Abstract
The influence of light regulation on the growth and enzyme production of three endolichenic fungal isolates, i.e. Pseudopestalotiopsis theae (EF13), Fusarium solani (EF5), and Xylaria venustula (PH22), was determined. The isolates were exposed to blue, red, green, yellow, white fluorescent light (12 h light–12 h dark photoperiod) (test), and 24 h dark (control) conditions. Results revealed that the alternating light–dark conditions resulted in the formation of dark rings in most fungal isolates but was absent in PH22. Red light induced sporulation while yellow light elicited higher biomass in all isolates (0.19 ± 0.01 g, 0.07 ± 0.00 g, and 0.11 ± 0.00 g, for EF13, PH22, and EF5, respectively) as compared to incubation in the dark. Results also showed that blue light induced higher amylase activity in PH22 (15.31 ± 0.45 U/mL) and L-asparaginase activity in all isolates (0.45 ± 0.01 U/mL, 0.55 ± 0.39 U/mL, and 0.38 ± 0.01 U/mL, for EF13, PH22, and EF5, respectively) compared to both control conditions. Green light enhanced the production of xylanase (6.57 ± 0.42 U/mL, 10.64 ± 0.12 U/mL, and 7.55 ± 0.56 U/mL for EF13, PH22, and EF5, respectively) and cellulase (6.49 ± 0.48 U/mL, 9.57 ± 0.25 U/mL, and 7.28 ± 0.63 U/mL, for EF13, PH22, and EF5, respectively). In contrast, red light was the least effective light treatment as production of enzymes was the least, with lower levels of amylase, cellulase, xylanase, and L-asparaginase detected. To conclude, all three endolichenic fungi are light-responsive, with fungal growth regulated with the use of red light and yellow light, and manipulation of enzyme production via blue and green light.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fungi produce a variety of natural compounds such as extracellular enzymes (e.g. amylase, cellulase, xylanase) and secondary metabolites that are useful as therapeutics and for other various applications. In this study, a rare group of fungi known as endolichenic fungi is explored for their natural compounds. Endolichenic fungi are found in lichens. They are similar to endophytes, where they exist inside their host tissues without causing any symptoms (Kellogg and Raja 2016). Endolichenic fungi have been found to produce beneficial compounds that are with antimicrobial, antioxidant, and anticancer properties (Kannagara et al. 2009). Their metabolite profiles have been investigated and approximately 140 novel metabolites have been discovered from endolichenic fungi (Kellogg and Raja 2016). Some of the well-known bioactive compounds include novel ambuic acid derivatives (anti-proliferative compounds) isolated from Pestalotiopsis sp., dehydroherbarin (antibacterial compounds) from Corynespora sp., and enzymes such as cellulase, protease, and xylanase from Colletotrichum sp., Pestalotiopsis sp., and Xylaria sp., which are used as anti-cancer agents, antibiotics, and enzymes in food industry, respectively (Paranagama et al. 2007; Ding et al. 2009; Kannangara et al. 2009).
One of the main challenges of harnessing beneficial compounds from fungi is the low quantities obtained, attributed to the sub-optimal growth conditions (Nielsen and Nielsen 2017). Factors such as pH, temperature, and nutrients are known to influence the production of valuable compounds. Keller et al. (1997) observed lesser production of mycotoxin in Aspergillus nidulans and A. parasticus when cultured in medium with low pH. Bhattacharyya and Jha (2011) obtained higher production of antimicrobial metabolites from Aspergillus strain TSF 146 incubated at 25 °C and pH 5.5. In addition, carbon and nitrogen sources also have a significant effect on production of metabolites in fungi. Jain and Gupta (2012) discovered that supplementation with dextrose (carbon source) enhanced antimicrobial activity of extracts from Penicillium sp. Hence, it is highly suggestive that manipulation of growth conditions may present possible strategies to improve the yield of compounds.
Of the many growth conditions known to influence growth of fungi, light is the least studied and commonly overlooked as fungi are not autotrophic and do not rely on light as a source of energy. Nevertheless, light is a crucial environmental signal that regulates the developmental and physiological processes in fungi (Calvo et al. 2002; Gan and Ting 2019). Contrary to beliefs, these studies revealed that various light spectra regulate common functions in fungi such as growth, and the production of pigments and enzymes. This was demonstrated by Maqsood et al. (2014), in which the presence of light enhanced mycelial growth of Colletotrichum gloeosporioides compared to incubation in the dark. Irradiation with red light induced formation of asci and spores in Monascus (Miyake et al. 2005), while white, blue, and red light promoted asexual stage of Aspergillus glaucus HB-19 compared to cultures incubated in darkness (Miyake et al. 2005; Cai et al. 2013). Light treatments have also been reported to induce enzyme and metabolite production by fungi as this is associated with growth (Tisch and Schmoll 2010; Nielsen and Nielsen 2017). With white fluorescent light, some fungi, such as Trichoderma viride, produce more 1,3-β-glucan synthase enzyme (increased by 130%) (Farkas and Nemcovic 2001).
Existing studies on the influence of light on fungi were primarily focused on model fungal species such as Neurospora crassa, Aspergillus nidulans, and Trichoderma reesei (Calvo et al. 2002; Tisch and Schmoll 2010). However, the influence of light beyond the model species is not well established. In this study, endolichenic fungi are examined for their growth and enzyme production in response to light treatment. Endolichenic fungi are gaining interest in recent years due to their potential in the production of enzymes and compounds with antioxidant and antimicrobial activity, with great potential for application. The three endolichenic fungi (Pseudopestalotiopsis, Xylaria, and Fusarium) used in this study have been reported to produce bioactive compounds (Santiago et al. 2021) and may benefit from light treatments. This study, therefore, aims to explore the possibility in using light (blue, red, yellow, and green light) as an effective and affordable regulator to improve the growth (circadian rings, sporulation, and biomass) and production of beneficial enzymes (i.e. amylase, cellulase, xylanase, and L-asparaginase) in these endolichenic fungi (Pseudopestalotiopsis theae, Fusarium solani, and Xylaria venustula).
Materials and methods
Endolichenic fungi
Three endolichenic fungal isolates (Pseudopestalotiopsis theae, Fusarium solani, and Xylaria venustula) were obtained as pure cultures from stock cultures in the laboratory. Of these isolates, P. theae (EF13) and F. solani were isolated from the fruticose lichen Usnea pectinata, whereas X. venustula (PH22) was isolated from Usnea baileyi. The isolates have been assigned with their various accession numbers from GenBank as follows: EF13 as Pseudopestalotiopsis theae (MG 881833), EF5 as Fusarium solani (MG 881825), and PH22 as Xylaria venustula (MH 370742) (Santiago et al. 2021). Fungal cultures were established by inoculating mycelial plugs of stock cultures onto potato dextrose agar (PDA) and incubated at 28 ± 2 °C until use.
Light exposure conditions
Six different light conditions were set-up to provide the various light spectrum and their corresponding wavelengths and photosynthetic photon flux density (PPFD): blue (460 nm; PPFD 70.52 µmol/m2s), green (540 nm; PPFD 64.47 µmol/m2s), yellow (580 nm; PPFD 65.52 µmol/m2s), red (660 nm, 2384 lx; PPFD 78.69 µmol/m2s), white fluorescent light (390 to 700 nm; PPFD 6.86 µmol/m2s), and dark (control). To achieve this, each growth chamber was equipped with two plant growing light tubes (T8 light emitting diodes (LED)) which emitted the desired specific wavelengths. The wattage and input voltage of each coloured LED light tubes were 9W and 220 V, respectively. The LED light tubes were purchased from JLL Electrical Sdn Bhd (Kuala Lumpur, Malaysia) and Chen Xi Deng Shi Co. Ltd (ShenZhen, China). The LED light tubes were fixed into customized wooden boxes (65 cm × 35 cm × 30 cm) which served as growth chambers for incubation. The white fluorescent light condition was achieved using white fluorescent light (ambient light), while cultures incubated under dark condition were placed into the growth chamber without switching on the light. The white fluorescent light and dark conditions were used as control. The temperature of the growth chamber was approximately 28 ± 2 °C throughout the study. For cultures that were subjected to light treatments and white fluorescent light conditions, the fungal cultures were exposed to corresponding light spectrum for a photoperiod of 12 h light/12 h dark, while cultures that were subjected to the dark condition were covered with aluminium foil (Murthy et al. 2015).
Influences of light on growth and development
To determine the influence of light spectrum on fungal growth and development, the following parameters were determined—colony morphology, sporulation, and growth (biomass). To determine the colony morphology, 7-day old cultures were observed and described, paying attention to the pigmentation and formation of concentric (circadian) rings. As for sporulation, 15 mycelial plugs for each isolate were placed into a 15 mL falcon tube containing 5 mL of distilled water. The mixture was agitated at 200 rpm for 30 min. The number of spores was counted using a hemacytometer (Bhunjun et al. 2021) as in Eq. 1.
For biomass estimation, broth cultures were established by inoculating 5 mycelial plugs into 40 mL potato dextrose broth (PDB) and incubated for 7 days in the growth chamber under different light conditions. All the broth cultures were incubated under static conditions. After incubation, the mycelium (biomass) was filtered with sterile cheesecloth, collected, and oven-dried (65 °C) on a filter paper until a constant weight was obtained. The dry weight of mycelial biomass was calculated as in Eq. 2 (Wang et al. 2016a, b):
Influence of light on the production of extracellular enzymes
Four important extracellular enzymes (amylase, L-asparaginase, cellulase, and xylanase) were included in this study. The isolates were first subjected to the qualitative colorimetric assay (agar plate method) to screen for the production of enzymes. The agar plate method is based on the utilization of the supplemented substrate in the agar by the fungi, which leads to colour change in the agar or when reacted with reagents (Farris et al. 2016). For the quantitative assessment, the measurement of enzyme activities was first established by inoculating five mycelial plugs into 40 mL of PDB. Then, the inoculated broth cultures were incubated under six different light conditions for 7 days under static conditions. After incubation, the broth cultures were filtered through sterile filter paper (Whatman No. 1, Tisch, USA) and the culture filtrate was collected and used for the quantitative assays. The qualitative and quantitative methods for each specific enzymes are described in the following sections.
Amylase activities
For qualitative method, a 5-mm fungal mycelial plug was inoculated onto modified PDA supplemented with 1% (w/v) soluble starch (R and M Chemical, UK). The plates were incubated at 28 ± 2 °C for 3 days. After incubation, the agar was flooded with 1% iodine solution and the formation of a clear zone around the fungal colonies was observed to confirm the presence of amylase (Sunitha et al. 2012). For the quantitative assay, the dinitrosalicyclic acid (DNS) method by Sunitha et al. (2012) was used. Briefly, 0.5 mL of the collected filtrate (crude enzyme) was added to 0.5 mL of 1% (w/v) soluble starch and 1 mL of 1 mol/L of sodium acetate buffer (pH 6.0). The reaction mixture was then incubated at 37 °C for 30 min. The DNS reagent (1 mL) (Sigma-Aldrich, USA) was then added to the mixture to terminate the reaction. Distilled water was added to give a final volume of 5 mL of the reaction mixture. The reaction mixture was read at 540 nm using the multimode microplate reader (Spark®, Switzerland). Glucose was used as the standard to construct the standard curve (R2 = 0.9967) and one unit (IU) of amylase is expressed as the release of 1 µmol of reducing sugar per min under assay conditions.
Cellulolytic activities
The qualitative screening of cellulolytic activities was performed as described by Adesina and Onilude (2013). A 5-mm mycelial plug of isolates was inoculated onto the modified PDA plates supplemented with 1% of carboxymethyl cellulose (CMC) (Calbiochem, US) and incubated for 3 days at 28 ± 2 °C. After incubation, the agar plates were flooded with 0.4% Congo red and destained with 1 mol/L sodium chloride (NaCl). The formation of the clear zone around the mycelial plug confirmed the presence of cellulase. As for quantitative assay, the cellulolytic activity was measured based on the methods by Sridevi and Charya (2011). Briefly, 1 mL of the collected filtrate (crude enzyme) was added to 1 mL of 1% carboxymethylcellulose (CMC) and incubated at 50 °C for 15 min. The reaction in the mixture was then stopped by adding 3 mL of DNS reagent. Then, the mixture was incubated at 100 °C for 15 min and the absorbance was measured at 540 nm using the multimode microplate reader (Spark®, Switzerland). Glucose was used as the standard to construct the standard curve (R2 = 0.9979) and one unit (IU) of cellulase is expressed as the release of 1 µmol of reducing sugar per min under assay conditions.
Xylanase activities
To detect the presence of xylanase, a 5-mm mycelial plug of the fungal isolates was inoculated onto the PDA plates supplemented with 1% of birchwood xylan (Sigma, Germany). The agar plates were then incubated at 28 ± 2 °C for 5 days. After incubation, the agar plates were then flooded with 0.1% Congo red and destained with 1 mol/L NaCl. The formation of a clear zone around the colonies was observed to confirm the presence of xylanase (Sridevi and Charya 2011). For the quantitative assay, xylanase was determined based on the method by Sridevi and Charya (2011). Briefly, 1 mL of collected filtrate (crude enzymes) was added to 1 mL of 1% (w/v) birchwood xylanase in 1 mL of 0.05 mol/L citrate buffer (pH 5). Mixtures were then incubated in a water bath at 55 °C for 10 min. Then, 3 mL of DNS reagent was added to the reaction mixture and incubated at 100 °C for 15 min. Absorbance was measured at 540 nm using the multimode microplate reader (Spark®, Switzerland). Glucose was used as the standard to construct the standard curve (R2 = 0.9979) and one unit (IU) of xylanase is expressed as the release of 1 µmol of reducing sugar per min under assay conditions.
L-asparaginase activities
The qualitative assay for L-asparaginase was screened by first inoculating a fungal plug onto PDA containing 10 g/L L-asparagine (Merck, USA) and 2.5% phenol red dye (Sigma-Aldrich, USA), and incubated at 28 ± 2 °C for 5 days. The formation of a pink zone around the colony indicates the presence of L-asparaginase (Chow and Ting 2015). For the quantitative assay, L-asparaginase activity was determined using the method by Chow and Ting (2015). Briefly, 200 µL of the crude extract was mixed with 200 µL of Tris HCL (pH 7.2) and 400 µL of 0.04 mol/L L-asparagine. The mixture was then incubated at 37 °C for 1 h. To stop the reaction, 300 µL of 15% (w/v) trichloroacetic acid (TCA) was added. The mixture was then centrifuged at 10,000 rpm for 5 min. The supernatant (100 µL) was pipetted into fresh tubes containing 100 µL of distilled water and 300 µL of Nessler’s reagent (Merck, Germany). The mixture was incubated at room temperature (26 ± 2 °C) for 5 min before absorbance reading at 450 nm using the multimode microplate reader (Spark®, Switzerland). One unit of asparaginase is expressed as the amount of enzyme that catalyses the formation of 1 μmol of ammonia per min at 37 ± 2 °C. Ammonium sulphate was used as a standard to construct the standard curve (R2 = 0.9983) in this assay.
Statistical analysis
All assays were performed in triplicates and the data were analysed statistically using the software Statistical Package for Social Sciences (SPSS) Version 22.0. Any significant difference between samples was determined by one-way analysis of variance (ANOVA) and the means were compared using post hoc Tukey test (HSD, p < 0.05).
Results
Influence of light on growth
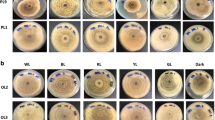
Light treatment induced variable responses from endolichenic fungi, which were observed through growth measurements (biomass), sporulation, and phenotypic characteristics (circadian rings). Irradiation with various light treatments (blue, red, yellow, and green) triggered the formation of dark concentric circadian rings in isolates Pseudopestalotiopsis theae (EF13) and Fusarium solani (EF5) but not in Xylaria venustula (PH22). Interestingly, these banding patterns were also absent from all the fungal cultures kept in the control environment (i.e. white fluorescent and dark conditions). These results suggest that the EF13 (Pseudopestalotiopsis theae) and EF5 (Fusarium solani) isolates are photo-responsive; however, the PH22 isolate (Xylaria venustula) was not photo-responsive, as the circadian rings were absent for cultures in the light treatments, as well as in conditions of control. Among the four different light treatments, the most intense circadian rings were found in fungal cultures exposed to red light, followed by blue light, green light, and yellow light (Figs. 1, 2, and 3).
In addition to circadian rings, the irradiation of light treatments significantly influenced the growth (biomass) of fungi as well, compared to white fluorescent light and dark conditions. It was observed that exposure to yellow and blue light led to a significant increase in growth (higher biomass) in isolate EF13 (0.19 ± 0.02 g and 0.18 ± 0.02 g, respectively) compared to cultures incubated in white fluorescent light and dark incubation conditions (0.17 ± 0.02 g and 0.14 ± 0.02 g, respectively) (Fig. 4). Both yellow and blue light did not implicate growth of EF5 as the biomass produced (0.11 ± 0.02 g and 0.11 ± 0.02 g, respectively) was comparable with the biomass produced by cultures incubated in white fluorescent light and in the dark (0.13 ± 0.01 g and 0.08 ± 0.01 g, respectively) (Fig. 4). On the contrary, growth of both EF13 and EF5 was suppressed when the isolates were exposed to green light or when kept in darkness. Under green light exposure, growth was inferior with low biomass recorded at only 0.13 ± 0.01 g and 0.07 ± 0.01 g for EF 13 and EF 5, respectively (Fig. 4). This poor growth was also noted for cultures incubated in the dark (0.14 ± 0.02 g and 0.08 ± 0.01 g for EF13 and EF5, respectively). Incubation in dark conditions was also detrimental compared to white fluorescent light incubation, with lower biomass observed in cultures kept in the dark (mean biomass of 0.06 ± 0.01 to 0.14 ± 0.02 g) (Fig. 4). Of the three endolichenic isolates, PH22 was the least sensitive to light treatments, where the light conditions, including white fluorescent and dark incubation, did not significantly influence growth with biomass of 0.06 ± 0.01 to 0.09 ± 0.01 g observed. In short, yellow, blue, and white fluorescent light is relatively appropriate to enhance biomass, whereas incubation using red, green light, or dark incubation conditions tends to suppress fungal biomass.
Biomass (g) of three endolichenic fungi (ELF), Pseudopestalotiopsis theae (EF13), Fusarium solani (EF5), and Xylaria venustula (PH22) that were incubated under six different light conditions (white fluorescent, blue light, red light, yellow light, green light, and in dark) for 7 days. Values are means of triplicates. Means with the same letters within the isolate are not significantly different at HSD(0.05). Bars indicate the standard error of means (± SEM)
The sporulation of all fungal isolates was influenced by light regulation as well, with varying degrees of sporulation demonstrated. Red light induced the highest number of spores in isolate PH22 (10.58 ± 1.94 × 104 spores per mL), EF5 (5.50 ± 0.87 × 104 spores per mL), and EF13 (5.17 ± 0.29 × 104 spores per mL) (Fig. 5). The other light spectra elicited different sporulation responses in isolates. Green light was moderately effective on the sporulation of EF5, in which green light induced a higher number of spores (4.33 ± 2.08 × 104 spores per mL) compared to both white fluorescent light and dark incubation (1.83 ± 1.05 × 104 spores per mL and 2.58 ± 0.14 × 104 spores per mL, respectively). Although green light was comparable to the effectiveness of red light in inducing sporulation of EF5 (4.33 ± 2.08 × 104 spores per mL and 5.50 ± 0.87 × 104 spores per mL, respectively), it did not influence sporulation in EF13 and PH22 (Fig. 5). On the other hand, blue and yellow light were detrimental to the sporulation in fungi, as indicated by its significantly lower spore count (mean spore count ranging from 0.50 ± 0.25 × 104 spores per mL to 1.50 ± 0.43 × 104 spores per mL and 0.00 ± 0.00 × 104 spores per mL to 123.50 ± 2.39 × 104 spores per mL, respectively) (Fig. 5). Furthermore, all the fungal isolates were observed to sporulate fairly well when incubated in white fluorescent or dark conditions, with mean spore count of 1.17 ± 0.38 × 104 spores per mL to 162.92 ± 3.00 × 104 spores per mL for white fluorescent light, and 1.75 ± 2.17 × 104 spores per mL to 48.67 ± 4.65 × 104 spores per mL for dark incubation (Fig. 5). In general, red light is considered useful to enhance the production of spores in fungi, while yellow light suppresses sporulation in fungi.
Spore count (× 10.4 spore per mL) of three endolichenic fungi (ELF), Pseudopestalotiopsis theae (EF13), Fusarium solani (EF5), and Xylaria venustula (PH22) that were incubated under six different light conditions (white fluorescent, blue light, red light, yellow light, green light, and in dark) for 7 days. Values are means of triplicates. Means with the same letters within the isolate are not significantly different at HSD(0.05). Bars indicate the standard error of means (± SEM)
Influences of light on enzymatic activities of endolichenic fungi
It was discovered that all fungal isolates in this study can produce amylase, cellulase, L-asparaginase, and xylanase. Results revealed that light exposure influenced the amylase activity in fungi. It was observed that white fluorescent light, blue light, and incubation in the dark effectively regulated the amylase activity of most isolates. Both white fluorescent light and dark incubation favoured the production of amylase in isolates EF13 and EF5, where higher amylase activities were observed in the cultures that were incubated under white fluorescent light and dark incubation conditions (8.81 ± 0.76 U/mL and 8.27 ± 0.40 U/mL for EF13, and 9.59 ± 0.29 U/mL and 10.19 ± 0.24 U/mL for EF5), respectively (Fig. 6). On the other hand, blue light elicited the highest production of amylase in PH22, which was recorded at 15.31 ± 0.45 U/mL (Fig. 6). All three isolates responded well to yellow light as well with comparable levels of amylase activities for both PH22 and EF5 (14.18 ± 0.62 U/mL and 9.75 ± 0.48 U/mL, respectively) as compared to cultures from the dark incubation (13.53 ± 0.06 U/mL and 10.19 ± 0.24 U/mL, respectively) (Fig. 6). Unlike blue and yellow light, the green and red light treatments were detrimental to amylase activities in all three isolates. Under green light treatment, isolates EF13, PH22, and EF5 recorded significantly lower amylase activities of 6.48 ± 0.76 U/mL, 9.86 ± 0.93 U/mL, and 6.82 ± 0.58 U/mL, respectively). However, treatment with red light was the least effective as amylase activities were the lowest for all three isolates, at 5.06 ± 0.15 U/mL (EF13), and 7.34 ± 0.45 U/mL (PH22), and 5.41 ± 0.41 U/mL (EF5) (Fig. 6). In short, blue and yellow lights are considered useful for amylase production, just as incubation in white fluorescent light or in darkness, while red and green light were detrimental to amylase activities.
Amylase activity (U/mL) of three endolichenic fungi, Pseudopestalotiopsis theae (EF13), Fusarium solani (EF5), and Xylaria venustula (PH22) that were incubated under six different light conditions (white fluorescent, blue light, red light, yellow light, green light, and in dark) for 7 days. Values are means of triplicates. Means with the same letters within the isolate are not significantly different at HSD(0.05). Bars indicate the standard error of means (± SEM)
This study revealed that green light treatment was beneficial in regulating the cellulase activity in fungi (Fig. 7). Both EF13 and EF5 produced the highest cellulase activity when the fungal cultures were incubated under green light, with mean cellulase activity of 6.09 ± 0.43 U/mL and 6.82 ± 0.59 U/mL, respectively. On the contrary, the other light treatments (blue, red, and yellow light) were less effective in inducing cellulase production in the isolates (except for isolate PH22), as the cellulase activities were significantly lower (ranging from 4.13 ± 0.07 to 4.53 ± 0.33 U/mL and 4.57 ± 0.77 to 5.73 ± 0.37 U/mL, for EF13 and EF5, respectively) (Fig. 7). Yellow light suppressed cellulase activities in both EF13 (4.49 ± 0.11 U/mL) and EF5 (5.73 ± 0.37 U/mL). Lower cellulase activity was also observed for all isolates exposed to blue light (mean cellulase activity of 4.53 ± 0.34 to 9.19 ± 0.53 U/mL) as compared to cultures incubated under white fluorescent light (mean cellulase activity of 6.05 ± 0.26 to 9.33 ± 0.39 U/mL) and in dark conditions (mean cellulase activity of 4.70 ± 0.36 to 8.78 ± 0.14 U/mL) (Fig. 7). Red light was found to be the most detrimental light spectrum to all isolates, where the lowest cellulase activities were observed for EF13, PH22, and EF5 with 4.13 ± 0.07 U/mL, 8.55 ± 0.21 U/mL, and 4.56 ± 0.77 U/mL, respectively. Of the three endolichenic fungi, PH22 was not particularly sensitive to varying light spectra as cellulase activities were not significantly different under light conditions, including white fluorescent and dark incubations, with mean cellulase activity ranging from 8.55 ± 0.21 to 9.48 ± 0.60 U/mL (Fig. 7).
Cellulase activity (U/mL) of three endolichenic fungi, Pseudopestalotiopsis theae (EF13), Fusarium solani (EF5), and Xylaria venustula (PH22), under different light conditions, that were incubated under six different light conditions (white fluorescent, blue light, red light, yellow light, green light, and in dark) for 7 days. Values are means of triplicates. Means with the same letters within the isolate are not significantly different at HSD(0.05). Bars indicate the standard error of means (± SEM)
Among the various light treatments, green light was the most effective in regulating xylanase activities for endolichenic fungi. Significantly higher xylanase activities were observed for EF13 (6.57 ± 0.42 U/mL), PH22 (10.64 ± 0.12 U/mL), and EF5 (7.55 ± 0.06 U/mL), as compared to cultures incubated under white fluorescent light (6.34 ± 0.17 U/mL for EF13, 9.73 ± 0.51 U/mL for PH22, and 6.72 ± 0.09 U/mL for EF5) (Fig. 8). On the contrary, exposure to blue, red, and yellow light was less effective than green light. These light treatments recorded significantly lower xylanase activities than cultures incubated in white fluorescent light or in the dark (Fig. 8). Exposure to blue light triggered lower levels of xylanase activity in EF13 (4.75 ± 0.32 U/mL), PH22 (9.41 ± 0.36 U/mL), and EF5 (5.40 ± 0.15 U/mL) compared to white fluorescent light. Red light was also detrimental to xylanase activities in all isolates, particularly EF5 (5.36 ± 0.09 U/mL). Yellow light was the least effective in regulating xylanase activities. Under yellow light, both EF13 and PH22 produced the lowest xylanase activities, which was recorded at 4.62 ± 0.17 U/mL and 9.12 ± 0.47 U/mL, respectively (Fig. 8). Interestingly, white fluorescent light appeared to have more positive influence on xylanase activities compared to incubation in the dark. It was observed that exposure to white fluorescent light induced comparable levels of xylanase activities for PH22 (9.73 ± 0.51 U/mL), whereas levels in EF5 (6.72 ± 0.09 U/mL) and EF13 (6.34 ± 0.17 U/mL) were significantly higher than xylanase activities, demonstrated by cultures incubated in the dark (6.31 ± 0.27 U/mL and 5.39 ± 0.26 U/mL, respectively) (Fig. 8). In short, treatments with green and white fluorescent light were effective in inducing xylanase activities in PH22, EF5, and EF13.
Xylanase activity (U/mL) of three endolichenic fungi, Pseudopestalotiopsis theae (EF13), Fusarium solani (EF5), and Xylaria venustula (PH22) under different light conditions that were incubated under six different light conditions (white fluorescent, blue light, red light, yellow light, green light, and in dark) for 7 days. Values are means of triplicates. Means with the same letters within the isolate are not significantly different at HSD(0.05). Bars indicate the standard error of means (± SEM)
Results showed that light exposure was beneficial for the regulation of L-asparaginase activity in fungi. Blue light elicited significantly higher L-asparaginase activity in PH22 (0.56 ± 0.04 µmol/mL per min), EF13 (0.45 ± 0.01 µmol/mL per min), and EF5 (0.34 ± 0.01 µmol/mL per min) (Fig. 9). Exposure to yellow light was beneficial as well, where high L-asparaginase activity was observed for isolate PH22 (0.48 ± 0.04 µmol/mL per min) and EF5 (0.30 ± 0.02 µmol/mL per min), which were comparable to cultures treated with blue light (Fig. 9). Both red and green light were, however, less effective in regulating the L-asparaginase activity in fungi. Under green light exposure, the L-asparaginase activity in EF13 (0.36 ± 0.01 µmol/mL per min), PH22 (0.38 ± 0.05 µmol/mL per min), and EF5 (0.26 ± 0.02 µmol/mL per min) was not significantly different from the cultures incubated under white fluorescent light (0.37 ± 0.02 µmol/mL per min, 0.38 ± 0.05 µmol/mL per min, and 0.30 ± 0.03 µmol/mL per min, respectively). Incubation under red light also did not favour L-asparaginase production in the fungal isolates, particularly EF5, where the lowest L-asparaginase activity was observed for EF5 (0.24 ± 0.02 µmol/mL per min) (Fig. 9). Compared to incubation under white fluorescent light, incubation in the dark elicited lower levels of L-asparaginase activity (mean of 0.26 ± 0.02 to 0.35 ± 0.01 µmol/mL per min) (Fig. 9). It is suggestive that light may be a crucial factor to induce the production of L-asparaginase, and blue and yellow lights (in some instances) may positively influence L-asparaginase activity for fungi.
L-asparaginase activity (µmol/mL per min) of three endolichenic fungi, Pseudopestalotiopsis theae (EF13), Fusarium solani (EF5), and Xylaria venustula (PH22) under different light conditions that were incubated under six different light conditions (white fluorescent, blue light, red light, yellow light, green light, and in dark) for 7 days. Values are means of triplicates. Means with the same letters within the isolate are not significantly different at HSD(0.05). Bars indicate the standard error of means (± SEM)
Discussion
Influence of light on fungal growth and development
This study indicated that light could significantly influence the growth and development of fungi. The formation of concentric circadian rings was evident in most fungal isolates exposed to light treatments (except PH22), suggesting that these isolates are generally photo-responsive fungi. Similar growth patterns were found in other photo-responsive fungi such as Aspergillus niger, Neurospora crassa, and Botrytis cinerea, validating that circadian patterns could be a common feature displayed by photo-responsive fungi (Canessa et al. 2013; Fuller et al. 2015; Schumacher 2017). On the contrary, PH22 remained unaffected by the alternating light–dark conditions, or any specific light spectra irradiated, suggesting that PH22 might be an isolate that is less sensitive to light. Another possible explanation for the absence of circadian ring formation in PH22 may be due to the slow growth characteristics of PH22, hence, making the detection of circadian rings difficult.
Among the different light spectra (blue, green, red, and yellow light), red light elicited the most intense circadian rings and was critical in inducing sporulation. Such results are expected as most of the existing literature reported red light as one of the most effective light sources in enhancing fungal sporulation. A similar outcome was observed in other fungal species such as N. crassa, A. niger, Alternaria solani, and B. cinerea, where asexual sporulation was induced under stimulation of red light (Mooney and Yager 1990; Wang et al. 2016a, b; Igbalajobi et al. 2019). The circadian ring symbolizes the response to circadian rhythm developed under constant external cues, such as light (as in this study) and temperature (Franco et al. 2017). When exposed to light irradiation, the growth of fungi is retarded and conidiation is induced, resulting in the formation of a ring of conidia (circadian ring) (Murthy et al. 2015). Although the exact red light signalling pathway and influence on sporulation is unclear, it has been proposed that red-light photoreceptors may directly alter the genes responsible for the transcription of sporulation in fungi, resulting in enhanced sporulation (Chen et al. 2010; Martinez-Soto and Ruiz-Herrera 2017; Yu and Fischer 2019). Blumenstein et al. (2005) and Wang et al. (2016a, b) reported that the asexual sporulation in N. crassa and A. nidulans decreased when the phytochrome genes (FphA, phy-1, and phy-2) were knocked out, further supporting the hypothesis that the red light photoreceptor can directly affect the transcription of the sporulation genes.
Our study also revealed that blue, yellow, and green light were also able to induce formation of circadian rings but were detrimental towards fungal sporulation. These observations were similar to Tan and Epton (1973) and Campbell et al. (2003), where the irradiation of these light spectra suppressed sporulation in B. cinerea and Pyrenophora seminiperda. Sporulation is one of the major photo-responses in fungi and is often used as an indicator of photo-responsiveness of fungi in early assessments. Sporulation is also a crucial process in regulating the production of enzymes, as both processes are often associated with each other (Tisch and Schmoll 2010). Preliminary studies reported that metabolites essential for inducing sporulation or maintaining spores such as enzymes and pigments would be strongly affected prior to or during sporulation (Sekiguchi and Gaucher 1977; Calvo et al. 2002; Tish and Schmoll 2010). For instance, Takano et al. (2000) revealed that the production of melanin was significantly increased in Colletotrichum lagenarium when the conidiation was induced. These suggested the possibility of using sporulation as an effective alternative strategy to regulate the production of target compounds.
Our study has shown that yellow light is key to enhancing growth in terms of biomass. The findings were contrary to some studies, which reported that green and yellow light led to a reduction of biomass in fungi (Velmurugan et al. 2010; Soumya et al. 2014; Wang et al. 2016a, b). One of the possible reasons for the contradicting results observed in this study could be that different fungal species were used. Light responses in fungi are mostly species-dependent, as sensitivity to light depends on the photoreceptors (Tisch and Schmoll 2010; Hosseinpour et al. 2017). Our study also revealed that cultures incubated under white fluorescent light produced greater biomass than those in the dark. In fact, white fluorescent light is a full spectrum light source that covers the majority of the visible colours and wavelengths (approximately 400 to 700 nm) (Cheng et al. 2012; Fuller et al. 2015). Hence, it could be postulated that the effects of white fluorescent light could have triggered a response to monochromatic light or the combined effects of several monochromatic light, depending on the sensitivity of the photoreceptors (Cheng et al. 2012). Estrada and Avalos (2009) further supported this hypothesis, where exposure to white fluorescent light was found to regulate the expression of opsA (a green light photoreceptor encoding gene) in Fusarium fujikuroi.
Influence of light on enzymes
In this study, it was revealed that light can be a crucial factor in regulating enzyme production in fungi. Results showed that blue light positively regulated the production of amylase by isolate PH22 and L-asparaginase activities by isolates EF13, PH22, and EF5. Zhu and Wang (2005) have also reported the effects of blue light on amylase production. In their study, glucoamylase activities increased two-fold in Aspergillus niger cultured under blue light compared to those incubated in the dark. Illumination with blue light has also influenced the light-oxygen-voltage (LOV) domain photoreceptors in Bacillus subtilis. The LOV domain photoreceptors respond to blue light by enhancing glucose release, resulting in higher amylase production (Zhang et al. 2017; Elumalai et al. 2019). Although the effectiveness of blue light in regulating amylase production in fungi is not well understood compared to bacteria, several LOV domain photoreceptors have been found in fungi. These include VIVID (VVD), White Collar-1 (WC-1), and Envoy-1 (ENV-1) that are found to be well-conserved in several endophytic fungi (Purschwitz et al. 2006; Lokhandwala et al. 2015). This suggested that fungi may adopt similar mechanisms as bacteria in enhancing amylase production. This was further supported by several studies that proved that exposure to light could alter the glucose metabolism in fungi (Hill 1976; Schrenkenbach et al. 1981; Cetz-Chel et al. 2016; Zhang et al. 2017).
Blue light was also able to induce the production of L-asparaginase. This discovery is relatively novel as L-asparaginase has not been documented for its production under light regulation. In fact, among the four enzymes studied, L-asparaginase was the least studied compared to amylase, cellulase, and xylanase. Nevertheless, this study has revealed that blue light effectively regulated the production of L-asparaginase for most isolates. The interaction mechanisms under light illumination for L-asparaginase remains to be explored. Preliminary comparisons with light-fungal studies have revealed that light treatments usually regulate the expression of enzymes by controlling the cyclic adenosine monophosphate (cAMP) levels in the fungi via photoreceptors or carbon catabolite proteins (Casas-Flores et al. 2006). Rozalska and Mikuchi (1992) reported that L-asparaginase production was highly regulated with the addition of cAMP. Thus, it is hypothesized that high cAMP levels upon exposure to blue light may have favoured the production of L-asparaginase. The increased levels of L-asparaginase due to exposure to blue light may also be related to inhibitory effects on glucose uptake (Schrenkenbach et al. 1981). The accumulated glucose becomes an excellent substrate to increase the production of L-asparaginase (Abdel-Raou et al. 2019; Yap et al. 2021).
Blue light was, however, less effective in influencing the production of cellulase and xylanase activities. For both cellulase and xylanase, green light was more effective. A similar outcome was observed in the study conducted by Araujo et al. (2021), in which the production of both cellulase and xylanase in Pleurotus spp. were significantly enhanced under the presence of green light. The regulation of green light on both cellulase and xylanase activities could be an indirect cascading effect from the activation of green light photoreceptor, a type of G-protein coupled receptors involved in the formation of cAMP (Brown 2004; Fuller et al. 2015). Several studies have found that there is a correlation between high cAMP concentration with high xylanase and cellulase production (Morosoli et al. 1989; Kulkarni et al. 1999; Nogueira et al. 2015). Green light was, however, less effective in inducing amylase and L-asparaginase production. The exact mechanism of light regulation for amylase production remains to be studied. In the study of Pawlik et al. (2019), the uptakes of carbon sources in Cerrena unicolor were greatly reduced under irradiation of green light. Hence, it can be postulated that the green light exposure could inhibit the glucose uptake in fungi, consequently leading to suppressed production of amylase to avoid overproduction of glucose.
The other light treatments, i.e. yellow and red lights, have contrasting influences on enzyme production. Yellow light is the least-studied light spectrum among the various light spectrum, but several studies do exist. Velmurugan et al. (2010) reported that with yellow light irradiation, both Monascus purpureus and Penicillum purpurogenum produced the lowest pigment yield compared to cultures incubated under other light spectrums (blue, red, green, white fluorescent light, and in darkness). On the contrary, yellow light seemed to elicit amylase and L-asparaginase production in isolates used in this study, suggesting that yellow light could be used to regulate the production of the respective enzymes. The effect of yellow light was also reported by Hosseinpour et al. (2017), who observed increase of biomass (0.135 g) and melanin production (0.98 absorbance) of Exophiala crusticola. Additionally, photoreceptors in fungi that are sensitive toward yellow light are still not known. As such, it is difficult to hypothesize how yellow light triggers the changes in enzyme production as light responses are strongly regulated by the photoreceptors in fungi. Similarly, red light resulted in the least enzymatic activities for amylase and L-asparaginase. The association between red light and enzyme production in fungi is also not well studied. However, it can be postulated that reducing enzymatic activities could be due to high sporulation induced by red light. Hill (1976) reported that the uptake of nutrients and substrates would be reduced as growth is retarded during the sporulation period. Consequently, the hydrolytic enzymes may be suppressed to limit the degradation of the substrates (Tisch and Schmoll 2010). Betina and Zajacova (1978) found that the conidiation of Trichoderma viride was limited when the glucose level was high. This hypothesis may be supported by the findings in this study in which sporulation of most of the isolates was strongly enhanced by irradiation of red light, and this may have conversely affected the enzyme production.
Comparing between white fluorescent light and dark incubation conditions, incubation under white fluorescent light elicited the production of cellulase, xylanase, and L-asparaginase, at a more superior level than incubation in the dark condition, further supporting the hypothesis that light is crucial for enzyme production. This supports the study by Stappler et al. (2017) that light-dependent genes responsible for cellulase production in Trichoderma reesei were positively regulated upon activation of photoreceptor BLR1 and BLR2 coupled with the G-protein pathway. In addition, several studies have also proven that the regulation of xylanase genes could be light-dependent (Gyalai-Korpos et al. 2010; Beier et al. 2020). Therefore, it is highly suggestive that white fluorescent light may be better than incubation in the dark to induce the production of certain enzymes.
Conclusion
This study has revealed that different light spectra elicited different response and a varying degree of influence on the growth and enzymatic activities of endolichenic fungi (Pseudopestalotiopsis theae (EF13), Fusarium solani (EF5), and Xylaria venustula (PH22). It was revealed that red light is effective in promoting sporulation, while yellow light induced positive fungal growth (enhanced biomass). Blue light is highly recommended to induce the production of amylase by isolate PH22 and L-asparaginase by isolates EF13, PH22, and EF5, while green light was more efficient to induce production of xylanase by isolates EF13, PH22, and EF5. Exposure to the specific light spectra can therefore be used as a tool to optimize growth and production of valuable enzymes.
Data Availability
Data can be made available by the authors upon reasonable request.
References
Abdel-Raou M, Aldeweik HM, Elbendary EY (2019) Impact of physical and chemical factors on L-asparaginase enzyme purified from soil bacteria. J Biol Sc 20:48–55
Adesina FC, Onilude AA (2013) Isolation, identification and screening of xylanase and glucanase-producing microfungi from degrading wood in Nigeria. Afr J Agric Res 8:4414–4421
Araujo NL, Avelino KV, Halabura MIW, Marim RA, Kassem ASS, Linde GA, Colauto NB, Doo-Valle JS (2021) Use of green light to improve the production of lignocellulose-decay enzymes by Pleurotus spp. in liquid cultivation. Enzyme Microb Technol 149:109860. https://doi.org/10.1016/j.enzmictec.2021.109860
Beier S, Hinterdobler W, Bazafkan H, Schillinger L, Schmoll M (2020) CLR1 and CLR2 are light dependent regulators of xylanase and pectinase genes in Trichoderma reesei. Fungal Genet Biol 136:103315. https://doi.org/10.1016/j.fgb.2019.103315
Betina V, Zajacova J (1978) Regulation of periodicity and intensity of photo-induced conidiation of Trichoderma viride. Folia Microbiol 23:453–459
Bhattacharyya PN, Jha DK (2011) Optimization of cultural conditions affecting growth and improved bioactive metabolites production by a subsurface Aspergillus strain TSF 146. Int J Appl Biol Pharm 2:133–143
Bhunjun CS, Phillips AJL, Jayawardena RS, Promputtha I, Hyde KD (2021) Importance of molecular data to identify fungal plant pathogens and guidelines for pathogenicity testing based on Koch’s postulates. Pathogens 10:1096. https://doi.org/10.3390/pathogens10091096
Blumenstein A, Vienken K, Tasler R, Purschwitz J, Veith D, Frankenberg-Dinkel N, Fischer R (2005) The Aspergillus nidulans phytochromes FphA represses sexual development in red light. Curr Biol 15:1833–1838
Brown LS (2004) Fungal rhodopsins and opsin-related proteins: eukaryotic homologues of bacteriorhodopsin with unknown functions. Photochem Photobiol Sci 3:555–565
Cai MH, Fang Z, Niu CP, Zhou XS, Zhang YX (2013) Light regulation on growth, development and secondary metabolism of marine-derived filamentous fungi. Folia Microbiol 58:537–546
Calvo AM, Wilson RA, Bok JW, Keller NP (2002) Relationship between secondary metabolism and fungal development. Microbiol Mol Biol Rev 66:447–459
Campbell MA, Medd RW, Brown JB (2003) Optimizing conditions for growth and sporulation of Pyrenophora semeniperda. Plant Pathol 52:448–454
Canessa P, Schumacher J, Hevia MA, Tudzynski P, Larrondo LF (2013) Assessing the effects of light on differentiation and virulence of the plant pathogen Botrytis cinerea: characterization of the white-collar complex. PLOS 8:e84223. https://doi.org/10.1371/journal.pone.0084223
Casas-Flores S, Rios-Momberg M, Rosales-Saavedra T, Martinez-Hernandez P, Olmedo-Monfil V, Herrera-Estrella A (2006) Cross-talk between a fungal blue light perception system and the cyclic AMP signalling pathway. Eukaryot Cell 5:499–506
Cetz-Chel JE, Balcazar-Lopez D, Esquivel-Naranjo EU, Herrera-Estrella A (2016) The Trichoderma atroviride putative transcription factor Blu7 controls light responsiveness and tolerance. BMC Genom 17:e2016. https://doi.org/10.1186/s12864-016-2639-9
Chen HC, Dunlap JC, Loros JL (2010) Neurospora illuminates fungal photoreception. Fungal Genet Biol 47:922–929
Cheng CW, Chen CK, Chang CJ, Chen LY (2012) Effect of colour LEDs on mycelia growth of Aspergillus ficuum and phytase production in photo-fermentations. J Photochem Photobiol B 106:81–86
Chow YY, Ting ASY (2015) Endophytic L-asparaginase-producing fungi from plants associated with anticancer properties. J Adv Res 56:869–876
Ding G, Li Y, Fu SB, Liu SC, Wei JC, Che YS (2009) Ambuic acid and torreyanic acid derivatives from the endolichenic fungus Pestalotiopsis sp. J Nat Prod 72:182–186
Elumalai P, Lim JM, Park YJ, Cho M, Shea PJ, Oh BT (2019) Enhanced amylase production by a Bacillus subtilis strain under blue light emitting diodes. Prep Biochem Biotech 49:143–150
Estrada AF, Avalos J (2009) Regulation and targeted mutation of opsA, coding for the nop-1 opsin orthologue in Fusarium fujukuroi. J Mol Biol 387:59–73
Farkas V, Nemcovic M (2001) Cell wall composition and polysaccharide synthase change following photoinduction in Trichoderma viride. Acta Bot Hung 52:281–288
Farris MH, Ford KA, Doyle RC (2016) Qualitative and quantitative assays for detection and characterization of protein antimicrobials. J Vis Exp 110:e53819. https://doi.org/10.3791/53819
Franco DL, Canessa P, Bellora N, Risau-Gusman S, Olivares-Yanes C, Perez-Lara R, Libkind D, Larrondo LF, Marpegan L (2017) Spontaneous circadian rhythms in a cold-adapted natural isolate of Aureobasidium pullalans. Sci Rep 7:13812–13837
Fuller KK, Loros JJ, Dunlap JC (2015) Fungal photobiology: visible light as a signal for stress, space and time. Curr Genet 61:275–288
Gan PT, Ting ASY (2019) Light mediation as a strategy to induce production of valuable microbial compounds. In: Microbial Technology for the Welfare of Society, Springer Singapore, Singapore, pp 101–123
Gyalai-Korpos M, Nagy G, Mareczky Z, Schuster A, Reczey K, Schmoll M (2010) Relevance of the light signalling machinery of cellulase expression in Trichoderma reesei (hypocrea jecorina). BMC Res Notes 3:330. https://doi.org/10.1186/1756-0500-3-330
Ha SY, Jung JY, Park JH, Lee DH, Choi JW, Yang JK (2020) Effect of light-emitting diodes on cordycepin production in submerged Cordyceps militaris cultures. J Mushroom 18:10–19
Hill EP (1976) Effect of light on growth and sporulation of Aspergillus ornatus. J Gen Microbiol 95:39–44
Hosseinpour L, Zareei M, Boroujeni ZB, Yaghoubi R, Hashemi SJ (2017) Effect of different incubation temperatures, times, and colored lights on fungal biomass and black pigment (melanin) production in Exophiala crusticola. Infect Epidemiol Microbiol 3:90–95
Igbalajobi O, Yu ZZ, Fischer R (2019) Red and blue light sensing in the plant pathogen Alternaria alternata depends on phytochrome and the white collar protein LreA. Appl Environ Microbiol 10:e00371-e419. https://doi.org/10.1128/mBio.00371-19
Jain P, Gupta S (2012) Effect of carbon and nitrogen sources on antimicrobial metabolite production of endophytic fungus Penicillium sp. against human pathogen. J Pharm Res 5:4325–4328
Kannagara BTSDP, Rajapaksha RSCG, Paranagama PA (2009) Nature and bioactive of endolichenic fungi in Pseudocyphellaria sp, Parmotrema sp. and Usnea sp. at Hakgala montane forest in Sri Lanka. Lett Appl Microbiol 48:203–209
Keller NO, Nesbitt C, Sarr B, Phillips TD, Burow GB (1997) pH regulation of sterigmatocystin and aflatoxin biosynthesis in Aspergillus spp. Phytopathology 87:643–648
Kellogg JJ, Raja HA (2016) Endolichenic fungi: a new source of rich bioactive secondary metabolites on the horizon. Phytochem Rev 16:271–293
Kulkarni N, Shendye A, Rao M (1999) Molecular and biotechnological aspects of xylanases. FEMS Microbiol Rev 23:411–456
Lokhandwala J, Hopkins HC, Rodriguez-Iglesias A, Dattenbock C, Schmoll M, Zoltowski BD (2015) Structural biochemistry of a fungal LOV domain photoreceptor reveals an evolutionarily conserved pathway integrating light and oxidative stress. Structure 23:116–125
Maqsood A, Rehman A, Ahmad I, Nafees M, Ashraf I, Qureshi R, Jamil I, Rafay M, Hussain T (2014) Physiological attributes of fungi associated with stem end rot of mango (Mangifera indica L.) cultivars in postharvest fruit losses. Pak J Bot 46:1915–1920
Martinez-Soto D, Ruiz-Herrera J (2017) Functional analysis of the MAPK pathways in fungi. Rev Iberoam Micol 34:192–202
Miyake T, Mori A, Kii T, Okuno T, Usui Y, Sato F, Sammoto H, Watanabe A, Kariyama M (2005) Light effects on cell development and secondary metabolism in Monascus. J Ind Microbiol Biotechnol 32:103–108
Mooney JL, Yager LN (1990) Light is required for conidiation in Aspergillus nidulans. Genes Dev 4:1473–1482
Morosoli R, Durand S, Boucher F (1989) Stimulation of xylanase synthesis in Cryptococcus albidus by cyclic AMP. FEMS Microbiol Lett 57:57–60
Murthy PS, Suzuki S, Kusumoto KI (2015) Effect of light on the growth and acid protease production of Aspergillus oryzae. Food Sci Technol 21:631–635
Nielsen JC, Nielsen J (2017) Development of fungal cell factories for the production of secondary metabolites: linking genomics and metabolism. Synth Syst Biotechnol 2:5–12
Nogueira KMV, Costa MDN, Paule RGD, Mendonca-Natividade FC, Ricci-Azevedo R, Silva RN (2015) Evidence of cAMP involvement in cellobiohydrolase expression and secretion by Trichoderma reesei in presence of the inducer sophorose. BMC Microbiol 15:195. https://doi.org/10.1186/s12866-015-0536-z
Paranagama PA, Wijeratne EMK, Burn AM, Marron MT, Gunatilaka MK, Arnold AE, Gunatilaka AAL (2007) Heptaketides from Corynespora sp. inhabiting cavern beard lichen, Usnea cavernosa: first report of metabolites of and endolichenic fungus. J Nat Prod 70:1700–1705
Pawlik A, Ruminowicz-Stefaniuk M, Frac M, Mazur A, Wielbo J, Janusz G (2019) The wood decay fungus Cerrena unicolor adjusts its metabolism to grow on various types of wood and light conditions. Plos One 14:e0211744. https://doi.org/10.1371/journal.pone.0211744
Purschwitz J, Muller S, Kastner C, Fischer R (2006) Seeing the rainbow: light sensing in fungi. Curr Opin Microbiol 9:566–571
Rozalska M, Mikucki J (1992) Staphylococcal L-asparaginase: catabolic repression of synthesis. Pol J Microbiol 41:145–150
Santiago KAA, Dela Cruz TEED, Ting ASY (2021) Diversity and bioactivity of endolichenic fungi in Usnea lichens of the Philippines. Czech Mycol 73:1–19
Schreckenbach T, Walckhoff B, Verfuerth C (1981) Blue light receptor in a white mutant of Physarum polycephalum mediates inhibition of spherulation and regulation of glucose metabolism. PNAS 78:1009–1013
Schumacher J (2017) How light affects the life of Botrytis. Fungal Genet Biol 106:26–41
Sekiguchi J, Gaucher M (1977) Conidiogenesis and secondary metabolism in Penicillum urticae. Appl Environ Microbiol 33:147–158
Soumya K, Swathi L, Sreelatha GL, Sharmila T (2014) Light influences pigment, biomass and morphology in Chaetomium cupreum- SS02- a photoresponse study. Int J Curr Microbiol Appl Sci 3:53–64
Sridevi B, Charya MAS (2011) Isolation, identification and screening of potential cellulase-free xylanase producing fungi. Afr J Biotechnol 10:4624–4630
Stappler E, Dattenbock C, Tisch D, Schmoll M (2017) Analysis of light and carbon specific transcriptomes implicates a class of G-protein-coupled receptors in cellulose sensing. mSphere 2:e00089–17. https://doi.org/10.1128/mSphere.00089-17
Sunitha VH, Ramesha A, Savitha J, Srinivas C (2012) Amylase production by endophytic fungi Cylindocephalum sp. isolated from medicinal plant Alpinia calcarate. Braz J Micriobiol 43:1213–1221
Takano Y, Kikuchi T, Kubo Y, Hamer JE, Mise K, Furusawa I (2000) The Colletotrichum lagenarium MAP kinase gene CMK1 regulates diverse aspects of fungal pathogenesis. Mol Plant Microbe Interact 13:374–383
Tan KK, Epton AH (1973) Effect of light on the growth and sporulation of Botrytis cinerea. Trans Br Mycol Soc 61:145–157
Tisch D, Schmoll M (2010) Light regulation of metabolic pathway in fungi. Appl Microbiol 85:1259–1277
Velmurugan P, Lee YH, Venil CK, Perumalsamy L, Chae JC, Oh BT (2010) Effect of light on growth, intracellular and extracellular pigment production by five pigment-producing filamentous fungi in synthetic medium. J Biosci Bioeng 109:346–350
Wang LL, Dai Y, Chen WP, Shao YC, Chen FS (2016a) Effects of light intensity and color on the biomass, extracellular red pigments, and citrinin production of Monascus ruber. J Agric Food Chem 64:9506–9514
Wang Z, Li N, Li JG, Dunlap JC, Trail F, Townsend JP (2016b) The fast-evolving phy-2 gene modulates sexual development in response to light in the model fungus Neurospora crassa. mBio 7:e02148. https://doi.org/10.1128/mBio.02148-15
Yap LS, Lee WL, Ting ASY (2021) Optimization of L-asparaginase production from endophytic Fusarium proliferatum using OFAT and RSM and its cytotoxic evaluation. J Microbiol Methods 191:e106358. https://doi.org/10.1016/j.mimet.2021.106358
Yu ZZ, Fischer R (2019) Light sensing and responses in fungi. Nat Rev 17:25–36
Zhang XX, Gao YY, Yin Y, Cai MH, Zhou XS, Zhang YX (2017) Regulation of different polyketide biosynthesis by green light in an endophytic fungus of mangrove leaf. 3 Biotech 7:363. https://doi.org/10.1007/s13205-017-0996-y
Zhu JC, Wang XJ (2005) Effect of blue light on conidiation development and glucoamylase enhancement in Aspergillus niger. Wei Sheng Wu Xue Bao 45:275–278
Funding
The authors extend their gratitude to the Monash University Malaysia for the funding and facilities provided to complete the study.
Author information
Authors and Affiliations
Contributions
Material preparation, data collection, and analysis were performed by Gan Peck Ting. The first draft of the manuscript was written by Gan Peck Ting and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gan, P.T., Lim, Y.Y. & Ting, A.S.Y. Influence of light regulation on growth and enzyme production in rare endolichenic fungi. Folia Microbiol 68, 741–755 (2023). https://doi.org/10.1007/s12223-023-01050-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12223-023-01050-2