned above. Nevertheless, 1x blb had smaller flowers and narrower leaflets than 2x blb. Compared with 1x ver1 and 1x ver2, 1x ver3 had narrower leaves during its in vitro stage and the early growing stage in the pot; it is thought to be a narrow-leaf mutant as described for one of the monoploid S. verrucosum clones (Irikura 1975b). However, the narrow leaves of 1x ver3 were then contracted and broadened at the later growing stages, as shown in Fig. 1. 1x ver3 did not flower, while 1x ver1 and 1x ver2 grew normally and flowered (Fig. 1). In general, S. stoloniferum and S. acaule set abundant berries even in a pollinator-free greenhouse. However, 2x sto and 2x acl did not produce any naturally setting berries.
Morphology. Haploid plants had smaller flowers and narrower leaflets than the parental species. 1x ver3 had narrower leaves during its in vitro stage and the early growing stage in the pot; then the leaves were contracted and broadened at the later growing stages, as shown in this picture. See Table 1 for clonal codes
2x blb, 1x blb, 4x sto, 2x sto and 2x pnt had W-type cytoplasm (Table 2). 1x ver1, 1x ver2, and 1x ver3 had D-type cytoplasm. 2x acl had M-type cytoplasm, while DM had P-type cytoplasm. These results are in accordance with those previously reported for these species (Hosaka and Sanetomo 2012, 2014).
2x blb showed relatively good pollen stainability (43.2%) (Table 2, Fig. 2). Interestingly, 1x blb, a doubled monoploid, showed slightly better pollen stainability (56.5%) though not statistically tested. Two of three monoploid clones of S. verrucosum flowered but exhibited poor pollen shedding, and the pollen was completely sterile (Fig. 2). 2x sto and 2x acl were also completely pollen sterile (Fig. 2).
Heterozygosity Levels
After discarding ambiguous SNPs from the 30,991 obtained SNPs, 19,889 SNPs, of which 11,511 were monomorphic among the ten samples, were used to compare heterozygosity (Table 3).
1x blb, 1x ver1, 1x ver2, 1x ver3, and DM were completely homozygous, supporting the conclusion that they were derived from monoploid gametes through anther culture. According to our previous data (Hosaka and Sanetomo 2020), the heterozygosity of the South American cultivated diploid species S. phureja (clone phu 1.22) was 8.5–8.9%. Compared with this heterozygosity level, the Mexican diploid species 2x blb and 2x pnt showed extremely low heterozygosity levels (0.75% and 0.34%, respectively). Dihaploid plants of S. stoloniferum and S. acaule showed relatively high heterozygosity, at 14.4% and 10.8%, respectively. 4x sto exhibited the highest heterozygosity, at 14.8%, and 96.0% of the heterozygous loci were in a duplex condition (AABB). Of the heterozygous loci in 2x sto, 97.8% were duplex in 4x sto (data not shown).
Chromosomal Positions of the Heterozygous Loci
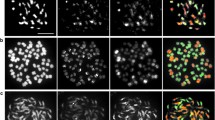
Based on the locations of the SNPs in the potato reference genome (DM ver. 4.03), the surveyed SNPs covered entire chromosomes, and their genotypes are depicted in Fig. 3. Heterozygous SNPs were rarely found in pericentric regions in 2x pnt and 2x blb. Heterozygous blocks can be observed on the south arms of chromosomes 1 and 2 in 2x blb. The other heterozygous SNPs did not form a block in 2x pnt or 2x blb. 1x blb exhibited only AA and BB genotypes, and the two genotypes were evenly distributed across all the chromosomes. Heterozygous SNPs in 4x sto, 2x sto, and 2x acl were localized across all the chromosomes, and no notable differences were found either among samples or among chromosomes.
Parents and the Gametic Progeny
Of the 19,403 comparable SNPs between 2x blb and 1x blb, 45 SNPs showed AA in 2x blb, whereas they showed BB in 1x blb. In contrast, 29 SNPs showed BB in 2x blb, whereas they showed AA in 1x blb. The presence of these discordant SNPs indicates that 2x blb was not a true anther parent of 1x blb but was rather a sibling, with the same accession number (PI 243507). Similarly, of the 19,668 comparable SNPs between 4x sto and 2x sto, 19 SNPs showed discordant results, indicating that 4x sto was not a true anther parent of 2x sto but was rather a sibling, with the same accession number (PI 161178).
According to Irikura’s notes, 1x ver1, 1x ver2, and 1x ver3 were derived from the same S. verrucosum accession (PI 160228). The SNP data indicated that 1x ver2 and 1x ver3 were identical at all the 19,865 comparable SNPs and that both were different from 1x ver1 at 39 SNP loci. The positions of these different loci were located randomly across the genome (data not shown).
Similarities to DM and Among Materials
The similarity to DM was estimated by the frequency of the A (= DM) allele (Table 3). The highest A-allele frequency (89.2–89.5%) was obtained from the previously analyzed clone phu 1.22 (Hosaka and Sanetomo 2020), which is classified with DM as the same species, S. phureja. The second-highest A-allele frequency (75.7%) was obtained from the South American species S. acaule (2x acl). Among the Mexican species, the A-allele frequencies were quite similar (72.1–73.5%) irrespective of the ploidy level or genome constitution.
Using the numerical values for the respective genotypes (AA = 3, AB = 2, and BB = 1) in each of 19,889 SNPs, the genetic relatedness among diploid and monoploid materials was revealed through PCoA. 1x ver3 was eliminated because it was likely a duplicate of 1x ver2, as mentioned above. To visualize the similarities among genotypes, the distribution plot of the principal component scores for the first and second components (cumulative contribution rate of 74.7%) is shown in Fig. 4a. 1x ver1 and 1x ver2 were the most closely related to each other, as expected. Then, 1x blb, 2x blb, and 2x pnt were closely related to each other. 2x sto was plotted at a position intermediate between a group of 1x ver1 and 1x ver2 and a group of 1x blb, 2x blb and 2x pnt. 2x acl was also plotted between the two groups but was closer to DM than 2x sto was. When the third and fourth components were used (an added contribution rate of 19.4%, Fig. 4b), 2x acl was distinct from the others, and 2x pnt was clearly separated from 1x blb and 2x blb.
Genetic relatedness among genotypes determined according to principal coordinate analysis (PCoA). Distribution plots using the first and second components (a) and the third and fourth components (b). See Table 1 for clonal codes
Discussion
Long-term Preservation of In Vitro Plants
Although Irikura’s haploid plants have been cultured for almost a half century, their species identity and ploidy levels were confirmed morphologically, cytologically and by SNP analysis. The observation of complete pollen sterility in 1x ver1, 1x ver2, 2x sto, and 2x acl (Table 2) is in accordance with their irregular meiotic chromosome pairings: 0.155 bivalents + 11.69 univalents in monohaploid S. verrucosum; 0.01 trivalents + 4.03 bivalents + 15.92 univalents in dihaploid S. stoloniferum (Irikura1976); and 10.3 bivalents + 3.4 univalents in ATDH-1 (Yamada et al. 1998a). As Irikura noted, 1x blb is a naturally chromosome-doubled monoploid. Discordance was observed only in the identity of three monoploid S. verrucosum clones. 1x ver2 and 1x ver3 were similar according to the SNP array analysis but different in terms of morphology (normal vs. narrower leaflets). Irikura (1975b) indicated the possibility that the narrow-leaf mutant was redifferentiated from the pollen callus of S. verrucosum. If so, both 1x ver2 and 1x ver3 might be derived from the same pollen callus, although human error, such as the mislabeling of culture tubes or the incorrectly performed sampling of leaves for DNA extraction, could not be excluded.
Heterozygosity
S. stoloniferum is highly self-fertile and forms only bivalent chromosomes at meiosis (Matsubayashi 1955; Irikura 1976; Pendinen et al. 2008). Nevertheless, this species showed the highest heterozygosity, and most of the heterozygous loci were in a duplex condition. The heterozygosity of 2x sto was only slightly lower than that of 4x sto, and 97.8% of the heterozygous loci in 2x sto were those that were in a duplex condition in 4x sto. Additionally, the heterozygous loci of 4x sto and 2x sto were distributed across entire chromosomes (Fig. 3). These results suggest that the different alleles in the locus on homoeologous chromosomes are genetically fixed; this condition is termed ‘fixed heterozygosity’ (MacKey 1970). The high heterozygosity in 2x acl also likely resulted from the fixed heterozygosity in the loci on homoeologous chromosomes (Camadro et al. 1992; Yamada et al. 1998b). Compared with the South American diploid species (phu 1.22), Mexican diploid species (2x blb and 2x pnt) had less than one-tenth lower heterozygosity, which was similarly reported by Hardigan et al. (2015). This under-estimation of heterozygosity in the Mexican diploid species was apparently caused by ascertainment bias (Hardigan et al. 2015; Bamberg and del Rio 2020), because the presently used SNPs were those primarily detected among cultivars (Hamilton et al. 2011; Vos et al. 2015).
Genomic Relationships
S. pinnatisectum and S. bulbocastanum have pinnate and simple leaves, respectively, and are morphologically distinct from each other (Hawkes 1990; Spooner et al. 2004). Nevertheless, meiosis of the F1 hybrids was almost regular, with a low percentage of univalent; this indicates that the two species have basically similar genomes and possess only cryptic structural differences (Magoon et al. 1958; Matsubayashi 1991). The present study also revealed that the two species were closely related to each other but distantly related to S. verrucosum and S. phureja DM. Resequencing projects (short-read assembly to the DM genome) also revealed that the Mexican diploid species, excluding S. verrucosum, were distantly related to the South American species, i.e., the A-genome species (Hardigan et al. 2017; Li et al. 2018). Furthermore, Li et al. (2018) reported that although S. verrucosum and S. phureja are diploid A-genome species (Swaminathan and Howard 1953; Matsubayashi 1991), the two species were separated into different clades of the South American species complex (S. brevicaule complex) (Li et al. 2018). The PCoA plot indicated that 2x sto was intermediate between the A-genome species S. verrucosum and the Mexican species S. pinnatisectum and S. bulbocastanum (Fig. 4), supporting an allotetraploid origin of S. stoloniferum; an A genome derived from S. verrucosum (Spooner and Castillo 1997; Rodríguez and Spooner 2009; Sanetomo and Hosaka 2013) and a B genome derived from S. pinnatisectum, S. bulbocastanum or related species (Irikura 1976; Pendinen et al. 2008; Wang et al. 2008; Rodríguez and Spooner 2009). The PCoA plot indicated that 2x acl was also intermediate between the A-genome species S. verrucosum and the Mexican species S. pinnatisectum and S. bulbocastanum but was the closest to DM. Thus, one of the 2x acl genomes is undoubtedly the A genome, but the second genome seems to be neither the A genome nor that of S. pinnatisectum or S. bulbocastanum; however, Nakagawa and Hosaka (2002) did not support an amphidiploid origin of S. acaule involving two distinct species. Overall, the genetic relationship among the present materials was in good agreement with previously available knowledge.
Usefulness of These Materials
To date, whole genomes have been sequenced for cultivated potato species (Potato Genome Sequencing Consortium 2011; Kyriakidou et al. 2020; Zhou et al. 2020; van Lieshout et al. 2020) and the wild diploid species S. commersonii (Aversano et al. 2015) and S. chacoense (Leisner et al. 2018). All of these carry the A genome (Matsubayashi 1991). Only a draft genome has been published for the Mexican diploid species S. pinnatisectum (Tiwari et al. 2021). Through recent advances such as third-generation (or long-read) sequencing (TGS) technologies, high-quality genome assemblies with high resolution can be achieved due to the longer length of the reads. However, the construction of haplotype-resolved genomes for highly heterozygous species remains a challenge (Kyriakidou et al. 2018). Instead, completely homozygous genotypes are desirable for efficiently and accurately sequencing the whole genomes. The monohaploid S. verrucosum and chromosome-doubled monohaploid S. bulbocastanum are completely homozygous, as confirmed in this study. The dihaploid S. stoloniferum and S. acaule are likely heterozygous in loci between different genomes but homozygous in all loci within the genome. Thus, these materials are suitable for whole-genome sequencing, which is currently ongoing. Whole-genome information would be useful for exploring basic and applied topics such as self- and unilateral cross-compatibility, late blight resistance, and the genomic relationship between the A genome and the genomes of Mexican diploid species.
Availability of Data and Material
All data are included in the manuscript. Plant materials are available upon request.
Code Availability
Not applicable.
References
Aversano, R., F. Contaldi, M.R. Ercolano, V. Grosso, M. Iorizzo, F. Tatino, L. Xumerle, A. Dal Molin, C. Avanzato, A. Ferrarini, M. Delledonne, W. Sanseverino, R.A. Cigliano, S. Capella-Gutierrez, T. Gabaldón, L. Frusciante, J.M. Bradeen, and D. Carputo. 2015. The Solanum commersonii genome sequence provides insights into adaptation to stress conditions and genome evolution of wild potato relatives. The Plant Cell 27: 954–968.
Bamberg, J.B., and A. del Rio. 2020. Assessing under-estimation of genetic diversity within wild potato (Solanum) species populations. American Journal of Potato Research 97: 547–553.
Bamberg, J., A. Kielar, A. del Rio, and D. Douches. 2021. Making hybrids with the wild potato Solanum jamesii. American Journal of Potato Research 98: 187–193.
Camadro, E.L., R.W. Masuelli, and M.C. Cortés. 1992. Haploids of the wild tetraploid potato Solanum acaule ssp. acaule: Generation, meiotic behavior, and electrophoretic pattern for the aspartate aminotransferase system. Genome 35: 431–435.
Cappadocia, M., D.S.K. Cheng, and R. Ludlum-Simonette. 1984. Plant regeneration from in vitro culture of anthers of Solanum chacoense Bitt. and interspecific diploid hybrids S. tuberosum L. × S. chacoense Bitt. Theoretical and Applied Genetics 69: 139–143.
Dinu, I.I., R.J. Hayes, R.G. Kynast, R.L. Phillips, and C.A. Thill. 2005. Novel inter-series hybrids in Solanum, section Petota. Theoretical and Applied Genetics 110: 403–415.
Eijlander, R., W. ter Laak, J.G.Th. Hermsen, M.S. Ramanna, and E. Jacobsen. 2000. Occurrence of self-compatibility, self-incompatibility and unilateral incompatibility after crossing diploid S. tuberosum (SI) with S. verrucosum (SC): I. Expression and inheritance of self-compatibility. Euphytica 115: 127–139.
Foroughi-Wehr, B., H.M. Wilson, G. Mix, and H. Gaul. 1977. Monohaploid plants from anthers of a dihaploid genotype of Solanum tuberosum L. Euphytica 26: 361–367.
Hamernik, A.J., M. Ramon, and R.E. Hanneman Jr. 2001. Modified conventional breeding methods to efficiently transfer unique late blight resistance from 2x (1EBN) Mexican species to 2x (2EBN) and 4x (4EBN) breeding lines. American Journal of Potato Research 78: 456.
Hamilton, J.P., C.N. Hansey, B.R. Whitty, K. Stoffel, A.N. Massa, A. Van Deynze, W.S. De Jong, D.S. Douches, and C.R. Buell. 2011. Single nucleotide polymorphism discovery in elite North American potato germplasm. BMC Genomics 12: 302.
Hardigan, M.A., J. Bamberg, C.R. Buell, and D.S. Douches. 2015. Taxonomy and genetic differentiation among wild and cultivated germplasm of Solanum sect. Petota. The Plant Genome 8 (1). https://doi.org/10.3835/plantgenome2014.06.0025.
Hardigan, M.A., F.P.E. Laimbeer, L. Newton, E. Crisovan, J.P. Hamilton, B. Vaillancourt, K. Wiegert-Rininger, J.C. Wood, D.S. Douches, E.M. Farré, R.E. Veilleux, and C.R. Buell. 2017. Genome diversity of tuber-bearing Solanum uncovers complex evolutionary history and targets of domestication in the cultivated potato. Proceedings of the National Academy of Sciences of the United States of America 114: E9999–E10008.
Hawkes, J.G. 1990. The potato - evolution, biodiversity and genetic resources. London: Belhaven Press.
Hermsen, J.G.Th., and M.S. Ramanna. 1969. Meiosis in different F1-hybrids of Solanum acaule Bitt. × S. bulbocastanum Dun. and its bearing on genome relationship, fertility and breeding behaviour. Euphytica 18: 27–35.
Hermsen, J.G.Th., and M.S. Ramanna. 1976. Barriers to hybridization of Solanum bulbocastanum Dun. and S. verrucosum Schlechtd. and structural hybridity in their F1 plants. Euphytica 25: 1–10.
Hermsen, J.G.Th., and J. Verdenius. 1973. Selection from Solanum tuberosum Group Phureja of genotypes combining high-frequency haploid induction with homozygosity for embryo-spot. Euphytica 22: 244–259.
Hosaka, K., and R.E. Hanneman Jr. 1998. Genetics of self-compatibility in a self-incompatible wild diploid potato species Solanum chacoense. 2. Localization of an S locus inhibitor (Sli) gene on the potato genome using DNA markers. Euphytica 103: 265–271.
Hosaka, K., and R. Sanetomo. 2012. Development of a rapid identification method for potato cytoplasm and its use for evaluating Japanese collections. Theoretical and Applied Genetics 125: 1237–1251.
Hosaka, K., and R. Sanetomo. 2014. Application of a PCR-based cytoplasm genotyping method for phylogenetic analysis in potato. American Journal of Potato Research 91: 246–253.
Hosaka, K., and R. Sanetomo. 2020. Creation of a highly homozygous diploid potato using the S locus inhibitor (Sli) gene. Euphytica 216: 169.
Hougas, R.W., S.J. Peloquin, and A.C. Gabert. 1964. Effect of seed-parent and pollinator on frequency of haploids in Solanum tuberosum. Crop Science 4: 593–595.
Irikura, Y. 1975a. Induction of haploid plants by anther culture in tuber-bearing species and interspecific hybrids of Solanum. Potato Research 18: 133–140.
Irikura, Y. 1975b. Cytogenetic studies on the haploid plants of tuber-bearing Solanum species. 1. Induction of haploid plants of tuber-bearing Solanums (in Japanese). Research Bulletin of the Hokkaido National Agricultural Experiment Station 112: 1–67.
Irikura, Y. 1976. Cytogenetic studies on the haploid plants of tuber-bearing Solanum species. 2. Cytological investigations on haploid plants and interspecific hybrids by utilizing haploidy (in Japanese). Research Bulletin of the Hokkaido National Agricultural Experiment Station 115: 1–80.
Irikura, Y., and S. Sakaguchi. 1972. Induction of 12-chromosome plants from anther culture in a tuberous Solanum. Potato Research 15: 170–173.
Jansky, S., and A. Hamernik. 2009. The introgression of 2x 1EBN Solanum species into the cultivated potato using Solanum verrucosum as a bridge. Genetic Resources and Crop Evolution 56: 1107–1115.
Kyriakidou, M., H.H. Tai, N.L. Anglin, D. Ellis, and M.V. Stromvik. 2018. Current strategies of polyploid plant genome sequence assembly. Frontiers in Plant Science 9: 1660.
Kyriakidou, M., S.R. Achakkagari, J.H.G. López, X. Zhu, C.Y. Tang, H.H. Tai, N.L. Anglin, D. Ellis, and M.V. Strömvik. 2020. Structural genome analysis in cultivated potato taxa. Theoretical and Applied Genetics 133: 951–966.
Leisner, C.P., J.P. Hamilton, E. Crisovan, N.C. Manrique-Carpintero, A.P. Marand, L. Newton, M. Gina, G.M. Pham, J. Jiang, D.S. Douches, S.H. Jansky, and C.R. Buell. 2018. Genome sequence of M6, a diploid inbred clone of the high-glycoalkaloid-producing tuber-bearing potato species Solanum chacoense, reveals residual heterozygosity. The Plant Journal 94: 562–570.
Li, Y., C. Colleoni, J. Zhang, Q. Liang, Y. Hu, H. Ruess, R. Simon, Y. Liu, H. Liu, G. Yu, E. Schmitt, C. Ponitzki, G. Liu, H. Huang, F. Zhan, L. Chen, Y. Huang, D. Spooner, and B. Huang. 2018. Genomic analyses yield markers for identifying agronomically important genes in potato. Molecular Plant 11: 473–484.
Lightbourn, G.J., and R.E. Veilleux. 2007. Production and evaluation of somatic hybrids derived from monoploid potato. American Journal of Potato Research 84: 425–435.
Lokossou, A.A., H. Rietman, M. Wang, P. Krenek, H. van der Schoot, B. Henken, R. Hoekstra, V.G.A.A. Vleeshouwers, E.A.G. van der Vossen, R.G.F. Visser, E. Jacobsen, and B. Vosman. 2010. Diversity, distribution, and evolution of Solanum bulbocastanum late blight resistance genes. Molecular Plant-Microbe Interactions 23: 1206–1216.
MacKey, J. 1970. Significance of mating systems for chromosome and gametes in polyploids. Hereditas 66: 165–176.
Magoon, M.L., D.C. Cooper, and R.W. Hougas. 1958. Cytogenetic studies of some diploid Solanums section Tuberarium. American Journal of Botany 45: 207–221.
Matsubayashi, M. 1955. Studies on the species differentiation in the section Tuberarium of Solanum. III. Behavior of meiotic chromosomes in F1 hybrid between S. longipedicellatum and S. schickii, in relation to its parent species. Science Report, Hyogo University of Agriculture 2: 25–31.
Matsubayashi, M. 1982. Species differentiation in Solanum, sect. Petota. XI. Genomic relationships between S. acaule and certain diploid Commersoniana species. Science Report, Faculty of Agriculture, Kobe University 15: 23–33.
Matsubayashi, M. 1991. Phylogenetic relationships in the potato and its related species. In Chromosome engineering in plants: Genetics, breeding, evolution Part B, ed. T. Tsuchiya and P.K. Gupta, 93–118. Amsterdam: Elsevier.
Meyer, R., F. Salamini, and H. Uhrig. 1992. Biotechnology and plant breeding: relevance of cell genetics in potato improvement. Proceedings of the Royal Society of Edinburgh. Section B: Biological Sciences 99: 11–21.
Mix, G. 1983. Production of dihaploid plantlets from anthers of autotetraploid genotypes of Solanum tuberosum L. Potato Research 26: 63–67.
Nakagawa, K., and K. Hosaka. 2002. Species relationships between a wild tetraploid species, Solanum acaule Bitter, and its related species as revealed by RFLPs of chloroplast and nuclear DNA. American Journal of Potato Research 79: 85–98.
Ortiz, R., and S.J. Peloquin. 1994. Use of 24-chromosome potatoes (diploids and dihaploids) for genetical analysis. In Potato genetics, ed. J.E. Bradshaw and G.R. Mackay, 133–154. Wallingford: CAB International.
Pendinen, G., T. Gavrillenko, J. Jiang, and D.M. Spooner. 2008. Allopolyploid speciation of the Mexican tetraploid potato species Solanum stoloniferum and S. hjertingii revealed by genomic in situ hybridization. Genome 51: 714–730.
Peterson, B.A., S.H. Holt, R.E. Laimbeer, A.G. Doulis, J. Coombs, D.S. Douches, M.A. Hardigan, C.R. Buell, and R.E. Veilleux. 2016. Self-fertility in a cultivated diploid potato population examined with the Infinium 8303 potato single-nucleotide polymorphism array. Plant Genome 9: 3.
Potato Genome Sequencing Consortium. 2011. Genome sequence and analysis of the tuber crop potato. Nature 475: 189–195.
Rodríguez, F., and D.M. Spooner. 2009. Nitrate reductase phylogeny of potato (Solanum sect. Petota) genomes with emphasis on the origins of the polyploid species. Systematic Botany 34: 207–219.
Sanetomo, R., and K. Hosaka. 2013. A recombination-derived mitochondrial genome retained stoichiometrically only among Solanum verrucosum Schltdl. and Mexican polyploid wild potato species. Genetic Resources and Crop Evolution 60: 2391–2404.
Sopory, S.K., E. Jacobsen, and G. Wenzel. 1978. Production of monohaploid embryoids and plantlets in cultured anthers of Solanum tuberosum. Plant Science Letters 12: 14–54.
Spooner, D.M., and R.T. Castillo. 1997. Reexamination of series relationships of South American wild potatoes (Solanaceae: Solanum sect. Petota): Evidence from chloroplast DNA restriction site variation. American Journal of Botany 84: 671–685.
Spooner, D.M., R.G. van den Berg, A. Rodríguez, J. Bamberg, R.J. Hijmans, and S.I.L. Cabrera. 2004. Wild potatoes (Solanum section Petota; Solanaceae) of North and Central America. Systematic Botany Monographs Vol 68. Ann Arbor: The American Society of Plant Taxonomists.
Swaminathan, M.S. 1954. Nature of polyploidy in some 48-chromosome species of the genus Solanum section Tuberarium. Genetics 39: 59–76.
Swaminathan, M.S., and H.W. Howard. 1953. The cytology and genetics of the potato (Solanum tuberosum) and related species. Bibliographia Genetica 16: 1–192.
Tiwari, J.K., S. Rawat, S.K. Luthra, R. Zinta, S. Sahu, S. Varshney, V. Kumar, D. Dalamu, N. Mandadi, M. Kumar, S.K. Chakrabarti, A.R. Rao, and A. Rai. 2021. Genome sequence analysis provides insights on genomic variation and late blight resistance genes in potato somatic hybrid (parents and progeny). Molecular Biology Reports 48: 623–635.
Uhrig, H. 1985. Genetic selection and liquid medium conditions improve the yield of androgenetic plants from diploid potatoes. Theoretical and Applied Genetics 71: 455–460.
Uijtewaal, B.A., G.J. Huigen, and J.G.Th. Hermsen. 1987. Production of potato monohaploids (2n=x=12) through prickle pollination. Theoretical and Applied Genetics 73: 751–758.
van Breukelen, E.W.M., M.S. Ramanna, and J.G.Th. Hermsen. 1975. Monohaploids (n=x=12) from autotetraploid Solanum tuberosum (2n=4x=48) through two successive cycles of female parthenogenesis. Euphytica 24: 567–574.
van Lieshout, N., A. van der Burgt, M.E. de Vries, M. ter Maat, D. Eickholt, D. Esselink, M.P.W. van Kaauwen, L.P. Kodde, R.G.F. Visser, P. Lindhout, and R. Finkers. 2020. Solyntus, the new highly contiguous reference genome for potato (Solanum tuberosum). G3 Genes Genomics Genetics 10: 3489–3495.
Veilleux, R.E., J. Booze-Daniels, and E. Pehu. 1985. Anther culture of a 2n pollen producing clone of Solanum phureja Juz. & Buk. Canadian Journal of Genetics and Cytology 27: 559–564.
Vos, P.G., J.G.A.M.L. Uitdewilligen, R.E. Voorrips, R.G.F. Visser, and H.J. van Eck. 2015. Development and analysis of a 20K SNP array for potato (Solanum tuberosum): An insight into the breeding history. Theoretical and Applied Genetics 128: 2387–2401.
Wang, M., S. Allefs, R.G. van den Berg, V.G.A.A. Vleeshouwers, E.A.G. van der Vossen, and B. Vosman. 2008. Allele mining in Solanum: Conserved homologues of Rpi-blb1 are identified in Solanum stoloniferum. Theoretical and Applied Genetics 116: 933–943.
Wenzel, G., O. Schieder, T. Przewozny, S.K. Sopory, and G. Melchers. 1979. Comparison of single cell culture derived Solanum tuberosum L. plants and a model for their application in breeding programs. Theoretical and Applied Genetics 55: 49–55.
Yamada, T., S. Misoo, T. Ishii, Y. Ito, K. Takaoka, and O. Kamijima. 1997. Characterization of somatic hybrids between tetraploid Solanum tuberosum L. and dihaploid S. scaule. Breeding Science 47: 229–236.
Yamada, T., K. Hosaka, K. Nakagawa, N. Kaide, S. Misoo, and O. Kamijima. 1998a. Nuclear genome constitution and other characteristics of somatic hybrids between dihaploid Solanum acaule and tetraploid S. tuberosum. Euphytica 102: 239–246.
Yamada, T., K. Hosaka, N. Kaide, K. Nakagawa, S. Misoo, and O. Kamijima. 1998b. Cytological and molecular characterization of BC1 progeny from two somatic hybrids between dihaploid Solanum acaule and tetraploid S. tuberosum. Genome 41: 743–750.
Zhou, Q., D. Tang, W. Huang, Z. Yang, Y. Zhang, J.P. Hamilton, R.G.F. Visser, C.W.B. Bachem, C.R. Buell, Z. Zhang, C. Zhang, and S. Huang. 2020. Haplotype-resolved genome analyses of a heterozygous diploid potato. Nature Genetics 52: 1018–1023.
Acknowledgements
I thank E. Kakuta and W. Takenaka for the maintenance of the in vitro plants. This research was financially supported in part by a JSPS Grant-in-Aid for Scientific Research for Young Scientists (Grant Number JP19K15813) and by Calbee Inc., Hokkaido Potato Growers Association, Kewpie Corp., KENKO Mayonnaise Co., Ltd., and the Japan Snack Cereal Foods Association.
Author information
Authors and Affiliations
Contributions
RS and KH conducted entire experiments and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Additional information
This paper is dedicated to late Dr. Yukio Irikura (died at the age of 72 on June 1, 2002), who created most of the plant materials used in this study. The Hawkes (1990) classification system is tentatively adopted throughout the text.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sanetomo, R., Hosaka, K. Re-evaluation of Monohaploid Solanum verrucosum and S. bulbocastanum (2n = x = 12) and Dihaploid S. stoloniferum and S. acaule (2n = 2x = 24), All Derived from Anther Culture. Am. J. Potato Res. 98, 333–343 (2021). https://doi.org/10.1007/s12230-021-09847-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12230-021-09847-y