Abstract
Potato is a heterozygous autotetraploid crop propagated as tubers. Diploid potatoes, which are mostly self-incompatible due to gametophytic self-incompatibility, are often used to reduce genetic complexity. The discovery of the S locus inhibitor (Sli) gene has created a way to develop diploid inbred lines and perform F1 hybrid breeding in potato. However, residual heterozygosity found in advanced-generation selfed progenies has posed the question of whether a minimum level of heterozygosity is necessary to maintain self-fertility. We continued selfing and finally identified a highly homozygous diploid potato among tenth-generation selfed progeny, which was homozygous at all 18,579 genome-wide single nucleotide polymorphism (SNP) markers surveyed. The S10 plants suffered severe inbreeding depression in terms of fertility and vigor, showing a small number of mature flowers and extremely slow growth. Although asexual techniques such as anther culture followed by chromosome doubling can result in completely homozygous diploid potatoes, all previously derived plants were male sterile. In contrast, continuous selfing using Sli swept out all lethal alleles and selected for self-fertility, which generated a highly homozygous diploid potato retaining male and female fertility and tuberization ability under long days.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Potato (tetraploid form of Solanum tuberosum L.) is a heterozygous tuber crop. Heterotic effects on vigor and yield are maintained and used for clonal propagation via tubers. However, genetic analyses are difficult due to autotetraploidy and heterozygosity. It is thought that many deleterious or lethal genes exist as heterozygotes in the potato genome which would be expressed by selfing. Thus, inbreeding depression in terms of viability and fertility has limited continuous selfing in potato (Krantz 1924; De Jong and Rowe 1971).
The majority of the diploid potatoes of cultivated and wild species are self-incompatible due to the gametophytic self-incompatibility system controlled by the S locus (Pushkarnath 1942; Pandey 1962; Cipar et al. 1964a). However, exceptional self-compatible diploid variants have often been reported (Pushkarnath 1942; Cipar et al. 1964b; De Jong and Rowe 1971; Olsder and Hermsen 1976; De Jong 1977; Cappadocia et al. 1986; Birhman 1986). Hanneman (1985) found self-compatible clones of S. chacoense Bitt., a wild diploid (2n = 2x = 24) tuber-bearing Solanum species, and generated a number of self-compatible lines by continued self-crosses. One of his clones, chc 525-3, was analyzed for self-compatibility, by which the S locus inhibitor (Sli) gene was identified (Hosaka and Hanneman 1998a). The Sli gene is a single dominant gene mapped to the distal end of potato chromosome 12 (Hosaka and Hanneman 1998b). Recently, the candidate region for Sli was narrowed to a 333 kb region on chromosome 12 (Clot et al. 2020). The Sli gene was transferred to cultivated diploid potatoes by crossing, which created a way to develop highly homozygous diploid potato lines (Phumichai et al. 2005). Using inbred lines, F1 hybrid breeding would become possible (Birhman and Hosaka 2000). Since then, the Sli gene has been used to generate inbred lines for F1 hybrid breeding (Lindhout et al. 2011). Breeding at the diploid level is more efficient than breeding at the tetraploid level. True potato seed (TPS) of inbred lines can be rapidly increased, stored in a refrigerator almost indefinitely, and transported easily and does not carry any economically important fungal, oomycete, or bacterial pathogens or major potato viruses. Consequently, TPS and F1 hybrid breeding have many advantages over traditional breeding and seed tuber production and have significantly impacted the scientific and industrial communities (Lindhout et al. 2011; Jansky et al. 2016).
However, inbreeding depression is a serious problem because it reduces plant vigor and fertility (Krantz 1924, 1946; Mendiburu and Peloquin 1977; Ross 1986; Golmirzaie et al. 1998a, b; Phumichai et al. 2005). We initially introduced Sli from clone F1-1, an interspecific hybrid between chc 525-3 and S. phureja clone 1.22 (hereinafter, phu 1.22), into Andean cultivated diploid potatoes and performed continued selfing (Birhman and Hosaka 2000). A second self-crossed clone, 96H14-10 (S2), was accidentally crossed not with self-pollen but with bulked pollen of the other S2 plants, from which a vigorous, highly self-fertile clone (97H32-6) was selected (Phumichai et al. 2006) and further subjected to continuous selfing. The intensiveness of this effort, which depended on self-generation, varied according to time, situation, and willingness. However, after three decades since the initial cross between chc 525-3 and phu 1.22 in 1987 or after two decades since 97H32-6 was selected, we finally obtained nearly or completely homozygous clones among tenth self-generation plants. The earlier stage of the inbreeding process was reported in Phumichai et al. (2005) and Phumichai and Hosaka (2006). In this paper, the inbreeding process for later generations is reported, and the whole process toward genetic fixation is investigated using genome-wide single nucleotide polymorphism (SNP) markers.
Materials and methods
Plant materials
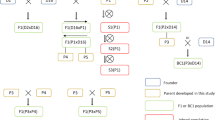
Plant materials and their pedigrees are represented in Fig. 1. The generation derived by selfing is denoted by “S”, while the generation derived by crossing between two genotypes is denoted by “F”. The clone phu 1.22 is from a cultivated diploid species, S. phureja (PI 225682) and a famous haploid inducer (Kotch and Peloquin 1987). The clone chc 525-3 is a self-compatible clone of a wild diploid species, S. chacoense, and was already in the S7 generation (Hanneman 1985 and personal communication). The Sli gene was identified in this clone (Hosaka and Hanneman 1998a). Note that M6 (= chc 523-3), a vigorous fertile inbred clone of S. chacoense recently released by Jansky et al. (2014), was a selfed progeny of “84 chc 616-8”, while chc 525-3 was a selfed progeny of “84 chc 616-3”. Thus, the same S5 clone generated the S6 family “84 chc 616”, of which two S6 genotypes were selfed, resulting in chc 525-3 and M6. The clone chc 525-3 was selfed, deriving S8 family 11H129. This family was evaluated for self-compatibility (data not shown), of which 24 self-compatible genotypes were used for SNP analysis. 93H100 is a diploid family derived by open pollination in a field where many accessions of S. phureja and several accessions of S. stenotomum were grown together. Fifty-seven plants of family 93H100 were pollinated with the pollen from F1-1, and the hybrid seeds were mixed together. 94H87-16 is one of 1125 plants of family 94H87, raised from these mixed seeds (Birhman and Hosaka 2000). After an accidental sib-cross among S2 plants, 97H32-6 was selected, which is a vigorous plant and a superior Sli gene donor frequently used in our laboratory (Phumichai et al. 2006; Sanetomo et al. 2014). The clone 97H32-6 was regarded as an initial genotype (S0) for continuous selfing in this study. Plants of the S6 generation and their selfed progenies up to the S11 generation were grown and evaluated. In addition, DM (DM 1–3 516 R44) was used for the SNP analysis. The clone DM resulted from chromosome doubling of a monoploid derived by anther culture of S. phureja (Lightbourn and Veilleux 2007) and was used for whole-genome sequencing, leading to a reference potato genome (The Potato Genome Sequencing Consortium 2011).
Crossing
Plants were grown in a pollinator-free greenhouse or a screenhouse under a 16 h daylength with supplementary lights. Anthers and petals were removed from flower buds one or two days prior to opening, and freshly collected pollen was applied immediately to the stigmas. Berries were collected one month after pollination. After another month of maturation, seeds were collected, dried and stored at 4 °C until use.
Raising seedlings
Seeds were soaked in 2000 ppm gibberellic acid (GA3) solution for 48 h, rinsed in tap water, sown in a cell tray filled with potting soil, and covered with vermiculite. After the second leaves were well expanded, the young seedlings were first transplanted into black vinyl pots (10.5 cm diameter) and then into 5-inch (15 cm diameter) or 7-inch (21 cm diameter) pots for further growth.
SNP data
Total DNA was extracted from fresh leaves by the method described in Hosaka and Hanneman (1998b). A total of 1–2 micrograms of dried DNA was sent to GeneSeek (Neogen Corporation, NE, USA) to obtain 22K potato V3 SNP array data genotyping 21,027 SNP loci. Three data sets were separately obtained. Data set 1 contained most of the samples from the parents to the S10 progenies. Data set 2 contained 17H145-5 (S10) and its selfed progeny (S11). Data set 3 contained chc 525-3 (S7 S. chacoense) and its selfed progeny (S8). Each data set contained DM as a quality control. The obtained data sets were first separately filtered in the same way as described below. Additional filtering is described in the Results. Heterozygous SNPs in DM (completely homozygous) and SNPs with > 10% missing values were first discarded. Then, ambiguous SNPs such as those suggested by Peterson et al. (2016) and those originating from chromosome 0 or chloroplast DNA were discarded. Heterozygous SNPs across all samples were also discarded. These SNPs were likely resulted from genotype-calling failures, because contradictory genotypes were observed between the data sets. DM was re-evaluated by discarding contradictory data and supplementing missing data to the greatest extent possible using the three sets of DM data. SNPs with no data for DM were discarded. All genotypes were represented as either AA (identical to that of DM), AB, BB or missing.
Results
Inbreeding process from S6 to S11
Viability- and self-fertility-related traits in plants from S6 to S11 are represented in Supplementary Table 1. Due to limited capacity, the population size considerably differed among generations (85 plants of four families in S6, five plants of one family in S7, 28 plants of two families in S8, 396 plants of 12 families in S9, 50 plants of four families in S10, and 14 plants of one family in S11). Particularly, the S7 plants were first grown in vitro for another purpose, from which five plants were transplanted to potting soil and further multiplicated by stem-cutting for the present inbreeding study. In addition, growing conditions such as season, temperature, and daylength were not constant over all generations. Thus, the percentages of germinated plants (32.3–100%), mature plants (14.0–100%), self-pollinated plants (0–100%), and selfers (0–100%) differed greatly among families and generations and might not be comparable between selfed generations. Selfer frequencies were not drastically decreased. However, as inbreeding proceeded, we observed a general tendency toward inbreeding effects, such as slower growth, a longer growing period, bud-dropping, a lower number of mature flowers, a smaller amount of pollen grains, a lower number of seeds per berry, and a requirement for better growing conditions for flowering. If well-matured flowers were self-crossed, they often set berries. We sometimes found dwarf and/or semidwarf (intermediate morphology between normal and dwarf) plants (Fig. 2a, b), which never flowered.
a, b Normal and dwarf genotypes segregating in S8 family 11H5 (a) and S9 family 16H163 (b). c-h Characterization of S10 plants of families 17H145, 17H146, 17H147, and 17H148. Two plants of each of the four S10 families (c). d Two normal (left) and two semidwarf (right) plants in 17H146, which produced normal flowers (e) and antherless flowers with light yellow-margined leaves (f), respectively. g One tuber each for five genotypes of each family. h One tuber each for all genotypes of family 17H146. The six tubers shown in the uppermost column were those with semidwarf phenotypes
Characterization of S10 plants
The most recently characterized plants were S10 plants. The seeds of four S10 families (17H145, 17H146, 17H147, and 17H148) were sown on September 3, 2017, after two days of treatment with GA3. Out of 187 seeds sown, a total of 122 germinated, of which 84 (68.9%) germinated by September 11 (8 days after seed-sowing), 32 by September 15 (12 days), and 6 by September 26 (23 days). Eighty-three seedlings were first transplanted into black vinyl pots on November 8 (66 days), and then, 50 of them were transplanted into 5-inch pots on January 13, 2018 (132 days). Final transplanting into 7-inch pots (15 for 17H145, 21 for 17H146, 9 for 17H147, and 5 for 17H148) was performed on February 23, 2018 (173 days). The reduction in number from seeds to final plants was primarily due to plant mortality. As the plants grew, the lower leaves dried until they died, probably because the poor root system could not provide a sufficient water supply to the entire aboveground part. It was noticed that the plants were more attractive to aphids, so frequent insecticide sprays were necessary. By the flowering stage, morphological differences between families became clear. As shown in Fig. 2c, all plants of family 17H147 were apparently taller than the others. Within-family variation in morphology was not recognized in any families except 17H146. Six of the 21 plants in family 17H146 were smaller in shape, produced light yellow-margined leaves (Fig. 2d, f) and set flower buds later. Their flowers were antherless or had only premature anthers (Fig. 2f).
Almost all the plants formed young buds, but those formed in the earlier flowering period were exclusively dropped. Mature flowers appeared from the end of March until early May (200–250 days after seed-sowing, Fig. 2e). During this period, self-pollination was performed. The number of pollen grains and ease of pollen shedding from the anther varied by plant and pollination timing and even by anther within the same flower. A much smaller number of pollen grains could be collected and applied immediately to the stigmas. No plants in families 17H147 and 17H148 formed mature flowers. Of the 30 normal plants in families 17H145 and 17H146, 19 were able to pollinate, and 12 of them set berries (Supplementary Table 2). The berry-setting rate in the successful crosses ranged from 6.7 to 66.7%. The mean number of seeds per berry ranged from 9 to 47.5. These variations seemed to be caused primarily by the microenvironmental conditions of the plants.
All plants were almost simultaneously defoliated, the tubers were harvested on June 26, 2018 (296 days after seed-sowing), and the total weight for each genotype was measured (Supplementary Table 3). The mean yields were 214.3 g/plant for family 17H145, 180.6 g/normal plant or 51.1 g/semidwarf plant for family 17H146, 142.7 g/plant for family 17H147, and 252.4 g/plant for family 17H148. Yield differences among families and between normal and semidwarf plants of family 17H146 were significant according to one-way ANOVA (P < 0.001). The tubers were oblong in shape, had deep eyes and appeared similar between genotypes (Fig. 2g). The tubers of the semidwarf plants were smaller than those of the normal plants in the same family (Fig. 2h).
Selection of informative SNPs
Three separately obtained SNP data sets were first filtered in the same way as described in the Methods. In Data set 1, SNPs with missing data for 97H32-6 (S0) were discarded because heterozygosity between S0 and later-generation plants was to be compared, which resulted in 18,579 informative SNPs. Data set 2 provided 19,333 informative SNPs. In Data set 3, missing data for chc 525-3 (S7) were supplemented based on the genotypes of its S8 population, which resulted in the largest number of informative SNPs (n = 19,567).
SNP monitoring during the inbreeding process up to S6
SNP genotypes are summarized in Table 1. The process of genetic fixation from parents to S6 progeny is displayed for each of the 12 chromosomes in Fig. 3a, and heterozygosity by generation and by chromosome is represented in Table 2. DM is a completely homozygous clone derived from the cultivated diploid species S. phureja. Hence, phu 1.22 showed the highest similarity to DM (85.3%, Table 1) and a percent heterozygosity of 8.5% across all SNPs, ranging from 5.1% on chromosome 8 to 12.3% on chromosome 4 (Table 2).
Overall view of chromosomes toward genetic fixation. Each chromosome is arranged from north (left) to south (right). a SNP genotypes of parents and reduced heterozygosity from S0 (97H32-6) to S6 (8H1-3). AA is the DM-type genotype. b Parental origin for each SNP locus in the completely homozygous S10 clone 17H145-5: homozygous with likely the chc 525-3-specific allele (chc), obviously the alternative allele (non-chc), or undeterminable (?). Homozygous portions exclusively fixed to one of the chc 525-3 alleles are indicated by arrows (see text)
As chc 525-3 is from a wild species, it showed the lowest similarity (73.2%) to DM. Since it was an S7 plant, the overall heterozygosity was as low as 4.5%, which was nearly the same as that observed in M6 (4.8%, Leisner et al. 2018). However, heterozygosity differed considerably by chromosome: chromosomes 3 and 5 were nearly or completely homozygous, whereas chromosomes 8 and 9 were heterozygous along their whole lengths (heterozygosity of 20.9% and 7.4%, respectively) and chromosomes 1, 2, 4, 6, 7, and 11 contained heterozygous blocks (Fig. 3a). The chromosome-wide heterozygosity of chromosomes 8 and 9 and heterozygous blocks on chromosomes 1, 2, 5, 7, 10, and 12 were similarly found in M6 (Marand et al. 2019). However, the chromosome-wide heterozygosity of chromosome 4 observed in M6 was considerably disrupted in chc 525-3. Additional heterozygous blocks were found on chromosomes 6 and 11 in chc 525-3, while the heterozygous blocks at the distal ends of chromosomes 3 and 6 in M6 were not detected in chc 525-3.
As expected, the interspecific hybrid, F1-1, showed the highest heterozygosity (20.8%), and the heterozygous loci covered the entire lengths of all 12 chromosomes (Fig. 3a).
The S0 plant, 97H32-6, was more similar to DM (75.9%, Table 1) than F1-1 because 97H32-6 was a backcross progeny of F1-1 to S. phureja. Its overall heterozygosity was reduced to 12.6%, and chromosomes 10 and 12 were more homozygous (heterozygosity of 4.0% and 0.6%, respectively).
After six rounds of selfing, the heterozygosity was reduced to 1.4% in the S6 plant, 8H1-3 (Table 2). As with chc 525-3, large portions of chromosomes 8 and 9 were still heterozygous in 8H1-3. Small portions of chromosomes 1, 4 and 6 were also heterozygous (Fig. 3a). The average rate of reduction in heterozygosity from S0 to S6 was 31.0% [= 1–(1.37/12.64)^(1/6)] per generation (theoretically 50% with no selection).
SNP genotyping of advanced selfed generations
The genotypes for all heterozygous loci in S6 plant 8H1-3 were visually displayed for the S7, S8, and S9 plants and all plants in four S10 families (17H145, 17H146, 17H147, and 17H148) (Fig. 4). Two SNPs on chromosome 1 and 4 SNPs on chromosome 4 were heterozygous in the S6 plant, which were homozygous in one of the two S7 plants. However, these SNPs were heterozygous in the other S7 plant and segregating among its S10 plants. Heterozygous loci with 18 SNPs on chromosome 6 were still segregating in two S10 families. Among these 18 SNP loci distributed in 2.6 Mb, nine recombination points were detected from S6 to S9. On chromosome 8, 174 heterozygous SNPs were distributed in 48.5 Mb along the entire chromosome. These SNPs were fixed to homozygotes in the S8 parent for family 17H146 and the S9 parent for family 17H147, while they were still segregating in families 17H145 and 17H148. Fifteen recombination points were detected in pericentric regions. On chromosome 9, 58 SNPs distributed in 38.1 Mb were heterozygous in S6. These SNPs were fixed to homozygotes in three of four S10 families. Eleven recombination points were detected, which were randomly distributed on chromosome 9. All the heterozygous SNPs in S6 seemed randomly fixed either to AA or BB homozygotes in the progenies.
Whole-chromosome-level and recalcitrant residual heterozygosity in 8H1-3 (S6) and their fixation in its selfed progenies. Red arrowheads indicate completely homozygous plants, while black arrowheads indicate semidwarf plants. Recombination points that occurred in the parent are shown by pink triangles
Finally, in the S10 generation, all SNPs were homozygous in 12 S10 plants (shown by red arrowheads on the right in Fig. 4): two in family 17H145 (clonal identities 17H145-5 and 17H145-8), five in family 17H146 (-5, -6, -12, -16, and -17), four in family 17H147 (-1, -3, -5, and -6), and one in family 17H148 (-2). The SNP genotypes of the six semidwarf, male-sterile plants of family 17H146 were compared with those of the other normal plants. However, there were no common loci that might be associated with the semidwarf phenotype (Fig. 4). The average rate of reduction in heterozygosity from S6 to S10 was 42.2% {=1–[(0.25 + 0.18 + 0.01 + 0.17)/4/1.37]^(1/4)} per generation.
17H145-5, a completely homozygous plant in all SNPs, was similar to DM at 83.9% of SNPs according to Data set 1 or 82.3% of SNPs according to Data set 2 (Table 1). Whether the SNP genotype was derived from chc 525-3 was further investigated. Since the 93H100-derived parent was not available, we assumed that its genotype would be similar to those of phu 1.22 and DM if the latter two had identical genotypes. For example, if the genotypes for a given SNP were BB in chc 525-3, AA in phu 1.22, AB in F1-1, and BB in 17H145-5, the B allele of 17H145-5 likely originated from chc 525-3 (chc). If the genotypes were BB (or AA) in chc 525-3 and AA (or BB) in 17H145-5, the A (or B) allele of 17H145-5 clearly originated from a plant other than chc-525-3 (non-chc). In most of the other genotype combinations, SNP origin was undeterminable. As shown in Fig. 3b, most chc alleles were fixed to paracentric regions, except for those on chromosomes 10 and 12, where chc alleles were fixed chromosome-wide and already fixed in 97H32-6 (S0). Blocks of fixation for chc alleles were observed on chromosomes 1, 2, 4, 6, 7, and 9.
Characterization of S11 plants
Of the completely homozygous S10 plants in all SNPs, only 17H145-5 produced seeds by selfing (Supplementary Table 2). Thirty-six seeds were sown on March 12, 2019, after two days of treatment with GA3, of which 21 germinated by April 1 (20 days after seed-sowing). Sixteen of them were transplanted into black vinyl pots on April 11 and then into 7-inch pots on May 15 (64 days). Fourteen S11 plants were grown to maturity in a screenhouse (family 19H2). Their aboveground morphologies were identical, and they started to form buds at the same time in early July (120 days). However, all young buds were dropped due to continued rainy days. The plants were cut back and continued to grow. Finally, some of them formed mature flowers at the end of March 2020 and self-pollinated. Four plants produced small berries and a total of 54 seeds. These seeds were sown in vitro, and 38 of them germinated. These S12 plants will be available for distribution from the US Potato Genebank in Sturgeon Bay, Wisconsin.
The 14 S11 plants of family 19H2 and its parental S10 clone, 17H145-5, were compared using 19,333 informative SNPs (Table 2). All 14 plants were identical and homozygous at all SNP loci and identical to the parental S10 clone.
Characterization of S8S. chacoense inbreds
In Data set 3, chc 525-3 was heterozygous at 1156 SNPs, with 5.9% heterozygosity (Table 2). In its selfed progeny (11H129) consisting of 24 plants, the heterozygosity ranged from 1.4 to 5.4% among plants and from 0 to 15.4% among chromosomes, with an average of 3.4% (Table 2). The rate of reduction in heterozygosity was 42.3%.
Segregation in the 11H129 family for only the heterozygous loci in chc 525-3 is displayed in Supplementary Table 4. One of two homozygotes was absent among 24 plants for 92 SNPs on nine chromosomes. Interestingly, most of these distorted loci were located in two segments: 44 SNPs in an 8.02 Mb segment, flanked by PotVar0060614 and solcap_snp_c1_11562, on chromosome 8 and 28 SNPs in a 1.15 Mb segment, flanked by PotVar0053460 and solcap_snp_c2_5652, at the distal end of chromosome 12.
Distorted segments transmitted to S10 progenies
The two distorted segments in family 11H129 were located on chromosomes 8 and 12 of the S10 clone, 17H145-5, as shown in Fig. 3b. Of the 8.02 Mb segment distorted on S. chacoense chromosome 8, the 7.07 Mb region contained both homozygotes for the respective loci among the advanced selfed progenies, while the remaining 0.95 Mb region, flanked by solcap_snp_c2_47459 and PotVar0134850, was identical among all advanced progenies and with a S. chacoense homozygote (Supplementary Table 5).
For the 1.15 Mb segment on chromosome 12, homozygous genotypes in S8 S. chacoense were all similar to those of 97H32-6 (Fig. 5) and the advanced progenies. Thus, these genotypes composed one of the haplotypes in chc 525-3 (haplotype c), which was likely transmitted to F1-1. All missing genotypes in F1-1 could be inferred from the parental genotypes, and then, two haplotypes for chc 525-3 (haplotypes c and d) and two haplotypes for phu 1.22 (haplotypes a and b) were also inferred (Fig. 5). Consequently, it was found that one of the haplotypes of phu 1.22 was similar to that of DM (haplotype a), which was united with haplotype c to form F1-1. Then, haplotype c was fixed in the S0 plant. In this short region of 1.15 Mb, at least seven, and possibly nine, recombination points were detected among 24 plants in family 11H129 (Fig. 5).
Heterozygous SNPs in chc 525-3 formed a heterozygous block at the distal end of chromosome 12 and segregated in the selfed progeny 11H129 (24 plants). Missing data and haplotypes a, b, c, and d were inferred from the parental genotypes. In the region in a rectangle, only one homozygote was found. The candidate regions for Sli (Clot et al. 2020) and la3 (Zhang et al. 2020) are shown
Discussion
Completely homozygous diploid potato
Peterson et al. (2016) posed the question of whether a minimum level of heterozygosity is necessary to maintain self-fertility. We identified 12 S10 plants that were completely homozygous at all 18,579 genome-wide SNPs surveyed, and 100% homozygosity was verified by the lack of genetic segregation at 19,366 SNPs among 14 S11 plants. Considering a haploid genome of 844 Mb (The Potato Genome Sequencing Consortium 2011) and a SNP frequency of 0.68% in M6 (Leisner et al. 2018), 5.7 million SNPs might exist in M6 and chc 525-3 as well (both are S7 clones of S. chacoense). The present study identified only a portion of the heterozygous SNPs in chc 525-3 (1156), leading to the possibility that heterozygous portions remain undetected, even in S11 plants. Whole-genome sequencing is necessary to prove complete homozygosity in S11 plants. Even so, we suggest that a completely homozygous diploid potato retaining self-fertility can be created by continued selfing using the Sli gene.
97H32-6 (S0) is a highly vigorous plant that naturally produces tens of berries and many tubers under long days. In the earlier selfed generations from this clone, the selfer frequency steadily decreased from 82.5% in S1 to 26.7% in S4 (Phumichai et al. 2005). As the selfed generations advanced, the selfed progenies were cryptically improved for fertility (Phumichai and Hosaka 2006). Consequently, the number of self-pollinated plants and the selfer frequency did not become truly low but remained high in some families (Supplementary Table 1). However, advanced plants of selfed generations became less vigorous and grew slowly. The S10 plants took approximately ten months to grow from seed to tuber. The number of mature flowers was greatly affected by environmental and developmental conditions. Therefore, diploid potato suffered severe inbreeding depression in terms of fertility and vigor, as generally recognized in outcrossing species (Jones 1918; Krantz 1924, 1946; Mendiburu and Peloquin 1977; Ross 1986; Golmirzaie et al. 1998a, b; Charlesworth and Willis 2009).
Use of the Sli gene
This study verified that the Sli gene consistently functioned in every selfed generation. Previously, we demonstrated that the Sli gene can be transferred by hybridization to various genotypes and that it functions to induce self-compatibility (Birhman and Hosaka 2000; Phumichai et al. 2006), which has led to the development of a new breeding technology, i.e., diploid breeding or F1 hybrid breeding (Lindhout et al. 2011; Jansky et al. 2016; de Vries et al. 2016). The Sli gene might have an additional function: breaking an interspecific hybridization barrier with a highly reproductively isolated species, S. pinnatisectum (Sanetomo et al. 2014). Selfing F1 plants by the function of Sli has provided an opportunity to reveal the true genetics of tuber shape, skin traits and flesh color (Endelman and Jansky 2016; Meijer et al. 2018) and resistance to common scab and cold-induced sweetening (Braun et al. 2017). Inspired by the successful use of Sli, other self-compatible variants have been reported (Jansky et al. 2014; Peterson et al. 2016; Haynes and Guedes 2018; Zhang et al. 2019; Clot et al. 2020). Recently, Clot et al. (2020) disclosed that a similar DNA sequence of the Sli gene was shared among well-known self-compatible diploid clones. Genome editing by the clustered regularly interspaced short palindromic repeat (CRISPR)-associated protein 9 (Cas9) system was used to knock out the self-incompatibility gene S-RNase and induce self-compatible diploid lines (Ye et al. 2018; Enciso-Rodriguez et al. 2019). Nevertheless, Sli-based selfing is a useful, easy and robust tool for inducing self-compatibility, which will ensure its wide applicability in potato genetics and breeding.
Residual heterozygosity in advanced selfed generations
The rate of reduction in heterozygosity per generation was consistent, measuring 38.4% from S0 to S4 in a previous study (Phumichai et al. 2005) and 31.0% from S0 to S6 and slightly higher (42.2%) from S6 to S10 in this study. The lower reduction rate than the theoretical 50% is likely due to preferred heterotic effects on fecundity and viability and to unpreferable exposure of recessive lethal or deleterious genes (Cho et al. 1998; McMullen et al. 2009; Zhang et al. 2019).
Whole-chromosome-level heterozygosity was found on chromosomes 8 and 9 in S6 plants and chc 525-3 in this study and in M6 in previous studies (Leisner et al. 2018; Marand et al. 2019), which was associated with low levels of recombination (Gore et al. 2009; McMullen et al. 2009; Leisner et al. 2018). Interestingly, a genome-wide association study using a panel of diverse tetraploid potato genotypes revealed that chromosome 8 showed extremely conserved linkage disequilibrium due to low levels of recombination and suggested that chromosome 8 has been exposed to selection pressures that differ from those of other chromosomes during the last 150 years of breeding (Sharma et al. 2018). Therefore, the chromosome-level heterozygosity on chromosome 8 may be a general feature in potato and may play a major role in heterosis (McMullen et al. 2009).
Marand et al. (2019) suggested that persistent heterozygosity in shorter heterozygous blocks embedded within homozygous regions (recalcitrant heterozygosity) might be associated with the production of functional gametes. However, although the completely homozygous plants in all SNPs prominently showed slow growth, a poor root system and low fecundity, they were still sexually and asexually (by tubers) fertile. Therefore, residual heterozygosity is likely a transient phenomenon in a continued inbreeding process.
Segregation distortion
Hosaka and Hanneman (1998a) suggested that a recessive lethal allele was tightly coupled with Sli at the end of chromosome 12 because even after seven rounds of selfing, chc 525-3 harbored Sli in a heterozygous state. However, in an F2 population from a Sli-possessing clone or further selfed progeny populations, distorted segregation in the direction of the Sli-possessing clone at the distal end of chromosome 12 was reported (Birhman and Hosaka 2000; Meijer et al. 2018). The recessive lethal allele, inferred based on the complete absence of one homozygous genotype, was identified in the 57.2–57.8 Mb interval on chromosome 12 in DM and S. chacoense 39-7 (Endelman et al. 2019). We identified a 1.15 Mb segment in the 59.0–60.1 Mb interval on chromosome 12 in S8 S. chacoense, in which one of two homozygotes was completely absent. Although the critical position was slightly different, the same recessive lethal allele was likely involved in chc 525-3. Zhang et al. (2019) resequenced whole genomes of selfed progenies from clones E, RH89-039-16, and C151 and identified five lethal alleles (la1, la2, la3, ar1, and ws1) and four deleterious alleles affecting growth and vigor (ym, yl1, pa1, and pa2). One of the lethal alleles, lethal allele 3 (la3), resides in the 58.5–60.3 Mb interval on chromosome 12 of RH89-039-16. The candidate regions for Sli (Clot et al. 2020) and la3 (Zhang et al. 2020) and the 1.15 Mb segment are displayed in Fig. 5, indicating that Sli and la3 overlap in this segment. Inferred from the common DNA sequence of Sli present in RH89-039-16 and chc 525-3 (Clot et al. 2020), it is clear that the lethal gene harbored in the 1.15 Mb segment of chc 525-3 is la3. However, haplotype c of chc 525-3, carrying Sli and a nonlethality gene (La3), was fixed to a homozygote (Fig. 5). The homozygous state for Sli in 97H32-6 (Phumicahi et al. 2006) and M6 (Jansky et al. 2014) supported a coupling phase for linkage between Sli and La3, which is contradictory to our previous hypothesis (Hosaka and Hanneman 1998a). Endelman et al. (2019) reported a recombination hotspot (12 of 35 progeny had recombination events) between 57.6 and 59.2 Mb at the distal end of chromosome 12. We also observed a recombination hotspot (at least 7 of 24 progeny had recombination events) in the 1.15 Mb segment. Further analysis is needed to disclose the relationships between Sli, la3, and the recombination hotspot.
The 0.95 Mb segment, in which one of two homozygotes was completely absent, was identified in the 40.1–41.1 Mb interval on chromosome 8. Although another known lethal gene (la1) resides in the 2.4–6.4 Mb interval on chromosome 8 (Zhang et al. 2019), the 0.95 Mb segment might contain a novel lethal gene.
Dwarfism
In almost all generations, dwarf and semidwarf plants were observed within some families (Supplementary Table 1). A recessive dwarfing gene, ga2, was identified at the distal end of chromosome 7 in the parental clones, phu 1.22 and F1-1 (Kimura and Hosaka 2002). However, chromosome 7 was completely homozygous in the S6 generation (Fig. 3a). Semidwarf phenotypes detected in the S10 generation were not associated with any SNP markers (Fig. 4). Thus, it is unlikely that ga2 was expressed for dwarfism during selfed generations. Bamberg and Miller (2012) could not detect dwarfs in phu 1.22-derived progeny, suggesting that our phu 1.22 is a clonal sport not genetically identical to the stocks they tested. Thus, we speculate that the morphological abnormalities such as dwarfism, semidwarfism, and antherlessness observed in our selfed progenies appeared by epigenetic alteration, as reported in rice (Akimoto et al. 2007; Miura et al. 2009) and Arabidopsis thaliana (Saze and Kakutani 2007). Aberrant segregations and paternal-or atavism-like transmission have been reported for DNA methylation-sensitive random amplified polymorphic DNA (RAPD) markers in the present advanced selfed progenies (Nakamura and Hosaka 2010). Alternatively, hidden heterozygous loci might be exposed by selfing because we recently noticed that dwarfs also appeared among S8 and S9 progenies of chc 525-3 (unpublished).
Conclusions
After a long-term crossing experiment taking place over 30 years, a nearly or completely homozygous diploid potato was created. Although asexual techniques such as anther culture followed by chromosome doubling or prickle pollination followed by protoplast fusion can result in completely homozygous diploid potatoes (Karp et al. 1984; Uijtewaal et al. 1987; M’Ribu and Veilleux 1990; Meyer et al. 1993), all previously derived plants, including DM, were male sterile because of the homozygous state of undesirable alleles imposed by anther culture followed by chromosome doubling in a single cycle without the opportunity for fertility selection (Paz and Veilleux 1999; Peterson et al. 2016). Continuous selfing has swept out all lethal alleles and selected for self-fertility (Phumichai and Hosaka 2006), leading to the generation of a homozygous diploid potato retaining male and female fertility and tuberization ability under long days. Although the S11 plants are weak in terms of vigor and fecundity due to severe inbreeding depression, they are undoubtedly useful genetic resources for various purposes, such as elucidating the mechanisms of heterosis, self-compatibility, interspecific crossability, tuberization, lethality, and other features.
References
Akimoto K, Katakami H, Kim H-J, Ogawa E, Sano CM, Wada Y, Sano H (2007) Epigenetic inheritance in rice plants. Ann Bot 100:205–217
Bamberg J, Miller CJr (2012) Comparisons of ga1 with other reputed gibberellin mutants in potato. Am J Potato Res 89:142–149
Birhman RK (1986) Pollination mechanisms in Solanum chacoense. Acta Bot Indica 16:89–91
Birhman RK, Hosaka K (2000) Production of inbred progenies of diploid potatoes using an S-locus inhibitor (Sli) gene, and their characterization. Genome 43:495–502
Braun SR, Endelman JB, Haynes KG, Jansky SH (2017) Quantitative trait loci for resistance to common scab and cold-induced sweetening in diploid potato. Plant Genome 10:3
Cappadocia M, Cheng DSK, Ludlum-Simonette R (1986) Self- compatibility in doubled haploids and their F1 hybrids, regenerated via anther culture in self-incompatible Solanum chacoense Bitt. Theor Appl Genet 72:66–69
Charlesworth D, Willis J (2009) The genetics of inbreeding depression. Nat Rev Genet 10:783–796
Cho J, McCouch S, Kuiper M, Kang M-R, Pot J, Groenen J, Eun M (1998) Integrated map of AFLP, SSLP and RFLP markers using a recombinant inbred population of rice (Orysa sativa L.). Theor Appl Genet 97:370–380
Cipar MS, Peloquin SJ, Hougas RW (1964a) Inheritance of incompatibility in hybrids between Solanum tuberosum haploids and diploid species. Euphytica 13:163–172
Cipar MS, Peloquin SJ, Hougas RW (1964b) Variability in the expression of self-incompatibility in tuber-bearing diploid Solanum species. Am Potato J 41:155–162
Clot CR, Polzer C, Prodhomme C, Schuit C, Engelen CJM, Hutten RCB, van Eck HJ (2020) The origin and widespread occurrence of Sli-based self-compatibility in potato. Theor Appl Genet 133:2713–2728
De Jong H (1977) Self-compatibility in inbred cultivated diploid potatoes. Incompatibility Newsl 8:16–17
De Jong H, Rowe PR (1971) Inbreeding in cultivated diploid potatoes. Potato Res 14:74–83
de Vries M, ter Maat M, Lindhout P (2016) The potential of hybrid potato for East-Africa. Open Agr 1:151–156
Enciso-Rodriguez F, Manrique-Carpintero NC, Nadakuduti SS, Buell CR, Zarka D, Douches D (2019) Overcoming self-incompatibility in diploid potato using CRISPR-Cas9. Front Plant Sci 10:376
Endelman JB, Jansky SH (2016) Genetic mapping with an inbred line-derived F2 population in potato. Theor Appl Genet 129:935–943
Endelman J, Jansky SH, Butler N, Christensen G (2019) Genetic evidence of a recessive lethal allele on potato chromosome 12. Am J Potato Res 96:331
Golmirzaie AM, Ortiz R, Atlin GN, Iwanaga M (1998a) Inbreeding and true seed in tetraploid potato. I. Selfing and open pollination in Andean landraces (Solanum tuberosum Gp. Andigena). Theor Appl Genet 97:1125–1128
Golmirzaie AM, Bretschneider K, Ortiz R (1998b) Inbreeding and true seed in tetraploid potato. II. Selfing and sib-mating in heterogeneous hybrid populations of Solanum tuberosum. Theor Appl Genet 97:1129–1132
Gore MA, Chia JM, Elshire RJ, Sun Q, Ersoz ES, Hurwitz BL, Peiffer JA, McMullen MD, Grills GS, Ross-Ibarra J, Ware DH, Buckler ES (2009) A first-generation haplotype map of maize. Science 326:1115–1117
Hanneman REJr (1985) Self fertility in Solanum chacoense. Am Potato J 62:428–429
Haynes KG, Guedes ML (2018) Self-compatibility in a diploid hybrid population of Solanum phureja-S. stenotomum. Am J Potato Res 95:729–734
Hosaka K, Hanneman REJr (1998a) Genetics of self-compatibility in a self-incompatible wild diploid potato species Solanum chacoense. I. Detection of an S locus inhibitor (Sli) gene. Euphytica 99:191–197
Hosaka K, Hanneman REJr (1998b) Genetics of self-compatibility in a self-incompatible wild diploid potato species Solanum chacoense. 2. Localization of an S locus inhibitor (Sli) gene on the potato genome using DNA markers. Euphytica 103:265–271
Jansky SH, Chung YS, Kittipadukal P (2014) M6: a diploid potato inbred line for use in breeding and genetics research. J Plant Regist. https://doi.org/10.3198/jpr2013.05.0024crg
Jansky SH, Charkowski AO, Douches DS, Gusmini G, Richael C, Bethke PC, Spooner DM, Novy RG, De Jong H, De Jong WS, Bamberg JB, Thompson AL, Bizimungu B, Holm DG, Brown CR, Haynes KG, Sathuvalli VR, Veilleux RE, Miller JC Jr, Bradeen JM, Jiang J (2016) Reinventing potato as diploid inbred line-based crop. Crop Sci 56:1412–1422
Jones DF (1918) The effect of inbreeding and crossbreeding upon development. Proc Natl Acad Sci USA 4:246–250
Karp A, Risiott R, Jones MGK, Bright SWJ (1984) Chromosome doubling in monohaploid and dihaploid potatoes by regeneration from cultured leaf explants. Plant Cell Tiss Org Cult 3:363–373
Kimura T, Hosaka K (2002) Genetic mapping of a dwarfing gene found in Solanum phureja clone 1.22. Am J Potato Res 79:201–204
Kotch GP, Peloquin SJ (1987) A new source of haploid germplasm for genetic and breeding research. Am Potato J 64:137–141
Krantz FA (1924) Potato breeding methods. Minn Agric Exp Stn Tech Bull 25:1–32
Krantz FA (1946) Potato breeding methods. III. A suggested procedure for potato breeding. Techn Bull Minn Agric Exp Sta 173
Leisner CP, Hamilton JP, Crisovan E, Manrique-Carpintero NC, Marand AP, Newton L, Pham GM, Jiang J, Douches DS, Jansky SH, Buell CR (2018) Genome sequence of M6, a diploid inbred clone of the high-glycoalkaloid-producing tuber-bearing potato species Solanum chacoense, reveals residual heterozygosity. Plant J 94:562–570 (erratum: Plant J 96:482)
Lightbourn GJ, Veilleux RE (2007) Production and evaluation of somatic hybrids derived from monoploid potato. Am J Potato Res 84:425–435
Lindhout P, Meijer D, Schotte T, Hutten RCB, Visser RGF, van Eck HJ (2011) Towards F1 hybrid seed potato breeding. Potato Res 54:301–312
Marand AP, Jansky SH, Gage JL, Hamernik AJ, de Leon N, Jiang J (2019) Residual heterozygosity and epistatic interactions underlie the complex genetic architecture of yield in diploid potato. Genetics 212:317–332
McMullen M, Kresovich S, Villeda H, Bradbury P, Li H, Sun Q, Flint-Garcia S, Thornsberry J, Acharya C, Bottoms C, Brown P, Browne C, Eller M, Guill K, Harjes C, Kroon D, Lepak N, Mitchell SE, Peterson B, Pressoir G, Romero S, Rosas MO, Salvo S, Yates H, Hanson M, Jones E, Smith S, Glaubitz JC, Goodman M, Ware D, Holland JB, Buckler ES (2009) Genetic properties of the maize nested association mapping population. Science 325:737–740
Meijer D, Viquez-Zamora M, van Eck HJ, Hutten RC, Su Y, Rothengatter R, Visser RGF, Lindhout WH, van Heusden AW (2018) QTL mapping in diploid potato by using selfed progenies of the cross S. tuberosum × S. chacoense. Euphytica 214:121
Mendiburu AO, Peloquin SJ (1977) Bilateral sexual polyploidization in potatoes. Euphytica 26:573–583
Meyer R, Salamini F, Uhrig H (1993) Isolation and characterization of potato diploid clones generating a high frequency of monohaploid or homozygous diploid androgenetic plants. Theor Appl Genet 85:905–912
Miura K, Agetsuma M, Kitano H, Yoshimura A, Matsuoka M, Jacobsen SE, Ashikari M (2009) A metastable DWARF1 epigenetic mutant affecting plant stature in rice. Proc Natl Acad Sci USA 106:11218–11223
M’Ribu HK, Veilleux RE (1990) Effect of genotype, explant, subculture interval and environmental conditions on regeneration of shoots from in vitro monoploids of a diploid potato species, Solanum phureja Juz. & Buk.. Plant Cell Tiss Org Cult 23:171–179
Nakamura S, Hosaka K (2010) DNA methylation in diploid inbred lines of potatoes and its possible role in the regulation of heterosis. Theor Appl Genet 120:205–214
Olsder J, Hermsen JGTh (1976) Genetics of self-compatibility in dihaploids of Solanum tuberosum L. 1. Breeding behaviour of two self-compatible dihaploids. Euphytica 25:597–607
Pandey KK (1962) Interspecific incompatibility in Solanum species. Amer J Bot 49:874–882
Paz MM, Veilleux RE (1999) Influence of culture medium and in vitro conditions on shoot regeneration in Solanum phureja monoploids and fertility of regenerated doubled monoploids. Plant Breed 118:53–57
Peterson BA, Holt SH, Laimbeer PE, Doulis AG, Coombs J, Douches DS, Hardigan MA, Buell CR, Veilleux RE (2016) Self-fertility in a cultivated diploid potato population examined with the Infinium 8303 potato single-nucleotide polymorphism array. Plant Genome 9:3
Phumichai C, Hosaka K (2006) Cryptic improvement for fertility by continuous selfing of diploid potatoes using Sli gene. Euphytica 149:251–258
Phumichai C, Mori M, Kobayashi A, Kamijima O, Hosaka K (2005) Toward the development of highly homozygous diploid potato lines using the self-compatibility controlling Sli gene. Genome 48:977–984
Phumichai C, Ikeguchi-Samitsu Y, Fujimatsu M, Kitanishi S, Kobayashi A, Mori M, Hosaka K (2006) Expression of S-locus inhibitor gene (Sli) in various diploid potatoes. Euphytica 148:227–234
Pushkarnath (1942) Studies on sterility in potatoes. 1. The genetics of self- and cross-incompatibilities. Indian J Genet Pl Breed 2:11–36
Ross H (1986) Potato breeding-problems and perspectives. Verlag Paul Parey, Berlin
Sanetomo R, Akino S, Suzuki N, Hosaka K (2014) Breakdown of a hybridization barrier between Solanum pinnatisectum Dunal and potato using the S locus inhibitor gene (Sli). Euphytica 197:119–132
Saze H, Kakutani T (2007) Heritable epigenetic mutation of a transposon-flanked Arabidopsis gene due to lack of the chromatin-remodeling factor DDM1. EMBO J 26:3641–3652
Sharma SK, MacKenzie K, McLean K, Dale F, Daniels S, Bryan GJ (2018) Linkage disequilibrium and evaluation of genome-wide association mapping models in tetraploid potato. G3 8:3185–3202
The Potato Genome Sequencing Consortium (2011) Genome sequence and analysis of the tuber crop potato. Nature 475:189–195
Uijtewaal BA, Jacobsen E, Hermsen JGTh (1987) Morphology and vigour of monohaploid potato clones, their corresponding homozygous diploids and tetraploids and their heterozygous diploid parent. Euphytica 36:745–753
Ye M, Peng Z, Tang D, Yang Z, Li D, Xu Y, Zhang C, Huang S (2018) Generation of self-compatible diploid potato by knockout of S-RNase. Nat Plants 4:651–654
Zhang C, Wang P, Tang D, Yang Z, Lu F, Qi J, Tawari NR, Shang Y, Li C, Huang S (2019) The genetic basis of inbreeding depression in potato. Nat Genet 51:374–378
Acknowledgements
We thank late Dr. R. K. Birhman and Dr. C. Phumichai for their contributions to this long-term experiment. This research was supported partly by a JSPS Grant-in-Aid for Scientific Research (C) (Grant Number JP19K05962) and by Calbee Inc., Hokkaido Potato Growers Association, Kewpie Corp., KENKO Mayonnaise Co., Ltd., and the Japan Snack Cereal Foods Association.
Author information
Authors and Affiliations
Contributions
KH and RS carried out the crossing experiment. KH conducted SNP analysis and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hosaka, K., Sanetomo, R. Creation of a highly homozygous diploid potato using the S locus inhibitor (Sli) gene. Euphytica 216, 169 (2020). https://doi.org/10.1007/s10681-020-02699-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10681-020-02699-3