Abstract
In this study, silver@graphene oxide nanocomposites (Ag@GO NCs) derived from extracts of Antarctic lichens Usnea antarctica and Umbilicaria antarctica were synthesized and their antimicrobial activity against to fish pathogen bacteria were evaluated. According to the results of characterization test, spherical Ag NPs were well dispersed on the surface of graphene oxide. While the diameters of Usnea antartica extract-based NCs were observed at an average of 28 nm, the diameters of Umbilica antartica extract-based NCs were found 40 nm. The absorption points of Usnea antartica and Umbilicaria antartica extract-based Ag@GO NCs were detected at 445 nm. The highly stable structures of NCs synthesized with both lichen extracts were exhibited with zeta potential. In addition, functional groups that play a role in the synthesis were revealed by FT-IR analysis and its crystal structure was revealed by XRD analysis. The lichen-based NCs have excellent antimicrobial activity against to Staphylococcus aureus, Aeromonas hydrophila, Pseudomonas aeruginosa, and Yersinia ruckeri strains. As a result, we suggest that Ag@GO NCs were synthesized with both lichen extracts by an effective, inexpensive, and eco-friendly method and are applicable for antimicrobial activity studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introductıon
There are a lot of studies available in the literature that nanoparticles (NPs) have unique properties compared to bulk forms as an advantage of the surface–volume relationship (Khan et al. 2019). Due to their different structural, physical, and chemical properties, nanoparticles have attracted the attention of researchers in terms of synthesis process and industrial applications. With the biological synthesis method developed as an alternative to the physical and chemical synthesis process of nanoparticles, nanoparticles are synthesized through biomaterials such as various plant (Hassan et al. 2022), seaweed (Princy and Gopinath 2021; González-Ballesteros et al. 2018), and fungi (Kaplan et al. 2021; Bafghi et al. 2021) extracts.
With the developments in the field of biotechnology, silver-containing nanostructures are important in the industrial field with their unique physical, chemical, and biological properties. Among the metallic nanoparticles (mNPs), silver nanoparticles (Ag NPs) (Akintelu et al. 2021; Yaqoob et al. 2020) have the potential to be used in many applications. Due to its widely use in the industry, the need to synthesize Ag NPs with a cheap, effective, and eco-friendly method has emerged, and studies have been carried out on the biosynthesis of Ag NPs and their application areas. Nazari et al. (2020) reported that nanomaterials synthesized by biological method have lower toxicity compared to chemical synthesized nanostructures. The potential of use of Ag NPs synthesized by various bio-extracts for antioxidant (Turunc et al. 2021), wound healing (Kaplan et al. 2021), anticancer (Wang et al. 2021), catalytic, (Princy and Gopinath 2021), and sensor (Zamarchi and Vieira 2021) applications has been reported. The most interesting properties of biosynthesized Ag NPs are their antimicrobial activity and many studies have been performed for this area (Vanlalveni et al. 2021; Jadoun et al. 2021).
The most interesting properties of biosynthesized Ag NPs are their antimicrobial activity and many studies have been done for this purpose. For instance, minimum inhibition concentration (MIC) of Ag NPs synthesized using Punica granatum peel extract against Staphylococcus aureus, Bacillus subtilius, Escherichia coli, and Pseudomonas aeruginosa strains were determined at 50, 43, 38, and 44 µg/ml, respectively (Dut Jasuja et al. 2014). In another study, the MIC of Rosmarinus officinalis leaf extract-based Ag NPs against S. aureus, B. subtilis, E. coli, and Pseudomonas aeruginosa strains were recorded at 773, 1546, 193, and 386 µg/ml, respectively (Ghaedi et al. 2015). Moteriya and Chanda (2020) emphasized that Caesalpinia pulcherrima-based Ag NPs exhibit highly effective antimicrobial activity against B. subtilis, B. cereus, S. aureus, Corallium rubrus, E. coli, P. aeruginosa, Klebsiella pneumoniae, Candida albicans, and C. glabrata.
Besides the advantages it has, Ag NPs have disadvantages such as their tendency to agglomerate and low stabilization properties. To eliminate this disadvantageous situations, studies for the immobilization and applications of free NPs to the substrate such as graphene oxide (GO) continue (Zhu et al. 2021, Ahmad et al.). GO, biocompatible, is a graphene-derived 2-dimensional material that allows bonding to the structure of NPs through the reactive groups in its structure (Zhu et al. 2021, Ahmad et al.). Zhu et al. (2021) suggested that GO could be used to enrich the antimicrobial activities of Ag NPs. Ahamed et al. (2022) reported that Ag@GO nanocomposites (NCs) synthesized with orange peel extract showed twice anticancer activity against human lung cancer and breast cancer cells compared to free Ag NPs, and were also biocompatible with normal cells. Emima Jorensia et al. (2021) observed that Ag@GO NCs synthesized using Punica granatum peel extract had more effective antimicrobial activity than free Ag NPs. In addition, it has been documented that P. aeruginosa-based Ag@GO NCs have antimicrobial activity against E. coli (Potbhare et al. 2020), Syzgium cumini extract-based Ag@GO NCs against E. coli, P. aeruginosa, Bacillus sp., and Staphylococcus sp. strains (Thomas et al. 2020).
Lichens are symbiotic associations between fungi and green algae or cyanobacteria and they are very rich in chemical compounds. More than 700 secondary compounds are known in lichens and the great majority are found on medullary hyphae with relatively few in the cortices. Lichens produce various secondary compounds which often vary in response to environmental gradients (Swanson et al. 1996; Bjerke et al. 2004; Vatne et al. 2011), in particular in relation to solar radiation (Bjerke and Dahl 2002; McEvoy et al. 2006, 2007), and nitrogen availability (Solhaug and Gauslaa 2012). As they are the most dominant organisms in the terrestrial vegetation of Antarctica, lichens have abilities to cope with multiple environmental stresses, including low temperatures, desiccation, and high solar radiation exposure (Schroeter and Scheidegger 1995; Kappen 2000; Lud et al. 2001; Gautam et al. 2011). In this study, Ag@GO NCs were synthesized using the lichen samples belonging to Usnea antarctica and Umbilicaria antarctica collected from Antarctica and their antimicrobial activities against Staphylococcus aureus, Aeromonas hydrophila, Pseudomonas aeruginosa, and Yersinia ruckeri strains have been revealed. These two lichen species are very successfully adapted to survive in the harsh environmental conditions of Antarctica and they are also very common in the white continent. It is thought that the findings will be a guide for the application of biomaterials based from Antarctic lichens in biomedical fields.
2 Materials and methods
2.1 Collections of lichen samples
Lichen samples belonging to Usnea antarctica were collected on small pepples from James Ross Island located in the North east of Antarctic Peninsula. This species has maximum cover on the rocks of this island. The samples belonging to Umbilicaria antarctica were collected on big rocks from Galindez Island which is located in the west of Antarctic Peninsula. About 20 g of each lichens were collected in January 2017. The locations of these islands and the photographs of the lichens are provided in Fig. 1. After collecting the lichens, they were left for drying in an open area for 1 week where air circulance is present.
2.2 Green synthesis of Ag@GO nanocomposites and their characterization
Ag@GO NCs were synthesized by the method applied by Ocsoy and their study group with slight modification (Ocsoy et al. 2017). 10 g dried lichen samples were holded in deionized water (100 ml, 85 °C) and filtered. Ag NPs were obtained by mixing the lichen extract and Ag NO3 (5 × 10–3 M) in a magnetic stirrer at a ratio of 1:9 until a color change was observed. Then, Ag NP (2 ml) and lichen extract (2 ml) were added to GO (2 ml, 0.1 mg/ml), respectively. Single-layer GO (0.5–3 μm) was obtained from Graphene Supermarket (Ronkonkoma, NY). After about 5 min, NaCl solution (0.09 M, 2.4 ml) was added dropwise to the solution. After about 15 s, 5 ml of NaCl solution (0.29 M) was added dropwise and mixed for 30 min, AgNP/GO particles were separated by centrifugation (5 min at 3000 rpm) and then dried in an oven (1 night at 70 °C). Ag@GO NCs were characterized by evaluating their characteristic light absorption points, surface charges, morphology, crystal structures, and the presence of secondary metabolites in their structure. The sample is located at the interface to image diffuse electrons in the SEM and is commonly used for imaging lighter atoms in an electron microscope. A concentrated 50 µl Ag@GO aqueous solution is dripped onto the carbon tape-coated stub and dried overnight to obtain clear images. The resulting Ag@GO nanocomposites are used in ZEISS EVO LS10 SEM at an operating voltage of 25 kV to obtain SEM images. X-ray diffraction (XRD) is used to obtain information about the crystallographic structure or elemental composition of the materials obtained. Fourier transform infrared spectroscopy (FT-IR) is used to characterize the presence of adsorbates on the nanomaterial surface.
2.3 Antimicrobial activity of Ag@GO nanocomposites
Antimicrobial properties of Ag@GO NCs were determined by broth microdilution technique. Staphylococcus aureus, Aeromonas hydrophila, Pseudomonas aeruginosa, and Yersinia ruckeri strains were grown in Müeller Hinton broth and the microorganism density was adjusted to 0.5 McFarland. The medium and 100 µg/ml of the samples to be tested for activity were added to the wells, and finally, 5 µg/ml microorganisms were added to each well. In addition, 10 µg/ml was taken from each well and cultivated on tryptic soy agar quantitatively and the percent inhibition rates of the samples on microorganisms were determined (Ocsoy et al. 2017).
3 Results and discussion
3.1 Characterization of Ag@GO NCs
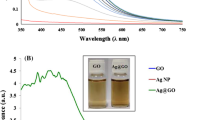
The characteristic light points of Ag@GO NCs synthesized using Umbilicaria antarctica and Usnea antarctica extract were detected at 445 nm (Fig. 2). The peak at 295 nm detected for Ag@GO NCs synthesized with both lichen extracts indicates the presence of GO. Zhu et al. (2021) noted the presence of Ag NPs in the NCs structure with the peak observed in NCs at 400 nm. Ahmad et al. (2021) reported the presence of GO and Ag NPs with surface plasmon resonance (SPR) vibrations observed at 300 and 420 nm, respectively. Similar to our findings, in another study, characteristic peaks of Ag@rGO NCs were determined at 264 and 425 nm (Wu et al. 2011). In previous studies, characteristic light absorption points of Ag NPs were determined in the range of 380–450 nm, depending on the morphology, diameter, and aggregation state of the NP (Princy and Gopinath 2021; Kaplan et al. 2021; Turunc et al. 2021; Wang et al. 2021). The data we obtained by UV characterization of Ag@GO NCs synthesized with lichen extracts are in agreement with the previous studies.
Zeta potential test is an important analysis applied to comment on the stability of nanomaterials based on the surface charge of nanomaterials. The surface charges of Umbilicaria antarctica-based Ag@GO NCs (Fig. 3) and Usnea antarctica-based Ag@GO NCs (Fig. 4) were detected at −28 and −31 mV, respectively. The negative surface charge of nanoparticles ensures stable structure by preventing aggregation of the biomaterial synthesized with the biocoating agent (Turunc et al. 2021). In previous studies, it has been reported that NPs with a surface charge lower than −25 mV are high stable (Heydari and Rashidipour 2015). In line with this information, it can be said that the secondary metabolites in both lichen extracts provide the synthesis of highly stable NCs.
With electron microscope images, it was determined that Usnea antarctica and Umbilicaria antarctica extract-based Ag NPs had an average size of 28 and 40 nm, respectively, and tended to agglomerate slightly (Figs. 5 and 6). Ag NPs synthesized with Fucus gardeneri extract were determined to be around 19 nm (Princy and Gopinath 2021). In another study, it was reported that the Ag NPs in the synthesized GO layers were around 40–50 nm, and the NP sizes increased relatively with increasing Ag concentration (Liu et al. 2017). As a result of the reaction of 1 and 10 mM AgNO3 with Catharanthus roseus extract, the average diameter of the Ag NPs observed in the GO layers was approximately 10.48 and 7 nm, respectively, while the Ag NPs formed as a result of the reaction with 100 mM AgNO3 were reported to be distributed in quite different sizes (Rohaizad et al. 2020). According to the literature review, the size of Ag NPs in biosynthesis differs according to the reduced metal concentration and the biological material from which the extract is obtained.
The presence of secondary metabolites that play a role in the reduction of metals and coating of NCs was determined by FT-IR analysis. FT-IR analyses of Umbilicaria antartica and Usnea antartica-based Ag@GO NCs are given in Figs. 7 and 8, respectively. Spectrums observed at 878, 1060, 1247, 1373, and 1694 cm−1 by FT-IR analysis of Ag@GO NCs synthesized with Umbilicaria antartica extract indicate the presence of carbonyl, alkoxy groups (CO), epoxy groups (C–H), C–O deformation, and aromatic rings (C=C), respectively. The peaks at 1987 and 2046 cm−1 correspond to aromatic compounds (C–H). Other components, such as O–H deformation, CH2, and –OH groups, were detected at 2895, 2972, and 3612 cm−1, respectively. Our findings are consistent with the previous studies (Sahu et al. 2015; Lorestani et al. 2015; Hooda and Sharma 2020). Absorption peaks of Usnea antartica extract-based Ag@GO NCs at 904, 1061, 1382, 2107, 2317, 2894, and 2964 cm−1 correspond to alken (C=C), alkoxy, C–O deformation, (C=O), (HCO3−), O–H deformation, and CH groups, respectively (Sahu et al. 2015; Lorestani et al. 2015; Hooda and Sharma 2020).
The crystal structure of Ag NPs synthesized with lichen extracts was determined by XRD analysis. The diffractions of Umbilicaria antarctica (Fig. 9) vs Usnea antarctica (Fig. 10) extract-based Ag NPs at 2 theta 28°, 32°, 38°, 44°,46°, 54°, 57° 64°, and 77° correspond to (2 1 0), (1 2 2), (1 1 1), (2 0 0), (2 3 1), (1 4 2), (2 4 1), (2 2 0), and (3 1 1) planes, respectively. This data consistent with planes of JCPDS, file No. 04-0783 (Meng 2015). With this analysis, the crystal structure of Ag NPs immobilized in GO was demonstrated.
3.2 Antimicrobial activity of Ag@GO NCs
It was observed that AgNP and Ag@GO NCs showed significant antimicrobial activity against S. aureus, Y. ruckeri, A. hydrophila, and P. aeruginosa strains (Table 1). The MIC values of Ag@GO NCs and Ag NPs synthesized with both lichen extracts against S. aureus strain were determined at 30 and 60 ppm, respectively, and Ag@GO NCs exhibited more effective antimicrobial activity than Ag NPs. While it was observed that Ag@GO NCs exhibited the highest antimicrobial activity against Y. ruckeri strain (30 ppm), Ag NPs synthesized with Usnea antartica extract exhibited the lowest activity (60 ppm). Ag@GO NCs synthesized with Usnea antartica extract exhibited the highest activity (15 ppm) against A. hydrophila, and it was noted that NPs and Umbilicaria antartica-based Ag@GO NCs exhibited similar levels (30 ppm) of antimicrobial activity. Ag@GO NCs synthesized with both lichen extracts have more effective antimicrobial properties than Ag NPs against the P. aeruginosa strain. According to our result, Ag@GO NCs had more effective antimicrobial activity than Ag NPs.
In a study where Ag@GO NC exhibited more effective antimicrobial activity against E. coli strains than Ag NP, researchers suggested that this was due to the nonspecific binding of bacteria to graphene (Liu et al. 2011). It has been reported that Ag@GO NCs synthesized with Punica granatum extract have more effective antimicrobial activity than Ag NPs synthesized with the same extract (Emima Jeronsia et al. 2021). In another study evaluating the antimicrobial activities of Ag NP and Ag@GO NCs synthesized from grape seed, it was found that the antimicrobial activity increased with increasing Ag concentration in the synthesis of Ag@GO NCs and Ag@GO NCs had greater antimicrobial activity compared to Ag NP (Liu et al. 2017). The researchers explained their data with Ag@GO NC’s destruction of the bacterial wall and oxidative damage caused by Ag ions. In the literature, the high antimicrobial activity of Ag GO NC compared to Ag NP has been explained: (1) the synergistic effect of GO layers and Ag ions; (2) the damage to the cell wall of the formed reactive oxygen species (ROS), and our opinion is in this direction as well (Ahmad et al. 2021; Ma et al. 2011; Wierzbicki et al. 2019).
4 Conclusion
First time in this study, Antarctic lichens were used for synthesis of graphene oxide-decorated Ag NCs synthesis. Ag NPs well dispersed on the graphene oxide surface were displayed by SEM images. It was noted that Usnea antarctica extract-based spherical Ag@NCs had an average diameter of 28 nm and a good stability (−31 mV) structure. It was observed that Umbilicaria antarctica extract-based Ag@GO NCs were 40 nm, and had good stability (−28 mV). Crystallinity of Ag@GO NCs and functional groups were detailed using XRD and FT-IR analyses, respectively. The rich antimicrobial activity of Ag@GO NC based on two Antarctic lichen extracts was explained by ROS. As a result, Ag@GO NCs with antimicrobial activity were synthesized by eco-friendly method (biosynthesis) to avoid the disadvantages of traditional (physical and chemical) synthesis methods.
Data availability
Data are available on request from the authors.
References
Ahamed M, Akhtar MJ, Khan MAM, Alhadlaq HA (2022) A novel green preparation of Ag/RGO nanocomposites with highly effective anticancer performance. Polymers 13:3350. https://doi.org/10.3390/polym13193350
Ahmad MA, Aslam S, Mustafa F, Arshad U (2021) Synergistic antibacterial activity of surfactant free Ag–GO nanocomposites. Sci Rep 11:196. https://doi.org/10.1038/s41598-020-80013-w
Akintelu SA, Olugbeko SC, Folorunso AS, Oyebamiji AK, Folorunso FA (2021) Potentials of phytosynthesized silver nanoparticles in biomedical fields: a review. Int Nano Lett 11:273–293. https://doi.org/10.1007/s40089-021-00341-1
Bafghi MH, Safdari H, Darroudi M, Sabouri Z, Zargar M, Zarrinfar H (2021) Evaluation and comparison of the effects of biosynthesized selenium and silver nanoparticles using plant extracts with antifungal drugs on the growth of Aspergillus and Candida species. Rend Lincei Sci Fis Nat 32:791–880. https://doi.org/10.1007/s12210-021-01021-0
Bjerke JW, Dahl T (2002) Distribution patterns of usnic acid-producing lichens along local radiation gradients in West Greenland. Nova Hedwigia 75:487–506. https://doi.org/10.1127/0029-5035/2002/0075-0487
Bjerke JW, Joly D, Nilsen L, Brossard T (2004) Spatial trends in usnic acid concentrations of the lichen Flavocetraria nivalis along local climatic gradients in the Arctic (Kongsfjorden, Svalbard). Polar Biol 27:409–417. https://doi.org/10.1007/s00300-004-0590-8
Dut Jasuja N, Kumar Gupta D, Reza M, Joshi SC (2014) Green Synthesis of AgNPs stabilized with biowaste and their antimicrobial activities. Braz J Microbiol 45:1325–1332. https://doi.org/10.1590/S1517-83822014000400024
Emima Jeronsia J, Ragu R, Jerline Mary A, Jerome Das S (2021) Elucidating the structural, anticancer, and antibacterial traits of Punica granatum peel extracts-mediated Ag and Ag/GO nanocomposites. Microsc Res Tech 85:44–55. https://doi.org/10.1002/jemt.23883
Gautam S, Singh J, Pant AB (2011) Effect of UV-B radiations on the pigments of two Antarctic lichens of Schirmacher Oasis, East Antarctica. Polar Res 32:279–287. https://doi.org/10.2478/v10183-011-0019-3
Ghaedi M, Yousefinejad M, Safarpoor M, Khafri HZ, Purkait MK (2015) Rosmarinus officinalis leaf extract mediated green synthesis of silver nanoparticles and investigation of its antimicrobial properties. J Ind Eng Chem 25:167–172. https://doi.org/10.1016/j.jiec.2015.06.020
González-Ballesteros N, González-Rodríguez JB, Rodríguez-Argüelles MC, Lastra M (2018) New application of two Antarctic macroalgae Palmaria decipiens and Desmarestia menziesii in the synthesis of gold and silver nanoparticles. Polar Sci 15:49–54. https://doi.org/10.1016/j.polar.2017.10.004
Hassan AMS, Mahmoud AS, Ramadan MF, Eissa MA (2022) Microwave-assisted green synthesis of silver nanoparticles using Annona squamosa peels extract: characterization, antioxidant, and amylase inhibition activities. Rend Fis Acc Lincei 33:83–91. https://doi.org/10.1007/s12210-022-01049-w
Heydari R, Rashidipour M (2015) Green synthesis of silver nanoparticles using extract of oak fruit hull (jaft): Synthesis and ın vitro cytotoxic effect on MCF-7 cells. Int J Breast Cancer 2015:846743. https://doi.org/10.1155/2015/846743
Hooda F, Sharma M (2020) Green synthesis, characterization and antibacterial activity of ıron oxide nanoparticles. Plant Arch 20:1196–1200
Jadoun S, Arif R, Jangid NK, Meena RK (2021) Green synthesis of nanoparticles using plant extracts: a review. Environ Chem Lett 19:355–374. https://doi.org/10.1007/s10311-020-01074-x
Kaplan Ö, Gökşen Tosun N, Özgür A, Erden Tayhan S, Bilgin S, Türkekul İ, Gökce İ (2021) Microwave-assisted green synthesis of silver nanoparticles using crude extracts of Boletus edulis and Coriolus versicolor: Characterization, anticancer, antimicrobial and wound healing activities. J Drug Deliv Sci Technol 64:102641. https://doi.org/10.1016/j.jddst.2021.102641
Kappen L (2000) Some aspects of the great success of lichens in Antarctica. Antarct Sci 12:314–324. https://doi.org/10.1017/S0954102000000377
Khan I, Saeed K, Khan I (2019) Nanoparticles: Properties, applications and toxicities. Arab J Chem 12:908–931. https://doi.org/10.1016/j.arabjc.2017.05.011
Liu L, Liu J, Wang Y, Yan X, Sun DD (2011) Facile synthesis of monodispersed silver nanoparticles on graphene oxide sheets with enhanced antibacterial activity. New J Chem 35:1418–1423. https://doi.org/10.1039/C1NJ20076C
Liu CC, Xu H, Wang L, Qin X (2017) Facile one-pot green synthesis and antibacterial activities of GO/Ag Nanocomposites. Acta Metal Sin-Engl 30:36–44. https://doi.org/10.1007/s40195-016-0517-8
Lorestani F, Shahnavaz Z, Alias MP, Y, Manan NSA, (2015) One-step hydrothermal green synthesis of silver nanoparticle-carbon nanotube reduced-graphene oxide composite and its application as hydrogen peroxide sensor. Sens Actuators B Chem 208:389–398. https://doi.org/10.1016/j.snb.2014.11.074
Lud D, Huiskes AHL, Moerdijk TCW, Rozema J (2001) The effects of altered levels of UV-B radiation on an Antarctic grass and lichen. İn: Rozema J, Manetas Y, Björn LO (eds) Responses of plants to UV-B radiation. Advances in vegetation science, Springer, Dordrecht. https://doi.org/10.1007/978-94-017-2892-8_9
Ma J, Zhang J, Xiong Z, Young Y, Zhao XS (2011) Preparation, characterization and antibacterial properties of silver-modified graphene oxide. J Mater Chem 21:3350–3352. https://doi.org/10.1039/C0JM02806A
McEvoy M, Nybakken L, Solhaug KA, Gauslaa Y (2006) UV triggers the synthesis of the widely distributed secondary compound usnic acid. Mycol Prog 5:221–229. https://doi.org/10.1007/s11557-006-0514-9
McEvoy M, Solhaug KA, Gauslaa Y (2007) Solar radiation screening in usnic acid containing cortices of the lichen Nephroma arcticum. Symbiosis 43:143–150
Meng Y (2015) A sustainable approach to fabricating Ag nanoparticles/PVA hybrid nanofiber and ıts catalytic activity. Nanomaterials 5:1124–1135. https://doi.org/10.3390/nano5021124
Moteriya P, Chanda S (2020) Green synthesis of silver nanoparticles from Caesalpinia pulcherrima leaf extract and evaluation of their antimicrobial, cytotoxic and genotoxic potential (3-in-1 system). J Inorg Organomet Polym 30:3920–3932. https://doi.org/10.1007/s10904-020-01532-7
Nazari F, Jafarirad S, Movafeghi A, Kosari-Nasab M, Mohajel Kazemi E (2020) Toxicity of microwave-synthesized silver reduced graphene oxide nanocomposites to the microalga Chlorella vulgaris: Comparison with the hydrothermal method synthesized counterparts. J Environ Sci Health Part A 55:639–649. https://doi.org/10.1080/10934529.2020.1726142
Ocsoy I, Temiz M, Celik C, Altinsoy B, Yilmaz V, Duman F (2017) A green approach for formation of silver nanoparticles on magnetic graphene oxide and highly effective antimicrobial activity and reusability. J Mol Liq 227:147–152. https://doi.org/10.1016/j.molliq.2016.12.015
Potbhare AK, Umekar MS, Chouke PB, Bagade MB, Tarik Aziz SK, Abdala AA, Chaudhary RG (2020) Bioinspired graphene-based silver nanoparticles: fabrication, characterization and antibacterial activity. Mater Today Proc 29:720–725. https://doi.org/10.1016/j.matpr.2020.04.212
Princy KF, Gopinath A (2021) Green synthesis of silver nanoparticles using polar seaweed Fucus gardeneri and its catalytic efficacy in the reduction of nitrophenol. Polar Sci 30:100692. https://doi.org/10.1016/j.polar.2021.100692
Rohaizad A, Shahabuddin S, Shahid MM, Rashid NM, Hir ZAM, Ramly MM, Awang K, Siong CW, Aspanut Z (2020) Green synthesis of silver nanoparticles from Catharanthus roseus dried bark extract deposited on graphene oxide for effective adsorption of methylene blue dye. J Environ Chem Eng 8:103955. https://doi.org/10.1016/j.jece.2020.103955
Sahu D, Sarkar N, Sahoo G, Mohapatra P, Swain SK (2015) Silver ımprinted graphene nanocomposites: synthesisis and morphological study. Appl Sci Adv Mat Int 1:224–227
Schroeter B, Scheidegger C (1995) Water relations in lichens at subzero temperatures: structural changes and carbon dioxide exchange in the lichen Umbilicaria aprina from continental Antarctica. New Phytol 131:273–285. https://doi.org/10.1111/j.1469-8137.1995.tb05729.x
Solhaug KA, Gauslaa Y (2012) Secondary lichen compounds as protection against excess solar radiation and herbivores. In: Lüttge U, Beyschlag W, Büdel B, Francis D (eds) Progress in botany 73. Springer, Berlin, Heidelberg, pp 283–304
Swanson A, Fahselt D, Smith D (1996) Phenolic levels in Umbilicaria americana in relation to enzyme polymorphism, altitude and sampling date. Lichenologist 28:331–339. https://doi.org/10.1006/lich.1996.0030
Thomas R, Unnikrishnan J, Nair AV, Daniel EC, Balachandran M (2020) Antibacterial performance of GO-Ag nanocomposite prepared via ecologically safe protocols. Appl Nanosci 10:4207–4219. https://doi.org/10.1007/s13204-020-01539-z
Turunc E, Kahraman O, Binzet R (2021) Green synthesis of silver nanoparticles using pollen extract: characterization, assessment of their electrochemical and antioxidant activities. Anal Biochem 621:114123. https://doi.org/10.1016/j.ab.2021.114123
Vanlalveni C, Lallianrawna S, Biswas A, Selvaraj M, Changmai B, Rokhum SL (2021) Green synthesis of silver nanoparticles using plant extracts and their antimicrobial activities: a review of recent literature. RSC Adv 11:2804–2837. https://doi.org/10.1039/D0RA09941D
Vatne S, Asplund J, Gauslaa Y (2011) Contents of carbon based defence compounds in the old forest lichen Lobaria pulmonaria vary along environmental gradients. Fungal Ecol 4:350–355. https://doi.org/10.1016/j.funeco.2011.03.007
Wang Y, Chinnathambi O, Nasif O, Ali A (2021) Green synthesis and chemical characterization of a novel anti-human pancreatic cancer supplement by silver nanoparticles containing Zingiber officinale leaf aqueous extract. Arab J Chem 14:103081. https://doi.org/10.1016/j.arabjc.2021.103081
Wierzbicki M, Jaworski S, Sawosz E, Jung A, Gielerak G, Jaremek H, Łojkowski W, Woźniak B, Stobiński L, Małolepszy A, Chwalibog A (2019) Graphene oxide in a composite with silver nanoparticles reduces the fibroblast and endothelial cell cytotoxicity of an antibacterial nanoplatform. Nanoscale Res Lett 14:320. https://doi.org/10.1186/s11671-019-3166-9
Wu T, Liu S, Luo Y, Lu W, Wang L, Sun X (2011) Surface plasmon resonance-induced visible light photocatalytic reduction of graphene oxide: Using Ag nanoparticles as a plasmonic photocatalyst. Nanoscale 3:2142–2144. https://doi.org/10.1039/C1NR10128E
Yaqoob AA, Umar K, Ibrahim MNM (2020) Silver nanoparticles: various methods of synthesis, size affecting factors and their potential applications–a review. Appl Nanosci 10:1369–1378. https://doi.org/10.1007/s13204-020-01318-w
Zamarchi F, Vieira IC (2021) Determination of paracetamol using a sensor based on green synthesis of silver nanoparticles in plant extractJ. Pharm Biomed Anal 196:113912. https://doi.org/10.1016/j.jpba.2021.113912
Zhu J, Ni H, Hu C, Zhu Y, Cai J, Liu S, Gao J, Yang H, Liu H (2021) Rapid synthesis and characterization of silver-loaded graphene oxide nanomaterials and their antibacterial applications. R Soc Open Sci 8:201744. https://doi.org/10.1098/rsos.201744
Acknowledgements
This study is supported by TÜBİTAK 118Z587 coded project.
Author information
Authors and Affiliations
Contributions
F.D.K. ssynthesized and characterized nanoparticles, wrote the main manuscript, prepared figures M.G.H. collected and obtained extract of lichen samples, wrote the main manuscript AD. synthesized and characterized nanoparticles, study antimicrobial activity, wrote the main manuscript İÖ. synthesized and characterized nanoparticles All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no competing interests exist.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Koca, F.D., Demırbas, A., Halıcı, M.G. et al. Bio-synthesis of graphene oxide-decorated silver nanocomposites using Usnea antarctica and Umbilicaria antarctica from Antarctic Peninsula and evaluation of their antimicrobial activities. Rend. Fis. Acc. Lincei 35, 513–522 (2024). https://doi.org/10.1007/s12210-024-01246-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12210-024-01246-9